Abstract
A number of investigators have suggested that exposure to low-dose radiation may pose a potentially serious health risk. However, the majority of these studies have focused on the short-term rather than long-term effects of exposure to fixed source radiation, and few have examined the effects of internal contamination. Additionally, very few studies have focused on exposure in juveniles, when organs are still developing and could be more sensitive to the toxic effects of radiation. To specifically address whether early-life radiation injury may affect long-term immune competence, we studied 14-day-old juvenile pups that were either 5 Gy total-body irradiated or injected internally with 50 μCi soluble 137Cs, then infected with influenza A virus at 26 weeks after exposure. After influenza infection, all groups demonstrated immediate weight loss. We found that externally irradiated, infected animals failed to recover weight relative to age-matched infected controls, but internally 137Cs contaminated and infected animals had a weight recovery with a similar rate and degree as controls. Externally and internally irradiated mice demonstrated reduced levels of club cell secretory protein (CCSP) message in their lungs after influenza infection. The externally irradiated group did not recover CCSP expression even at the two-week time point after infection. Although the antibody response and viral titers did not appear to be affected by either radiation modality, there was a slight increase in monocyte chemo-attractant protein (MCP)-1 expression in the lungs of externally irradiated animals 14 days after influenza infection, with increased cellular infiltration present. Notably, an increase in the number of regulatory T cells was seen in the mediastinal lymph nodes of irradiated mice relative to uninfected mice. These data confirm the hypothesis that early-life irradiation may have long-term consequences on the immune system, leading to an altered antiviral response.
INTRODUCTION
The events at Fukushima Daiichi continue to raise public concern about exposure to low-dose radiation. It is certainly true that exposure to high-dose radiation remains a significant health hazard as it can lead to damaging effects on precursor cell populations. Potential sources of irradiation include areas with high levels of naturally radioactive rocks and salts, medical therapeutic devices, nuclear power plant accidents and terrorist attacks. Exposure can also occur from either external or internal sources, the latter through inhalation or ingestion (1). However, the complexity and range of pathogenic outcomes related to radiation exposure have made it difficult to ascribe specific long-term effects from such exposures to later life morbidities in human populations. Thus, there is a need to develop animal models for risk assessment, as well as to enable the development of countermeasures against radiological damage (1, 2).
It is widely acknowledged that children are especially vulnerable to exposures from a variety of toxic insults as their organs and tissues are still developing (3, 4). Indeed, studies conducted on survivors of fallout from atomic bombs in Japan have revealed a chronic dysregulation in immune function (5). Our group has focused its recent research efforts on identifying the late effects of external irradiation on the adult and neonate lung and, specifically, the irradiated lung’s response to delayed immune challenge (6–8). We have shown that external radiation exposure of the adult lung alone leads to impaired lung function and increased susceptibility to influenza infection long after radiation exposure and that club cells, a putative stem cell population, are particularly affected in this model (7, 9, 10). Furthermore, data suggest that club cell secretory protein (CCSP) plays a role in recovery from such injury in later life (8). We have also reported that total-body irradiation of neonatal mice where all organ systems including the lung and hematopoietic systems are exposed, leads to increased morbidity and altered pulmonary immune response to later life infection with influenza virus (6). Given that regenerative cell populations, such as club cells, promulgate tissue repair, it is critical for us to understand how they are affected by radiation damage, especially during early development.
In the data reported here, we have extended our studies of neonatal animals to mice that were irradiated at day 14 of life, and describe morbidity results after influenza infection at 26 weeks after exposure. Of note, postnatal day 14 in mice corresponds to the timeframe of 6–8 years old in human development (11). In addition, since internal irradiation is of concern in areas where nuclear power plant disasters have led to inhalation or ingestion of contaminated foods, we also examined the effects of early internal radiation contamination on the late response to infection. We confirmed that external irradiation during the juvenile period has long-lasting implications for later life response to respiratory viral infections, whereas a less severe and differentially distinct immune response follows juvenile internal radiation contamination.
MATERIALS AND METHODS
Mice and Irradiation
All protocols involving animal work were reviewed and approved by the University of Rochester Medical Center (URMC) Institutional Animal Care and Use Committee. C57BL/6 mice were bred in the URMC animal vivarium using purchased breeder pairs (Jackson Laboratory, Bar Harbor, ME). All pups used in this study remained with their dam until 28 days of age. At 14 days old, mice were injected intraperitoneally with phosphate buffered saline (PBS) or 50 μCi of soluble 137Cs. Prior to injection, the acidic soluble 137Cs was neutralized with NaOH to a pH of approximately 7. Alternatively, the external treatment group was 5 Gy irradiated with fixed-source 137Cs at a rate of 1.7 Gy/min. All work performed with 137Cs-injected animals was performed under the supervision of the URMC Radiation Safety Unit. Of note, due to housing restrictions with respect to the internally contaminated animals, independent, age-matched control groups were established for the external versus internal studies.
