Abstract
Previous studies reported a relatively low prevalence of transmitted drug resistance (TDR) in South Korea (<5%). A genotypic resistance test was performed on 131 treatment-naive HIV-1-infected individuals from February 2013 to February 2014. Eleven individuals (8.4%) presented TDR, of whom eight had K103N, revealing a significant increase in K103N TDR compared to previous studies (p<0.001). Using phylogenetic analysis, we identified three distinct clustering pairs with genetic relativeness and a total of five independent strains among the eight K103N cases. Our findings suggest that multiple sources of K103N occurred, most likely as a consequence of increased efavirenz use in South Korea.
As more potent antiretroviral therapy (ART) regimens become available, virological failures as a result of resistance acquisition during treatment of human immunodeficiency virus (HIV)-1 are becoming less common.1 However, the presence of transmitted drug resistance (TDR) is one of the most important issues to be considered during the selection of initial ART regimens, and many treatment guidelines recommend genotypic resistance testing before the implementation of ART.2 The increased use of ART in both developed and developing countries has led to an increase in the incidence of drug resistance, even among ART-naive HIV-infected individuals.3
Globally, the prevalence of TDR has been higher in Western countries in which ART was introduced iteratively via monotherapy, dual therapy, and, ultimately, triple drug ART.4 However, the prevalence of TDR in relation to a nucleoside analogue reverse transcriptase inhibitor (NRTI) is globally stable or even decreasing, and we are observing increasing TDR in developing countries, most likely due to the enhanced availability of ART in these regions.5 In South Korea, one study including 50 subjects reported a prevalence of 8.0% with three NRTIs and one protease inhibitor (PI) TDR strain in early 2000,6 but subsequent larger studies all found a lower prevalence of TDR (less than 5%) among ART-naive HIV-1-infected individuals.7–12
Here, we report a study of ART-naive HIV-1-infected individuals who were recruited at the National Medical Center, Seoul, South Korea from February 2013 to February 2014. All included individuals were over the age of 18 years and of Korean nationality. HIV-1 genotyping was performed using the ViroSeq HIV-1 Genotyping System version 2.0 (Abbott Laboratories. Abbott Park, IL), as previously described.9 Complete protease (amino acids 1–99) and partial reverse transcriptase (amino acids 1–335) genes of the pol region were aligned using Bioedit 7.2.5 software, and an approximate-maximum-likelihood phylogenetic tree including all generated sequences was built with Fasttree 2.1. HIV subtypes were determined using the REGA HIV-1 subtyping online tool (www.bioafrica.net/subtypetool). The presence of TDR was determined using the Stanford HIV Drug Resistance Database (Version 7.0) and the World Health Organization HIV Surveillance Drug Resistance Mutation list.13
Epidemiological, clinical, and laboratory data were collected through medical chart review. The study protocol and standardized case record forms were approved by the institutional review board. Statistical analysis was performed using SPSS version 16 (IBM Corporation, Armonk, NY). Fisher's exact test was used to assess differences between groups. p values were two-sided and considered significant at a level of <0.05.
