Abstract
OBJECTIVE:
To investigate whether serum bicarbonate (HCO3) levels can be used to accurately diagnose diabetic ketoacidosis (DKA) and classify its severity in children with new-onset diabetes mellitus (NODM).
METHODS:
Retrospective study of all patients with NODM presenting to Boston Children’s Hospital from October 1, 2007, to July 1, 2013. DKA was defined as blood glucose ≥200 mg/dL, venous pH (vpH) <7.3, and urine ketones ≥2+, and severe DKA as vpH <7.1. Linear regression was used to assess serum HCO3 as a predictor of vpH, and logistic regression to evaluate serum HCO3 as a predictor of DKA and severe DKA.
RESULTS:
Of 690 study cohort subjects (47% girls, age 10.8 ± 4.3 years, 76.7% white), 19.4% presented with DKA. The relationship between serum HCO3 and vpH was log-linear (r = 0.87, 95% CI 0.85–0.89, P < .001). HCO3 predicted vpH (R2 0.75, P < .001) using the formula vpH = 6.81301 + (0.17823*ln[HCO3]) and DKA and severe DKA (c-statistic 0.97 [95% CI 0.96–0.99, P < .001] and 0.99 [95% CI 0.991–0.999, P < .001], respectively). HCO3 cutoffs of <18 and <8 mmol/L had sensitivities of 91.8% and 95.2%, and specificities of 91.7% and 96.7%, respectively, to diagnose DKA and severe DKA. Findings were similar in a validation cohort of 197 subjects.
CONCLUSIONS:
Serum HCO3 concentration alone can substitute for vpH to diagnose DKA and classify severity in children with NODM. It is suggested as an alternative to reliance on vpH, especially in settings in which access to vpH measurement is limited.
What’s Known on This Subject:
Diabetic ketoacidosis (DKA) is a common and serious first manifestation of diabetes mellitus in children. During initial evaluation, the venous blood pH is frequently used to make the diagnosis and classify the severity of DKA.
What This Study Adds:
This study demonstrates that the serum bicarbonate concentration is a simple and accurate predictor of DKA and its severity and can be used in lieu of venous pH measurement, especially in resource-poor settings where access to pH measurement is limited.
Diabetes mellitus in youth is common, with an estimated worldwide annual incidence of 80 000 children younger than 15 years1 and the incidence is rising.2 Diabetic ketoacidosis (DKA) at presentation of new-onset diabetes mellitus (NODM) occurs in approximately 15% to 70% of patients in Europe and North America.3 The incidence of DKA at presentation is higher in younger children, ethnic minorities, families of lower socioeconomic status, and regions with a lower prevalence of diabetes,3–7 which includes many resource-limited countries.
In many pediatric academic medical centers in the United States, the standard initial diagnostic evaluation for children with NODM and especially those suspected to have DKA includes a panel of laboratory tests to measure venous blood gas (VBG) and the concentrations of plasma glucose, serum electrolytes, serum bicarbonate (HCO3), serum urea nitrogen (SUN), creatinine, hemoglobin A1c (HbA1c), and serum or urinary ketones. Acidemia is defined as arterial pH <7.35 or venous pH (vpH) <7.3, and traditionally, a vpH <7.3 or a serum HCO3 <15 mmol/L is used to confirm the diagnosis of DKA, with lower values of both indicating greater severity of the condition. Venous pH <7.1 or serum HCO3 <5 mmol/L has been used to define severe DKA.3,8 These definitions are incorporated into hospital practice guidelines, and emergency department physicians and pediatricians often base management decisions, for example, admission to hospital and use of intravenous versus subcutaneous insulin therapy, on the vpH value. However, in remote or limited resource settings, VBG is not readily available.
A recent study of pediatric patients seen in an emergency department suggested that the venous HCO3 concentration accurately predicts abnormal vpH in children with DKA.9 Similarly, in adults, venous HCO3 concentrations predicted arterial pH with a high degree of sensitivity and specificity.10 The objective of our study was to evaluate the ability of serum HCO3, measured at presentation, as part of a routine basic metabolic panel, to accurately predict DKA and classify its severity in a large population of youth with NODM.
