Abstract
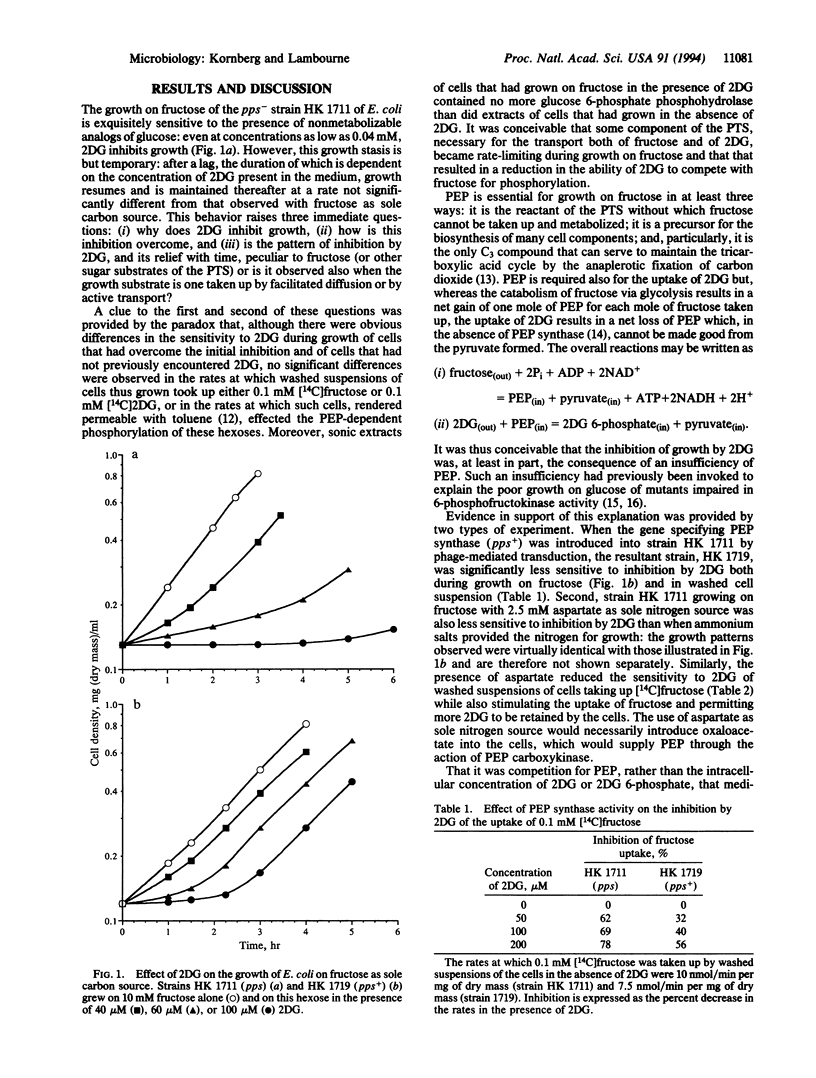
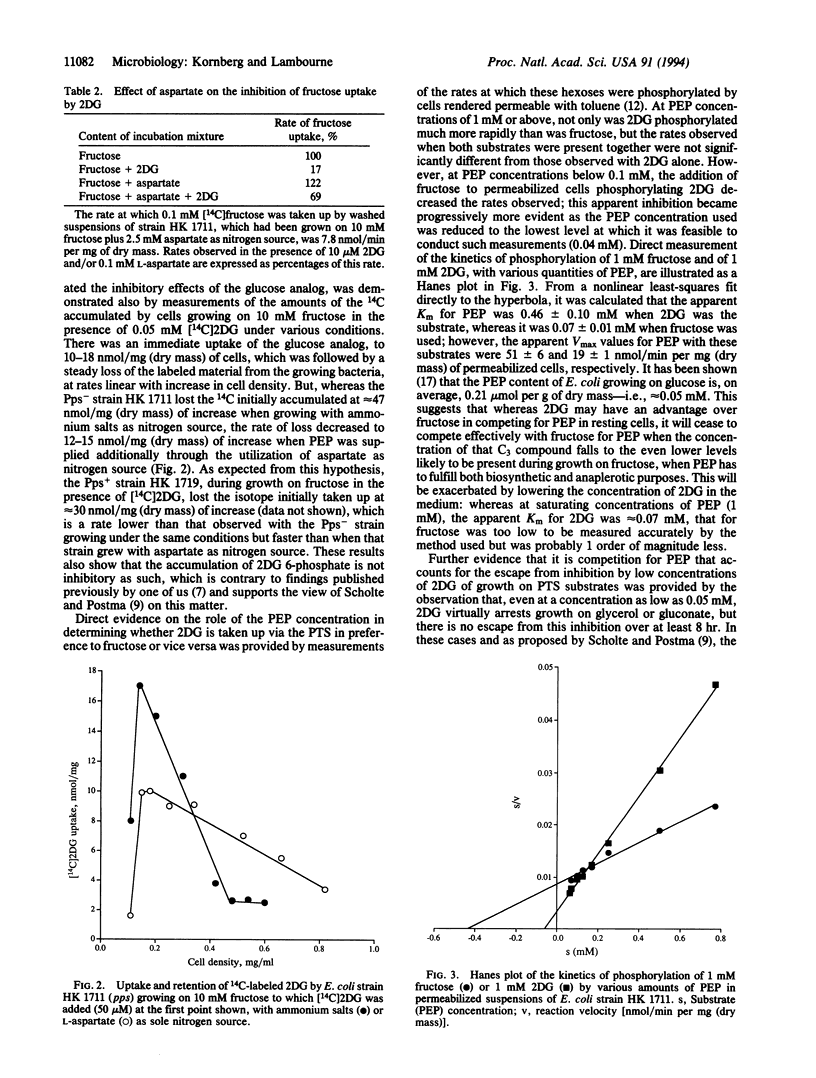
Nonmetabolizable glucose analogs inhibit the growth of Escherichia coli on a wide variety of carbon sources. This phenomenon was investigated with particular reference to the effect of 2-deoxyglucose (2DG) on growth on fructose as sole carbon source. When the inhibitor is supplied in sufficiently low concentrations, the initial arrest of growth is overcome; this relief of inhibition is aided by means that increase the availability of phosphoenolpyruvate (PEP) to the growing cells, such as the use of L-aspartate instead of ammonium chloride as sole nitrogen source for growth, and the introduction of the pps+ allele into a pps- strain. Studies with [14C]2DG showed that the analog or its 6-phosphate as such did not inhibit growth but that 2DG exerted its effect by competing for intracellular PEP and lowering its concentration below that needed to sustain growth. Direct measurements of the PEP-dependent phosphorylation of 2DG and of fructose by permeabilized E. coli showed that the apparent Km for PEP was nearly 7 times higher for 2DG that it was for fructose, although the apparent Vmax for 2DG was nearly 3 times that for fructose; this explains the ability of cells to overcome the inhibition by low, but not by high, concentrations of 2DG.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D., Kornberg H. L. Regulation of fructose uptake by glucose in Escherichia coli. J Gen Microbiol. 1975 Sep;90(1):157–168. doi: 10.1099/00221287-90-1-157. [DOI] [PubMed] [Google Scholar]
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Location of a gene specifying phosphopyruvate synthase activity on the genome of Escherichia coli, K12. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):281–292. doi: 10.1098/rspb.1967.0066. