Abstract
Glycaemic control, in particular at postprandial period, has a key role in prevention of different diseases, including diabetes and cardiovascular events. Previous studies suggest that postprandial high blood glucose levels (BGL) can lead to an oxidative stress status, which is associated with metabolic alterations. Cinnamon powder has demonstrated a beneficial effect on postprandial glucose homeostasis in animals and human models. The purpose of this study is to investigate the effect of cinnamon tea (C. burmannii) on postprandial capillary blood glucose level on nondiabetic adults. Participants were given oral glucose tolerance test either with or without cinnamon tea in a randomized clinical trial. The data revealed that cinnamon tea administration slightly decreased postprandial BGL. Cinnamon tea ingestion also results in a significantly lower postprandial maximum glucose concentration and variation of maximum glucose concentration (p < 0.05). Chemical analysis showed that cinnamon tea has a high antioxidant capacity, which may be due to its polyphenol content. The present study provides evidence that cinnamon tea, obtained from C. burmannii, could be beneficial for controlling glucose metabolism in nondiabetic adults during postprandial period.
1. Introduction
Postprandial glucose level (PBG) has been reported to be an important factor in glycaemic control [1, 2]. The importance of postprandial glycaemia regulation is in accordance with epidemiological studies, which have demonstrated that postprandial hyperglycaemia is a predictor of diabetes and cardiovascular events [3, 4]. The postprandial state can stimulate the reactive oxygen species (ROS) production leading to an oxidative stress status. This status involves molecular mechanism for development of different complications associated with hyperglycaemia [5–7]. Moreover, postprandial oxidative stress can be accompanied by postprandial inflammation and endothelial dysfunction as reported by Ceriello et al. [8].
Postprandial glucose concentration refers to plasma glucose concentration after eating, which can be evaluated using a reference test standardized, 75 g oral glucose tolerance test (OGTT) [9]. During OGTT, plasma glucose level is obtained by secretion/action of insulin [10]. However, many other factors can also influence glucose metabolism including timing, quantity, and meal composition [11–13].
Many traditional plants and spices possess medicinal properties, such as control blood glucose levels. Cinnamon is one of these spices that has been demonstrated to be effective in improving glycaemia [14, 15] both in healthy and diabetic subjects. In healthy subjects, a study revealed that 5 g of cinnamon powder lowered PBG after OGTT [16] and another showed that 6 g added to a rice pudding improved PBG area under the curve (AUC) [17]. In type 2 diabetic subjects, cinnamon revealed that it can exert a hypoglycaemic effect, decreasing PBG and fasting blood glucose level (FBG) [18]. These beneficial effects seem to be related to its water-soluble polyphenols. An in vitro study showed that polyphenols possess an insulin-like action [19]. In addition, these cinnamon bioactive compounds revealed high antioxidant properties in human and animal models on oxidative stress through inhibiting lipid peroxidation [20, 21].
The aim of the present study is to investigate the effect of a cinnamon tea (6 g C. burmannii/100 mL) on postprandial capillary blood glucose level on nondiabetic adults.
2. Material and Methods
2.1. Subjects
Following ethical committee approval 30 nondiabetic adults with ages between 20 and 53 years were selected from the local community to participate in this study. A written informed consent was obtained from each volunteer after explaining the aim and experiment risk procedures. Inclusion criteria included subjects aged 18 or more, both genders with nondiabetic condition (fasting blood glucose level < 100 mg/dL [22]). Exclusion criteria comprised individuals who use medication for glycaemia control and have gastrointestinal symptoms or diseases. The study also excluded subjects who have altered medication, pregnancy, lactation, and allergy to cinnamon.
2.2. Study Design
Thirty nondiabetic adults were selected and randomly allocated in 2 groups (n = 15): control group, oral glucose tolerance test (OGTTcontrol) alone, and intervention group, OGTT followed by cinnamon tea administration (OGTTcinnamon). The participants were asked not to ingest any cinnamon at the day before the intervention.
