Small arteries and arterioles constrict in response to increases in intraluminal pressure through a process called “myogenic tone” or the “myogenic response” (McCarron et al., 1989). Development of myogenic tone has profound physiological implications; it contributes substantially to regulation of systemic blood pressure, maintenance of capillary/tissue perfusion, and autoregulation of blood flow to specific organs, such as kidneys and brain (Davis, 2012). Thus, understanding the mechanisms that govern the myogenic response is germane to our understanding of the cardiovascular system as a whole. The current thinking is that myogenic tone is regulated by a “push/pull” relationship between vasoconstrictor and vasodilator mechanisms, both of which are regulated through focal increases in intracellular Ca2+ concentration within cellular microdomains (Hill-Eubanks et al., 2011). The interaction and proximity of these two vasoregulatory pathways are critical to the regulation of myogenic tone and proper vascular function. However, even with the breadth and extent of investigation into the myogenic response, a definitive model of the structure, localization, and composition of these cellular-signaling domains remains elusive.
Karlin (2015) describes a new model of an arterial smooth muscle cell that, unlike previous models (Bennett et al., 2005; Kapela et al., 2008), incorporates five interconnected Ca2+ compartments and 37 separate protein components into two distinct microdomains. When tested, the proposed model accurately recapitulated the changes in membrane potential and intracellular Ca2+ observed experimentally in response to increases in intraluminal pressure. Moreover, when used to simulate pressure-independent responses to vasoconstrictors (ATP, α-adrenergic agonists) and vasodilators (nitric oxide, epoxyeicosatrienoic acids, β-adrenergic agonists), this model continued to accurately represent the changes in smooth muscle cell membrane potential and intracellular Ca2+ recorded experimentally under the same conditions.
Mechanisms of pressure-induced dilation and constriction
Of the mechanisms that regulate the development of myogenic tone in smooth muscle, the best defined is the major vasodilatory pathway that opposes myogenic constriction. This pathway involves large conductance Ca2+-activated K+ (BK) channels and voltage-dependent Ca2+ channels (VDCCs) in the plasma membrane, which are closely aligned with one another and with ryanodine receptors (RyRs) on the sarcoplasmic reticulum (Brayden and Nelson, 1992; Nelson and Quayle, 1995; Nelson et al., 1995; Jaggar et al., 2000). Intravascular pressure-induced membrane depolarization increases the open probability of VDCCs, thereby elevating Ca2+ influx and intracellular Ca2+ concentration (Nelson et al., 1990; Rubart et al., 1996; Knot and Nelson, 1998). This increase in intracellular Ca2+ leads to an increase in the activity of clusters of RyRs in the adjacent sarcoplasmic reticulum, causing a transient focal increase in Ca2+ known as a Ca2+ spark (Nelson et al., 1995). Ca2+ sparks, in turn, activate nearby BK channels to cause membrane hyperpolarization and closure of VDCCs, which ultimately leads to vasodilation (Jaggar et al., 2000).
The upstream molecular players in the pathway leading to myogenic constriction are less well characterized, but it is generally accepted that increases in intraluminal pressure leads to the activation of cation channels in smooth muscle cells to cause membrane depolarization. Evidence collected to date suggests that the most likely candidates for these cation channels are members of the transient receptor potential (TRP) superfamily (Welsh et al., 2002). One such channel is TRPM4, a member of the melastatin subfamily of TRP channels that primarily conducts Na+, and as such causes membrane depolarization and subsequent Ca2+ influx through VDCCs (Earley and Brayden, 2015). However, other mechanosensitive TRP channels present in smooth muscle could also locally increase cytosolic Ca2+ concentrations in response to stretch (Bulley et al., 2012). Whether resulting from the opening of VDCCs or of TRP channels, localized increases in Ca2+ serve as part of a feed-forward mechanism that opens adjacent Ca2+-activated Cl− channels (CaCCs) to augment membrane depolarization, Ca2+ influx, global intracellular Ca2+ concentration, and vasoconstriction (Jaggar et al., 2000; Bulley et al., 2012).