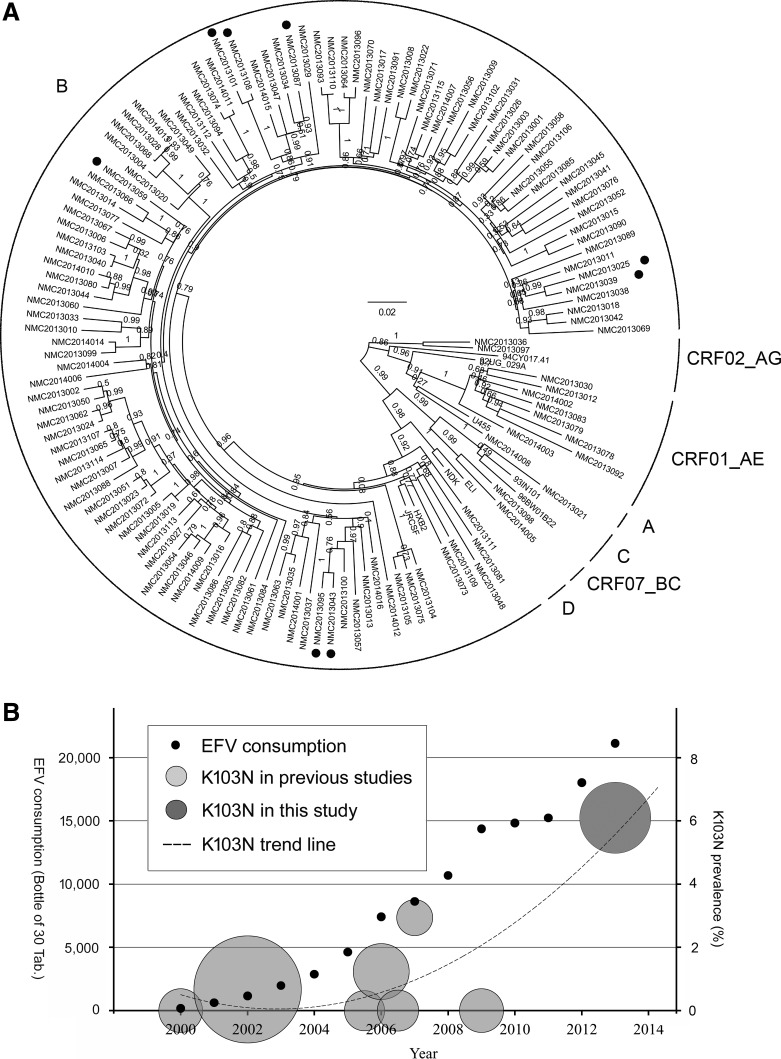
A total of 131 eligible individuals were enrolled during the study period; 94.5% were male, and approximately two-third reported their HIV risk factor as men who have sex with men. Most were infected with subtype B (89.3%) followed by CRF01_AE (6.1%). The presence of TDR was identified in 11 patients (8.4%) who were all infected with HIV-1 subtype B. The most common drug resistance mutation was K103N (72.7%), which was found among eight individuals (6.1%), while the prevalence of TDR for NRTI, nonnucleoside analogue reverse transcriptase inhibitor (NNRTI), and PI was 0.8%, 6.9%, and 1.5%, respectively (Table 1). Among all viral strains carrying the K103N mutation, three distinct possible transmission pairs were identified by phylogenetic analysis with a high bootstrap value (>98%) and low genetic distance (<0.04, Fig. 1A).14 No further genetic relationship supporting clonal spread was observed among any K103N-carrying strain, and no difference was observed in the topology when the phylogenetic tree was constructed with the K103 position sequences of the total study population removed (data not shown).
Table 1.
Characteristics of Korean Treatment-Naive HIV-1-Infected Patients
| Characteristics | N=131 |
|---|---|
| Age (median, year) | 31 (IQR 25–40) |
| Male sex (%) | 125 (95.4) |
| Known exposure category (%) | |
| Men who have sex with men | 49 (37.4) |
| Heterosexual contact | 27 (20.6) |
| Intravenous drug use | 0 |
| Transfusion | 0 |
| No record | 55 (42.0) |
| Interval between HIV diagnosis and analysis (median, month) | 2.3 (IQR 0.9–8.5) |
| CD4+T cell count (median, cells/mm3) | 298 (IQR 191–423) |
| Plasma log10 viral load (median, copies/ml) | 4.32 (IQR 3.88–4.95) |
| HIV-1 subtype (%) | |
| A1 | 1 (0.8) |
| B | 117 (89.3) |
| C | 1 (0.8) |
| CRF01_AE | 8 (6.1) |
| CRF02_AG | 2 (1.5) |
| CRF07_BC | 2 (1.5) |
| NRTI SDRM | |
| D67N/K219Q | 1 (0.8) |
| NNRTI SDRM | |
| K103N | 8a (6.1) |
| K101E | 1 (0.8) |
| PI SDRM | |
| M46L | 1a (0.8) |
| F53Y | 1 (0.8) |
One strain was harboring K103N and M46L simultaneously.