2.3. Subject Groups Characterization
At baseline (before interventions), general characteristic data, such as anthropometric data, medical condition, and pharmacological therapy, were collected using a questionnaire development by investigator. Participants were also questioned about usual cinnamon intake. A 24-hour dietary recall was taken preceding each intervention to compare food intake at the day before the intervention between groups. The Food Processor SQL (version 10.5.0) programme was used to analyse the nutritional composition of the food.
2.4. Oral Glucose Tolerance Test (OGTT)
The glucose (dextrose) was weighed (75 g) using an analytical balance and dissolved in 200 mL of water, according to American Dietetic Association [22]. Following overnight fasting (12 h) blood glucose level was measured using a capillary drop blood, before intervention (t 0). In control group, subjects ingested glucose solution (200 mL) alone (OGTTcontrol). In intervention group subjects ingested 100 mL cinnamon tea (OGTTcinnamon) immediately after glucose solution (200 mL) intake. Blood samples were collected, for each participant, at 30 (t 30), 60 (t 60), 90 (t 90), and 120 (t 120) minutes in control and intervention groups. Sterilized lancet, glucose meter equipment, and strips for glucose meter (FreeStyle Abbott Diabetes Care) were used for blood glucose level measurement.
2.5. Chemicals and Equipment for Antioxidant Capacity Studies
Ferric chloride (III) hexahydrate (FeCl3·6H2O), Folin-Ciocalteu (2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), TPTZ 2,4,6-tri(2-pyridyl)-s-triazine, methanol (CH3OH), nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT) 2-amino-2-hydroxymethyl-propane-1,3-diol (tris), and phenazine methosulfate (PMS) were purchased from Sigma-Aldrich, gallic acid-1-hydrate (C6H2(OH)3COOH·H2O) was purchased from Acros Organics, and sodium carbonate (Na2CO3) was purchased from ICS Science group. All reagents were pro analysis grade.
All the absorbance measurements were performed in a Perkin-Elmer Lambda 25. The regents were weighed in an analytical balance (Sartorius, ±0.0001 g) and all the solutions were done with distilled water.
2.6. Cinnamon Tea Preparation
The Cinnamomum burmannii bark was purchased from Sucrame Company (Portugal) with Indonesia origin. Sticks of cinnamon (60 g) were soaked into 1000 mL of water. After 24 h at room temperature, cinnamon solution was heated for 30 min at 100°C and then filtered at room temperature. This method was adapted by Shen and coauthors [23]. After the cinnamon tea preparation a 100 mL individual dose was distributed to each participant. For chemical analysis, a hydromethanolic extract (50 : 50) was performed with cinnamon tea previously obtained.
2.7. Total Phenolic Content Determination
The total phenolic concentration in the extract was determined according to Folin-Ciocalteu method [24] employing gallic acid as standard. The results were expressed as mg for gallic acid equivalent (GAE)/g of extract. A volume of 375 μL of cinnamon extract and 4 mL of sodium carbonate were added to 5 mL of Folin-Ciocalteu reagent. After 15 min the absorbance was measured at 765 nm. This test was performed for 8 replicates.
2.8. Antioxidant Assay Using Ferric Reducing Antioxidant Power (FRAP) Method
The method for determination of ferric reducing effect was based on the reduction, at low pH, employing a colourless ferric complex (Fe3+) to a blue-coloured ferrous complex (Fe2+) by electron-donating antioxidants action in 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) presence [25]. A fresh solution was prepared by mixing 25 mL of acetate buffer (300 mM, pH = 3.6) into 2.5 mL of TPTZ solution (10 mM) to HCl (40 mM) and 2.5 mL of FeCl3·6H2O solution (20 mM). The solution was heated at 37°C. Samples (150 μL) were introduced in tubes with 2850 μL of the FRAP solution and were maintained in the dark condition for 30 min. The absorbance was measured at 593 nm. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as standard and the results were expressed in μmol Trolox/L. This test was performed for 6 replicates.
