Abstract
Enteral immunomodulatory nutrition is considered as a promising therapy for the treatment of acute lung injury and acute respiratory distress syndrome (ALI/ARDS). However, there are still some divergences, and it is unclear whether this treatment should be recommended for patients with ALI/ARDS. Therefore, we conducted this systematic review and meta-analysis to assess the efficacy and safety of an enteral immunomodulatory diet on the clinical outcomes of ALI/ARDS patients. Methods: We retrieved potentially relevant clinical trials though electronic databases. All trials of enteral immunomodulatory diet for ALI/ARDS were included. Analyses of the overall all-cause mortality, 28-day ventilator-free days and 28-day intensive care unit (ICU) free days were conducted. Results: In total six controlled trials were evaluated. The pooled results did not show a significant reduction in the risk of all-cause mortality (M-H RR (the overall Mantel-Haenszel relative risk), 0.81 (95% CI, 0.50–1.31); p = 0.38; 6 trials, n = 717) in ALI/ARDS patients treated with the immunomodulatory diet. This treatment also did not extend the ventilator-free days and ICU-free days. However, patients with high mortality might benefit from this treatment. Conclusions: The enteral immunomodulatory diet could not reduce the severity of the patients with ALI/ARDS. Whereas, for ALI/ARDS patients with high mortality, this treatment might reduce the all-cause mortality, but its use should be treated with discretion.
Keywords: enteral nutrition, immunomodulatory diet, acute respiratory distress syndrome, acute lung injury, critical care, mortality
1. Introduction
Since its first description in 1967, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) have been known as common and lethal diseases. With mortality ranging from 25%–40% [1], ALI/ARDS is a life-threatening disorder that cannot be ignored. It is mainly caused by predisposing disorders such as pneumonia, aspiration, shock, and severe sepsis [2]. Benefiting from the exploration of the pathophysiology of ALI/ARDS, we know that after having been affected by these diseases, neutrophils will infiltrate into the alveolar space and pulmonary mesenchyme, where they will release pro-inflammatory cytokines and eventually cause ALI/ARDS [2], which is characteristic of leakage of edema fluid and mismatch of ventilation and perfusion [2,3].
Although we know much about the pathophysiologic change of ALI/ARDS, very little improvement in patient outcomes has been achieved. The main treatment is supportive care, including maintaining oxygenation and avoiding complications [1,2]. There are no specific and effective treatments for ALI/ARDS [4], although many ventilation strategies and medicines have been tried. Thus, it is urgent to find an effective treatment for ALI/ARDS. Over the past two decades, some trials [5,6,7] and meta-analyses [8,9] have suggested that the enteral use of an immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) might be a promising therapy.
This immunomodulatory diet is mainly combined with anti-inflammatory elements (such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and gamma-linolenic acid (GLA)) and antioxidants (such as vitamin C, vitamin E and beta-carotene). It has been reported that Omega-3 (EPA and DHA) could modulate inflammatory processes, such as by reducing leukotriene production [10,11] and decreasing the synthesis of prostaglandin E2 [12]. It can also reduce the permeability of the alveolar-capillary membrane [13]. As for the antioxidants, they can scavenge free radicals, as we all know, and thus reduce the inflammation [14].
Using enteral nutrition for ALI/ARDS patients has been demonstrated to improve oxygenation and extend 28-day ventilator-free days and 28-day intensive care unit (ICU) free days [5,7]. It has even been associated with reduced mortality [6,7]. Some meta-analyses [8,9] have also shown its effect. However, one trial conducted by Rice et al. [15] revealed that an enteral inflammation-modulating diet did not improve the outcomes of ALI/ARDS patients and might be harmful. This conclusion compelled us to re-evaluate the effectiveness and safety of this treatment.
Therefore, we conducted this systematic review and meta-analysis to re-evaluate the effectiveness and safety of enteral use of the immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) vs. standard enteral nutrition on the mortality and clinical outcomes in patients with ALI/ARDS and to guide further research in this area.
2. Methods
The work, including the literature search, study selection and data extraction, was conducted according to standard strategies described below. Two reviewers (CCL and LYB) completed this work independently, and all discrepancies were solved by discussion or consultation with the senior reviewer (FGJ). Ethical approval was not required to conduct this meta-analysis.