Activity Measurements (μCi) of Mice Injected with Radioactive Cesium (137Cs)
Activity [in microCuries (μCi)] was measured in the mouse pups and their respective dams starting at 6 h post injection, continued daily for one week, then three times per week up to 18 days post injection. Mice were assayed using an Canberra InSpector™ 1000 portable multichannel analyzer (MCA) (Meriden, CT) with a sodium iodide probe; daily background and a source check were also performed with the MCA. The check source was a 20 gram liquid source in a glass vial containing 9.3 μCi of 137Cs. The counting chamber was shielded with approximately 2 inches of lead, with additional lead sheeting of approximately one-half inch thickness to reduce background scatter radiation. The probe was held vertically, approximately 9 cm above the sample by means of a polyvinyl chloride fitting. Mice were immobilized in 50 ml conical tubes, which were then inserted into the counting chamber where individual measurements (counts/2 min) were made. While counts were underway, bedding and water was changed in each cage to remove excreted 137Cs. At the end of the assessment period, all activity data were supplied to Dr. Michael Stabin, Vanderbilt University (Nashville, TN) who calculated the mean accumulated dose to the mouse pups as being 2.5–2.7 Gy delivered over 50 days.3 Since it was difficult to make accurate estimations of the time and distance that the pups were exposed to contaminated dams and littermates in close proximity, we were unable to account for additional exposure of the pups to external radiation from cage mates.
Infection
Twenty-six weeks postirradiation, anesthetized mice were infected with influenza virus as described previously (6) using 100 HAU of influenza A/HKx31 (H3N2) virus. After infection, mice were weighed daily as a measure of morbidity. Subsets of mice were harvested at day 6, 9 and 14 post infection for whole-lung gene expression and immunohistochemical analysis.
Processing of Lung Tissue
Lungs were processed in several ways. At the time of harvest, the left lobe was inflated with zinc-buffered formalin, tied off using suture material and subsequently processed for immunohistochemistry (6). The right lower lobe was flash frozen and used to determine influenza viral load. The rest of the lung was placed into TRIzol® reagent (Life Technologies, Grand Island, NY) and used for isolating RNA according to the manufacturer’s protocol. RNA was used for subsequent quantitative PCR analysis.
Quantitative Real-Time Polymerase Chain Reaction
The concentration of isolated RNA was determined using the Gene Quant RNA/DNA kit (Pharmacia Biotech Inc., Piscataway, NJ). cDNA was synthesized from 1 μg of total lung RNA using the first strand synthesis protocol from a GeneAmp® kit (Applied Biosystems®, Carlsbad, CA). Probe sets for monocyte chemoattractant protein-1 (MCP1), CCSP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. CCSP and MCP-1 gene expression levels were obtained using SYBR® green. Expression levels of CCSP were calculated relative to GAPDH and normalized using the delta CT method. Copy numbers of MCP-1 and GAPDH were calculated with a quantitative method using a standard curve. All assays were run on an iCycler with iCycler iQ™ Multi-Color Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA).
Immunohistochemistry
Paraffin blocks were sectioned at 5 μm and slides were placed in a 60°C dry incubator overnight and then transferred to xylene for deparaffinization. Tissue was then rehydrated through a series of xylene and alcohol washes using standard protocols. Antigen retrieval was then accomplished using the DAKO antigen retrieval kit (Dako North America Inc., Carpinteria, CA) at 96°C for 30 min. Slides were treated for 30 min with Peroxidaze 1 (Biocare Medical, Concord, CA) to inhibit endogenous peroxidase and nonspecific antibody binding, respectively. Primary anti-CCSP antibody (Abcam®, Cambridge, UK) was diluted 1:2,000 and added to slides for 20 min at room temperature. Slides were then washed and incubated with rabbit anti-rodent horseradish peroxidase (HRP) polymer (Biocare Medical) for 30 min at room temp. After a wash, slides were incubated with Betazoid DAB chromagen (Biocare Medical) until signal was detectable. Slides were then stained with hematoxylin and eosin using standard protocols, treated with Cytoseal mounting media (Richard-Allan™ Scientific/Thermo Scientific, Rockford, IL) and coverslipped. Images were taken using a Nikon Eclipse 80i microscope (Nikon® Instruments Inc., Melville, NY) with digital imaging head. A 20× objective was used and pictures were taken with a DS-QiMc camera (Nikon® Instruments Inc.). The Nis-Elements BR acquisition software (Nikon® Instruments Inc.) was used to capture pictures.
Viral Load Measurements
Lung tissue was thawed and homogenized in sterile PBS/0.5% bovine serum albumin (BSA) using a Dounce homogenizer. Assays were performed as previously described (12). Briefly, serial dilutions of homogenate were incubated with confluent MDCK cell monolayer and overlaid with an agarose/Dulbecco’s modified Eagle medium/trypsin mixture in duplicate. After 48 h, the agarose overlay was removed and the plates were stained using crystal violet. The number of plaques were then counted and averaged over duplicate wells. The average number was multiplied by the dilution factor and then normalized to the corresponding weight of lung tissue used in the assay.
Anti-Influenza Antibody Responses
Ninety-six well U-bottom plates were coated overnight at 4°C with a lysate of MDCK cells that were infected with influenza A/HKx31 virus diluted 1:350 in PBS (13, 14). Plates were then washed (PBS/0.05% Tween® 20) and blocked with PBS/0.5% BSA for 30 min at room temperature. Serum was diluted at 1:64 and then 1:2 in PBS/0.5% BSA. Serum was added to the antigen-coated 96-well plates and incubated overnight at 4°C. Plates were then washed and incubated with a 1:500 dilution of HRP-conjugated goat anti-mouse IgG Fc (IgA/IgM/IgG Fab absorbed; SouthernBiotech, Birmingham, AL). Plates were washed and developed using 1-Step ABTS solution (Pierce/ThermoScientific, Rockford, IL) for 10 min. Absorbance at 650 nm was then determined using a Spectramax® plate reader (Molecular Devices, Sunnyvale, CA). Background was subtracted from raw values and graphed.