2.9. Superoxide Anion Radicals Scavenging Activity (O2 ∙−)
Superoxide anion was generated by reacting phenazine methosulfate (PMS), nicotinamide adenine dinucleotide hydride (NADH), and oxygen causing reduced NBT in Formazan [26, 27]. A volume of 0.5 mL of sample was added to 2 mL of a solution containing NADH (189 μM) and NBT (120 μM) with Tris-HCl (40 mM, pH = 8). The reaction started after the addition of 0.5 mL of PMS (60 mM). Control sample was measured using only distilled water. After 5 min of incubation, control absorbance was measured at 560 nm at room temperature. Absorbance was measured for different concentrations of cinnamon samples in order to represent a curve of % of inhibition versus phenolic concentration. The percentage of superoxide anion inhibition was calculated using the following equation:
| (1) |
2.10. Statistical Analysis
Statistical analysis was performed using Excel and SPSS Statistics (Statistical Package for Social Sciences) version 20.0 software for Windows. Data are presented as mean ± SD. Repeated Measures ANOVA of mixed type was used to assess the difference between the 2 groups for postprandial BGL at different times.
Independent samples t-test was used to assess the difference between the 2 groups for total caloric value, carbohydrates, protein and lipid, C max, ΔC max, and AUCIncremental values.
All statistical tests were performed at the 5% level of significance.
3. Results
3.1. General Characteristics of Participants
The general characteristics of the study participants are shown in Table 1, reporting age, anthropometrics parameters, medical conditions, and pharmacologic therapy. The BMI data for both genders revealed that most of participants have regular weight [28]. Regarding body fat mass (FM) the results show that the sample subjects are also in normal range in both genders [28], but female had significantly more fat mass than male. In addition, female subject had significantly (p < 0.05) less muscular mass than male.
Table 1.
Characteristics of the study participants (n = 30). Data represented mean (±SEM).
| Control group | Cinnamon group | |
|---|---|---|
| Mean (±SEM) | Mean (±SEM) | |
| Age (years) | ||
| Males | 35.3 (±6.7) | 30.2 (±3.7) |
| Females | 38.3 (±3.5) | 34.3 (±3.1) |
| BMI (Kg/m2) | ||
| Males | 23.6 (±1.2) | 24.9 (±0.7) |
| Females | 24.8 (±1.1) | 24.1 (±0.7) |
| FM (%) | ||
| Males | 15.1 (±2.1) | 17.2 (±0.8) |
| Females | 27.6 (±1.7) | 28.0 (±1.7) |
| MM (%) | ||
| Males | 52.7 (±2.1) | 67.1 (±1.5) |
| Female | 42.1 (±1.7) | 41.8 (±1.3) |
BMI: body mass index; FM: fat mass; MM: muscular mass.
In what concerns the ingestion of total energy intake and macronutrient composition regarding carbohydrates, protein and lipid at the day before the intervention, the 2 groups can be considered homogeneous since they did not reveal significant differences (p > 0.05) (Table 2).
Table 2.
Dietary analysis of total energy intake (TEI), carbohydrates (CD), protein (P), and lipid (L) intake at the day before OGTT(control) and at the day before OGTT(cinnamon) by participants. Data are mean ± SEM; n = 15, each group.
| Dietary Parameters |
Day before OGTT(control)
Mean (±SEM) |
Day before OGTT(cinnamon)
Mean (±SEM) |
p |
|---|---|---|---|
| TEI (Kcal) | 1708.01 (±97.04) | 1736.51 (±113.74) | 0.850 |
| CD (g) | 216.04 (±19.32) | 225.40 (±15.33) | 0.707 |
| P (g) | 75.66 (±6.15) | 77.67 (±6.49) | 0.823 |
| L (g) | 58.54 (±4.6) | 58.47 (±6.61) | 0.993 |
Independent sample t-test was used for statistical analysis.
3.2. Postprandial Blood Glucose Level
Blood glucose levels (BGL) were measured for the 2 groups (OGTT(control) and OGTT(cinnamon)) (Table 3). Statistical analysis revealed that there is no interaction between the independent and repeated measures factors (p = 0.209), which means that it is not possible to infer about differences in BGL in different moments.
Table 3.