2.1. Search Strategy
An extensive computer search of the relevant literature was performed by the two reviewers independently using databases including MEDLINE (PubMed), Embase and the Cochrane Central Register of Controlled Trials. We also retrieved potentially relevant literature manually, including conference abstracts published in the American Journal of Respiratory and Critical Care Medicine, Critical Care Medicine and Chest. All articles and conference abstracts about enteral nutrition therapies for patients with ALI or ARDS were identified regardless of language. The search terms we used were critically ill patients, acute lung injury, ALI, acute respiratory distress syndrome, ARDS, mechanical ventilation, sepsis, immunomodulatory diet, fish oil, antioxidants, omega-3 fatty acids, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and γ-linolenic acid (GLA).
2.2. Study Selection
Studies were included if they fulfilled all of the inclusion criteria. (1) Participants: patients had to be diagnosed with ALI/ARDS or have respiratory failure that required mechanical ventilation. (2) Type of studies: studies were eligible only if they were randomized controlled trials. (3) Type of interventions: studies used enteral nutrition therapies (omega-3 fatty acids, γ-linolenic acid and antioxidants). Studies were excluded if they did not provide outcomes related to mortality, 28-day ventilator-free days or 28-day ICU-free days. Crossover studies were also excluded.
2.3. End Points and Data Extraction
The primary end point was all-cause mortality, and the secondary end points were 28-day ventilator-free days, 28-day ICU-free days and adverse effects. For all-cause mortality, we used 28-day mortality. If 28-day mortality could not be acquired, we used ICU or hospital mortality instead. We also extracted and collected the relevant information about each study, such as the characteristics of the studies, characteristics of the participants, enteral immunomodulatory therapy strategies and types of outcomes.
2.4. Quality Assessment
The quality levels of the included trials were also evaluated independently by two authors (CCL and LYB). We assessed the risk of bias (including selection bias, performance bias, attrition bias, detection bias, reporting bias and other bias) using the assessment table recommended by the Cochrane Reviewers’ Handbook [16]. We also evaluated the methodological quality of the included trials using the Modified Jadad Scale [17], where the full score is 7, and scores of 4–7 are regarded as high quality and 1–3 as low quality.
2.5. Data Processing and Statistical Analysis
First, we examine the heterogeneity of the included studies using the I2 statistic and Chi2 test, with significant heterogeneity if p ≤ 0.10 for the Chi2 test or I2 ≥ 50%. If significant heterogeneity was obtained, we would use the random-effects model for the following analysis; otherwise, the fixed-effects model would be used.
Second, we pooled the treatment effects of enteral nutrition on the all-cause mortality to estimate the summary effect. As the mortality outcome was dichotomous, we calculated the relative risk (RR) and 95% confidence interval (CI) of every included trial and then pooled them to estimate the overall Mantel-Haenszel (M-H) RR and the 95% CI. For the continuous variables, we calculated the standardized mean difference (SMD). To test the robustness of the results, we performed a sensitivity analysis by excluding each individual study and re-analyzing. The funnel plot was calculated to evaluate the publication bias.
The results were considered statistically significant if (1) the two-sided p-value ≤ 0.05, (2) the confidence interval for RR did not include 1, and (3) the confidence interval for SMD did not include 0. The data synthesis and sensitivity analyses were performed using Review Manager (version 5.1).
