All known K+ channels use the same molecular architecture to achieve K+ selectivity. Selectivity filters, even from distantly related K+ channels, appear nearly identical in atomic resolution crystal structures; remarkably, the deviation in the coordinates of K+-coordinating backbone carbonyls is less than the uncertainty in the models themselves (Zhou et al., 2001; Jiang et al., 2002; Nishida and MacKinnon, 2002; Long et al., 2007; Brohawn et al., 2012). Although retaining an exquisite selectivity for K+, this highly conserved filter allows passage of a significant flux of ions. The large conductance calcium-activated K+ channel (also known as Big Potassium, BK, Maxi-K, KCNMA1, and Slo1) has a single-channel conductance near 300 pS at physiological K+ concentrations, or >500 pS in saturating [K+] (Brelidze and Magleby, 2004). Most other K+ channels have markedly lower conductances, and none of the mammalian voltage-gated (Kv) family of K+ channels has a conductance approaching that of BK (Gutman et al., 2005). Given the extreme conservation of the selectivity filter structure among K+ channels, it is likely that the selectivity filters of all K+ channels are capable of such flux. In fact, numerous point mutations distal to the selectivity filter can increase the unitary conductance of Kv channels (Lopez et al., 1994; Sukhareva et al., 2003). It would appear that nature has governed the conductance of these channels, muffling their thunderous flux. In this issue, Díaz-Franulic et al. in David Naranjo’s laboratory systematically assess how physical and chemical determinants outside of the selectivity filter govern conductance of a Kv channel.
The article “Pore dimensions and the role of occupancy in unitary conductance of Shaker K channels” addresses a fundamental biophysical question: What physical features of Shaker’s inner cavity limit ionic flux?
To investigate this question, the authors measured unitary conductance while using three biochemical perturbations: adding sugar, adding K+, and electrostatically charging the channel’s inner cavity.
Sugar
Díaz-Franulic et al. (2015) measured the effects of sucrose-induced viscosity changes on channel conductance. Increasing sucrose concentration enables measurement of the resistance of different segments in the permeation path (Brelidze and Magleby, 2005). This perturbation slows the diffusion of K+, essentially by getting in their way, such that diffusion is retarded. The effect of sucrose on diffusion manifests in bulk solution, but not in narrow cavities of the ion-permeation path that the large carbohydrate molecules cannot access. By deconvolving the effects of sucrose on the unitary conductance of Shaker channels, the authors were able to calculate the channel’s inner pore conductance, independent of resistance from the solution leading up to pore entrances.
K+
Increasing [K+] increases the conductance of the Shaker channel (Heginbotham and MacKinnon, 1993; Moscoso et al., 2012). This is because K+-binding sites in the permeation path are not fully occupied at physiological K+ concentrations. At higher concentrations, K+ can saturate sites in the inner cytoplasmic cavity of the channel’s pore, alleviating limitations of ion throughput resulting from low site occupancy. Measurement of single-channel currents in increasing concentrations of K+ can identify the impact of K+ site occupancy on maximal conductance. Díaz-Franulic et al. use this technique to distinguish site occupancy from ion throughput by raising [K+] to high levels during each perturbation.
Charge
To address whether the electrostatics of the inner cavity limit conductance, Díaz-Franulic et al. mutated the inner cavity. Their overriding theory is that the hydrophobic residues of the inner cavity create a suboptimal environment for hydrated K+ ions, and this damps K+ flux. To make the inner cavity more cationophilic, they replaced residues in and near the cavity with aspartate, which is expected to be negatively charged at neutral pH. In turn, this anionic density is expected to increase K+ occupancy of the inner cavity by electrostatic attraction. As a result of the fourfold symmetry of the channel, each aspartate addition creates a charged ring. In BK, such charged rings of glutamates increase channel conductance (Brelidze et al., 2003; Carvacho et al., 2008). In bacterial MthK channels, rings of glutamates in the internal cavity increase conductance and K+ occupancy in the cavity (Shi et al., 2011). Moreover, electrophysiological measurements from the Naranjo laboratory suggest that insertion of an aspartate in Shaker’s internal cavity increases occupancy by K+ (Moscoso et al., 2012). In the current report, the authors measured single-channel conductance properties of Shaker with aspartates inserted over a wider region of the S6 transmembrane helix. They replaced four residues with aspartate, individually and in combinations (Figs. 1 and 2). These perturbations of viscosity, K+-binding site saturation, and channel electrostatics enabled dissection of the physical details of Shaker’s permeation path. Measurements of unitary conductance over a wide range of voltages laid the foundation for three interesting conclusions:
Figure 1.

