Abstract
BACKGROUND AND OBJECTIVES:
Prospective data are lacking to determine which children might benefit from prompt neuroimaging after unprovoked seizures. We aimed to determine the prevalence of, and risk factors for, relevant intracranial abnormalities in children with first, unprovoked seizures.
METHODS:
We conducted a 6-center prospective study in children aged >28 days to 18 years with seemingly unprovoked seizures. Emergency department (ED) clinicians documented clinical findings on a standardized form. Our main outcome was the presence of a clinically relevant intracranial abnormality on computed tomography (CT) or MRI, defined as those that might change management, either emergently, urgently, or nonurgently.
RESULTS:
We enrolled 475 of 625 (76%) eligible patients. Of 354 patients for whom cranial MRI or CT scans were obtained in the ED or within 4 months of the ED visit, 40 (11.3%; 95% confidence interval [CI]: 8.0–14.6%) had clinically relevant intracranial abnormalities, with 3 (0.8%; 95% CI: 0.1–1.8%) having emergent/urgent abnormalities. On logistic regression analysis, a high-risk past medical history (adjusted odds ratio: 9.2; 95% CI: 2.4–35.7) and any focal aspect to the seizure (odds ratio: 2.5; 95% CI: 1.2–5.3) were independently associated with clinically relevant abnormalities.
CONCLUSIONS:
Clinically relevant intracranial abnormalities occur in 11% of children with first, unprovoked seizures. Emergent/urgent abnormalities, however, occur in <1%, suggesting that most children do not require neuroimaging in the ED. Findings on patient history and physical examination identify patients at higher risk of relevant abnormalities.
What’s Known on This Subject:
Weak recommendations exist to guide emergent neuroimaging decisions in children with first, unprovoked seizures. The prevalence of and risk factors associated with clinically relevant abnormalities on neuroimaging have not been well defined in prospective studies.
What This Study Adds:
Clinically relevant intracranial abnormalities on neuroimaging occur in 11% of children with first, unprovoked seizures. Emergent/urgent abnormalities, however, occur in <1%, suggesting that most of these children do not require emergent neuroimaging. Specific clinical findings identify patients at higher risk.
Annually, 25 000-40 000 children in the United States sustain a first seizure that is not associated with a precipitant such as fever or trauma (ie, an unprovoked seizure).1–3 Approximately 8% to 31% of children with first, unprovoked seizures have abnormalities upon neuroimaging and <1% to 8% have abnormalities warranting intervention.4–12 The wide range of estimates results from the varying populations studied, differing outcome definitions, and the varied use of MRI.4–12 The majority of patients undergo acute neuroimaging, most frequently computed tomography (CT).6 The use of CT exposes the child to substantial radiation, with the risk of inducing malignancy.13–15
Several clinical predictors appear to increase the prevalence of intracranial abnormalities in children with unprovoked seizures.5–11,16–18 Most previous studies, however, have been retrospective, have not included standardized evaluations of patients, or the clinical findings were not elicited by acute care providers.4–12,16–21 Existing guidelines provide only weak recommendations regarding which children might benefit from prompt neuroimaging.9–11
Our primary aim was to determine the prevalence of, and risk factors for, clinically relevant intracranial abnormalities on neuroimaging in a well-described group of children who presented to medical care for the first time after having sustained unprovoked seizures. Our secondary aim was to derive a prediction model to identify children in this population at low risk of clinically relevant intracranial abnormalities.
Methods
Study Design
We performed a prospective study in children presenting with apparently unprovoked seizures to any of 6 urban, university-affiliated, pediatric emergency departments (EDs) in the United States from March 2005 through September 2007. The study was approved by each center’s institutional review board. We obtained written informed consent from the guardian at 1 site and obtained waivers of written informed consent at the remaining sites.
Eligibility
Children aged 29 days to 18 years were eligible if they presented to the ED for seizure evaluation and had (1) a first, apparently unprovoked seizure (ie, incident cases) or (2) a history of a previous, apparently unprovoked seizure for which the patient had not been medically evaluated (ie, prevalent cases). The select group of prevalent patients was included because clinicians must nonetheless decide if prompt neuroimaging is indicated.
We excluded patients for any of the following reasons: syncopal or breath-holding episodes, altered mental status without seizure symptoms, known neurologic disorders that complicated the neurologic examination, or absence seizures. We also excluded patients with head trauma within 24 hours of presentation, fever in the previous 24 hours (≥38.0°C), toxin ingestion, or known metabolic disorder predisposing to seizures. In addition, we excluded children if they had previous medical evaluations by neurologists for seizures or if transferred from an outside facility with neuroimaging already completed. Finally, we excluded patients if they had previous neuroimaging for seizures, but we did not exclude patients if the neuroimaging was performed for other purposes.
Assessments
An ED clinician (faculty physician, fellow physician, resident physician, nurse practitioner, or physician assistant) performed a standardized history and physical examination and recorded the findings on a structured data form. Clinicians assessed specific patient history and clinical findings in 4 domains: past and recent medical history, seizure-specific history, general physical examination, and neurologic examination. We chose the clinical findings on the basis of an extensive review of the literature and through detailed discussions with faculty physicians who were expert in pediatric and adult neurology.5–11
To assess interrater reliability of clinical findings, a second clinician performed an independent evaluation within 30 minutes of the first assessor on a convenience sample (47%) of those enrolled (details published previously).22 Clinician assessors were asked to complete the data form before they had knowledge of cranial CT or MRI results, if obtained. Clinical assessors were also asked to complete the physical examination, when possible, after the postictal phase or after the patient awakened from any sedative effects of medications administered.