3. Results
3.1. Study Selection and Quality Assessment
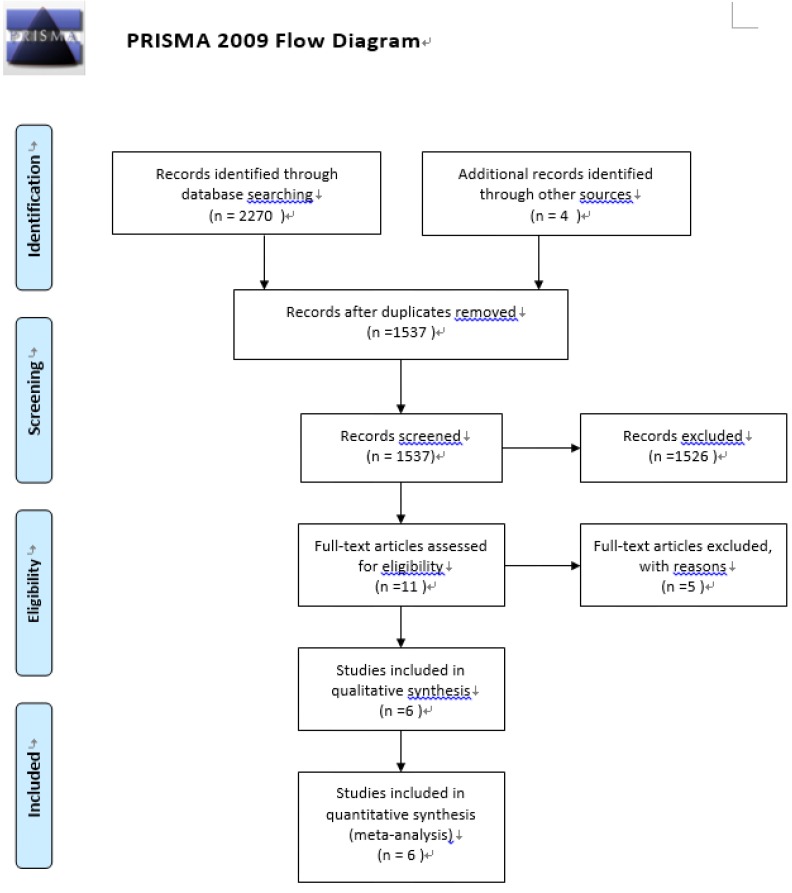
We identified six studies [5,6,7,15,18,19] that fulfilled our inclusion criteria out of 2274 potential articles though searching the relevant databases (see Figure 1). All of them were included in our analysis. Five relevant papers [20,21,22,23,24] were excluded based on the reasons described in Table S1. The major characteristics of the six included trials are summarized in Table 1. In short, the trials encompassed a total of 717 patients, with 365 patients in the experimental groups and 352 patients in the control groups. The mean age of the patients ranged from 51.0 to 65.1. The mortality of the control groups ranged from 12.5% to 57.14%. When stratified by the compositions of the immunomodulatory diet, two studies included treatment with EPA + GLA + antioxidants, and four studies included treatment with EPA + DHA + GLA + antioxidants. When stratified by the blind strategies, four trials were double-blind, one trial was single-blind and one trial was unblinded.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Table 1.
Characteristics of Included Trials.
| Parameter | Gadek et al., 1999 | Singe et al., 2006 | Pontes-Arda et al., 2006 | Grau-Carmona et al., 2011 | Rice et al., 2011 | Elamin et al., 2012 |
|---|---|---|---|---|---|---|
| Interventions | EPA + GLA + antioxidants | EPA + GLA + antioxidants | EPA + DHA + GLA + antioxidants | EPA + DHA + GLA + antioxidants | EPA + DHA + GLA + antioxidants | EPA + DHA + GLA + antioxidants |
| Control Diet | Isonitrogenous andisocaloric control diet | Isonitrogenous and isocaloric control diet | Isonitrogenous and isocaloric control diet | Isocaloric control diet | Isocaloric and isovolemic control diet | Isonitrogenous and isocaloric control diet |
| Treatment Duration | N/A | 14 days | N/A | N/A | 21 days | 7 days |
| Route | Gastric, duodenal, jejunalfeeding tube | Nasogastric, duodenal, jejunal tube | Eneral feeding | Gastric, jejunal tube | Bolus delivery | Nasogastric, nasoduodenal, nasojejunal, jejunostomytubes |
| Sample Size | ||||||
| Treatment Group | 51 | 46 | 55 | 61 | 143 | 9 |
| Control Group | 47 | 49 | 48 | 71 | 129 | 8 |
| Sex Ratio (Male:Female) | 52:46 | NA | 61:42 | 30:132 | 133:139 | 8:9 |
| Average Age (years) | 51 | 59.7 | 65.1 | 63 | 54.1 | 52.4 |
| No. of Participants Drop-out or Withdrawal | 48 | 5 | 62 | 28 | 0 | 5 |
| Blind Type | Double-blind | Unblind | Double-blind | Single-blind | Double-blind | Double-blind |
| Mordified Jadad Scale | 7 | 5 | 5 | 5 | 7 | 5 |
| Primary End Point | Time receiving ventilatorysupport | Change in oxygenation and breathing patterns | 28-day mortality | New organ dysfunction | Ventilator-free days | Oxygenation and modified Lung Injury Scores |
| Mortality Outcome Type | Mortality | 28-day mortality | 28-day mortality | 28-day mortality | 60-day or hospital mortality | 28-day mortality |
| Mortality | ||||||
| Treatment Group | 6/51 | 13/46 | 18/55 | 11/61 | 38/143 | 0/9 |
| Control Group | 9/47 | 28/49 | 25/48 | 11/71 | 21/129 | 1/8 |
| Mortality Rate of Control Group | 9/47 (19.15%) | 28/49 (57.14%) | 25/48 (52.08) | 11/71 (15.49) | 21/129 (16.28) | 1/8 (12.5) |
| PaO2/FiO2 Ratio (Day 7) | ||||||
| Treatment Group | N/A | 296.5 ± 165.3 (SD) | 224.4 | 217 | N/A | 178 |
| Control Group | N/A | 236.3 ± 79.8 (SD) | 150.5 | 190 | N/A | 201 |
Abbreviations: EPA, eicosapentaenoic acid; GLA, gamma-linolenic acid; DHA, docosahexaenoic acid; N/A, not available.