Methods
Study Design and Study Population
This was a cross-sectional retrospective review of all patients with NODM presenting to Boston Children’s Hospital (BCH) between October 1, 2007, and July 1, 2013. Inclusion criteria were NODM, serum HCO3 concentration measured within 15 minutes of an initial vpH measurement, and urinary ketone measurement within 4 hours of vpH measurement. These cutoffs were chosen a priori to avoid introduction of effects related to response to therapy. Prespecified exclusion criteria were a previous diagnosis of diabetes and neonatal diabetes. DKA was defined as blood glucose ≥200 mg/dL, vpH <7.3, and urine ketones ≥2+. Mild, moderate, and severe DKA were defined as vpH 7.2 to 7.29, 7.1 to 7.19, and <7.1, respectively. Subjects were divided temporally into a study cohort (January 1, 2009–July 1, 2013) and a validation cohort (October 1, 2007–December 31, 2008). The study was approved by the institutional review board at BCH.
Data Collection
We used the BCH inpatient Diabetes Quality Improvement Database to identify eligible subjects. This database includes all patients with diabetes admitted to BCH. Virtually all patients with NODM referred to BCH who require treatment with insulin are admitted to the hospital. We used Childrens 360, a data extraction tool linked to the electronic medical record, to extract subject data. We collected information on patient age at diagnosis, race/ethnicity, anthropometric measures, date and time of laboratory analyses, and laboratory data, including concentrations of plasma glucose, serum electrolytes and HCO3, serum and/or urine ketones, HbA1c, and VBG.
Outcome Measures
Our main outcome measure was the correlation between serum HCO3 concentration and vpH in a population of children and adolescents at the time of their initial diagnosis of diabetes mellitus. Secondary outcomes were the accuracy of serum HCO3 concentration to diagnose DKA and classify its severity, including a determination of serum HCO3 cutoffs that would best correlate with the traditionally used vpH cutoffs of <7.3 to define presence of DKA and <7.1 to define severe DKA.
Laboratory Analysis
Serum HCO3 concentration was measured by using quantitative enzyme-based determination on the Roche/Hitachi cobas c systems platform (Roche Diagnostics, Indianapolis, IN). The VBG, plasma glucose, and serum electrolytes were determined by using the Radiometer ABL 825 (Radiometer, Cleveland, OH). Urine ketone concentrations were measured by using Acetest tablets Bayer Healthcare (Whippany, NJ). HbA1c was measured by immunoassay by using the Roche Integra 800 platform (Roche Diagnostics, Indianapolis, IN). All measurements were performed in the BCH Clinical Chemistry Laboratory.
Statistical Analysis
Standard descriptive statistics were used to describe subject characteristics. These included percentages for categorical variables, and mean and standard deviation (SD) or median and interquartile range as appropriate, for continuous variables. Analyses of continuous data used Student’s t test and Wilcoxon rank sum test for normal and non-normal data, respectively, and χ2 tests to compare categorical variables. The relationship between serum HCO3 and vpH was assessed by Pearson correlation. Given that the relationship appeared log-linear rather than linear, and because vpH is a log of hydrogen ion concentration, serum HCO3 was log-transformed for further analyses. Linear regression was used to assess serum HCO3 as a predictor of vpH, and to create a formula to calculate the vpH as a function of serum HCO3. A priori, a strong correlation was defined as a Pearson correlation coefficient ≥0.7 and a very strong correlation as a coefficient ≥0.85.
For our secondary outcomes, logistic regression and receiver operating characteristic (ROC) curves were used to evaluate serum HCO3 concentration as a predictor of DKA or severe DKA. An area under the curve (AUC) of greater than 0.9 was chosen a priori as the minimum acceptable value.11 The Hosmer and Lemeshow test was used to assess how well the model was calibrated; that is, how well predicted DKA event rates matched expected event rates. Other variables included in the model by using a purposeful selection approach were age, race/ethnicity, serum osmolality, concentrations of plasma glucose, serum sodium, and SUN, SUN/creatinine ratio, and anion gap. Variables were retained if the c-statistic improved by >10% or if there was a change in any β coefficient of more than 20%. Characteristics for different serum HCO3 concentration cutoffs to predict presence of DKA and severe DKA from the model included sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The final analysis was then replicated in the validation cohort.