We conducted telephone follow-up calls 2 weeks and 4 months after enrollment to determine the results of any cranial CT or MRI subsequent to the ED visit and to determine the clinical course. If the legal guardian was unavailable by telephone, we conducted a mail survey.
During the 2-week telephone follow-up call, trained research coordinators or site investigators also obtained detailed narratives of the seizure events. These seizure assessments were reviewed by at least 1 epileptologist or the lead investigator, blinded to patient outcomes, to determine if a seizure actually occurred, whether there was a clear precipitant, and whether it was an incident or prevalent seizure. We excluded patients from the final analysis if the event was clearly not a seizure, the seizure was of the absence type, or there was a clear precipitant. In the final analysis, we included patients for whom we were not able to complete the detailed follow-up seizure assessment. To assess for enrollment bias, we reviewed ED patient logs to identify missed eligible patients.
Outcomes: Clinically Relevant Intracranial Abnormalities
Our main outcome was the presence or absence of a clinically relevant intracranial abnormality on neuroimaging. A priori, we defined neuroimaging results as normal, showing a benign finding, or having a clinically relevant intracranial abnormality (Table 2). We defined clinically relevant intracranial abnormalities as those that would potentially result in a change in management.5,6 We subdivided clinically relevant neuroimaging abnormalities into emergent/urgent and nonurgent categories. We considered emergent/urgent abnormalities as those typically warranting immediate or urgent therapeutic or diagnostic interventions, whether the abnormalities were felt to be the causes of or related to the seizures. Categorization of the findings was determined by review of the literature, input from 4 pediatric emergency faculty physicians, 2 epileptologists, and 1 pediatric neurosurgeon. Subsequently, pediatric emergency medicine and general pediatric physicians reviewed the outcomes for face validity.
TABLE 2.
Frequency of Clinically Relevant Intracranial Findings on Neuroimaging
| Value, n (%) or n (%; 95% CI) | |
|---|---|
| Any emergent/urgent or nonurgent intracranial abnormality | 40 (11.3; 8.0–14.6) |
| Emergent/urgent | 3 (0.8; 0.1–1.8) |
| Tumor or metastases | 2 (0.6) |
| Infarction | 1 (0.3) |
| Intracranial hemorrhage | 0 (0) |
| Cerebral edema | 0 (0) |
| Shift of midline structures, | 0 (0) |
| Abscess | 0 (0) |
| Cysticercosis with edema | 0 (0) |
| Obstructive hydrocephalus | 0 (0) |
| Nonurgenta | 37 (10.5; 7.3–13.6) |
| Abnormal myelination | 13 (3.7) |
| Cortical or subcortical hyperintensities | 9 (2.5) |
| Chiari I malformation | 5 (1.4) |
| Migration abnormality | 3 (0.8) |
| Mesial temporal sclerosis | 2 (0.6) |
| Hippocampal shape abnormalities | 2 (0.6) |
| White matter increased signal | 2 (0.6) |
| Cavernous or venous angioma | 2 (0.6) |
| Cysticercosis without edema | 1 (0.3) |
| Atrioventricular malformation | 1 (0.3) |
| Focal calcifications | 1 (0.3) |
| Gliosis | 1 (0.3) |
| Empty sella syndrome | 1 (0.3) |
| Focal encephalomalacia | 1 (0.3) |
| Calcifications of the meninges | 1 (0.3) |
| Porencephalic cyst | 1 (0.3) |
| Temporal lobe arachnoid cyst | 0 (0) |
| Blurred gray-white differentiation | 0 (0) |
N = 354. IQR, interquartile range.
Patients could have >1 finding.
Neuroimaging in the ED and as an outpatient was at the discretion of the treating clinicians. We used the results from MRI scans when clinicians performed both CT and MRI. For patients with equivocal neuroimaging findings on the initial radiologist reading, a radiologist at each site, masked to clinical data, reread the studies to provide definitive assessments. For those imaging studies stated on 2-week or 4-month telephone follow-up to have been conducted outside the participating centers, we requested a release of information from the parents and subsequently attempted to obtain the neuroimaging reports. For those without follow-up neuroimaging, we considered there to be no emergent intracranial abnormality if no specific (eg, neurosurgical) intervention had been performed at the 2-week or 4-month follow-up.
Analysis
We used proportions with 95% confidence intervals (CIs) to describe the prevalence of clinically relevant intracranial abnormalities. We calculated relative risks with 95% CIs to identify risk factors potentially associated with clinically relevant intracranial abnormalities.
For certain potential predictors, we created clinically sensible dichotomous composite variables. For example, we created the variable “any focal component to the seizure” as the composite of any seizure with either a focal motor component or one in which the head or eyes turned to one side during the seizure. In addition, we created the dichotomous variable “high-risk past medical history” to categorize predisposing conditions that may increase the risk of emergent/urgent intracranial abnormalities, including the presence of an intracranial ventricular shunt or a previous history of a brain tumor, other neoplasm, stroke, coagulopathy, sickle cell disease, or anatomic cardiac defect.