We evaluated the quality of the included trials using the Modified Jadad Scale and Cochrane’s risk of bias assessment table. As shown in Table S2, all of the included studies were high quality, and most of them had low risk of bias in the generation of random sequence, allocation concealment, incomplete outcome data and selective reporting. Only two trials were high risk in terms of the blinding of participants and personnel.
3.2. Effect on Mortality
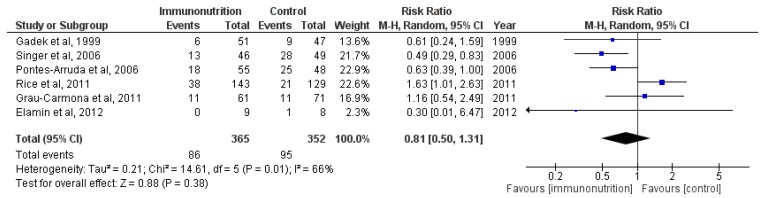
Because significant heterogeneity was found across the included trials (χ2 = 14.61, df = 5 (p = 0.01); I2 = 66%), we used the random-effects model to analyze the overall effect of immunomodulatory nutrition on mortality. As shown in Figure 2, there was no significant difference between the two groups (M-H RR, 0.81 (95% CI, 0.50–1.31); p = 0.38; six trials, n = 717) that is, the pooled result did not showed a significant reduction in the risk of all-cause mortality in ALI/ARDS patients treated with immunomodulatory nutrition. The overall mortality of the six trials was 25.24%, and the mortality of the experimental groups was 23.56% compared with 26.99% for the control groups.
Figure 2.
Forest plot of the association between enteral immunomodulatory diet and all-cause mortality among patients with ALI (acute lung injury)/ARDS (acute respiratory distress syndrome).
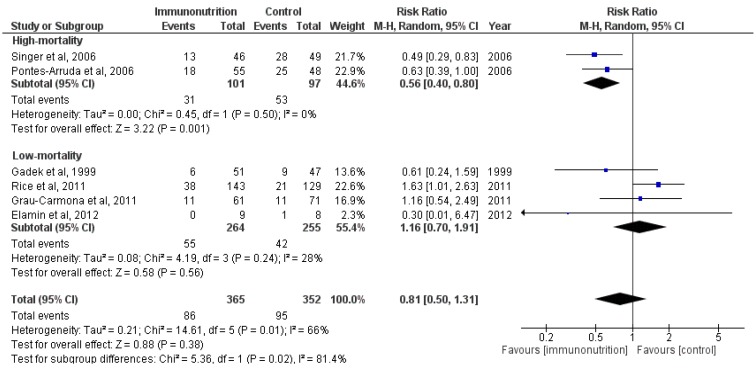
Because of the heterogeneity of the mortality in the control groups across the included trials, we conducted a subgroup analysis by stratifying the previous meta-analyses according to the mortality of the control groups. The analyses (M-H RR, 1.16 (95% CI, 0.70–1.91); p = 0.56; three trials, n = 97) revealed that for patients with low mortality, this treatment could not reduce the overall mortality in ALI/ARDS patients (see Figure 3). The results (M-H RR, 0.56 (95% CI, 0.40–0.80); p = 0.001; two trials, n = 198) indicated that patients with high mortality might benefit from this treatment, and there was a significant subgroup difference (χ2 = 5.36, df = 1 (p = 0.02); I2 = 81.4%). However, they were something that need our attention. The quality of the trials in this subgroup was lower than most of others (as shown in Table S2).