Sequence alignment of S6 regions of K+ channels discussed herein. Residues that increase unitary conductance when mutated to aspartate are colored red. Acidic residues that decrease unitary conductance when neutralized are gray.
Figure 2.
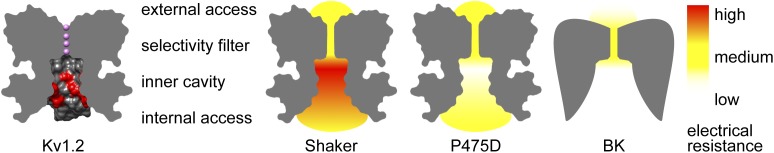
Illustration of K+ channel regions contributing to electrical resistance. (Far left) Vertical slice through the axis of symmetry of the Kv1.2 pore (Protein Data Bank accession no. 2R9R), with surface rendering of the inner cavity. Residues mutated to aspartate by Díaz-Franulic et al. (2015) are colored red; K+ is colored violet. The relative electrical resistance of permeation path segments in Shaker wild-type, Shaker P475D, and BK channels is schematized from left to right. Coloring crudely reflects the resistances reported by Díaz-Franulic et al.
(1) The dominating resistance of Shaker’s K+-permeation path is that of the inner cavity.
The inner pore of the Shaker channel has a resistance of 30 GΩ in 100 mM K+. However, in the high conductance P475D mutant, inner pore resistance dropped to 3 GΩ. Residue 475 is the constriction point that marks the intracellular end of the inner cavity. In light of its spatial isolation from the selectivity filter, the authors assumed that mutation at position 475 would not affect the conductance properties at the selectivity filter, and interpreted their measurements accordingly. They constructed a functional model of the Shaker permeation path as a series of resistors. This formalism revealed that there is a sucrose-inaccessible region of Shaker’s inner cavity that is at least 10-fold higher resistance than the selectivity filter in the wild-type channel. Thus, passing the inner cavity is the rate-limiting bottleneck for ions moving through the Shaker Kv channel (Fig. 2).
(2) Charge insertion can reduce inner cavity resistance to trivial values.
The unitary conductances measured after inserting aspartate residues at locations in and near the inner cavity indicate that resistance can be readily minimized. By inserting combinations of aspartate residues, the authors found that, although all insertions tested increased Shaker’s conductance, combinations of aspartates increased conductance only marginally. This suggests that the high resistance of the inner cavity can be reduced until it is no longer rate limiting for the flux of K+ ions. They found that the maximal conductance that can be achieved by inserting charge into the inner cavity plateaus at ∼200 pS. This is 10-fold larger than Shaker’s 20-pS conductance in physiological K+ concentrations.
(3) The hydrodynamic radius of a K+ ion entering the inner cavity is 3.8–4.1 Å.
Díaz-Franulic et al. devised a clever technique to measure the functionally relevant average hydrodynamic radius of K+ ions as they enter the internal mouth of the Shaker channel. As worked out previously with the BK channel (Brelidze and Magleby, 2005), measurements with high sucrose solutions can distinguish resistance in the channel pore from resistance encountered by ions approaching the pore. By comparing conductance with and without sucrose, the authors calculated a radius of capture of K+ ions: how much bigger the entrance of the cytoplasmic cavity is than a hydrated K+ ion. By assuming that the entrance to the cavity of an open Shaker channel will match the crystal structure of its mammalian homologue Kv1.2, they estimated the functional radius of K+ ions entering the pore. They concluded that the effective radius of the entering ion is 3.8–4.1Å, consistent with K+ retaining its first hydration shell of eight waters.