NRTI, nucleoside analogue reverse transcriptase inhibitor; SDRM, surveillance drug resistance mutation; NNRTI, nonnucleoside analogue reverse transcriptase inhibitor; PI, protease inhibitor.
FIG. 1.
Phylogenetic analysis of the pol gene and prevalence trend of K103N transmitted drug resistance (TDR). (A) Strains with a closed circle on the outer side of the taxon represent K103N TDR and they do not reveal a significant genetic relationship except for the three transmission pairs (NMC2013025, NMC2013039; NMC2013043, NMC2013095; and NMC2013101, NMC2013108). The circular brackets on the periphery of the tree indicate the subtypes as described in the text. (B) Small round dots depict the annual consumption of efavirenz in South Korea. Gray colored circles on the left and darker circle on the right represent K103N TDR in previous reports in South Korea and in this study, respectively. The center of the circle depicts the prevalence of K103N TDR and the area of the circle is equivalent to the sample size of each study. The dotted trend line of the prevalence of K103N TDR is a second-dimensional polynomial line inferred from the studies.
In our cohort, the prevalence of NRTI TDR was low (0.8%), which is consistent with previous studies revealing decreasing trends.5 However, we found a high prevalence of K103N (6.1%), which is a significant increase when compared to previous studies in South Korea (Table 2). The increase in NNRTI TDR is important because it is associated with virological failure of first-line ART when suboptimal NNRTI-based regimens are selected for such patients.15,16 Although there have been some reports about TDR being transmitted within clusters, including K103N,17–22 we identified just three distinctly clustering pairs that were carrying K103N. Taking into account the three described pairs, there were at least five independent K103N TDR strains in our study population, implying multiple sources of K103N transmission.
Table 2.
Increasing Trend of Transmitted Drug Resistance in South Korea
| TDRa(n, %) | 2000–2005 (n=350)6,12 | 2006–2010 (n=251)7–11 | This study (n=131) | p-value |
|---|---|---|---|---|
| K103N | 2 (0.6) | 2 (0.8) | 8 (6.1) | <0.001 |
| Overallb | 14 (4.0) | 4 (1.5) | 11 (8.5) | 0.006 |
Study period was determined as the median time of sample collection in each study.
Overall transmitted drug resistance (TDR) prevalence of prior studies was reestimated according to the 2009 WHO HIV Surveillance Drug Resistance Mutation list.
Another possibility would be the importation of TDR into South Korea, and recent studies reported a high prevalence of K103N TDR of over 5% in China whose exchange with South Korea in people and goods has markedly increased.23,24 However, we found a distinct clustering of Chinese subtype B strains (GenBank accession numbers KC988120–KC98815923) from those from South Korea in phylogenetic analysis and none of our K103N TDR strains was genetically related to the Chinese subtype B strains (data not shown). Therefore, we believe that the increasing prevalence of K103N TDR is likely a consequence of the widespread use of NNRTI in South Korea in the past decade as observed in other areas.25,26 In fact, whereas the number of people living with HIV/AIDS (PLHA) increased from 1,585 to 7,788 (4.9 times) from 2002 to 2012,27 the annual consumption of efavirenz increased much more from 1,156 to 18,026 bottles (15.6 times, Fig. 1B), and the ratio of increases in efavirenz data for consumption compared to PLHA was 3.2 (the consumption of efavirenz in South Korea was obtained from MSD Korea, Ltd.). Taken together, the increase in K103N TDR is most likely related to an increase in the use of NNRTI, especially efavirenz.
The increasing prevalence of NNRTI TDR has been reported among ART-naive individuals all over the world,25,28,29 and new potent drugs are emerging with excellent safety profiles and considerably fewer side effects, such as once-daily integrase inhibitors (elvitegravir) or second generation NNRTI (rilpivirine), so some have proposed that it is time to reconsider efavirenz as a first line treatment regimen.30 Although the proportion of ART-naive individuals starting an efavirenz-based first line regimen will likely decrease in the future, the risk of transmission of NNRTI-resistant strains may continue for a while, especially the K103N mutation, considering that efavirenz is one of the most commonly used anchor drugs around the world.