Figure 3.
Forest plot of the association between enteral immunomodulatory diet and all-cause mortality among patients with ALI/ARDS, stratified by discrepancy of mortality.
3.3. Effect on 28-Day Ventilator-Free Days and 28-Day ICU-Free Days
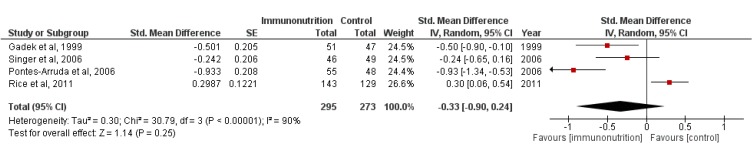
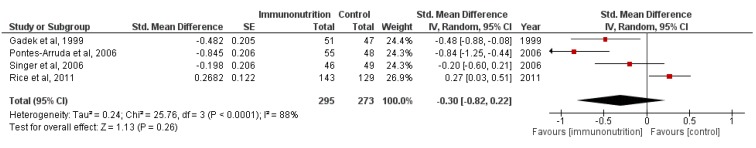
We also pooled the data about the 28-day ventilator-free days and 28-day ICU-free days. The outcomes of 568 participants from four trials were available when assessing the effect of enteral nutrition on ventilator-free days and ICU-free days. As shown in Figure 4 and Figure 5, enteral nutrition did not extend the ventilator-free days (M-H RR, −0.33 (95% CI, −0.90–0.24); p = 0.25; four trials, n = 568) and ICU-free days (M-H RR, −0.30 (95% CI, −0.82–0.22); p = 0.26; four trials, n = 568). Because of the significant heterogeneity of the included trials ((χ2 = 30.79, df = 3 (p < 0.00001); I2 = 90%) and (χ2 = 25.76, df = 3 (p < 0.0001); I2 = 88%)), the random-effects model was selected.
Figure 4.
Forest plot of the association between enteral immunomodulatory diet and 28-day ventilator-free days among patients with ALI/ARDS.
Figure 5.
Forest plot of the association between enteral immunomodulatory diet and 28-day ICU-free days among patients with ALI/ARDS.
3.4. Sensitivity Analyses
To test the robustness of the results, we conducted sensitivity analyses. We excluded each individual study, re-analyzing and comparing with the original results. When excluding the trial conducted by Rice T. et al. [15], the overall effect was M-H RR, 0.63 (95% CI, 0.47–0.85); p = 0.0.003; five trials, n = 445 (see Figure S1). When excluding other trials, the results were consistent with the previous one.