We conducted a backward elimination procedure (and confirmatory stepwise selection procedure) to build a multivariable logistic regression model, including those variables with a P < .10 on χ2 bivariable analyses, to identify independent risk factors associated with the presence of a clinically relevant intracranial abnormality. We confirmed the choice of the final model on the basis of the lowest Akaike information criterion values. We conducted 2 regression analyses, one including and one excluding those with high-risk past medical histories. We did so because many clinicians would obtain acute neuroimaging for these high-risk patients regardless of clinical findings on physical examination. In the logistic regression analyses, we only included the composite variables rather than the individual findings (eg, including “any focal aspect” to the seizure rather than individual focal aspects). Using SAS software (SAS Institute, Cary, NC), we imputed values for predictors with missing values by estimating the probability of a “yes” response, then sampling a Bernoulli distribution of these probabilities with a random seed and parameter “p” for each missing patient value.23
Finally, we performed recursive partitioning analysis to derive prediction models to identify children at low risk of clinically relevant intracranial abnormalities, attempting to maximize model sensitivity and negative predictive value. We included only those individual predictors that had a potential bivariable association with the outcome (ie, P < .10) and at least moderate interrater reliability (κ value of ≥0.4).24 We varied the assignment of high penalties (costs) for not identifying a patient with a clinically relevant intracranial abnormality, while attempting to maintain a good specificity. We conducted 10-fold internal cross-validation to protect against overfitting of the models. For each rule, we calculated sensitivity, specificity, positive predictive value, and negative predictive value with 95% CIs.
We calculated relative risks with 95% CIs with an online calculator (http://www.ebm.med.ualberta.ca/TherapyCalc.html). We performed the descriptive analysis using SPSS version 20 (IBM Corporation, Armonk, NY), the bivariable and multivariable regression analyses with SAS version 9.3 (SAS Institute, Cary, NC), and the recursive partitioning analysis with CART PRO 6.0 (Salford Systems, San Diego, CA).
Results
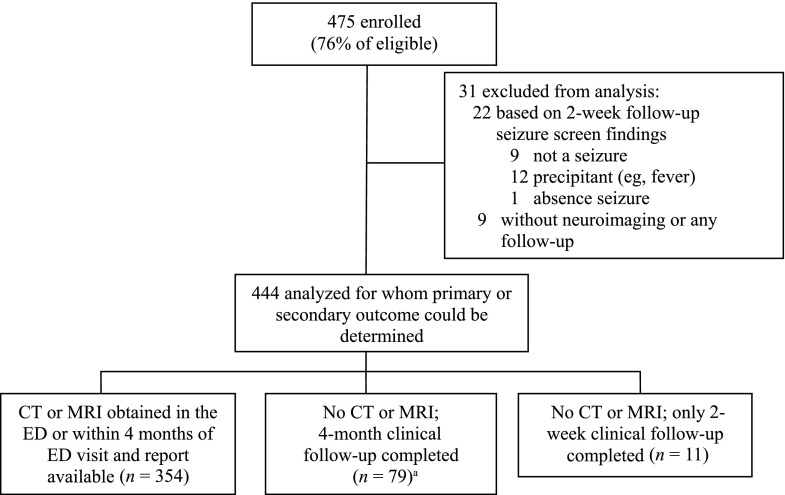
We enrolled 475 (76%) of 625 eligible children (Fig 1). Of the 444 included patients, 354 (79.7%) had CT or MRI scans performed within 4 months of the index ED visit. Cranial CT was the most frequent imaging study obtained in the ED (285 of 444 patients; 64.2%). Most patients (333 of 354; 94.1%) had all of their CT and/or MRI scans performed at participating sites. Of the 21 patients who had neuroimaging studies performed at outside sites, we obtained the neuroimaging reports for 11 (52.4%). The remaining 10 patients had MRI scans reported as normal by the parents; 5 had CT scans at the index ED visit that were also normal. Table 1 shows the patient characteristics and clinical management of those for whom CT or MRI scans were performed.
FIGURE 1.
Flow diagram. aOne patient had a cranial MRI but report was unavailable; 4-month follow-up was completed and the patient was included in this follow-up group.
TABLE 1.
Patient Characteristics and Clinical Management
| Characteristic | Patients With CT or MRI Scan (in ED or Obtained Within 4 Months of ED Visit and Have Known Results) (N = 354) |
|---|---|
| Median (IQR) age, mo | 76.5 (14.6–134.4) |
| Age, n (%) | |
| <24 months | 116 (32.8) |
| <12 months | 78 (22.0) |
| Male, n (%) | 194 (54.8) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 149 (42.1) |
| Black, non-Hispanic | 78 (22.0) |
| Hispanic | 83 (23.4) |
| Asian | 5 (1.4) |
| Other | 5 (1.4) |
| Missing | 34 (9.6) |
| Incident seizures,a n/N (%) | 281/286 (98.6) |
| ED disposition, n (%) | |
| Discharged to home | 231 (65.3) |
| Hospitalized | 123 (34.7) |
| Intensive care | 13 (3.7) |
| General inpatient service | 110 (31.0) |
| Cranial neuroimaging | |
| CT or MRI completed within 4 | |
| months of enrollment,b n (%) | 354 (79.7)c |
| CT only, n/N (%) | 167/354 (47.2) |
| MRI only, n/N (%) | 53/354 (14.9) |
| CT and MRI, n/N (%) | 134/354 (37.9) |
Denominator includes those patients who had 2-week follow-up completed and enough details provided by the parents to categorize index seizure as either an incident, unprovoked seizure (had not had one previously) or a prevalent seizure (had a previous unprovoked seizure but had neither been evaluated by a neurologist nor had neuroimaging).
Excludes 1 patient for whom MRI was completed but report was unavailable; patient was not included in further neuroimaging analysis.
A total of 285 of 354 (80.5%) underwent head CTs in the ED.