Mean blood glucose levels (mmol/L) obtained after oral glucose tolerance test (OGTT(control)) and after oral glucose tolerance test with cinnamon tea (OGTT(cinnamon)) at different moments: before OGTT (t 0) and after 30 (t 30), 60 (t 60), 90 (t 90), and 120 (t 120) minutes. Data are mean ± SEM; n = 15, each group.
| Time | OGTT(control)
Mean (±SEM) mmol/L |
OGTT(cinnamon)
Mean (±SEM) mmol/L |
|---|---|---|
| t 0 | 4.97 (±0.1) | 4.99 (±0.1) |
| t 30 | 10.14 (±0.4) | 8.87 (±0.4) |
| t 60 | 8.75 (±0.5) | 8.24 (±0.4) |
| t 90 | 7.66 (±0.5) | 7.29 (±0.3) |
| t 120 | 6.40 (±0.2) | 5.86 (±0.2) |
However, the data showed that the administration of cinnamon tea after OGTT slightly decreased BGL mean values compared to OGTT in the absence of cinnamon intake (Figure 1).
Figure 1.
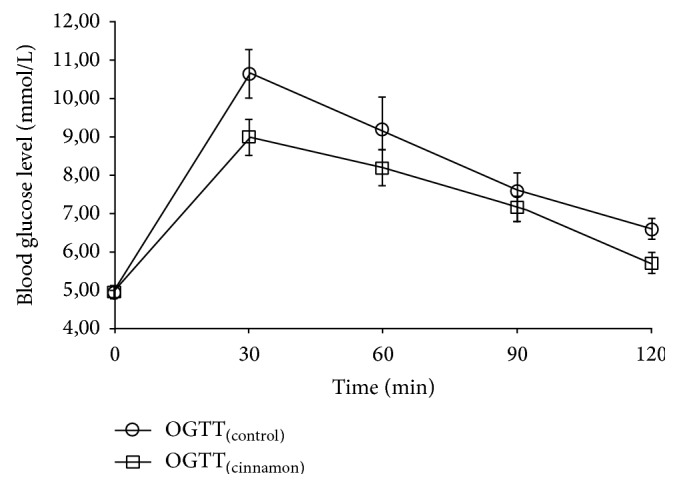
Mean (±SEM) time course of blood glucose levels (mmol/L) in nondiabetic subjects after OGTT(control) (○) and OGTT(cinnamon) (□).
The administration of cinnamon tea after OGTT resulted in a lower but not significantly postprandial blood glucose incremental area under the curve (AUCi) compared with OGTT(control). However, the variation of maximum glucose concentration and maximum glucose concentration mean values were significantly lower (p < 0.05) in OGTT(cinnamon) compared with OGTT(control) (Table 4).
Table 4.
Blood glucose level area under the curve (AUC), maximum glucose concentration (C max), and variation of maximum glucose concentration (ΔC max) in nondiabetic subjects at OGTT(control) and OGTT(cinnamon). Data are mean ± SEM (n = 15).
| OGTT(control) | OGTT(cinnamon) | p | |
|---|---|---|---|
| Mean (±SEM) | Mean (±SEM) | ||
| (mmol/L) | (mmol/L) | ||
| AUCi (0–120 min) | 403.73 (±48.5) | 297.47 (±33.9) | 0.084 |
| C max | 10.63 (±0.6) | 8.98 (±0.5) | 0.040∗ |
| ΔC max | 5.71 (±0.6) | 4.0 (±0.5) | 0.029∗ |
Independent samples t-test was assessed (∗differences for p < 0.05).
3.3. Total Phenol Content and Antioxidant Capacity
The data in Table 5 showed the total phenolic content, antioxidant capacity of cinnamon tea ingested by the participant. The superoxide anion radical scavenging activity (O2 ∙−) was measured at different concentration of C. burmannii tea. The results revealed that cinnamon tea has a strong inhibitory capacity, in a dose dependent manner, reaching 96% at 1143 mg/L gallic acid (half of the total phenols).
Table 5.