Electric field changes in Shaker’s inner cavity
If the relation between resistance and voltage drop is ohmic throughout the K+-permeation path, the segmental resistances reported by Díaz-Franulic et al. indicate that the voltage spanning Shaker’s inner cavity would be 10 times greater than the selectivity filter. Empirical measurements indicate that K+ or tetraethylammonium ions traverse <20% of the transmembrane electric field to reach their binding sites in Shaker’s inner cavity (Yellen et al., 1991; Choi et al., 1993; Thompson and Begenisich, 2001). It remains to be seen how the low voltage dependence of these ions can be reconciled with the high resistance of the inner cavity.
Comparison to BK
No matter how they mutated or cajoled Shaker, its currents never became quite as large as BK’s. Díaz-Franulic et al.’s model of Shaker conductance suggests that resistance in other regions becomes limiting once the inner cavity resistance becomes negligible. The backbone architecture of Kv channels may not be able to sustain as high a conductance as BK channels. Shaker’s calculated radius of K+ capture is smaller than BK’s, suggesting that the entrance to the internal cavity of BK channels is much wider than that of Kv channels (Brelidze and Magleby, 2005; Geng et al., 2011). Despite the size differences, it appears that physical features that contribute to the large conductance of BK channels can also enhance Shaker’s conductance. Careful work has revealed many of the structural features of the mouse BK channel, mSlo, that enable its ionic gusher. Key among these findings is that negatively charged rings formed by acidic residues near the inner cavity increase local ion concentration (Brelidze et al., 2003). When these charged rings are neutralized in BK, single-channel current decreases. Díaz-Franulic et al. found that when charged rings of aspartate residues were inserted at similar locations in Shaker, its conductance was raised by a similar mechanism, by increasing local [K+] around the inner mouth of the pore. Although the inner pore entrance to Kv channels is smaller than that of BK, entry and passage of ions through their inner cavities appear to be governed by similar chemistry.
Side-chain chemistry keeps Shaker’s currents small
A fascinating observation is that Kv channels limit the size of their unitary conductance. Díaz-Franulic et al. showed clearly that it is not only the size of the inner cavity that limits Shaker’s unitary conductance but also a lack of negative charge in this region. Their model explains why the conduction of Shaker channels is so small: the side-chain chemistry of the inner vestibule creates resistance an order of magnitude greater than that of any other part of the conduction pathway. Intriguingly, the conservation of uncharged amino-acid residues in the inner cavity of Kv channels suggests that evolutionary pressure keeps their conductance small (for a comparison of unitary conductance and S6 sequence see Moscoso et al., 2012, Table S1). Energy and chemical resources are required to express each individual ion channel. Yet nature has chosen to synthesize and maintain multiple small conductance Kv channels, rather than one large conductance channel capable of achieving the same total K+ flux. Given that dramatically larger conductance is physically achievable, and readily manifest with even a single-point mutation, it is notable that no eukaryotic Kv channel has a large unitary conductance.
Why have Kv channels evolved to be small?
There are at least two classes of plausible answers to this question:
(1) Large unitary conductance could be problematic in a cellular context.
Larger unitary conductance results in larger current variance as a channel is opened and closed. Perhaps a smaller conductance is advantageous in minimizing the current variance resulting from stochastic gating of Kv channels. Additionally, larger K+ conductance could result in undesirable changes in local K+ concentration. Electrophysiological consequences such as these could lead to evolutionary pressure to limit the conductance of Kv, but not BK, channels. Although stimulating to speculate about, testing these hypotheses is a daunting prospect, with little certainty that satisfying conclusions could be reached.
(2) Mutations that permit a large unitary conductance could have side effects irreconcilable with Kv gating.