Compared with enrolled patients, the missed eligible patients (n = 150) were younger (median age: 44.2 months) and slightly more likely to be admitted to the ICU (8 of 150; 5.3%; 3 were intubated and 1 was ventilator-dependent). The missed patients, however, were similar to those enrolled in the proportion hospitalized (49 of 150 [32.7%] vs 129 of 444 [29.0%]), the proportion with clinically relevant intracranial abnormalities (10 of 95 [10.5%] vs 40 of 354 [11.3%]), and the proportion with emergent/urgent intracranial abnormalities (1 of 95 [1.1%] vs 3 of 354 [0.8%]).
Prevalence of Clinically Relevant Intracranial Abnormalities
Of the 354 patients for whom MRI or CT scans were obtained in the ED or within 4 months of the ED visits, 40 (11.3%; 95% CI: 8.0–14.6%) had clinically relevant intracranial abnormalities, of whom 3 patients (0.8%; 95% CI: 0.1–1.8%) had abnormalities classified as emergent/urgent (Table 2). Of the 341 patients without high-risk past medical histories for whom CT or MRI scans were completed, 35 (10.3%; 95% CI: 7.1–13.6%) had clinically relevant intracranial abnormalities. Only 1 child underwent neurosurgery, a 16-year-old with a newly diagnosed astrocytoma.
Of the 90 patients who did not have neuroimaging performed at any time after enrollment but who had 2-week or 4-month follow-up completed, none had neurosurgical or other specific interventions performed after enrollment. Overall, of the 431 patients without high-risk past medical histories and who had either neuroimaging or follow-up completed, only 1 (0.2%; 95% CI: 0–0.7%), the child with an astrocytoma, had a neurosurgical procedure.
Risk Factors
In Tables 3 and 4, we present the relative risks of clinically relevant abnormalities on neuroimaging on the basis of the presence or absence of general and seizure-specific histories (Table 3) and physical examination findings (Table 4). The risk of clinically relevant abnormalities on neuroimaging did not differ substantially when we compared those aged <1 year with children aged 1 to 18 years (12.8% vs 10.9%) or those aged <2 years with children aged 2 to 18 years (13.8% vs 10.1%).
TABLE 3.
Risk of Clinically Relevant Intracranial Abnormalities Based on Patient History
| Risk of Abnormality if Clinical History Finding Present,a n/N (%) | Risk of Abnormality if Clinical History Finding Absent,a n/N (%) | RR (95% CI) | Missing | Response of “Unsure,” “Don’t Know,” “Preverbal,” “Nonverbal” | |
|---|---|---|---|---|---|
| General history | |||||
| Age <12 months | 10/78 (12.8) | 30/276 (10.9) | 1.2 (0.6–2.3) | 0 | 0 |
| High-risk past medical historyb | 5/13 (38.5) | 35/340 (10.3) | 3.7 (1.8–8.0)* | 0 | 1 |
| Exposure to cysticercosis | 0/5 (0) | 36/312 (11.5) | 0 | 8 | 29 |
| Nausea or vomiting before seizure | 6/47 (12.8) | 32/297 (10.8) | 1.2 (0.5–2.8) | 6 | 4 |
| Behavior change before seizure (sleeping more, less active, more emotional/irritable, unusual behavior) | 4/44 (9.1) | 34/304 (11.2) | 0.8 (0.3–2.2) | 6 | 0 |
| Headache on day of and before seizurec | |||||
| Any headache today | 3/40 (7.5) | 18/172 (10.5) | 0.7 (0.2–2.3) | 3 | 23 |
| Headache today of rapid onset | 0/11 (0) | 20/190 (10.5) | 0 | 5 | 32 |
| Headache today of rapid onset, first headache, or worst headache | 1/19 (5.3) | 19/189 (10.1) | 0.5 (0.1–3.7) | 3 | 27 |
| Previous history of headachesc | |||||
| Any history of headaches | 4/32 (12.5) | 17/184 (9.2) | 1.4 (0.5–3.8) | 4 | 18 |
| Previous headaches in morning or more frequent | 0/11 (0) | 20/203 (9.9) | 0 | 5 | 19 |
| Speech changec | 1/5 (20) | 23/221 (10.4) | 1.9 (0.3–11.6) | 3 | 9 |
| Dizzy, falling, or uncoordinatedc | 2/28 (7.1) | 20/197 (10.2) | 0.7 (0.2–2.8) | 2 | 11 |
| Vision changec (acuity, double/blurred vision, other) | 1/12 (8.3) | 19/208 (9.1) | 0.9 (0.1–6.3) | 2 | 16 |
| Seizure-specific history | |||||
| More than 1 seizure in previous 24 hours | 12/78 (15.4) | 26/267 (9.7) | 1.6 (0.8–3.0) | 3 | 6 |
| Longest seizure in previous 24 hoursd | |||||
| ≥5 min | 12/77 (15.6) | 27/228 (11.8) | 1.3 (0.7–2.5) | 17 | 32 |
| ≥15 min | 6/24 (25.0) | 33/281 (11.7) | 2.1 (1.0–4.6)** | 17 | 32 |
| Any focal aspect to seizure by historye | 24/133 (18.0) | 14/187 (7.5) | 2.4 (1.3–4.5)* | 4 | 30 |
| Motor aspect 1-sided at any point | 13/63 (20.6) | 19/236 (8.1) | 2.6 (1.3–4.9)* | 4 | 51 |
| Head turned to 1 side at any point | 13/58 (22.4) | 19/214 (8.9) | 2.5 (1.3–4.8)* | 5 | 77 |
| Eyes turned to 1 side at any point | 12/72 (16.7) | 18/195 (9.2) | 1.8 (0.9–3.6)** | 6 | 81 |
| Postictal duration ≥60 minutesf | 9/65 (13.8) | 29/250 (11.6) | 1.2 (0.6–2.4) | 4 | 35 |
N = 354. *P < .05, **P = .05–0.10 (χ2 analysis). RR, relative risk.