Total phenolic content, antioxidant capacity of cinnamon tea. Values are mean ± SEM.
| Chemical analysis | Mean (±SEM) |
|---|---|
| Total phenols (mg/L gallic acid, n = 8) | 2286,3 (±48,0) |
| Antioxidant capacity: FRAP assay (μmol Trolox/L, n = 6) | 11853,4 (±322,8) |
4. Discussion
Cinnamon capsule ingestion with either aqueous extract or cinnamon powder appears to improve fasting blood glucose level, independently of cinnamon species or extracts [15, 29]. Doubly linked polyphenol type-A polymers were identified, in the Ziegenfuss et al. study, as one of the possible bioactive compounds responsible for this effect [30]. In this study the administration of aqueous C. burmannii extract capsule (Cinnulin PF), with 1% of doubly linked polyphenol type-A polymers, improved fasting blood glucose levels, in prediabetes subjects. Moreover, in type 2 diabetic subjects or impaired fasting blood glucose, the administration of aqueous cinnamon extract also significantly reduced fasting blood glucose levels [31, 32].
In spite of consistent results regarding fasting blood glucose levels, the effect of cinnamon on postprandial glycaemia revealed heterogeneous results, which could be attributed to the bioactive compounds composition (which depends on extraction process, doses, species, and formulation), population samples, and study design employed in different studies [29].
The results of the present study demonstrated that cinnamon tea administration (6 g of C. burmannii into 100 mL water) slightly reduced PBG level after OGTT. The beneficial effects of this spice on glycaemia were reported after cinnamon powder ingestion where a significant reduction of PBG after 30 min of OGTT was observed [16, 17, 33]. However, Magistrelli and coauthors [33] showed no effect at 120 min after meal with cinnamon administration, compared with control meal. Other published data reported that cinnamon does not alter BGL at 120 minutes after OGTT [16, 17].
Although previous studies demonstrated that 3 g of cinnamon powder did not significantly alter AUC, C max and ΔC max BGL [34], the results from the present work showed that C. burmannii tea after OGTT significantly reduced C max (p = 0.040) and ΔC max (p = 0.029) compared with OGTT without cinnamon tea. This effect may be due to the high concentration employed in this study (6 g) compared with the other study, which uses 3 g.
Different molecular mechanisms have been suggested for the hypoglycaemic properties of this spice including reducing gastric empting [17], insulin-mimetic action [35, 36], which can lead to cellular glucose uptake [23], and reducing intestinal glycosidase activity. This effect on enzyme decreased breakdown of disaccharides into glucose, allowing a slow absorption of glucose and reducing PBG level [37].
The hypoglycaemic effect of cinnamon observed in the present study could also be attributed to the phenolic content of C. burmannii tea. According to literature, the molecular mechanism of action of cinnamon polyphenols includes the increase of insulin receptor-β protein in adipocytes suggesting acting beneficially in insulin signalling [35].
The data from this study suggest that the use of cinnamon tea can be beneficial to postprandial glucose levels; moreover its high phenolic content and antioxidant activity could also act beneficially. A significant relationship was found between antioxidant properties and total phenolic content in plants, suggesting that phenols are the bioactive compounds which contributed to their antioxidants activity [38]. In a study of Peng and coauthors, they show that proanthocyanidins of aqueous cinnamon extract can prevent the formation of advanced glycation-end product (AGE) [39]. AGE could be originated in high blood glucose levels conditions leading to reactive oxygen species production [5, 40]. Particularly, cinnamon tea employed in this study revealed a high activity of superoxide anion scavenging, which is in agreement with other published data demonstrating a strong scavenger capacity to free radicals in vitro models [41].