Ca2+-signaling microdomains and Ca2+ sensitivity
Strict regulation of global intracellular Ca2+ is of the utmost importance for the controlled development of myogenic tone because a relatively small change in Ca2+ concentration (from <100 to ∼400 nM global Ca2+) is sufficient to span the range from complete dilation to complete constriction (Knot and Nelson, 1998; Hill-Eubanks et al., 2011). As such, complex regulatory mechanisms orchestrate local Ca2+ dynamics within the smooth muscle cell to control both contractile and relaxant mechanisms. A logical way to achieve this regulation is to arrange the requisite component proteins into spatially distinct contractile and relaxant microdomains, which confine and insulate the necessary molecular players within an extremely limited space. In this arrangement, localized increases in intracellular Ca2+ can be high enough to activate pro-contractile and pro-relaxant mechanisms separately, without causing a short circuit between these mechanisms or increasing global cytosolic Ca2+ to an extent that could be deleterious to cell viability (Hill-Eubanks et al., 2011). Spatial constraints imposed within these microdomains also help impart signal fidelity and temporal control over the development of myogenic tone (Parekh, 2008).
However, microdomain proximity and component density are not the sole determinants of myogenic responsiveness, nor are they capable of controlling intracellular Ca2+ concentrations over such a narrow range. Rather, differences in the Ca2+ sensitivity of individual microdomain elements combine with microdomain arrangement to impart the resolution and precision necessary to control myogenic tone. The component with the highest sensitivity to Ca2+ is the CaCC, which is activated by high nanomolar to low micromolar concentrations of Ca2+ and thereby elicits membrane depolarization in some types of smooth muscle (Hartzell et al., 2005). At local Ca2+ concentrations of 1–5 µM, RyRs on the adjacent sarcoplasmic reticular membrane open; the resultant Ca2+ spark can raise local Ca2+ to a concentration high enough (1–100 µM) to activate BK channels at physiological membrane potentials (−40 mV) and hyperpolarize the cell (Nelson et al., 1995; Jaggar et al., 2000; Pérez et al., 2001). The combined proximity and differential Ca2+ sensitivity of these individual elements, as well as the separation of contractile and relaxant microdomains, leads to extremely strict regulation of Ca2+ influx through VDCCs and subsequent changes in global cytosolic Ca2+ (Fig. 1).
Figure 1.
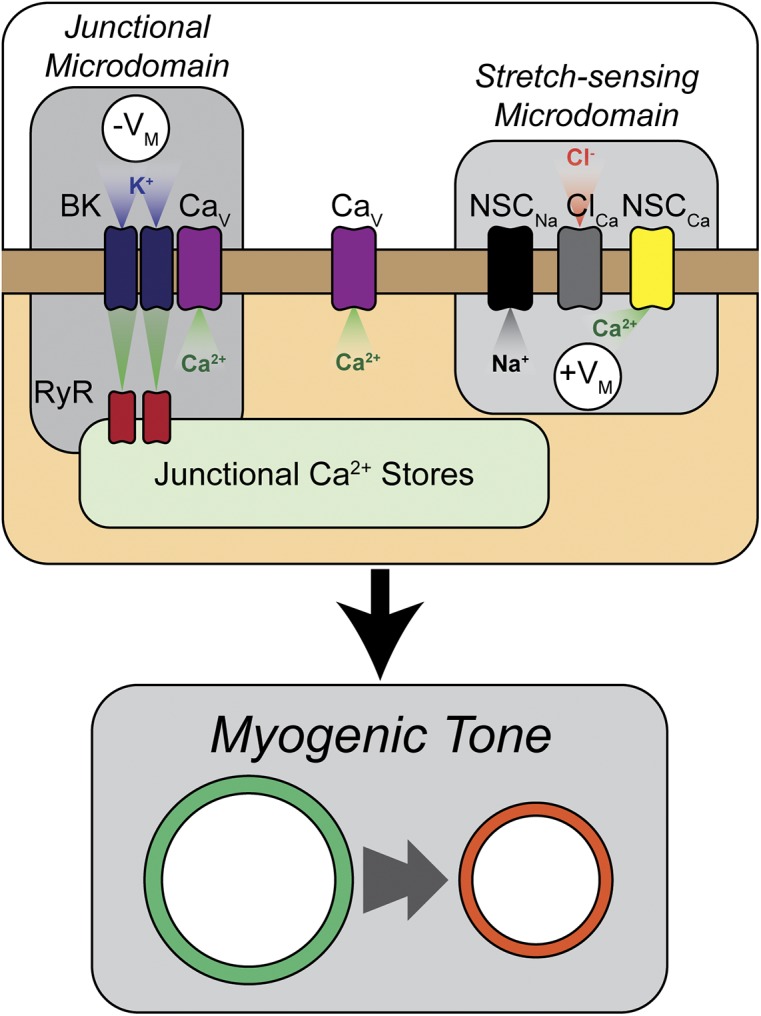
Arrangement of microdomains within a theoretical smooth muscle cell regulates myogenic tone. As modeled by Karlin (2015), myogenic tone development is regulated by the crosstalk between pro-contractile and pro-relaxant mechanisms that exist in close proximity within the smooth muscle cell. In the correct spatial arrangement, nonselective cation channels in stretch-sensing microdomains on the cell wall respond to deformation caused by increases in intraluminal pressure, allowing influx of Na+ (NSCNa) and Ca2+ (NSCCa). This leads to CaCC (ClCa) opening, depolarization of the cell membrane (+VM), and opening of VDCCs (CaV). In close proximity to stretch-sensing microdomains are pro-relaxant microdomains, formed at the interface between the junctional sarcoplasmic reticulum and the plasma membrane and containing RyRs and BK channels (BK). Increases in cytosolic Ca2+ cause RyR-mediated Ca2+ sparks that open BK channels to hyperpolarize the cell (−VM).