Denominators exclude patients for whom a data point was missing or marked as “unsure,” “don’t know,” “preverbal,” “nonverbal,” or “unable to assess.”
Defined as having a history of a brain tumor, other neoplasm, stroke, coagulopathy, sickle cell disease, anatomic cardiac defect, or presence of an intracranial ventricular shunt.
Excludes from analysis 116 patients younger than 2 years.
Seizure duration for the 305 patients with duration known was collected as follows: 29 (9.5%) with duration of <15 seconds, 71 (23.2%) with duration of 15 seconds to <1 minute, 128 (42.0%) with duration of 1 to <5 minutes, 53 (17.4%) with duration of 5 to <15 minutes, and 24 (7.9%) with duration of ≥15 minutes.
Defined as motor aspect 1-sided or head or eyes turned to 1 side at any point.
Postictal duration for the 315 patients with known duration of postictal phase was collected as follows: 64 (20.3%) with no postictal phase, 8 (2.5%) with postictal phase of <1 minute, 41 (13.0%) with postictal phase of 1 to <5 minutes, 106 (33.7%) with postictal phase of 5 to <30 minutes, 31 (9.9%) with postictal phase of 30 to <60 minutes, and 65 (20.6%) with postictal phase ≥60 minutes.
TABLE 4.
Risk of Clinically Relevant Intracranial Abnormalities Based on Physical Examination Findings
| Criterion | Risk of Abnormality if Physical Exam Finding Present,a n/N (%) | Risk of Abnormality if Physical Exam Finding Absent,a n/N (%) | RR (95% CI) | Missing | Response of “Unsure,” “Don’t Know,” “Preverbal,” “Nonverbal,” or “Not Done” |
|---|---|---|---|---|---|
| General physical examination | |||||
| Concerning skin findings (eg, café au lait) | 2/7 (28.6) | 38/347 (11.0) | 2.6 (0.8–8.7) | 0 | 0 |
| Neurologic examinationb | |||||
| Abnormal mental statusc | 10/62 (16.1) | 25/266 (9.4) | 1.7 (0.9–3.4) | 3 | 8 |
| Abnormal overall neurologic exam other than mental status | 7/33 (21.2) | 26/291 (8.9) | 2.4 (1.2–5.0)** | 2 | 13 |
| Any focal neurologic findings on exam (includes examination of pupil size, cranial nerves motor tone, motor strength, reflexes) | 5/16 (31.2) | 30/320 (9.4) | 3.3 (1.5–7.4)* | 3 | 0 |
| Abnormal muscle tone (focal or diffuse) | 5/18 (27.8) | 30/317 (9.5) | 3.3 (1.3–6.7)* | 2 | 2 |
| Abnormal motor strength (focal or diffuse) | 4/17 (23.5) | 28/307 (9.1) | 2.6 (1.0–6.5) | 2 | 13 |
| Abnormal sensation (focal or bilateral) | 0/3 | 22/246 (8.9) | 0 | 3 | 87 |
| Abnormal reflexes (focal or bilateral) | 1/11 (9.1) | 26/281 (9.3) | 1.0 (0.1–6.6) | 7 | 40 |
| Abnormal gait | 0/7 | 13/195 (6.7) | 0 | 6 | 131 |
| Abnormal cranial nerves (focal or bilateral) | 1/4 (25.0) | 28/290 (9.7) | 2.6 (0.5–14.7) | 4 | 41 |
| Unequal pupil size | 2/5 (40.0) | 33/328 (10.1) | 4.0 (1.3–12.2) | 4 | 2 |
| Abnormal posturing | 1/3 (33.3) | 28/314 (8.9) | 3.7 (0.7–19.2) | 19 | 3 |
| Abnormal cerebellar functiond | 0/2 | 11/184 (6.0) | 0 | 1 | 42 |
| Abnormal speechd | 0/6 | 14/196 (7.1) | 0 | 1 | 26 |
N = 354. *P < .05, **P = .05–0.10 (χ2 analysis). RR, relative risk.
Denominators exclude patients for whom a data point was missing or marked as “unsure,” “don’t know,” “preverbal,” “nonverbal,” or “unable to assess.”
Excludes 15 patients who were sedated, paralyzed, or intubated at the time of examination.
Mental status for the 328 patients with known mental status was collected as follows: 266 (81.1%) with normal mental status, 45 (13.7%) with mildly impaired mental status, and 17 (5.2%) with moderately/severely impaired mental status.
A priori excludes from all analysis patients either younger than 24 months or those sedated, paralyzed, or intubated at the time of examination (n = 125).
On multivariable analysis, a high-risk past medical history (adjusted odds ratio [OR]: 9.2; 95% CI: 2.4–35.7) and any focal aspect to the seizure (OR: 2.5; 95% CI: 1.2–5.3) were independently associated with clinically relevant abnormalities on neuroimaging; in addition, an overall abnormal neurologic examination (focal or nonfocal) had an adjusted OR of 2.4 (95% CI: 0.96–6.2). When those patients with high-risk past medical histories were removed from analysis, only the presence of a focal finding on neurologic examination was independently associated with the outcome (adjusted OR: 4.1; 95% CI: 1.3–13.6), while any focal aspect to the seizure had an adjusted OR of 1.9 (95% CI: 0.9–4.2). For both multivariable analyses, factors not found to be independently associated with the outcome included age <12 months and seizure lasting >15 minutes.