In summary, the increasing prevalence of HIV-1 TDR was observed among participants from a single center cohort in Seoul, South Korea, and K103N was the most commonly detected TDR mutation. Considering the lack of a genetic relationship for most of these strains in phylogenetic analysis, we hypothesize that this increase in K103N TDR is most likely associated with increased use of NNRTI rather than being secondary to the clonal spread of specific resistant strains or an inflow from an area with a high prevalence of K103N TDR. These results suggest that in Seoul, South Korea, a baseline genotypic resistance test before implementation of ART would be cost effective,31 considering the increasing occurrence of TDR in this area.
Sequence Data
Sequences in this study are available in GenBank under accession numbers KM820292–KM820422.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Audelin AM, Lohse N, Obel N, et al. : The incidence rate of HIV type-1 drug resistance in patients on antiretroviral therapy: A nationwide population-based Danish cohort study 1999–2005. Antivir Ther 2009;14(7):995–1000 [DOI] [PubMed] [Google Scholar]
- 2.Little SJ. and Smith DM: HIV treatment decisions and transmitted drug resistance. Clin Infect Dis 2005;41(2):233–235 [DOI] [PubMed] [Google Scholar]
- 3.Rubio A, Leal M, Pineda JA, et al. : Increase in the frequency of mutation at codon 215 associated with zidovudine resistance in HIV-1-infected antiviral-naive patients from 1989 to 1996. AIDS 1997;11(9):1184–1186 [DOI] [PubMed] [Google Scholar]
- 4.Wensing AM. and Boucher CA:. Worldwide transmission of drug-resistant HIV. AIDS Rev 2003;5(3):140–155 [PubMed] [Google Scholar]
- 5.Frentz D, Boucher CA, and van de Vijver DA: Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev 2012;14(1):17–27 [PubMed] [Google Scholar]
- 6.Park SW, Kim HB, Choi YJ, et al. : Genotypic resistance of antiretroviral drugs among drug-naive HIV type 1 patients with the background of long-term access-easy zidovudine therapy. AIDS Res Hum Retroviruses 2003;19(11):1039–1043 [DOI] [PubMed] [Google Scholar]
- 7.Kim MH, Song JE, Ahn JY, et al. : HIV antiretroviral resistance mutations among antiretroviral treatment-naive and -experienced patients in South Korea. AIDS Res Hum Retroviruses 2013;29(12):1617–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song JY, Lee JS, Jung HW, et al. : Primary anti-retroviral resistance in treatment-naive HIV-infected patients: A Korean HIV/AIDS cohort study. Infect Chemother 2009;41(4):230–232 [Google Scholar]
- 9.Chin BS, Choi JY, Han Y, et al. : Comparison of genotypic resistance mutations in treatment-naive HIV type 1-infected patients in Korea and China. AIDS Res Hum Retroviruses 2010;26(2):217–221 [DOI] [PubMed] [Google Scholar]
- 10.Bang JI, Song KH, Kim SH, et al. : Prevalence of primary antiretroviral resistance: Trends in Korea. AIDS Res Hum Retroviruses 2008;24(1):83–85 [DOI] [PubMed] [Google Scholar]
- 11.Kim SR, Rheu EK, Seol YM, et al. : Antiretroviral drug resistance among drug-naive HIV-1 infected patients. Korean J Med 2007;73(3):243–250 [Google Scholar]
- 12.Choi JY, Kim EJ, Park YK, et al. : National survey for drug-resistant variants in newly diagnosed antiretroviral drug-naive patients with HIV/AIDS in South Korea: 1999–2005. J Acquir Immune Defic Syndr 2008;49(3):237–242 [DOI] [PubMed] [Google Scholar]
- 13.Bennett D, Camacho R, Otelea D, et al. : Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009;4(3):e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks JI, Niznick H, Ofner M, et al. : Local phylogenetic analysis identifies distinct trends in transmitted HIV drug resistance: Implications for public health interventions. BMC Infect Dis 2013;13:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamers RL, Schuurman R, Sigaloff KC, et al. : Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: A multicentre cohort study. Lancet Infect Dis 2012;12(4):307–317 [DOI] [PubMed] [Google Scholar]