Flow Cytometry of Cells Isolated from Mediastinal Lymph Nodes
Mediastinal lymph nodes were collected and placed in PBS on ice, then disrupted by wetting a 40 μm cell strainer with a small volume of PBS + 10% fetal bovine serum (FBS; BD Biosciences, San Jose, CA), then disrupting the lymph nodes using the rubber portion of a syringe plunger until none of the structure remained. The filter was washed with 5 ml PBS + 10% FBS and the samples were placed on ice. The cells were then pelleted, brought up in 1 ml PBS + 10% FBS, counted, then 1 × 106 cells were pelleted and resuspended in 400 μl PBS + 10% FBS. Nonspecific antibody binding was blocked using Fc block at 1:500 in PBS + 10% FBS for 10 min on ice. Surface staining was then performed for 30 min at 4°C using CD127-BV421, Gr-1-APC, CD4-APC/Cy7, CD3-PE, CD8-PerCP (BD Biosciences), CD45-BV605 (Biolegend® Inc., San Diego, CA) and F4/80-PE/Cy7 (eBioscience Inc., San Diego, CA). After surface staining, cells were washed, stained with LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life Technologies) in PBS for 15 min at 4°C. For intracellular staining, cells were then permeablized and fixed using the Foxp3/ Transcription Factor Staining Buffer Set (eBiosciences) for 30 min at 4°C. Cells were incubated with permeablization buffer overnight and stained using FoxP3-AF488 and IFN-γ-BV786 (BD Biosciences) for 2 h at 4°C. Cells were then washed, resuspended in PBS and run on an 18 paramater LSRII flow cytometer (BD Biosciences). Bangs beads (Bangs Laboratories Inc., Fishers, IN) were incubated with 1 μl of detection antibody, which served as single color positive controls. A sequential gating strategy was used to identify specific T-cell populations. First, viable CD45+ cells were gated to select myeloid cells. T cells were next identified by gating on cells that were negative for F4/80 and Gr1. A gate was then placed on the CD3+CD4+ cells. Regulatory T cells (Tregs) were identified as Foxp3+ and CD127low.
Statistics
For quantitative assays, treatment groups were reported as mean ± standard error of the mean (SEM). Analysis of variance (ANOVA) with Tukey’s post test was performed for comparing gene expression levels within the internal and external experiments. Mouse weights were compared using a two-way ANOVA with a random effect for the repeated measures on mice. Analysis was performed with R version 3.0.2, 2013 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was established at P < 0.05.
RESULTS
Transfer of 137Cs from Internally Contaminated Mouse Pups to Dams
Relatively little is known about the clearance kinetics of radioactive salts from juvenile pups that receive an internal exposure of 137Cs. To address this point, we injected 14-day-old C57BL/6 pups with 50 μCi of 137Cs or PBS intraperitoneally. Mice were then individually placed inside a counting chamber and the activity was recorded (Fig. 1). A peak count was recorded at the first reading (36,747 ± 662 counts/2 min mean ± SEM) at 6 h (the first point represented on the graph) and radioactivity was still detectable at day 13 post injection (1,992 ± 415 counts/2 min). To analyze whether the contaminating material could be transferred from the pups to their mothers, dams were also assayed for radioactivity. A peak contamination in the dams was detected at day 7 (approximately 15,000 counts/2 min), with a gradual decay by day 13 (Fig. 1), indicating that, indeed, radioactive material was transferred from pups to their dams. Of note, this transfer of radioactivity to the dams may have a small effect on the kinetics of Cs elimination from the pups, however, the relevance of this finding to the human population is unclear since the dam contamination is likely a consequence of maternal grooming.
FIG. 1.
137Cs transfer between pups and dams after pup internal exposure. Fourteen-day-old pups were injected on day 0 with 50 μCi of 137Cs. Radioactivity was assessed at 6 h post injection and daily thereafter. Counts per 2 min were recorded. Data represent mean ± SEM, 3–6 pups per point. Data from one dam is shown.
Transferred Internal Contamination from Pups to Dams Leads to a Late Decrease in CCSP
Given that exposure of adult mice to external radiation leads to a late decrease in lung CCSP expression and decreased CCSP+ cells as detected by immunohistochemistry (8), we examined whether the dams contaminated internally led to a later decrease of CCSP expression in the lung. We sacrificed the dams from cages where pups were injected with 50 μCi soluble 137Cs or PBS at 12 and 26 weeks post exposure. We then isolated RNA from whole lungs and performed RT-PCR analysis to determine levels of GAPDH and CCSP expression. A ratio of CCSP/GAPDH was determined and graphed (Fig. 2A). A decrease in CCSP message was noted at both 12 weeks (73.5 ± 3.2 vs. 46.6 ± 11.2) and 26 weeks (65.6 ± 3 vs. 36.4 ± 9.2) post exposure among the dams with contaminated pups and those with uncontaminated litters. The 26-week post-exposure time point reached statistical significance (P = 0.038) suggesting that the earlier secondary internal contamination of adult animals may have led to late lung damage. Given that MCP-1 levels have been associated with decreased CCSP levels after influenza infection (6), we also assessed MCP-1 message levels in the lungs from the same animals (Fig. 2B). There were very low levels of MCP-1 observed in both the internally contaminated and control groups, which did not differ between groups, thus, there appeared to be no association between MCP-1 levels and decreased CCSP expression seen in adult animals secondarily exposed to internal 137Cs.
FIG. 2.
137Cs transferred to dams from contaminated pups leads to a late decrease in CCSP message. Dams of pups exposed to soluble 137Cs were euthanized at 12 and 26 weeks post exposure. Lungs were harvested and real-time PCR was used to detect CCSP (panel A) and MCP-1 (panel B) expression in lungs.