For all analyses, P < .05 was considered significant. Analyses were performed by using SAS Software Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Subjects
The NODM cohort consisted of a total of 987 subjects who had laboratory measurements performed at BCH at the time of diagnosis. Sixty-two subjects were excluded because of missing laboratory data: vpH (n = 61), serum HCO3 (n = 26), and/or urine ketones (n = 8). Of the remaining 925 subjects, 235 were excluded because VBG and serum HCO3 were not measured within 15 minutes of each other (n = 154), and/or urinary ketones were not measured within 4 hours of the initial VBG measurement (n = 119). Thirty-eight subjects did not meet the criteria for either time frame. Overall, 690 subjects met inclusion criteria and were included in the primary study analysis.
Of 284 subjects identified, 197 subjects (48% girls, 85% white, 16.8% DKA) were included in the validation cohort. Reasons for exclusion were similar to the study cohort (data not shown). Baseline characteristics for all subjects are shown in Table 1. There were no significant differences between the study and validation cohorts with respect to demographic characteristics, diabetes type, or severity of presentation. Laboratory characteristics for the study cohort are shown in Table 2.
TABLE 1.
Characteristics of Subjects
| Characteristic | Study Cohort, n = 690 | Validation Cohort, n = 197 | Pa |
|---|---|---|---|
| Girls, n (%) | 324 (47.0) | 95 (48.2) | .75 |
| Race/ethnicity, n (%) | .33 | ||
| White | 529 (76.7) | 163 (82.7) | |
| Asian | 12 (1.7) | 2 (1.0) | |
| Black | 49 (7.1) | 11 (5.6) | |
| Other | 55 (8.0) | 10 (5.1) | |
| Missing | 45 (6.5) | 11 (5.6) | |
| Age, y | 10.8 (4.3) | 10.3 (4.0) | .12 |
| BMI, z-score | −0.01 (1.32) | 0.02 (1.43) | .76 |
| Diabetes type, n (%) | .66 | ||
| Type 1 | 655 (94.9) | 186 (94.4) | |
| Type 2 | 24 (3.5) | 6 (3.1) | |
| Other | 11 (1.6) | 5 (2.5) | |
| DKA severity, n (%) | .53 | ||
| None | 556 (80.6) | 164 (83.2) | |
| Mild | 80 (11.6) | 19 (9.6) | |
| Moderate | 33 (4.8) | 6 (3.1) | |
| Severe | 21 (3.0) | 8 (4.1) |
Values are presented as mean (SD), or as n (%) where indicated.
Comparison of study versus validation cohorts.
TABLE 2.
Biochemical Characteristics of Subjects
| Characteristics | All, n = 690 | DKA, n = 134 | No DKA, n = 556 | Pa |
|---|---|---|---|---|
| vpH, median (IQR) | 7.36 (7.32–7.38) | 7.23 (7.15–7.27) | 7.37 (7.35–7.39) | <.001 |
| Serum HCO3, mmol/L, median (IQR) | 23 (19–26) | 11 (8–15) | 24 (22–26) | <.001 |
| Serum osmolality, mOsm/kg | 309 (13) | 321 (17) | 306 (10) | <.001 |
| Plasma glucose, mg/dL | 425 (167) | 470 (172) | 414 (168) | <.001 |
| SUN, mg/dL | 15 (5) | 15 (7) | 14 (5) | .03 |
| Creatinine, mg/dL | 0.48 (0.19) | 0.53 (0.24) | 0.46 (0.17) | .0003 |
| Serum SUN/Creat | 34 (17) | 31 (15) | 35 (17) | .04 |
| Anion gap, mmol/L | 17 (6) | 26 (6) | 15 (4) | <.001 |
| HbA1c, % | 11.3 (2.3) | 12.1 (1.8) | 11.1 (2.3) | <.001 |
| Urinary ketones ≥2+, n (%) | 464 (67.2) | 134 (100) | 330 (59.4) | <.001 |
All values are presented as mean (SD), except where indicated. IQR, interquartile range.