5. Conclusion
Data from this study provide evidence that cinnamon tea significantly decreased postprandial maximum glucose level in nondiabetic adults. The mechanism for cinnamon effect on glycaemia, based on slowing absorption of glucose through reducing intestinal glycosidase activity, cannot be applied to the present work since glucose solution was employed. One possible mechanism proposed to explain the effect of cinnamon tea on glycaemia may be related to the insulin action through the increasing of insulin receptor-β protein acting beneficially in insulin signalling. Further studies should be performed to investigate this mechanism.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Monnier L., Lapinski H., Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients. Diabetes Care. 2003;26(3):881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 2.Woerle H. J., Neumann C., Zschau S., et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes. Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Research and Clinical Practice. 2007;77(2):280–285. doi: 10.1016/j.diabres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Chien K.-L., Lee B.-C., Lin H.-J., Hsu H.-C., Chen M.-F. Association of fasting and post-prandial hyperglycemia on the risk of cardiovascular and all-cause death among non-diabetic Chinese. Diabetes Research and Clinical Practice. 2009;83(2):e47–e50. doi: 10.1016/j.diabres.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Lin H.-J., Lee B.-C., Ho Y.-L., et al. Postprandial glucose improves the risk prediction of cardiovascular death beyond the metabolic syndrome in the nondiabetic population. Diabetes Care. 2009;32(9):1721–1726. doi: 10.2337/dc08-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoguchi T., Li P., Umeda F., et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 6.Node K., Inoue T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovascular Diabetology. 2009;8, article 23:10. doi: 10.1186/1475-2840-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng F., Lu W., Jia C., Li H., Wang Z., Jia W. Relationships between glucose excursion and the activation of oxidative stress in patients with newly diagnosed type 2 diabetes or impaired glucose regulation. Endocrine. 2010;37(1):201–208. doi: 10.1007/s12020-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A., Taboga C., Tonutti L., et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106(10):1211–1218. doi: 10.1161/01.cir.0000027569.76671.a8. [DOI] [PubMed] [Google Scholar]
- 9.Bartoli E., Fra G. P., Schianca G. P. C. The oral glucose tolerance test (OGTT) revisited. European Journal of Internal Medicine. 2011;22(1):8–12. doi: 10.1016/j.ejim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Oka R., Yagi K., Sakurai M., et al. Insulin secretion and insulin sensitivity on the oral glucose tolerance test (OGTT) in middle-aged Japanese. Endocrine Journal. 2012;59(1):55–64. doi: 10.1507/endocrj.EJ11-0157. [DOI] [PubMed] [Google Scholar]
- 11.Wolever T. M. S., Mehling C. Long-term effect of varying the source or amount of dietary carbohydrate on postprandial plasma glucose, insulin, triacylglycerol, and free fatty acid concentrations in subjects with impaired glucose tolerance. American Journal of Clinical Nutrition. 2003;77(3):612–621. doi: 10.1093/ajcn/77.3.612. [DOI] [PubMed] [Google Scholar]
- 12.Brand-Miller J., Hayne S., Petocz P., Colagiuri S. Low—glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24(5):775–778. doi: 10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 14.Suksomboon N., Poolsup N., Boonkaew S., Suthisisang C. C. Meta-analysis of the effect of herbal supplement on glycemic control in type 2 diabetes. Journal of Ethnopharmacology. 2011;137(3):1328–1333. doi: 10.1016/j.jep.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 15.Allen R. W., Schwartzman E., Baker W. L., Coleman C. I., Phung O. J. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Annals of Family Medicine. 2013;11(5):452–459. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon T. P. J., Blannin A. K. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes, Obesity and Metabolism. 2007;9(6):895–901. doi: 10.1111/j.1463-1326.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 17.Hlebowicz J., Darwiche G., Björgell O., Almér L.-O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. American Journal of Clinical Nutrition. 2007;85(6):1552–1556. doi: 10.1093/ajcn/85.6.1552. [DOI] [PubMed] [Google Scholar]
- 18.Soni R., Bhatnagar V. Effect of cinnamon (Cinnamomum cassia) intervention on blood glucose of middle aged adult male with non insulin dependent diabetes mellitus (NIDDM) Ethno-Medicine. 2009;3:141–144. [Google Scholar]