Early External Irradiation Leads to Impaired Recovery after Infection with Influenza A Virus
Our group had previously examined the effects of external irradiation on newborn (4-day-old) pups that were subsequently exposed to influenza (6). We showed that whole-body irradiation exacerbated the loss of club cells expressing CCSP protein after influenza infection. In the current studies, we extended our investigations to a model that mimics juvenile (day 14) internal and external radiation exposure, and examined the long-term effects of radiation exposure. To this end, we allowed the exposed pups to recover for 26 weeks after either 50 μCi 137Cs contamination or 5 Gy external whole-body irradiation, after which they were infected with 100 HAU of influenza A virus (X31). Mice were subsequently weighed daily for two weeks and the ratio to their initial body weight was calculated (Fig. 3).
FIG. 3.

Early external irradiation leads to increased weight loss and impaired recovery versus internal contamination. Weights were recorded every day for two weeks after infection with 100 HAU of the influenza A virus X31 strain. Data are shown as percentage change from initial body weight and are mean ± SEM (n = 2–10 mice per group and time point).
Peak weight loss in influenza virus-infected mice occurred between day 5–6 for the internally contaminated group versus noncontaminated controls (81 ± 8% and 77 ± 9%). Both groups exhibited a near complete return to their baseline weights by day 14 post infection and appeared to convalesce normally after infection. In contrast, the animals that had received early external irradiation exhibited their peak weight loss at day 10 (55 ± 10%), suggesting an increased morbidity in response to influenza virus infection due to the effects of radiation. Their age-matched nonirradiated, but influenza virus-infected control group exhibited maximum weight loss at day 8 (78 ± 12%) and had recovered their baseline weight by day 11, whereas the previously exposed group failed to recover baseline weight by day 14 (75 ± 7%), suggesting that early external irradiation had inhibited complete convalescence from the respiratory viral infection. Thus, exposure to external radiation led to long-term sequelae with respect to recovery from respiratory viral infection, however the internally contaminated group convalesced normally after a late influenza virus infection.
Antibody Responses and Viral Clearance after Early Irradiation of Juvenile Mice
Since the animals that were exposed to external radiation failed to fully recover their body weight, we tested the hypothesis that these mice had lower levels of anti-influenza antibody compared to nonirradiated mice. Interestingly, similar levels of anti-influenza IgG antibodies were detected in all groups, suggesting that the humoral response had not been significantly impaired by the earlier irradiation (Fig. 4). The small, nonsignificant differences seen between the age-matched controls and externally irradiated mice were likely due to differences in group animal weights at the time of infection; we have no explanation for the overall lower body weights of the externally versus internally irradiated groups, beyond the observation that the groups were housed in separate areas of the vivarium due to restrictions on co-housing contaminated with uncontaminated animals.
FIG. 4.
Anti-influenza antibody is detectable in mice for all infected groups. Serum from infected mice was incubated on an ELISA plate coated with X31 influenza virus lysate over a range of dilutions. Antibody was detected using an IgG-specific detection antibody conjugated to HRP. Data are graphed as mean ± SEM. External n = 1–5 mice. Internal n = 3–5 mice.
We next examined whether viral load was different between the irradiated and nonirradiated control groups. We performed a plaque assay on homogenized lung tissue from day 6 and 9 infected mice and results were normalized to tissue weight. Viral load was comparable on day 6 between all 4 groups [controls (2 cohorts), internally contaminated and externally irradiated animals] (Fig. 5). However, by day 9 post infection, titers were reduced in all groups, with no significant difference observed between groups (data not shown).
FIG. 5.
Viral load is similar in all groups of irradiated and nonirradiated influenza virus-infected groups. Homogenates from one lung lobe from 2–3 animals on day 6 post infection were used in viral plaque assays. The number of plaques was normalized to weight of lung tissue used in the assay and graphed as mean ± SEM. No statistical differences were observed.
Alterations in CCSP and MCP1 Expression after Irradiation versus Infection
Given our previous observations that club cells are particularly sensitive to influenza infection in mice that were irradiated as newborns (4 days old), we assessed the effect of influenza infection on CCSP expression in mice that were irradiated as juveniles (14 days old) by qRT-PCR (Fig. 6A and B). Due to the role played by inflammatory cells during the innate phase of infection, we also examined expression levels of MCP-1, a chemokine known to attract macrophages and select T-cell populations (Fig. 6C and D). CCSP expression levels in whole-lung lysates were found to be similar among control and previously irradiated groups that were not infected. However, influenza infection led to a significant decrease in CCSP expression at day 6 and 9 in all infected groups (P < 0.01) relative to control (Fig. 6B). Of note, CCSP mRNA expression at day 14 remained reduced in the externally irradiated groups, regardless of infection with influenza A virus. Although the reduction in CCSP mRNA expression in the infected internally radioactively contaminated group was smaller than in the infected age-matched controls on day 6, this differential did not persist at the later time points.
FIG. 6.
Impaired recovery of CCSP expression in the internal irradiation group. One lung lobe per mouse was taken for RNA at each time point and treatment condition. qRT-PCR was used to quantitate absolute mRNA copy number of CCSP (panels A and B) and MCP-1 (panels C and D) and normalized to GAPDH copy number. Data represent mean ± SEM. n = 2–5 mice. *P < 0.05, **P < 0.01, ***P < 0.001.