Comparison of DKA versus no DKA.
Relationship Between Serum HCO3 Concentration and vpH
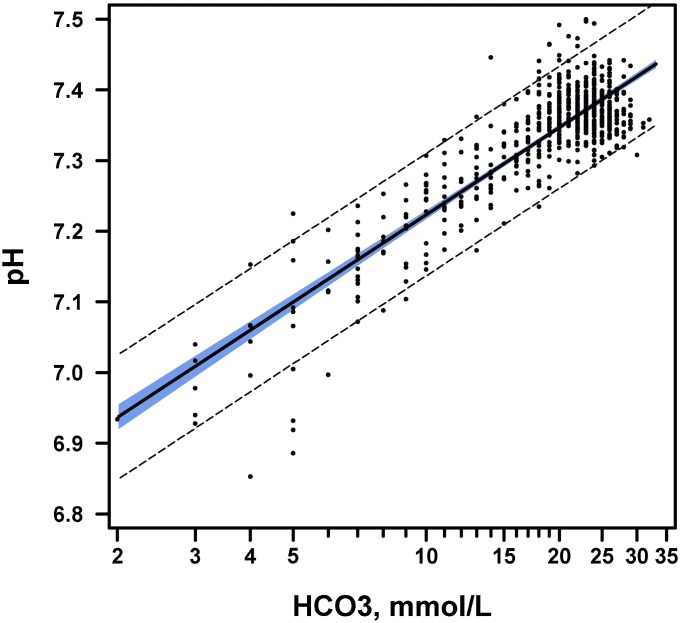
Serum HCO3 concentration correlated with vpH, r = 0.79 (95% confidence interval [CI] 0.76–0.82). The relationship appeared to be log-linear, and log transformation of serum HCO3 values showed a strong correlation between the log of HCO3 and vpH (Fig 1), r = 0.87 (95% CI 0.85–0.89). A linear regression model with serum HCO3 as the sole predictor of vpH yielded the following formula: vpH = 6.81301 + (0.17823*ln[HCO3]), R2 0.75, P < .001. The range of serum HCO3 concentrations with their corresponding vpH values is shown in Table 3.
FIGURE 1.
Correlation between serum HCO3 and venous pH. The log-linear correlation between serum HCO3 and venous pH, including 95% CIs, is shown. The x-axis displays the serum HCO3 concentration on a log scale. Outer band: 95% CI for a single predicted value. Inner band: 95% CI for predicted mean.
TABLE 3.
Corresponding Serum HCO3 and vpH Values
| Serum HCO3 | vpH |
|---|---|
| 1 | 6.813 |
| 2 | 6.937 |
| 3 | 7.009 |
| 4 | 7.060 |
| 5 | 7.100 |
| 6 | 7.132 |
| 7 | 7.160 |
| 8 | 7.184 |
| 9 | 7.204 |
| 10 | 7.223 |
| 11 | 7.240 |
| 12 | 7.260 |
| 13 | 7.270 |
| 14 | 7.283 |
| 15 | 7.296 |
| 16 | 7.307 |
| 17 | 7.318 |
| 18 | 7.328 |
Serum HCO3 Concentration as a Predictor of DKA
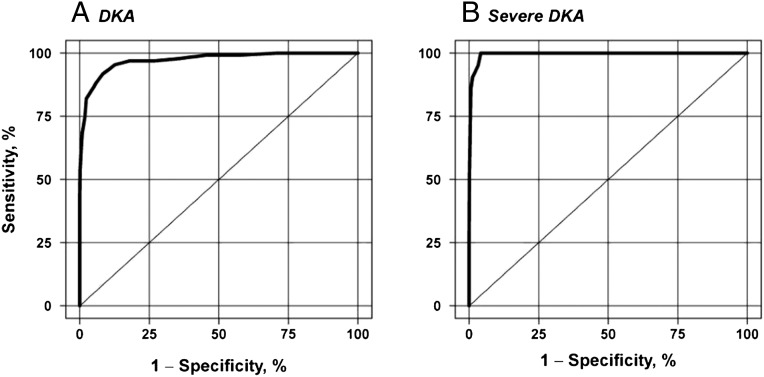
The ROC curve derived from the logistic regression model assessing serum HCO3 concentration as a predictor of DKA showed an AUC of 0.97 (95% CI 0.958–0.987, P < .001) (Fig 2A), with a maximum rescaled R2 of 0.79. The association of predicted probabilities and observed responses showed a concordance of 96.8%. No additional variables met criteria to be included as predictors or confounders. For the prediction of severe DKA, the AUC was 0.99 (95% CI 0.991–0.999, P < .001) (Fig 2B), with a maximum rescaled R2 of 0.82 and an association of predicted probabilities and observed responses showed a concordance of 99.4%. The Hosmer and Lemeshow χ2 statistic was nonsignificant for both DKA (P = .72) and severe DKA models (P = .85), indicating goodness of fit of both models. Sensitivity, specificity, NPV, and PPV of different serum HCO3 concentration cutoff values for diagnosis of DKA and for classification of severe DKA are shown in Table 4.
FIGURE 2.
ROC curves are shown for (A) serum HCO3 concentration as a predictor of DKA and (B) serum HCO3 concentration as a predictor of severe DKA. Each point on the curve represents a different HCO3 value, such that sensitivity and specificity vary with the HCO3 cutoff chosen.