Most, if not all, of the mutations that increase conductance of Kv channels produce additional changes in channel function. Increasing the negative charge density in Shaker’s inner cavity makes channels more susceptible to block by internal cations (Carvacho et al., 2008; Moscoso et al., 2012). These mutations also produce striking changes in gating (Sukhareva et al., 2003). It may be possible for evolution to compensate for any side effects of unitary conductance increase with further mutation of Kv channels. Yet it is notable that the hydrophobic residues at the internal cavity entrance that limit K+ conductance also form the constriction point of the voltage gate in Kv channels.
Inner cavity conformational changes are distinct in Shaker versus BK channels
Accumulating evidence indicates that the dynamics of the inner cavity are qualitatively different in Kv and BK channels. Open-channel blockers of Kv channels do not have the same open-channel dependence in BK channels (Wilkens and Aldrich, 2006; Tang et al., 2009). BK channels do not appear to couple gating to the same cytoplasmic portion of the S6 domain as Kv channels. Cysteine accessibility studies indicate that residues, which in the Shaker channel are inaccessible in the closed state (Liu et al., 1997), are accessible in closed BK channels (Zhou et al., 2011). All large conductance mutations studied by Díaz-Franulic et al. perturbed the gating equilibrium, favoring open states of the channel, with some charge insertions eliminating the ability of voltage to hold the channel closed (Hackos et al., 2002). Aspartates inserted into homologous positions in the BK inner cavity had different effects on gating. For example, the P475D mutation in Shaker lowered the resistance of the inner cavity and prevented the channel from staying closed at any voltage. The equivalent mutation in human BK, P320D, alters channel gating but relatively mildly, and the channel can still be closed by voltage (Chen et al., 2014). It could be that conformational changes unique to BK’s voltage-gating process allow channel gating to be compatible with large conductance, whereas the S6 gate of eukaryotic Kv channels relegates them to smallness.
Why the conductance of Kv channels is limited will likely remain grounds for speculation and commentary. Keeping with a tradition of hard-nosed JGP articles, the work of Díaz-Franulic et al. focuses on the physical underpinnings of electrophysiological phenomena. They provide an objective, firm foundation to the hypothesis that the inner cavity of the Shaker channel limits its conductance. With these solid empirical findings, our understanding of what governs the permeability of ion channels advances another step forward.
Acknowledgments
We thank Rod MacKinnon for helpful discussion and Kenton Swartz for critical reading.
D.C. Tilley received support from National Institutes of Health training grant T32HL086350.
The authors declare no competing financial interests.
Elizabeth M. Adler served as editor.
References
- Brelidze T.I., and Magleby K.L.. 2004. Protons block BK channels by competitive inhibition with K+ and contribute to the limits of unitary currents at high voltages. J. Gen. Physiol. 123:305–319. 10.1085/jgp.200308951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., and Magleby K.L.. 2005. Probing the geometry of the inner vestibule of BK channels with sugars. J. Gen. Physiol. 126:105–121. 10.1085/jgp.200509286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., Niu X., and Magleby K.L.. 2003. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc. Natl. Acad. Sci. USA. 100:9017–9022. 10.1073/pnas.1532257100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn S.G., del Mármol J., and MacKinnon R.. 2012. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 335:436–441. 10.1126/science.1213808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho I., Gonzalez W., Torres Y.P., Brauchi S., Alvarez O., Gonzalez-Nilo F.D., and Latorre R.. 2008. Intrinsic electrostatic potential in the BK channel pore: Role in determining single channel conductance and block. J. Gen. Physiol. 131:147–161. 10.1085/jgp.200709862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yan J., and Aldrich R.W.. 2014. BK channel opening involves side-chain reorientation of multiple deep-pore residues. Proc. Natl. Acad. Sci. USA. 111:E79–E88. 10.1073/pnas.1321697111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.L., Mossman C., Aubé J., and Yellen G.. 1993. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 10:533–541. 10.1016/0896-6273(93)90340-W [DOI] [PubMed] [Google Scholar]