Expression levels of MCP-1 were increased on day 6 in the lungs of all virus-infected mice compared to noninfected groups (P < 0.01) (Fig. 6C and D). By day 9 post infection, MCP-1 expression levels had returned to their pre-infection baseline in both the early internal contamination cohort and its age-matched infected controls, but remained significantly elevated in the infected externally irradiated and age-matched control groups (P < 0.01) (Fig. 6D). On day 14, a significantly elevated level of MCP-1 expression was seen only in the group that had earlier received external irradiation. Thus, the previously internally contaminated mice exposed to influenza behaved similarly to their noncontaminated counterparts and resolved inflammation by day 9. However, the externally irradiated group that experienced late influenza infection failed to completely resolve inflammation by day 14.
Exposure to Radiation Impairs Recovery of CCSP and Increases Cellular Infiltration after Infection
To interrogate lung pathology and further examine CCSP gene expression results, we formalin-fixed the left lobe from the lungs of each animal used for molecular analysis and stained for CCSP protein expression to qualitatively analyze histopathological changes and compare with the quantitative CCSP expression (Fig. 7). Light microscopic examination confirmed the RT-PCR data, showing that influenza virus infection had severely decreased CCSP expression in the large airways, which was most apparent at day 6 post infection in both internally contaminated and externally irradiated animals, as well as in the virus-infected sham-irradiated groups. In the infected internal contamination group, there was some recovery of CCSP+ cells by day 14, which was similar to that seen in both control groups, although no group had returned to baseline. With respect to the externally irradiated, virus infected animals, not only was there a decrease in CCSP+ cells, but an observable increase in cellular infiltration compared to the nonirradiated, influenza virus-infected controls (see Fig. 7) and signs of edema were evident as well.
FIG. 7.
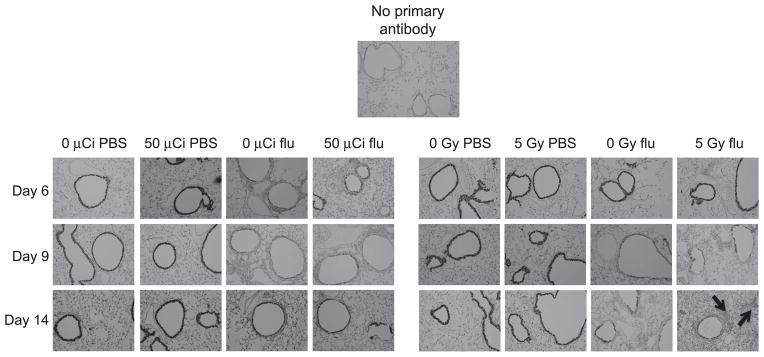
CCSP protein expression matches RNA profiles. Formalin-fixed lung tissue was stained for CCSP and counterstained with H&E. Note the increase in cellular infiltration in the 5 Gy external irradiation + flu panel versus 0 Gy + flu at day 14 post infection (black arrows).
External Irradiation of Juvenile Animals Causes an Increase in Frequency of Regulatory T Cells in the Mediastinal Lymph Node with Decreased FoxP3 Expression
Our observation that early external irradiation resulted in increased weight loss and lung damage in response to late influenza infection led us to examine whether the immune microenvironment was altered in these animals. Given that lung late effects (fibrosis) and regulatory T cell differentiation share common signaling pathways, and that regulatory T cells play a role in the resolution of inflammation after viral clearance, we assessed the frequency of regulatory T cells in the lymph nodes that drain the lung (15–18). Due to animal number limitations, we were only able to harvest mediastinal lymph nodes from mice that were externally irradiated 26 weeks earlier. In this pilot study, the mediastinal lymph nodes were stained for CD3, CD4, FoxP3 and CD127 using fluorophore-conjugated antibodies and their expression was analyzed by flow cytometry. CD3+CD4+ T cells were first identified (Fig. 8A) and these cells were then further gated for Tregs (CD127dimFoxP3+) and nonregulatory T cells (CD127brightFoxP3−) (Fig. 8B). We observed a significantly decreased frequency of CD3+CD4+ T cells in the irradiated group (Fig. 8C: 22.4 ± 1.4% in control vs. 15.55 ± 1.1% in irradiated mice). However, of the CD4+ T cells, a higher frequency stained as Tregs in irradiated animals versus controls (30.78 ± 2.1% vs. 19.0 ± 2.4%, respectively). Thus, early external irradiation led to long-term changes in the immune system, seen as decreased FoxP3 expression in the CD4+ T-cell population, which may offer an explanation for the observed increased inflammatory state after infection due to decreased immunosuppression.
FIG. 8.
Increased regulatory T cell frequencies are observed in mediastinal lymph nodes from externally irradiated mice. Panels A and B: Mediastinal lymph nodes removed at 26 weeks postirradiation were stained for regulatory T cells. Panel C: Graph showing the frequency of CD3+CD4+ cells (mean ± SEM). Panel D: The frequency of regulatory T cells (CD127lowFoxP3+) was determined from the CD3+CD4+ population. n = 4 mice per group.
DISCUSSION
Previous findings from our group have demonstrated that external irradiation to the lung alone in adult mice or whole-body irradiation of 4-day-old neonates leads to late morbidity in response to influenza infection (6–8). In the current study using our extended models, the data suggest that exposure to radiation during the juvenile stage of development may also lead to long-term consequences in the event of later life respiratory viral infection.