TABLE 4.
Evaluation of Serum HCO3 Concentration Cutoff Values
| HCO3, mmol/L | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|
| Diagnosis of DKA | ||||
| <15 | 82.1 | 97.7 | 89.4 | 95.8 |
| <16 | 84.3 | 96.2 | 84.3 | 96.2 |
| <17 | 88.1 | 94.2 | 78.7 | 97.0 |
| <18 | 91.8 | 91.7 | 72.8 | 97.9 |
| Classification of Severe DKA | ||||
| <5 | 52.4 | 99.9 | 91.2 | 98.5 |
| <6 | 85.7 | 99.4 | 81.8 | 99.6 |
| <7 | 90.5 | 98.8 | 70.1 | 99.7 |
| <8 | 95.2 | 96.7 | 47.6 | 99.9 |
Validation
Using the validation cohort, application of a serum HCO3 cutoff value of <15 and <18 mmol/L for the diagnosis of DKA yielded a sensitivity of 84.9% and 100%, specificity of 97% and 89.6%, PPV of 84.9% and 66%, and NPV of 97% and 100%, respectively. For the classification of severe DKA, a cutoff of <5 and <8 mmol/L yielded a sensitivity of 87.5% and 100%, specificity of 98.9% and 96.8%, PPV of 77.8% and 57.1%, and NPV of 99.5% and 100%, respectively.
Discussion
The results of our study confirm a very strong log-linear relationship between serum HCO3 concentration and vpH in patients with NODM. The findings suggest that serum HCO3 levels can reliably be used to diagnose and classify DKA in pediatric patients with NODM, and that VBG analysis is not required for the initial evaluation of these patients.
Several other studies have been performed in an attempt to simplify and render more efficient the initial diagnosis and subsequent management of DKA. Sheikh-Ali et al12 evaluated quantitative serum β-hydroxybutyrate (BOHB) as a diagnostic test for DKA on the basis that pH, HCO3, and anion gap are relatively nonspecific for the diagnosis of DKA. They suggested a BOHB level >3 mmol/L as a cutoff to diagnose DKA, but found a 20% discordance between BOHB and the conventional criteria by using pH <7.3, HCO3 <18 mmol/L, and glucose ≥200 mg/dL.12 At least 3 groups have suggested end-tidal CO2 as a simpler and less invasive diagnostic or monitoring tool for DKA.13–15 Although end-tidal CO2 had a strong linear correlation with vpH and HCO3 concentration, the agreement was not narrow enough to confirm DKA, especially in cases of mild acidosis,14,15 and its measurement has not been widely adopted. Another attempt to simplify the initial evaluation of patients with DKA has been with the measurement of electrolytes by using a VBG analyzer.16 Although this approach may be feasible in some settings, many remote and resource-limited settings are not equipped with VBG analyzers and may only have the capacity to measure serum electrolytes.