- 19.Anderson R. A., Broadhurst C. L., Polansky M. M., et al. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. Journal of Agricultural and Food Chemistry. 2004;52(1):65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 20.Ranjbar A., Ghaseminejhad S., Takalu H., Baiaty A., Rahimi F., Abdollahi M. Anti oxidative stress potential of Cinnamon (Cinnamomum zeylanicum) in operating room personnel; a before/after cross sectional clinical trial. International Journal of Pharmacology. 2007;3(6):482–486. doi: 10.3923/ijp.2007.482.486. [DOI] [Google Scholar]
- 21.Amin K. A., Abd El-Twab T. M. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: role of atorvastatine and cinnamon. International Journal of Clinical and Experimental Medicine. 2009;2(3):254–265. [PMC free article] [PubMed] [Google Scholar]
- 22.ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y., Fukushima M., Ito Y., et al. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Bioscience, Biotechnology and Biochemistry. 2010;74(12):2418–2425. doi: 10.1271/bbb.100453. [DOI] [PubMed] [Google Scholar]
- 24.Rama Prabha M., Vasantha K. Antioxidant, cytotoxicity and polyphenolic content of calotropis procera (ait.) r. br. flowers. Journal of Applied Pharmaceutical Science. 2011;1(7):136–140. [Google Scholar]
- 25.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 2006;19(6-7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 26.Morais Z. B., Pintão A. M., Costa I. M., Calejo M. T., Bandarra N. M., Abreu P. Composition and in vitro antioxidant effects of jellyfish catostylus tagi from sado estuary (SW Portugal) Journal of Aquatic Food Product Technology. 2009;18(1-2):90–107. doi: 10.1080/10498850802581799. [DOI] [Google Scholar]
- 27.Alam M. N., Bristi N. J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallagher D., Heymsfield S. B., Heo M., Jebb S. A., Murgatroyd P. R., Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. The American Journal of Clinical Nutrition. 2000;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 29.Rafehi H., Ververis K., Karagiannis T. C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes, Obesity and Metabolism. 2012;14(6):493–499. doi: 10.1111/j.1463-1326.2011.01538.x. [DOI] [PubMed] [Google Scholar]
- 30.Ziegenfuss T. N., Hofheins J. E., Mendel R. W., Landis J., Anderson R. A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. Journal of the International Society of Sports Nutrition. 2006;3(2):45–53. doi: 10.1186/1550-2783-3-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mang B., Wolters M., Schmitt B., et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. European Journal of Clinical Investigation. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 32.Roussel A.-M., Hininger I., Benaraba R., Ziegenfuss T. N., Anderson R. A. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. Journal of the American College of Nutrition. 2009;28(1):16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 33.Magistrelli A., Chezem J. C. Effect of ground cinnamon on postprandial blood glucose concentration in normal-weight and obese adults. Journal of the Academy of Nutrition and Dietetics. 2012;112(11):1806–1809. doi: 10.1016/j.jand.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 34.Hlebowicz J., Hlebowicz A., Lindstedt S., et al. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. The American Journal of Clinical Nutrition. 2009;89(3):815–821. doi: 10.3945/ajcn.2008.26807. [DOI] [PubMed] [Google Scholar]
- 35.Cao H., Polansky M. M., Anderson R. A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Archives of Biochemistry and Biophysics. 2007;459(2):214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Qin B., Dawson H. D., Schoene N. W., Polansky M. M., Anderson R. A. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition. 2012;28(11-12):1172–1179. doi: 10.1016/j.nut.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Kim S. H., Hyun S. H., Choung S. Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. Journal of Ethnopharmacology. 2006;104(1-2):119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 38.Dudonné S., Vitrac X., Coutiére P., Woillez M., Mérillon J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. Journal of Agricultural and Food Chemistry. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 39.Peng X., Cheng K.-W., Ma J., et al. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. Journal of Agricultural and Food Chemistry. 2008;56(6):1907–1911. doi: 10.1021/jf073065v. [DOI] [PubMed] [Google Scholar]
- 40.King G. L., Loeken M. R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochemistry and Cell Biology. 2004;122(4):333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 41.Su L., Yin J.-J., Charles D., Zhou K., Moore J., Yu L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chemistry. 2007;100(3):990–997. doi: 10.1016/j.foodchem.2005.10.058. [DOI] [Google Scholar]