Whole-body irradiation targets all organ systems, including the lung and immune system, both of which are considered to be dose limiting with respect to radiation therapy and are deemed to be relatively radiation sensitive. Nonetheless, the external radiation dose used in these studies is considered to be below the threshold for the induction of late lung damage (19). However, there are a number of investigators that believe that even in asymptomatic tissues, subclinical, cryptic damage may persist for months, if not years, postirradiation (2, 5, 7, 19). To assess long-term consequences of early-life exposure, mice were whole-body irradiated at 14 days of age, and after a 26-week recovery period, were then infected with 100 HAU of influenza A virus. We demonstrated that these animals do not regain body weight normally out to 14 days post infection (Fig. 3). However, the fact that normal antibody responses and viral clearance was observed in the irradiated group compared to age-matched controls suggested that the primary immune response was intact and was capable of mounting an antiviral response (Figs. 4 and 5). These results are consistent with data obtained when mice were irradiated during the neonatal period (6), however, it is possible that, upon further viral challenge, defects in the immune response could be revealed.
Consistent with our previous findings, CCSP message levels were reduced in the lungs of the externally irradiated animals after influenza infection; this was mirrored by protein expression, visualized in histological analysis and accompanied by increased cellular infiltrate in these mice (Fig. 7). Since the CCSP+ cells are more sensitive to viral insult, it is possible that these cells were expressing previous injury and that CCSP could be a potential biomarker for early radiation exposure. Furthermore, MCP-1 levels did not return to baseline by day 14 (Fig. 6), indicating a dysregulation in the resolution phase of the response. This matches well with our data demonstrating impaired weight gain in these mice, which could indicate a systemic increase in proinflammatory cytokines. Given that TNF-α is known to contribute to weight loss in mice, future investigations should examine plasma levels of TNF-α in relationship to the failure of externally irradiated mice to regain weight (20).
Although widely perceived as a lower risk, human exposure to internal radiation after, for example, nuclear power plant disasters has been well documented due to consumption of contaminated milk products and soft leafy vegetables (21–23). Furthermore, the perceived risk of large populations being exposed to radiation from internal contamination and/or external sources as a result of terrorist attacks has increased significantly since the terrorist attacks against the U.S. on September 11, 2001. However, despite the likely heterogeneity of an exposed population, the long-term effects of low-dose exposure on children are poorly understood. Given the recent findings from both the counterterrorism and space literature (24, 25), and the overall paucity of information on internal contamination, we examined the effects of an internal dose of soluble 137Cs on juvenile mice, again focusing on immune system function and the pulmonary response to a late respiratory viral challenge. Our data show that the majority of injected soluble 137Cs was cleared rapidly from the pups, although counts did not return to absolute background by 14 days after exposure, with low-level counts detected for over six weeks. This confirms the potential for prolonged low-dose systemic exposure after intake of a long-lived radioactive isotope, especially when isotopes, such as cesium or strontium, are preferentially taken up in soft tissue or bone. It is worth noting that we observed a transfer of radioactive material from contaminated pups to their dams, likely a consequence of maternal grooming, with the dams subsequently demonstrating reduced CCSP message in the absence of infection. However, we did not see any increase in MCP-1 levels in the lung of the dams, indicating that the loss of CCSP+ cells did not correlate with increased inflammation. Of note, there was no effect seen with respect to CCSP message levels in the animals that were either externally irradiated or internally contaminated as pups, which is in contrast to our observation in the dams. This would suggest that there may have been some regenerative capacity for damage when juveniles are internally contaminated that is not apparent in older adults (9, 10).
We chose to assess the response to viral infection in animals at 26 weeks after irradiation, which represents a time when fibrosis and pneumonitis are typically not induced over background levels in the C57BL/6J mouse strain (19, 26). Our previous work suggests that a dose of 12.5 Gy resulted in fibrosis at the 26-week time point, whereas a lower 5 Gy dose did not result in fibrosis as observed by histology (1, 7, 8). With respect to the ability of the internally contaminated juvenile mice to respond to late infection, overall, their response was closer to that of the sham-irradiated cohorts than the externally irradiated mice. This is likely a consequence of the significantly lower dose, calculated at approximately 2.5 Gy and attenuated over a 50-day exposure period compared to the single 5 Gy whole-body dose administered to the externally irradiated pups. We note that the loss of CCSP occurred only in groups that were infected with influenza A virus, compared to PBS controls, indicating that club cells are sensitive to the effects of viral infection. We also note a radiation effect in the influenza-infected group when mice were internally contaminated with 137Cs. At day 6, the internally contaminated group that was infected with influenza virus had higher levels of CCSP message versus noncontaminated but influenza-infected mice. However, at day 9 the internally irradiated group had a lower CCSP message level. The internal contamination group that was infected with influenza A virus exhibited loss of club cells with no increase in the severity of later life viral infection, indicating a decoupling of the pulmonary and immune system damages. Given a recent report that chronic low-level contamination with 137Cs leads to alterations in metabolism (27), future studies may need to focus on persistent exposure to Cs internal radiation followed by influenza infection, which we believe may lead to increased morbidity if followed to later time points (e.g., 46 weeks post contamination). Given that TNF-α, MCP-1 and other proinflammatory cytokines are known to contribute to weight loss and lung damage in mice infected with influenza virus, future studies should examine plasma levels of TNF-α and other proinflammatory cytokines (20, 28, 29), since such studies may reveal cytokine-targeted treatment strategies that could improve the long-term survival of irradiated humans, such as survivors of bone marrow transplantation.