- Díaz-Franulic I., Sepúlveda R.V., Navarro-Quezada N., González-Nilo F., and Naranjo D.. 2015. Pore dimensions and the role of occupancy in unitary conductance of Shaker K channels. J. Gen. Physiol. 146:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Niu X., and Magleby K.L.. 2011. Low resistance, large dimension entrance to the inner cavity of BK channels determined by changing side-chain volume. J. Gen. Physiol. 137:533–548. 10.1085/jgp.201110616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G.A., Chandy K.G., Grissmer S., Lazdunski M., McKinnon D., Pardo L.A., Robertson G.A., Rudy B., Sanguinetti M.C., Stühmer W., and Wang X.. 2005. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 57:473–508. 10.1124/pr.57.4.10 [DOI] [PubMed] [Google Scholar]
- Hackos D.H., Chang T.H., and Swartz K.J.. 2002. Scanning the intracellular S6 activation gate in the shaker K+ channel. J. Gen. Physiol. 119:521–532. 10.1085/jgp.20028569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., and MacKinnon R.. 1993. Conduction properties of the cloned Shaker K+ channel. Biophys. J. 65:2089–2096. 10.1016/S0006-3495(93)81244-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., and MacKinnon R.. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. 10.1038/417515a [DOI] [PubMed] [Google Scholar]
- Liu Y., Holmgren M., Jurman M.E., and Yellen G.. 1997. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 19:175–184. 10.1016/S0896-6273(00)80357-8 [DOI] [PubMed] [Google Scholar]
- Long S.B., Tao X., Campbell E.B., and MacKinnon R.. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 450:376–382. 10.1038/nature06265 [DOI] [PubMed] [Google Scholar]
- Lopez G.A., Jan Y.N., and Jan L.Y.. 1994. Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 367:179–182. 10.1038/367179a0 [DOI] [PubMed] [Google Scholar]
- Moscoso C., Vergara-Jaque A., Márquez-Miranda V., Sepúlveda R.V., Valencia I., Díaz-Franulic I., González-Nilo F., and Naranjo D.. 2012. K+ conduction and Mg2+ blockade in a shaker Kv-channel single point mutant with an unusually high conductance. Biophys. J. 103:1198–1207. 10.1016/j.bpj.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., and MacKinnon R.. 2002. Structural basis of inward rectification: Cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell. 111:957–965. 10.1016/S0092-8674(02)01227-8 [DOI] [PubMed] [Google Scholar]
- Shi N., Zeng W., Ye S., Li Y., and Jiang Y.. 2011. Crucial points within the pore as determinants of K+ channel conductance and gating. J. Mol. Biol. 411:27–35. 10.1016/j.jmb.2011.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhareva M., Hackos D.H., and Swartz K.J.. 2003. Constitutive activation of the Shaker Kv channel. J. Gen. Physiol. 122:541–556. 10.1085/jgp.200308905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Y., Zeng X.H., and Lingle C.J.. 2009. Closed-channel block of BK potassium channels by bbTBA requires partial activation. J. Gen. Physiol. 134:409–436. 10.1085/jgp.200910251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., and Begenisich T.. 2001. Affinity and location of an internal K+ ion binding site in shaker K channels. J. Gen. Physiol. 117:373–384. 10.1085/jgp.117.5.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens C.M., and Aldrich R.W.. 2006. State-independent block of BK channels by an intracellular quaternary ammonium. J. Gen. Physiol. 128:347–364. 10.1085/jgp.200609579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Jurman M.E., Abramson T., and MacKinnon R.. 1991. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 251:939–942. 10.1126/science.2000494 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Morais-Cabral J.H., Kaufman A., and MacKinnon R.. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. 10.1038/35102009 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Xia X.M., and Lingle C.J.. 2011. Cysteine scanning and modification reveal major differences between BK channels and Kv channels in the inner pore region. Proc. Natl. Acad. Sci. USA. 108:12161–12166. 10.1073/pnas.1104150108 [DOI] [PMC free article] [PubMed] [Google Scholar]