3.5. Adverse Effects
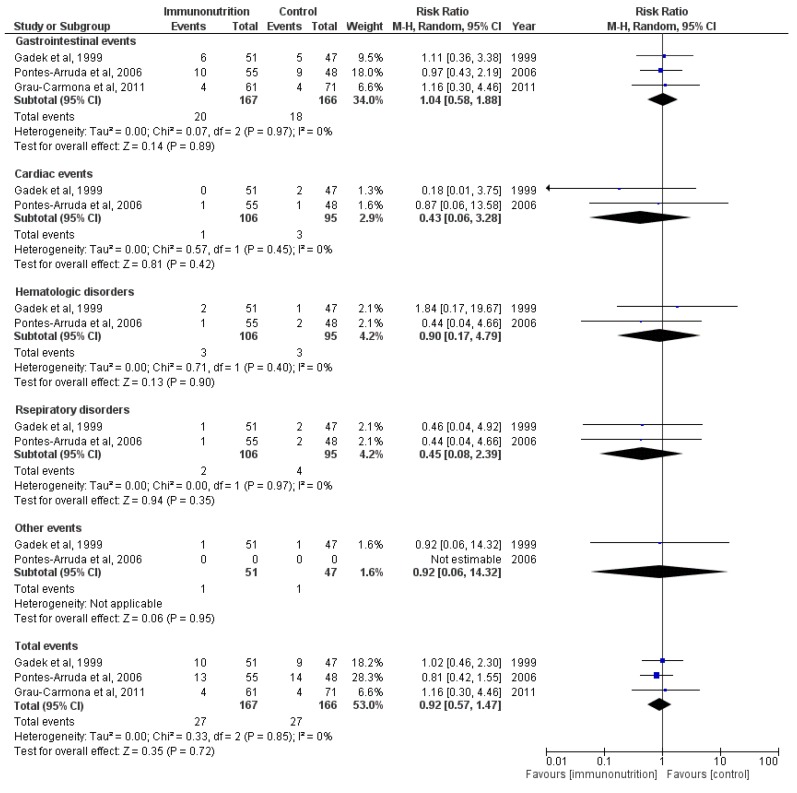
To test the safety of this treatment, we also analyzed the adverse effects of the enteral immunomodulatory diet. The majority of adverse events were gastrointestinal events such as diarrhea, dyspepsia and nausea. As shown in Figure 6, there was no significant difference between the two groups (M-H RR, 0.92 (95% CI, 0.57–1.47); p =0.72; three trials, n =333).
Figure 6.
Forest plot of the association between enteral immunomodulatory diet and adverse events among patients with ALI/ARDS.
3.6. Publication Bias
No evidence of publication bias was detected by funnel plots (see Figure S2).
4. Discussion
In conducting this systematic review, we searched the relevant literature comprehensively without language limitation. The pooled results from all six independently conducted trials revealed that an enteral immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) could not improve all-cause mortality, ventilator-free days or ICU-free days in patients with ALI/ARDS. Overall, patients could not benefit from enteral immunomodulatory diet, and its use should be treated with discretion.
It was believed previously that the immunomodulatory diet could suppress the elevated inflammatory reactions during ALI/ARDS [5], and patients could benefit from it [6]. Preclinical studies reported that Omega-3 (EPA and DHA) could reduce leukotriene synthesis and the production of prostaglandin E2, which could be beneficial in ALI/ARDS [3,11,13]. The antioxidants could also reduce the inflammation through scavenging free radicals [25]. Several clinical trials confirmed these results [5,6,7], and demonstrated an association between the usage of enteral immunomodulatory diet and improved outcomes in ALI/ARDS patients [5,6,7]. Two meta-analyses [8,9] also demonstrated this effect. However, some trials conducted recently achieved a contrary result [15,18], showing that enteral inflammation-modulating diet did not improve the outcomes and might be harmful. Our results were similar. However, some results needed extra attention. As shown in the characteristics of the included studies, the mortality of the control groups varied widely (from 12.50% to 57.14%), and the test for heterogeneity was also significant for mortality. This result may be due to the different severity of the illness and improved treatment strategies [2]. To decrease its influence on the final results, we used the random-effects model for analysis, and we also conducted a subgroup analysis stratified according to the mortality of the control groups. The result revealed that enteral immunomodulatory nutrition could only benefit ALI/ARDS patients with high mortality. For patients with low mortality, this treatment had no effect and might be harmful. From this perspective, it is important to clarify the indications of this treatment, and for future trials about this aspect, the enrolled patients could be restricted to severe cases. However, the quality of the two trials included in the high-mortality subgroup was lower than most of the others, and the results of these studies might be affected.
The drop-out proportions of most included studies were large. Undoubtedly, the reliabilities of the final results achieved by these trials were influenced by this factor [16]. The main reason that people left the studies was that the patients could not tolerate the rate of continuous enteral infusions because of gastrointestinal complications [5,7]. However, the study conducted by Rice T. et al. [15] solved this problem by using bolus delivery, namely small-volume supplementation, to deliver the supplements. The results indicated that this method was more tolerable. However, given 120 mL fluid once might increase the risk of aspiration, especially for patients who already have respiratory compromise.