Our group has previously reported that CCSP plays an important role in recovery from influenza infection in irradiated mice (8). Furthermore, this result is consistent with our previous findings showing that adult mice irradiated with an external thoracic dose of 15 Gy exhibited an increase in influenza-specific CD8+ T cells in the lungs at day 14 (7). Additionally, whole-body irradiation of neonatal mice followed by influenza infection 26 weeks later led to increased lymphoid follicle size within the lung (6). One possible explanation for this phenomenon is a defect in the ability of the immune system to resolve subsequent injury in the club cell population as a result of earlier radiation damage. Consistent with this idea is that even uninfected, but externally irradiated, mice exhibited a significant decrease in the proportion of CD4+ T cells in their mediastinal lymph nodes, with an increased fraction of these cells being Tregs (Fig. 8). These cells expressed lower levels of FoxP3, which is consistent with an impaired suppressive function during conditions of inflammatory disease (30–33). If Treg function is impaired, then despite the observation that there are a higher number of these cells, the net effect could be failure to control inflammation.
In general, our results suggest that early-life exposure to external radiation leads to alterations in both the immune and pulmonary systems. In the case of the immune system, it is possible that radiation exposure leads to immunosenescence and/or premature aging of the immune system. Lessons can be learned from atomic bomb survivors, whose immune systems exhibited long-term signs of increased systemic inflammation (5). One such report correlated decreased thymic output of T cells with increased levels of the proinflammatory marker, c-reactive protein and obesity in atomic bomb survivors (34). In our model, either whole-body or chest-only irradiation presumably targets the thymus, which could lead to a decrease in naïve T-cell output. A separate report showed a correlation between the level of radiation exposure and numbers of activated T cells seen decades later (35). It is well known that Tregs are dysregulated in the elderly, and impaired function could contribute to low levels of inflammation in aged irradiated individuals (36). Furthermore, impaired function in T cell can lead to consequences in the elderly with respect to vaccination, infection and autoimmune disease (37). If whole-body irradiation during childhood leads to accelerated immunosenescence and aging, then alterations in Treg function could contribute to the increased morbidity seen in response to influenza infection that our group has reported previously. Supporting this concept, a recently published report showed that Tregs promote recovery of mice after clearance of influenza virus (18). Furthermore, in a another report, it was suggested that the function of Tregs in obese individuals is impaired, which could contribute to the increased levels of inflammation seen in overweight individuals with chronic obstructive pulmonary disease (38).
Additional studies are needed to examine the relative ability of irradiated juvenile animals to respond to more virulent primary (e.g., PR8) or secondary infections and to determine whether undetected, subclinical damage is present in the immune systems of these mice. It is also important to determine the threshold at which the immune system is affected by internal contamination and how damage to the immune system is decoupled from the physical loss of club cells from the pulmonary milieu. Furthermore, the identification of biomarkers that could predict the damage to the club cell population and potential late effects of such damage would have implications for clinical practice with respect to thoracic radiation therapy, as well as countermeasure development for use after nuclear or radiological incidents. It is our hope that, by understanding how exposure to radiation leads to damage in the pulmonary and immune systems, new drug targets may be identified that could help protect against long-term morbidities due to external and/or internal radiation exposure, especially in vulnerable populations, such as children.
Acknowledgments
This work was supported by the Centers for Medical Countermeasures against Radiation Program, NIH/NIAID U19 grant AI091036. We gratefully acknowledge Drs. Luis Martinez and Aitor Nogales (University of Rochester Medical Center) for providing reagents for the detection of anti-influenza-specific plasma antibody levels and Dr. Michael Stabin (Vanderbilt University School of Medicine, Nashville, TN) for performing dosimetrical calculations for the internal cesium contamination studies.
Footnotes
Dr. Michael Stabin, personal communication.
References
- 1.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11:1386–94. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holsapple MP, O’Lone R. Juvenile immunotoxicology. Toxicol Pathol. 2012;40:248–54. doi: 10.1177/0192623311427708. [DOI] [PubMed] [Google Scholar]
- 4.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–13. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol. 2008;84:1–14. doi: 10.1080/09553000701616106. [DOI] [PubMed] [Google Scholar]
- 6.Johnston CJ, Manning CM, Rangel-Moreno J, Randall TD, Hernady E, Finkelstein JN, et al. Neonatal irradiation sensitizes mice to delayed pulmonary challenge. Radiat Res. 2013;179:475–84. doi: 10.1667/RR3242.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning CM, Johnston CJ, Reed CK, Lawrence BP, Williams JP, Finkelstein JN. Lung irradiation increases mortality after influenza A virus challenge occurring late after exposure. Int J Radiat Oncol Biol Phys. 2013;86:128–35. doi: 10.1016/j.ijrobp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning CM, Johnston CJ, Hernady E, Miller JN, Reed CK, Lawrence BP, et al. Exacerbation of lung radiation injury by viral infection: the role of Clara cells and Clara cell secretory protein. Radiat Res. 2013;179:617–29. doi: 10.1667/RR3279.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Li C, Minoo P. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am J Respir Cell Mol Biol. 2011;44:804–12. doi: 10.1165/rcmb.2009-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun R, Zhou Q, Ye X, Takahata T, Ishiguro A, Kijima H, et al. A change in the number of CCSP(pos)/SPC(pos) cells in mouse lung during development, growth, and repair. Respir Investig. 2013;51:229–40. doi: 10.1016/j.resinv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Burri P. Postnatal development and growth. In: Crystal RG, Barnes PJ, Weibel ER, editors. The lung: scientific foundations. 2. Philadelphia: Lippincott-Raven; 1997. pp. 1013–26. [Google Scholar]