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The direct synthesis of phosphoenolpyruvate from pyruvate by Escherichia coli. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):263–280. doi: 10.1098/rspb.1967.0065. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The role of phosphotransferase-mediated syntheses of fructose 1-phosphate and fructose 6-phosphate in the growth of Escherichia coli on fructose. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):105–119. doi: 10.1098/rspb.1974.0065. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Elvin C. M. Location and function of fruC, a gene involved in the regulation of fructose utilization by Escherichia coli. J Gen Microbiol. 1987 Feb;133(2):341–346. doi: 10.1099/00221287-133-2-341. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Fine control of sugar uptake by Escherichia coli. Symp Soc Exp Biol. 1973;27:175–193. [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Correlation between hexose transport and phosphotransferase activity in Escherichia coli. Biochem J. 1972 Mar;126(5):1241–1243. doi: 10.1042/bj1261241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of glucose uptake by Escherichia coli. FEBS Lett. 1972 Feb 15;20(3):270–272. doi: 10.1016/0014-5793(72)80084-x. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Role of phosphofructokinase in the utilization of glucose by Escherichia coli. Nature. 1970 Jul 4;227(5253):44–46. doi: 10.1038/227044a0. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W., Jacobson G. R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993 Sep;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl R. A., Vinopal R. T. Lack of glucose phosphotransferase function in phosphofructokinase mutants of Escherichia coli. J Bacteriol. 1976 May;126(2):852–860. doi: 10.1128/jb.126.2.852-860.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114(1):51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]