In this review, we demonstrated that ALI/ARDS patients could not benefit from enteral immunomodulatory diet through including some newly reported trials. However, we still need further exploration of the following issues. During sensitivity analyses, we found that the results were not very robust. The final conclusion was seriously affected by the trial conducted by Rice T. et al. When we excluded this study, re-analyzed and compared with the previous results, the opposite conclusion was obtained. This condition was more or less due to the discrepancy of the controlled nutrition, and the calorie intake was quite low in Rice T. et al.’s trials [26]. However, the reason is still unclear, and we should be aware that the conclusion is not certain. Further improved randomized clinical trials are needed.
Some limitations in this report should be mentioned. First, the heterogeneity tests of the all-cause mortality, ventilator-free days and ICU-free days were positive. Although we tried to reduce their influence methodologically (using a random-effects model and subgroup analyses), they might still cause some biases. Second, the sample sizes of the included trials were small, and only three trials had more than 100 patients available. Even worse, the drop-out proportions were large in the majority of the included trials. Third, there was also some variability in the patient types, outcome types, and route of intervention administration. When trying to solve this problem, we found clues indicating that the effects of enteral nutrition may be related to the severity of the ALI/ARDS. Finally, we did not assess the discrepancy of the ratio of partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FiO2 ratio) because of inadequate information. As one of the most frequently used indicators of oxygenation and respiratory function, the PaO2/FiO2 ratio is a good predictor of the condition of ALI/ARDS patients. Thus, further trials should report more information about it.
5. Conclusions
Overall, based on the existing data, the enteral immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) could not reduce the mortality of patients with ALI/ARDS and also could not extend the 28-day ventilator-free days or 28-day ICU-free days. However, the subgroup analysis showed that enteral immunomodulatory nutrition could benefit ALI/ARDS patients with high mortality, but it should be used with discretion. More well-designed clinical trials are urgently needed to verify this conclusion.
Acknowledgments
The conduct of this study was not funded. The authors thank Anan Yin for his support during revision of this manuscript.
Supplementary Information
Table S1: Studies excluded from the meta-analysis of clinical trials involving enteral nutrition treatment for ALI/ARDS.
Table S2: Risk of bias of the included studies.
Figure S1: Sensitivity results (overall effect when excluding the trial conducted by Rice T. et al.).
Figure S2: Funnel plot of the standard error by log relative risk of all-cause mortality.
Author Contributions
All authors contributed to the inception of the research question and study design. Congcong Li also contributed to the study selection, quality assessment and manuscript composition. Liyan Bo contributed to the study selection, quality assessment, and records review. Wei Liu and Xi Lu contributed to the data synthesis and data analysis. Faguang Jin and Xi Lu were responsible for the integrity of this work and contributed to the study design, final study selection and manuscript review. All authors contributed to drafting the manuscript and have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wheeler A.P., Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 2.Pierrakos C., Karanikolas M., Scolletta S., Karamouzos V., Velissaris D. Acute respiratory distress syndrome: Pathophysiology and therapeutic options. J. Clin. Med. Res. 2012;4:7–16. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller-Redetzky H.C., Felten M., Hellwig K., Wienhold S.M., Naujoks J., Opitz B., Kershaw O., Gruber A.D., Suttorp N., Witzenrath M. Increasing the inspiratory time and I:E ratio during mechanical ventilation aggravates ventilator-induced lung injury in mice. Crit. Care. 2015;19:23. doi: 10.1186/s13054-015-0759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhikari N., Burns K.E., Meade M.O. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: Systematic review and meta-analysis. Treat Respir. Med. 2004;3:307–328. doi: 10.2165/00151829-200403050-00005. [DOI] [PubMed] [Google Scholar]