Modeling myogenic responses in vascular smooth muscle cells
The model presented by Karlin (2015) is novel in its consideration of the individual Ca2+ dynamics within each microdomain, while simultaneously calculating the effects of the interaction between microdomains. This framework accurately modeled the changes in both intracellular Ca2+ and membrane potential in response to increases in intraluminal pressure (Knot and Nelson, 1998). It also accurately simulated the effects of BK channel and RyR blockade determined experimentally in pressurized arteries (Knot et al., 1998), suggesting that the model provides a reasonable representation of the relative contributions of these two channels. The Karlin model makes some interesting predictions regarding the nature of CaCCs and TRP channels, suggesting that these two entities must be in close proximity to one another to affect membrane potential without inadvertently increasing global cytosolic Ca2+ to a level capable of causing maximal contraction. It also suggests that the Na+/Ca2+ exchanger, regardless of mode or location, has little or no effect on short-term myogenic responses in vascular smooth muscle cells.
As with all models, the information generated from the simulation are only as precise as the data used to generate the simulation itself. Given the large number of components included in this new model (many of which lack experimentally verified biophysical parameters), several assumptions had to be made about the identity and nature of some key components that potentially affect the model’s accuracy. This is especially true with regard to the exact identity and regulation of stretch- and receptor-activated nonselective cation channels. Although they are assumed by Karlin to be members of the TRPM and TRPC subfamilies, respectively, concrete experimental evidence as to the exact nature and arrangement of these channels is still lacking (Earley and Brayden, 2015). Because of this gap in knowledge, Karlin astutely models these nonspecific cation currents in the broadest possible sense, in an attempt to maintain the robustness of the simulation while still allowing for future adaptation and clarification as new experimental evidence arises. Nonetheless, this experimental ambiguity is part of the challenge of modeling smooth muscle cell behavior, particularly as it relates to certain situations, such as predicting membrane potential and intracellular Ca2+ during VDCC blockade.
Overall, the model presented by Karlin (2015) represents a major step forward, as it is the first to accurately represent myogenic tone development in vascular smooth muscle. Furthermore, this model improves our understanding of intracellular Ca2+ microdomain–signaling dynamics as regulators of myogenic and agonist-induced vascular reactivity by enabling “experimental” manipulations that are either currently impossible or cost prohibitive. Given its reliability and robustness, Karlin’s model represents a valuable new tool for testing the potential arrangement and composition of Ca2+-signaling microdomains in a single smooth muscle cell, helping to direct and focus future physiological investigations into vascular smooth muscle cell function.