- 16.Wittkop L, Gunthard H, de Wolf F, et al. : Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect Dis 2011;11(5):363–371 [DOI] [PubMed] [Google Scholar]
- 17.Drescher SM, von Wyl V, Yang WL, et al. : Treatment-naive individuals are the major source of transmitted HIV-1 drug resistance in men who have sex with men in the Swiss HIV Cohort Study. Clin Infect Dis 2014;58(2):285–294 [DOI] [PubMed] [Google Scholar]
- 18.Grgic I, Lepej SZ, Lunar MM, et al. : The prevalence of transmitted drug resistance in newly diagnosed HIV-infected individuals in Croatia: The role of transmission clusters of men who have sex with men carrying the T215S surveillance drug resistance mutation. AIDS Res Hum Retroviruses 2013;29(2):329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezemer D, van Sighem A, Lukashov V, et al. : Transmission networks of HIV-1 among men having sex with men in the Netherlands. AIDS 2010;24(2):271–282 [DOI] [PubMed] [Google Scholar]
- 20.Antoniadou ZA, Kousiappa I, Skoura L, et al. : Short communication: Molecular epidemiology of HIV type 1 infection in northern Greece (2009–2010): Evidence of a transmission cluster of HIV type 1 subtype A1 drug-resistant strains among men who have sex with men. AIDS Res Hum Retroviruses 2014;30(3):225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner D, Amit S, Chalom S, et al. : Emergence of an HIV-1 cluster harbouring the major protease L90M mutation among treatment-naive patients in Tel Aviv, Israel. HIV Med 2012;13(4):202–206 [DOI] [PubMed] [Google Scholar]
- 22.Ruelle J, Ingels MG, Jnaoui K, et al. : Transmission network of an HIV type 1 strain with K103N in young Belgian patients from different risk groups. AIDS Res Hum Retroviruses 2013;29(10):1306–1309 [DOI] [PubMed] [Google Scholar]
- 23.Li L, Sun B, Zeng H, et al. : Relatively high prevalence of drug resistance among antiretroviral-naive patients from Henan, Central China. AIDS Res Hum Retroviruses 2014;30(2):160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao Y, Tian D, Zheng YY, et al. : Characteristics of HIV-1 natural drug resistance-associated mutations in former paid blood donors in Henan Province, China. PLoS One 2014;9(2):e89291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frentz D, Van de Vijver D, Abecasis A, et al. : Increase in transmitted resistance to non-nucleoside reverse transcriptase inhibitors among newly diagnosed HIV-1 infections in Europe. BMC Infect Dis 2014;14(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta RK, Jordan MR, Sultan BJ, et al. : Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: A global collaborative study and meta-regression analysis. Lancet 2012;380(9849):1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Infectious diseases surveillance yearbook. The Korea Centers for Disease Control and Prevention, 2012, p. 24 [Google Scholar]
- 28.Bartmeyer B, Kuecherer C, Houareau C, et al. : Prevalence of transmitted drug resistance and impact of transmitted resistance on treatment success in the German HIV-1 Seroconverter Cohort. PLoS One 2010;5(10):e12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Rodrigues N, Duran A, Bouzas MB, et al. : Increasing trends in primary NNRTI resistance among newly HIV-1-diagnosed individuals in Buenos Aires, Argentina. J Int AIDS Soc 2013;16:18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffi F, Pozniak AL, and Wainberg MA: Has the time come to abandon efavirenz for first-line antiretroviral therapy? J Antimicrob Chemother 2014;69(7):1742–1747 [DOI] [PubMed] [Google Scholar]
- 31.Weinstein MC, Goldie SJ, Losina E, et al. : Use of genotypic resistance testing to guide HIV therapy: Clinical impact and cost-effectiveness. Ann Intern Med 2001;134(6):440–450 [DOI] [PubMed] [Google Scholar]