To further understand the potential importance of neurologic factors that were variably significant on multivariable analyses, we explored the prevalence of clinically relevant intracranial abnormalities in patients without high-risk past medical histories based on the nature of the seizure (ie, focal or not), the presence of an abnormal overall neurologic examination (focal or nonfocal), and the presence of focal findings on neurologic examination (Table 5). Although combinations of these factors increased the prevalence of clinically relevant abnormalities, few patients who had these neuroimaging abnormalities had both focal seizures and either abnormal overall neurologic examinations or focal findings on neurologic examinations.
TABLE 5.
Prevalence of Clinically Relevant Abnormalities Based on Presence or Absence of Specific Clinical Findings in Patients Without a High-Risk Past Medical History
| Predictor(s) | Prevalence of Clinically Relevant Intracranial Abnormality, n/N (%; 95% CI) |
|---|---|
| Nonfocal seizure and normal neurologic examination (no focal or nonfocal abnormalities) | 11/161 (6.8; 3.9–11.9) |
| Both focal seizure and any abnormal neurologic examination findings (focal or nonfocal abnormalities) | 7/19 (36.8; 19.2–59.0) |
| Both focal seizure and focal findings on neurologic examination | 5/13 (38.5; 17.7–64.5) |
Patients for whom clinicians were unsure or unable to assess neurologic examination and patients who were either sedated or paralyzed were excluded.
All 3 patients with emergent/urgent neuroimaging abnormalities had focal aspects to their seizures. Two of the 3 patients also had high-risk past medical histories; 1 patient with a previous cancer (noncerebral) who had new central nervous system metastases and the other with congenital heart disease who had a cerebral infarction. The only patient without a high-risk past medical history who had an emergent/urgent abnormality had >1 seizure in the 24 hours before ED presentation and, during the seizure, her eyes turned to 1 side (ie, sign of focality). Of note, clinicians obtained ED CTs for 134 of 239 (56.1%) children who had neither high-risk histories, nor focal seizures, nor focal neurologic examination findings.
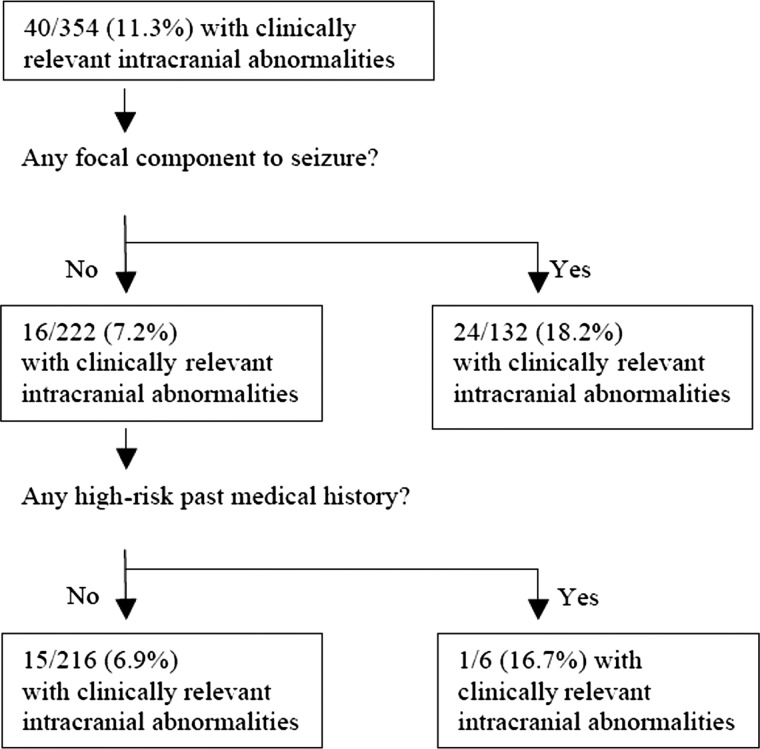
Figure 2 presents the results of the recursive partitioning analysis to identify patients at low risk of clinically relevant intracranial abnormalities on neuroimaging. No clinically sensible model was built that had a sensitivity >62.5%.
FIGURE 2.
Recursive partitioning model to identify children at low risk of clinically relevant intracranial abnormalities. Prediction rule sensitivity: 25/40 (62.5%; 95% CI: 45.5–76.9%). Prediction rule specificity: 201/314 (64.0%; 95% CI: 58.4–69.2%). Prediction rule negative predictive value: 201/216 (93.1%; 95% CI: 88.6–95.9%). Likelihood ratio (−): 0.51 (95% CI: 0.39–0.88). Likelihood ratio (+): 1.74 (95% CI: 1.31–2.30).
Discussion
In this large, multicenter study in a well-described cohort of children with unprovoked seizures, we identified the risk of clinically relevant intracranial abnormalities. Although the risk was ∼11%, abnormalities requiring emergent or urgent intervention occurred in <1%, particularly in those without high-risk past medical histories. In addition to a high-risk past medical history, a focal seizure and focal findings on neurologic examination are risk factors that increase the prevalence of clinically relevant abnormalities. Our results, along with previous data, strongly suggest that neuroimaging in the ED is required in the minority of children with seemingly unprovoked seizures, particularly if the child does not have a concerning past medical history, did not have a focal seizure, and does not have focal neurologic examination findings. In our study, more than half of the patients without high-risk past medical histories and with nonfocal seizures and nonfocal neurologic examinations underwent ED CTs.