The attempt to use venous HCO3 for the prediction of DKA seems intuitive given the Henderson-Hasselbach equation where pH = pK + log ([HCO3-]/[CO2]), establishing the pH as a function of the serum HCO3 concentration. Any pathophysiological state that affects the serum HCO3 concentration independent of the derangements caused by DKA poses a risk of misclassification if HCO3 is used to define DKA. Conditions that raise HCO3 levels, such as metabolic alkalosis from H+ loss caused by vomiting, and, conversely, those that lower HCO3 levels, such as renal dysfunction or HCO3 loss with diarrhea, could alter the observed relationship between HCO3 and vpH.
Only one previous study has assessed the utility of venous HCO3 for prediction of pH in children.9 Similar to our results, this study demonstrated a strong correlation between logarithm serum HCO3 concentrations and vpH. Whereas a relatively large percentage of that study population (∼39% of 300 patients) presented with DKA, only 8 subjects had severe DKA, thus possibly limiting the accuracy of the classification of severe DKA. The authors suggested the best statistical cutoff of 18.5 mmol/L for serum HCO3 to define the presence of DKA, and 10.5 mmol/L to define severe DKA. These cutoffs, particularly the latter, are higher than have been suggested in most of the pediatric literature, possibly a result of the small number of subjects with severe DKA.
We have provided a formula to calculate vpH from HCO3, which suggests HCO3 values of 15, 9, and 5 correspond to vpH levels of 7.3, 7.2, and 7.1, respectively (Table 3). Although these are consistent with pediatric guideline cutoffs3,17 of 15 and 5 mmol/L to diagnose DKA and severe DKA, respectively, these cutoffs had lower sensitivity (82.1% and 52.4%) compared with higher cutoffs, whereas specificity was only slightly higher (Table 4). Thus, HCO3 values that best correspond to vpH levels of 7.3 and 7.1 may not be the most appropriate cutoffs to use in situations in which a diagnosis of DKA or severe DKA should not be missed, such as in a young child or in settings with limited monitoring capability. We have shown sensitivities and specificities for a range of HCO3 values both for diagnosis of DKA and classification of its severity. To minimize the number of false-negatives, clinicians should choose a higher HCO3 cutoff; alternatively, choosing a lower HCO3 cutoff will minimize false-positives. The serum HCO3 range of 15 to 18 mmol/L is also consistent with the most recent American Diabetes Association adult guidelines, which include a bicarbonate range of 15 to 18 mmol/L (in addition to an arterial pH <7.25–7.3 and anion gap >10 mmol/L) for the diagnosis of DKA.18
Although minor differences exist for the criteria to diagnose DKA,3,8,17–19 the most commonly used definition in pediatrics is a combination of pH <7.3, HCO3 <15–18 mmol/L, ketonuria or ketonemia, and hyperglycemia. Some definitions add the anion gap18 as a measure of metabolic acidosis. In our study, serum HCO3 was the strongest predictor of the presence of DKA as defined by vpH <7.3 and presence of hyperglycemia and ketosis. Whereas the anion gap was also a significant predictor of DKA (AUC 0.94 [95% CI 0.926–0.962, P < .001]), it did not perform as well as the serum HCO3 concentration and did not meet the a priori criteria for inclusion in the final statistical model. This is not surprising given that the anion gap, although indicative of acidosis, is affected by several anions that are present in DKA, including lactate, BOHB, acetoacetate, free fatty acids, phosphate, and sulfate. Finally, none of the other measured biochemical parameters added to the value of the HCO3 to predict DKA. This is likely a reflection of the natural correlation between pH and HCO3, but supports the use of HCO3 as the sole predictor of DKA.