- 5.Gadek J.E., DeMichele S.J., Karlstad M.D., Pacht E.R., Donahoe M., Albertson T.E., Van Hoozen C., Wennberg A.K., Nelson J.L., Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit. Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Singer P., Theilla M., Fisher H., Gibstein L., Grozovski E., Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit. Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 7.Pontes-Arruda A., Aragao A.M., Albuquerque J.D. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit. Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 8.Pontes-Arruda A., Demichele S., Seth A., Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of outcome data. JPEN J. Parenter. Enter. Nutr. 2008;32:596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 9.Dee B.M., Bruno J.J., Lal L.S., Canada T.W. Effects of immune-enhancing enteral nutrition on mortality and oxygenation in acute lung injury and acute respiratory distress syndrome a meta-analysis. Hosp. Pharm. 2011;1:33–40. doi: 10.1310/hpj4601-33. [DOI] [Google Scholar]
- 10.El K.D., Gjorstrup P., Filep J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA. 2012;109:14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan S.A., Ali A., Khan S.A., Zahran S.A., Damanhouri G., Azhar E., Qadri I. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/502749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B., Gong X., Wan J.Y., Zhang L., Zhang Z., Li H.Z., Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm. Pharmacol. Ther. 2011;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Cox J.R., Phillips O., Fukumoto J., Fukumoto I., Tamarapu P.P., Arias S., Cho Y., Lockey R.F., Kolliputi N. Aspirin-Triggered Resolvin D1 Treatment Enhances Resolution of Hyperoxic Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2015 doi: 10.1165/rcmb.2014-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S., Zheng S., Liu Z., Tang C., Zhao B., Du J., Jin H. Endogeous sulfur dioxide protects against oleic acid-induced acute lung injury in association with inhibition of oxidative stress in rats. Lab. Investig. 2015;95:142–156. doi: 10.1038/labinvest.2014.147. [DOI] [PubMed] [Google Scholar]
- 15.Rice T.W., Wheeler A.P., Thompson B.T., DeBoisblanc B.P., Steingrub J., Rock P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banares R., Albillos A., Rincon D., Alonso S., Gonzalez M., Ruiz-del-Arbol L., Salcedo M., Molinero L.M. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: A meta-analysis. Hepatology. 2002;35:609–615. doi: 10.1053/jhep.2002.31354. [DOI] [PubMed] [Google Scholar]
- 18.Grau-Carmona T., Moran-Garcia V., Garcia-de-Lorenzo A., Heras-de-la-Calle G., Quesada-Bellver B., Lopez-Martinez J., Gonzalez-Fernandez C., Montejo-Gonzalez J.C., Blesa-Malpica A., Albert-Bonamusa I., et al. Effect of an enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid and anti-oxidants on the outcome of mechanically ventilated, critically ill, septic patients. Clin. Nutr. 2011;30:578–584. doi: 10.1016/j.clnu.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Elamin E.M., Miller A.C., Ziad S. Immune enteral nutrition can improve outcomes in medical-surgical patients with ARDS: A prospective randomized controlled trial. J. Nutr. Disord. Ther. 2012;2:109. doi: 10.4172/2161-0509.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson J.L., DeMichele S.J., Pacht E.R., Wennberg A.K. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants on antioxidant status in patients with acute respiratory distress syndrome. JPEN J. Parenter. Enter. Nutr. 2003;27:98–104. doi: 10.1177/014860710302700298. [DOI] [PubMed] [Google Scholar]
- 21.Pacht E.R., DeMichele S.J., Nelson J.L., Hart J., Wennberg A.K., Gadek J.E. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit. Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 22.Theilla M., Singer P., Cohen J., Dekeyser F. A diet enriched in eicosapentanoic acid, gamma-linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury: A randomized, prospective, controlled study. Clin. Nutr. 2007;26:752–757. doi: 10.1016/j.clnu.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Pontes-Arruda A., Martins L.F., de Lima S.M., Isola A.M., Toledo D., Rezende E., Maia M., Magnan G.B. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in the early treatment of sepsis: Results from a multicenter, prospective, randomized, double-blinded, controlled study: The INTERSEPT study. Crit. Care. 2011;15 doi: 10.1186/cc10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schott C.K., Huang D.T. omega-3 fatty acids, gamma-linolenic acid, and antioxidants: Immunomodulators or inert dietary supplements? Crit. Care. 2012;16:325. doi: 10.1186/cc11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W., Zhou S., Yao W., Gan X., Su G., Yuan D., Hei Z. Propofol prevents lung injury after intestinal ischemia-reperfusion by inhibiting the interaction between mast cell activation and oxidative stress. Life Sci. 2014;108:80–87. doi: 10.1016/j.lfs.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Chen W., Jiang H., Zhou Z.Y., Tao Y.X., Cai B., Liu J., Yang H., Lu C.D., Zeng J. Is omega-3 fatty acids enriched nutrition support safe for critical ill patients? A systematic review and meta-analysis. Nutrients. 2014;6:2148–2164. doi: 10.3390/nu6062148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.