Our estimates of the risk of clinically relevant abnormalities are generally similar to those in previous studies, with differences likely due to variability in populations studied and study methods.4–8,12,16,17,19 In 2 large previous prospective studies, in which patients were evaluated by neurologists, the risk of any clinically relevant abnormalities on imaging ranged from 21% to 31%, with the higher estimate noted in a study that exclusively used MRIs.5,12
We identified a somewhat lower estimate of emergent/urgent intracranial abnormalities on neuroimaging than previous studies. In 1 study of patients who were evaluated by neurologists, 4 of 411 children (0.9%) had intracranial lesions requiring intervention, 2 with brain tumors and 2 with neurocysticercosis.5 In a separate retrospective study of 475 children, investigators noted an 8% risk of “clinically significant” abnormalities, defined as neuroimaging findings that changed management or prognosis. That study included patients with traumatic injuries and did not differentiate emergent/urgent from nonurgent findings.6
The risk factors we found to be associated with clinically relevant intracranial abnormalities are clinically sensible, readily available, and consistent with several previous studies.5,7,8 A predisposing past medical condition, focal seizure, and abnormal neurologic examination each have been previously found to increase the risk of intracranial abnormalities.5,7,8 Although we found longer seizure duration (>15 minutes) to be associated with intracranial abnormalities on bivariable analysis, it did not remain significant in our multivariable analysis. Other investigations have variably found prolonged seizure duration as a potential predictor of intracranial abnormalities.5,17,18 In addition, we found no association between the presence of clinically relevant intracranial abnormalities and young age. Our results regarding age are similar to those of the largest similar previous study but conflict with previous (retrospective) studies that suggested that age <6 months was an important predictor.5,16,20 One recent study reported that younger age may help identify children with focal seizures at higher risk of emergent abnormalities.18
The clinical risk factors that were independently associated with intracranial abnormalities have been previously shown to have moderate-to-substantial interobserver agreement among pediatric emergency medicine physicians (eg, any seizure focality: κ = 0.58; 95% CI: 0.45–0.71; any focal neurologic findings on examination: κ = 0.66; 95% CI: 0.3–1.0).22 However, clinical findings are likely to be interpreted differently among physicians with different areas of expertise (eg neurologists, and ED physicians) and different levels of training (eg, faculty versus residents), as well as among clinicians with similar training.
We were unable to derive models that identified patients at very low risk of clinically relevant intracranial abnormalities with great accuracy. The factors in our final model are relatively similar (except for their inclusion of patient age) to those in one previously derived model.6 One likely reason that sensitive models are challenging to derive is the inclusive list of findings used to define clinically relevant abnormalities. Although the neuroimaging findings may be relevant to patient care, they include abnormalities of greater and lesser urgency to identify.10
Finally, although ED imaging may not be essential in most children with first, unprovoked seizures because the risk of emergent abnormalities is low, ED clinicians must also consider the availability of timely neurology follow-up when making management decisions, including imaging decisions. When neurology follow-up is prompt, the neurologist can determine whether an unprovoked seizure occurred, if the seizure type fits a pattern (eg, benign Rolandic epilepsy), and the need and urgency to perform an MRI. For situations in which neurology follow-up will likely be delayed (eg, months), the ED clinician will need to consider whether obtaining an MRI during the ED visit or scheduling an MRI is appropriate, because nonurgent findings can impact further management.
Because we did not collect the time from seizure to ED presentation, we are unsure that this time was not prolonged, which might affect the clinical evaluation. However, because parents are typically frightened by the seizure and because the seizure was the reason for ED presentation, we expect that the history and physical examination reflected typical acute evaluations. In addition, the missed eligible patients appeared to be somewhat more ill than enrolled patients; however, there was no meaningful difference in the proportion with relevant neuroimaging abnormalities.
Not all of the patients in the study received neuroimaging and, if they did, many of these scans were solely CTs. The lack of MRIs for all patients may have decreased our overall risk estimate for clinically relevant findings, although findings identified on MRI and not on CT would likely be nonurgent, with a remote likelihood that an emergent/urgent finding could be absent on CT yet present on MRI.
Although few in number in our sample (n = 13), we also included patients with high-risk past medical histories, who had a higher risk of relevant intracranial abnormalities. We included these patients because in addition to their past medical histories, their acute signs and symptoms (eg, seizure focality) likely help differentiate those with and without relevant intracranial abnormalities. Understanding their higher risk, we conducted analyses with and without these patients. Previous studies have not explicitly defined those diseases that constitute a high-risk past medical history. When those with high-risk past medical histories were removed from analysis, the risk of a clinically relevant intracranial abnormality changed little.
Finally, our intent was to focus on neuroimaging findings that might impact clinical management; as such, we did not attempt to determine whether these findings were the etiology of the seizures. These potential limitations, however, are balanced by the prospective nature of the study, the clinical evaluation by frontline providers at the time of ED presentation for the seizure, and the large cohort studied.
Conclusions
Approximately 11% of children with first, apparently unprovoked seizures have clinically relevant intracranial abnormalities on neuroimaging. Findings on patient history and physical examination can identify patients at higher risk of relevant abnormalities. Emergent/urgent abnormalities, however, occur in <1% of patients, suggesting that most of these children do not require neuroimaging, particularly CT, in the ED.
Acknowledgments
We thank Dr Emmanuel Pena, Ms Deborah York, and Mr Carl Brown for their tireless efforts necessary to complete this study. We also thank the clinicians, research coordinators, and nurses at each site who voluntarily provided their valuable time to help enroll patients into this study. Finally, we thank Steven Hollaren and Rajasekhar Ramakrishnan MS, ScD, for providing statistical support.