Acknowledgments
This work was supported by grants to M.T. Nelson from the National Institute of Diabetes and Digestive and Kidney Diseases (R37 DK-053832); National Heart, Lung, and Blood Institute (R01 HL121706 and PO1 HL-095488); Fondation Leducq; and the Totman Medical Research Trust. N.R. Tykocki was also supported by the National Heart, Lung, and Blood Institute (T32 HL007647-24) and the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK-103840-01A1).
The authors declare no competing financial interests.
Elizabeth M. Adler served as editor.
References
- Bennett M.R., Farnell L., and Gibson W.G.. 2005. A quantitative description of the contraction of blood vessels following the release of noradrenaline from sympathetic varicosities. J. Theor. Biol. 234:107–122. 10.1016/j.jtbi.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Brayden J.E., and Nelson M.T.. 1992. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 256:532–535. 10.1126/science.1373909 [DOI] [PubMed] [Google Scholar]
- Bulley S., Neeb Z.P., Burris S.K., Bannister J.P., Thomas-Gatewood C.M., Jangsangthong W., and Jaggar J.H.. 2012. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ. Res. 111:1027–1036. 10.1161/CIRCRESAHA.112.277145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.J. 2012. Perspective: Physiological role(s) of the vascular myogenic response. Microcirculation. 19:99–114. 10.1111/j.1549-8719.2011.00131.x [DOI] [PubMed] [Google Scholar]
- Earley S., and Brayden J.E.. 2015. Transient receptor potential channels in the vasculature. Physiol. Rev. 95:645–690. 10.1152/physrev.00026.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C., Putzier I., and Arreola J.. 2005. Calcium-activated chloride channels. Annu. Rev. Physiol. 67:719–758. 10.1146/annurev.physiol.67.032003.154341 [DOI] [PubMed] [Google Scholar]
- Hill-Eubanks D.C., Werner M.E., Heppner T.J., and Nelson M.T.. 2011. Calcium signaling in smooth muscle. Cold Spring Harb. Perspect. Biol. 3:a004549 10.1101/cshperspect.a004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar J.H., Porter V.A., Lederer W.J., and Nelson M.T.. 2000. Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278:C235–C256. [DOI] [PubMed] [Google Scholar]
- Kapela A., Bezerianos A., and Tsoukias N.M.. 2008. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: Agonist and NO stimulation. J. Theor. Biol. 253:238–260. 10.1016/j.jtbi.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Karlin A. 2015. Membrane potential and Ca2+ concentration dependence on pressure and vasoactive agents in arterial smooth muscle: A model. J. Gen. Physiol. 146:79–96. 10.1085/jgp.201511380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot H.J., and Nelson M.T.. 1998. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. 508:199–209. 10.1111/j.1469-7793.1998.199br.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot H.J., Standen N.B., and Nelson M.T.. 1998. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J. Physiol. 508:211–221. 10.1111/j.1469-7793.1998.211br.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron J.G., Osol G., and Halpern W.. 1989. Myogenic responses are independent of the endothelium in rat pressurized posterior cerebral arteries. Blood Vessels. 26:315–319. [DOI] [PubMed] [Google Scholar]
- Nelson M.T., and Quayle J.M.. 1995. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 268:C799–C822. [DOI] [PubMed] [Google Scholar]
- Nelson M.T., Patlak J.B., Worley J.F., and Standen N.B.. 1990. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 259:C3–C18. [DOI] [PubMed] [Google Scholar]
- Nelson M.T., Cheng H., Rubart M., Santana L.F., Bonev A.D., Knot H.J., and Lederer W.J.. 1995. Relaxation of arterial smooth muscle by calcium sparks. Science. 270:633–637. 10.1126/science.270.5236.633 [DOI] [PubMed] [Google Scholar]
- Parekh A.B. 2008. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J. Physiol. 586:3043–3054. 10.1113/jphysiol.2008.153460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez G.J., Bonev A.D., and Nelson M.T.. 2001. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am. J. Physiol. Cell Physiol. 281:C1769–C1775. [DOI] [PubMed] [Google Scholar]
- Rubart M., Patlak J.B., and Nelson M.T.. 1996. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J. Gen. Physiol. 107:459–472. 10.1085/jgp.107.4.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D.G., Morielli A.D., Nelson M.T., and Brayden J.E.. 2002. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 90:248–250. 10.1161/hh0302.105662 [DOI] [PubMed] [Google Scholar]