Patients had on average 4 VBGs measured, adding significant cost not only to the initial evaluation but to the entire DKA management process. In an era of increasing financial constraints, there is a potential not only for simplification but also for cost-saving in the evaluation of patients with DKA.
Particular strengths of our study include its large number of subjects, highly significant unambiguous results, and their immediate application to clinical practice. Sufficient data were available to develop a precise rule for the most recent time period (2009–2013, study cohort) and to demonstrate that it operated satisfactorily for a previous period (2007–2008, validation cohort). Dividing the data according to time allowed us to confirm the validity of our findings with respect to temporal variation.
Certain limitations of the study merit comment. First, this study was retrospective and limited to a single, large academic referral center. Although the results may be specific to the population studied, our demographics are representative of typical pediatric diabetes populations in the United States and Europe with regard to race/ethnicity, diabetes type, and age distribution. Although the inpatient database used to identify our subjects represents a comprehensive list of all inpatients presenting with NODM requiring insulin therapy, some patients with type 2 diabetes treated with oral antihyperglycemic agents may be treated as outpatients and would not be represented. However, as the focus of the analysis is on the diagnosis and classification of DKA, the composition of the sample as insulin-requiring patients supports the internal validity, and the large sample size and highly significant outcomes supports the generalizability to demographically similar populations.
Second, we could not differentiate between children who had been pretreated with intravenous fluids or insulin and those who had not received any treatment before arriving at BCH. However, a previous study that was able to compare subjects who had received insulin before hospital admission to insulin-naïve subjects did not find a difference with regard to the ability of serum HCO3 concentration to accurately predict the presence of DKA.13 We also could not exclude patients with comorbidities that may have influenced their acid-base status. Clinicians must account for the possible effect of underlying renal and pulmonary disorders when evaluating a patient in DKA.
In addition, we had to exclude a large number of subjects without near-simultaneous measurements of VBG and serum biochemistries, and ketone measurement within a few hours of diagnosis and treatment initiation. The excluded subjects differed from those included in our analyses: they had a higher prevalence of DKA (34.7% vs 19.4%, P < .001) and severe DKA (8.9% vs 3%, P < .001) and, not surprisingly, a higher frequency of electrolyte derangements and biochemical indicators of dehydration (Supplemental Table 5). These patients may have been sicker, and thus priority was not put on obtaining timely urine ketone measurements, the absence of which accounted for the largest number of patients excluded. However, when we included all patients in the analysis, regardless of the timing of their ketone measurement, none of the outcomes changed significantly (Supplemental Table 6).
Conclusions
Serum HCO3 concentration is a simple, reliable, and effective predictor of the presence and severity of DKA and can be used without the addition of a VBG to diagnose DKA and assess its severity. In clinical practice, a range of HCO3 cutoff values may be preferable to a single value for the diagnosis of DKA, based on the clinician’s priority to maximize either sensitivity or specificity. We suggest that such an approach should be considered in DKA management guidelines for both resource-limited and resource-rich settings. Future prospective, multicenter and, ideally, community-based studies should evaluate the use of these recommendations. It may also be of interest to examine the utility of serial serum HCO3 relative to BOHB concentrations for monitoring the response to treatment of DKA.
Acknowledgments
We thank Virginia Coburn and Julie Hosselbarth from the Business Intelligence Group at BCH for assistance with data extraction.