Glossary
- CI
confidence interval
- CT
computed tomography
- ED
emergency department
- OR
odds ratio
Footnotes
Dr Dayan conceived of and designed the study, including the data collection instruments; supervised the conduct of the study and data collection at his center; managed the data; completed data analyses; and drafted the initial manuscript; Drs Lillis, Bennett, Conners, Bailey, and Callahan supervised the conduct of the study and data collection at their sites and critically reviewed the manuscript; Dr Akman contributed to the study design, completed study procedures, and critically reviewed the manuscript; Dr Feldstein contributed to the study design and critically reviewed the manuscript; Mr Kriger conducted analyses and reviewed and revised the manuscript; Dr Hauser helped design the study, completed study procedures, and critically reviewed the manuscript; Dr Kuppermann conceived of and designed the study, supervised the conduct of the study, completed data analyses, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
The content in this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant KL2 TR000081. The study was also supported by cooperative agreement U03MC00007 from the Health Resources and Services Administration/Maternal and Child Health Bureau Emergency Medical Services for Children Program. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kaufman L, Hesdorffer D, Mu Kherjee R, Hauser WA. Incidence of first unprovoked seizures among children in Washington Heights, New York City, 1990–1994. Epilepsia. 1996;37:85 (abstract) [Google Scholar]
- 2.Verity CM, Ross EM, Golding J. Epilepsy in the first 10 years of life: findings of the Child Health and Education Study. BMJ. 1992;305(6858):857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993;34(3):453–468 [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics. 2000;106(3):527–532 [DOI] [PubMed] [Google Scholar]
- 5.Shinnar S, O’Dell C, Mitnick R, Berg AT, Moshe SL. Neuroimaging abnormalities in children with an apparent first unprovoked seizure. Epilepsy Res. 2001;43(3):261–269 [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Riviello JJ, Harper MB, Baskin MN. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111(1):1–5 [DOI] [PubMed] [Google Scholar]
- 7.Maytal J, Krauss JM, Novak G, Nagelberg J, Patel M. The role of brain computed tomography in evaluating children with new onset of seizures in the emergency department. Epilepsia. 2000;41(8):950–954 [DOI] [PubMed] [Google Scholar]
- 8.Garvey MA, Gaillard WD, Rusin JA, et al. Emergency brain computed tomography in children with seizures: who is most likely to benefit? J Pediatr. 1998;133(5):664–669 [DOI] [PubMed] [Google Scholar]
- 9.Hirtz D, Ashwal S, Berg A, et al. Practice parameter: evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, The Child Neurology Society, and The American Epilepsy Society. Neurology. 2000;55(5):616–623 [DOI] [PubMed] [Google Scholar]
- 10.Gaillard WD, Chiron C, Cross JH, et al. International League Against Epilepsy, Committee for Neuroimaging, Subcommittee for Pediatric . Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50(9):2147–2153 [DOI] [PubMed] [Google Scholar]
- 11.Harden CL, Huff JS, Schwartz TH, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology . Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69(18):1772–1780 [DOI] [PubMed] [Google Scholar]
- 12.Kalnin AJ, Fastenau PS, deGrauw TJ, et al. Magnetic resonance imaging findings in children with a first recognized seizure. Pediatr Neurol. 2008;39(6):404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warden CR, Brownstein DR, Del Beccaro MA. Predictors of abnormal findings of computed tomography of the head in pediatric patients presenting with seizures. Ann Emerg Med. 1997;29(4):518–523 [DOI] [PubMed] [Google Scholar]
- 17.Bautovich T, Numa A. Role of head computed tomography in the evaluation of children admitted to the paediatric intensive care unit with new-onset seizure. Emerg Med Australas. 2012;24(3):313–320 [DOI] [PubMed] [Google Scholar]
- 18.Aprahamian N, Harper MB, Prabhu SP, et al. Pediatric first time non-febrile seizure with focal manifestations: is emergent imaging indicated? Seizure. 2014;23(9):740–745 [DOI] [PubMed] [Google Scholar]
- 19.Landfish N, Gieron-Korthals M, Weibley RE, Panzarino V. New onset childhood seizures: emergency department experience. J Fla Med Assoc 1992;79(10):697–700 [PubMed] [Google Scholar]
- 20.King MA, Newton MR, Jackson GD, et al. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352(9133):1007–1011 [DOI] [PubMed] [Google Scholar]
- 21.Bui TT, Delgado CA, Simon HK. Infant seizures not so infantile: first-time seizures in children under six months of age presenting to the ED. Am J Emerg Med. 2002;20(6):518–520 [DOI] [PubMed] [Google Scholar]
- 22.Dayan PS, Lillis K, Bennett J, et al. Pediatric Emergency Department Northeast Team of the Pediatric Emergency Care Applied Research Network (PECARN) . Interobserver agreement in the assessment of clinical findings in children with first unprovoked seizures. Pediatrics. 2011;127(5). Available at: www.pediatrics.org/cgi/content/full/127/5/e1266 [DOI] [PubMed] [Google Scholar]
- 23.Little RJA, Rubin DB, eds. Statistical Analyses with Missing Data. 2nd ed. Hoboken: Wiley; 2002 [Google Scholar]
- 24.Cicchetti D, Bronen R, Spencer S, et al. Rating scales, scales of measurement, issues of reliability: resolving some critical issues for clinicians and researchers. J Nerv Ment Dis. 2006;194(8):557–564 [DOI] [PubMed] [Google Scholar]