- 12.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Baker SF, Guo H, Albrecht RA, Garcia-Sastre A, Topham DJ, Martinez-Sobrido L. Protection against lethal influenza with a viral mimic. J Virol. 2013;87:8591–605. doi: 10.1128/JVI.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogales A, Baker SF, Ortiz-Riano E, Dewhurst S, Topham DJ, Martinez-Sobrido L. Influenza A virus attenuation by codon deoptimization of the NS gene for vaccine development. J Virol. 2014;88:10525–40. doi: 10.1128/JVI.01565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong J, Roth M. Lung remodeling mechanisms in chronic lung diseases. Curr Opin Allergy Clin Immunol. 2014;14:69–76. doi: 10.1097/ACI.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Re SL, Yakoub Y, Devosse R, Uwambayinema F, Couillin I, Ryffel B, et al. Uncoupling between inflammatory and fibrotic responses to silica: evidence from MyD88 knockout mice. PloS One. 2014;9:e99383. doi: 10.1371/journal.pone.0099383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moser EK, Hufford MM, Braciale TJ. Late engagement of CD86 after influenza virus clearance promotes recovery in a FoxP3+ regulatory T cell dependent manner. PLoS Pathog. 2014;10:e1004315. doi: 10.1371/journal.ppat.1004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res. 1989;119:15–31. [PubMed] [Google Scholar]
- 20.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31:2566–73. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Tsubokura M, Kato S, Nomura S, Gilmour S, Nihei M, Sakuma Y, et al. Reduction of high levels of internal radio-contamination by dietary intervention in residents of areas affected by the Fukushima Daiichi nuclear plant disaster: a case series. PloS One. 2014;9:e100302. doi: 10.1371/journal.pone.0100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handl J, Beltz D, Botsch W, Harb S, Jakob D, Michel R, et al. Evaluation of radioactive exposure from 137Cs in contaminated areas of Northern Ukraine. Health Phys. 2003;84:502–17. doi: 10.1097/00004032-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Harada KH, Fujii Y, Adachi A, Tsukidate A, Asai F, Koizumi A. Dietary intake of radiocesium in adult residents in Fukushima prefecture and neighboring regions after the Fukushima nuclear power plant accident: 24-h food-duplicate survey in December 2011. Environ Sci Technol. 2013;47:2520–6. doi: 10.1021/es304128t. [DOI] [PubMed] [Google Scholar]
- 24.Cherry JD, Liu B, Frost JL, Lemere CA, Williams JP, Olschowka JA, et al. Galactic cosmic radiation leads to cognitive impairment and increased abeta plaque accumulation in a mouse model of Alzheimer’s disease. PloS One. 2012;7:e53275. doi: 10.1371/journal.pone.0053275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweet TB, Panda N, Hein AM, Das SL, Hurley SD, Olschowka JA, et al. Central nervous system effects of whole-body proton irradiation. Radiat Res. 2014;182:18–34. doi: 10.1667/RR13699.1. [DOI] [PubMed] [Google Scholar]
- 26.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 27.Grison S, Martin JC, Grandcolas L, Banzet N, Blanchardon E, Tourlonias E, et al. The metabolomic approach identifies a biological signature of low-dose chronic exposure to cesium 137. J Radiat Res. 2012;53:33–43. doi: 10.1269/jrr.11071. [DOI] [PubMed] [Google Scholar]
- 28.Zhao G, Liu C, Kou Z, Gao T, Pan T, Wu X, et al. Differences in the pathogenicity and inflammatory responses induced by avian influenza A/H7N9 virus infection in BALB/c and C57BL/6 mouse models. PloS One. 2014;9:e92987. doi: 10.1371/journal.pone.0092987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, et al. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol. 2013;87:6911–24. doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arandi N, Mirshafiey A, Jeddi-Tehrani M, Shaghaghi M, Sadeghi B, Abolhassani H, et al. Alteration in frequency and function of CD4+CD25+FOXP3+ regulatory T cells in patients with immune thrombocytopenic purpura. Iran J Allergy Asthma Immunol. 2014;13:85–92. [PubMed] [Google Scholar]
- 31.Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64:1539–46. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 32.Bending D, Pesenacker AM, Ursu S, Wu Q, Lom H, Thirugnanabalan B, et al. Hypomethylation at the Regulatory T cell-specific demethylated region in CD25hi T cells Is decoupled from FOXP3 expression at the inflamed site in childhood arthritis. J Immunol. 2014;193:2699–708. doi: 10.4049/jimmunol.1400599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilahun AY, Chowdhary VR, David CS, Rajagopalan G. Systemic inflammatory response elicited by superantigen destabilizes t regulatory cells, rendering them ineffective during toxic shock syndrome. J Immunol. 2014;193:2919–30. doi: 10.4049/jimmunol.1400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida K, Nakashima E, Kubo Y, Yamaoka M, Kajimura J, Kyoizumi S, et al. Inverse associations between obesity indicators and thymic T-cell production levels in aging atomic-bomb survivors. PloS One. 2014;9:e91985. doi: 10.1371/journal.pone.0091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaoka M, Kusunoki Y, Kasagi F, Hayashi T, Nakachi K, Kyoizumi S. Decreases in percentages of naive CD4 and CD8 T cells and increases in percentages of memory CD8 T-cell subsets in the peripheral blood lymphocyte populations of A-bomb survivors. Radiat Res. 2004;161:290–8. doi: 10.1667/rr3143. [DOI] [PubMed] [Google Scholar]
- 36.Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini-review. Gerontology. 2014;60:130–7. doi: 10.1159/000355303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol. 2012;24:350–5. doi: 10.1016/j.smim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan DB, Fernandez S, Price P, French MA, Thompson PJ, Moodley YP. Impaired function of regulatory T-cells in patients with chronic obstructive pulmonary disease (COPD) Immunobiology. 2014;219:975–9. doi: 10.1016/j.imbio.2014.07.005. [DOI] [PubMed] [Google Scholar]