Glossary
- AUC
area under the curve
- BCH
Boston Children’s Hospital
- BOHB
β-hydroxybutyrate
- CI
confidence interval
- DKA
diabetic ketoacidosis
- HbA1c
hemoglobin A1c
- HCO3
serum bicarbonate
- NODM
new-onset diabetes mellitus
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristics
- SUN
serum urea nitrogen
- VBG
venous blood gas
- vpH
venous pH measurement
Footnotes
Dr von Oettingen conceptualized and designed the study, carried out data collection and data analysis, and drafted the initial manuscript; Drs Wolfsdorf and Rhodes conceptualized and designed the study, supervised data collection and analysis, and critically reviewed and revised the manuscript; Dr Feldman supervised data analysis, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Rhodes receives research funding from Merck, and her spouse owned stock in Bristol-Myers Squibb, GlaxoSmithKline, and Sanofi and owns stock in Pfizer and Merck. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Rhodes receives research funding from Merck, and her spouse owned stock in Bristol-Myers Squibb, GlaxoSmithKline, and Sanofi and owns stock in Pfizer and Merck. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.International Diabetes Federation IDF World Diabetes Atlas 2013. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013 [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfsdorf JI, Allgrove J, Craig ME, et al. International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(suppl 20):154–179 [DOI] [PubMed] [Google Scholar]
- 4.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodacki M, Pereira JRD, Nabuco de Oliveira AM, et al. Ethnicity and young age influence the frequency of diabetic ketoacidosis at the onset of type 1 diabetes. Diabetes Res Clin Pract. 2007;78(2):259–262 [DOI] [PubMed] [Google Scholar]
- 6.Quinn M, Fleischman A, Rosner B, Nigrin DJ, Wolfsdorf JI. Characteristics at diagnosis of type 1 diabetes in children younger than 6 years. J Pediatr. 2006;148(3):366–371 [DOI] [PubMed] [Google Scholar]
- 7.Szypowska A, Skórka A. The risk factors of ketoacidosis in children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes. 2011;12(4 pt 1):302–306 [DOI] [PubMed] [Google Scholar]
- 8.Wolfsdorf J, Glaser N, Sperling MA, American Diabetes Association . Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150–1159 [DOI] [PubMed] [Google Scholar]
- 9.Nadler OA, Finkelstein MJ, Reid SR. How well does serum bicarbonate concentration predict the venous pH in children being evaluated for diabetic ketoacidosis? Pediatr Emerg Care. 2011;27(10):907–910 [DOI] [PubMed] [Google Scholar]
- 10.Nyenwe EA, Wan JY, Kitabchi AE. Venous serum bicarbonate concentration predicts arterial pH in adults with diabetic ketoacidosis. Endocr Pract. 2014;20(3):201–206 [DOI] [PubMed] [Google Scholar]
- 11.Hsu LM. Diagnostic validity statistics and the MCMI-III. Psychol Assess. 2002;14(4):410–422 [PubMed] [Google Scholar]
- 12.Sheikh-Ali M, Karon BS, Basu A, et al. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647 [DOI] [PubMed] [Google Scholar]
- 13.Garcia E, Abramo TJ, Okada P, Guzman DD, Reisch JS, Wiebe RA. Capnometry for noninvasive continuous monitoring of metabolic status in pediatric diabetic ketoacidosis. Crit Care Med. 2003;31(10):2539–2543 [DOI] [PubMed] [Google Scholar]
- 14.Fearon DM, Steele DW. End-tidal carbon dioxide predicts the presence and severity of acidosis in children with diabetes. Acad Emerg Med. 2002;9(12):1373–1378 [DOI] [PubMed] [Google Scholar]
- 15.Agus MSD, Alexander JL, Mantell PA. Continuous non-invasive end-tidal CO2 monitoring in pediatric inpatients with diabetic ketoacidosis. Pediatr Diabetes. 2006;7(4):196–200 [DOI] [PubMed] [Google Scholar]
- 16.Menchine M, Probst MA, Agy C, Bach D, Arora S. Diagnostic accuracy of venous blood gas electrolytes for identifying diabetic ketoacidosis in the emergency department. Acad Emerg Med. 2011;18(10):1105–1108 [DOI] [PubMed] [Google Scholar]
- 17.Dunger DB, Sperling MA, Acerini CL, et al. European Society for Paediatric Endocrinology. Lawson Wilkins Pediatric Endocrine Society . European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113(2). Available at: www.pediatrics.org/cgi/content/full/113/2/e133 [DOI] [PubMed] [Google Scholar]
- 18.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfsdorf J, Craig M, Daneman D, et al. Diabetes in childhood and adolescence. In: Colagiuri S, Hanas R, Donaghue K, Klingensmith G, Swift P, eds. Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence. Brussels, Belgium: International Diabetes Federation; 2011:70–81 [Google Scholar]