The endeavor to develop human epidermal growth factor receptor 2 (HER2) –targeting agents for cancer therapy now spans more than two decades, with four drugs already on the market and numerous others in the pharmaceutical pipelines. The interest in this mode of cancer therapy continues to intensify, particularly in the era of personalized medicine, because HER2 amplification underlies the biology of subsets of a large variety of cancers, including breast, gastric, esophageal, endometrial, ovarian, colorectal, bladder, head and neck, and others.
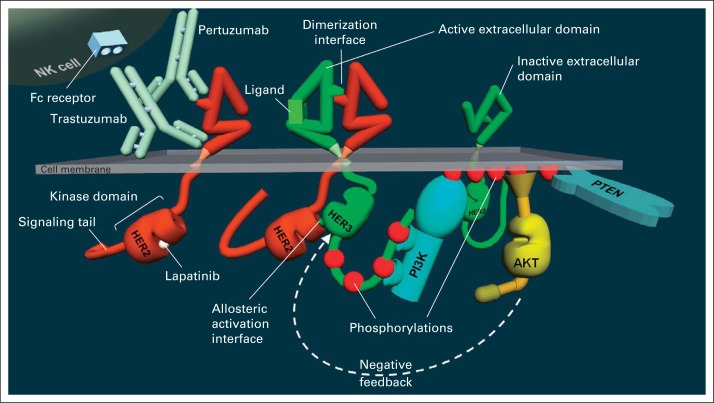
HER2 is a receptor tyrosine kinase located at the cell membrane with a large extracellular domain (ECD) and an intracellular catalytic kinase domain (KD) and signaling tail. Signal generation by HER2 occurs through heterodimerization with its HER family siblings (epidermal growth factor receptor, HER3, HER4), particularly HER3 (Fig 1). This process is prompted by ligand binding to HER3, which reconfigures its ECD exposing the interface that mediates dimerization with HER2. The proximation leads to the allosteric activation of the HER2 KD by the HER3 KD. The activated HER2 KD then phosphorylates the c-tail of HER3, leading to recruitment of several proteins and initiating a series of parallel signaling cascades that ultimately execute the phenotypic changes in cell behavior.
Fig 1.
Structure of the human epidermal growth factor receptor 2 (HER2) and HER3 receptors and their mode of activation through dimerization and activation of PI3K/Akt signaling and binding sites of trastuzumab, pertuzumab, and lapatinib, showing both an inactive and ligand-activated HER3. Binding of ligand reconfigures the extracellular domain of HER3, exposing the dimerization interface. The extracellular domain of HER2 is always in the active configuration and does not require ligand. The phosphorylated signaling tail of HER3 binds and activates PI3K, leading to phosphorylation of membrane lipids, which is reversed by the phosphatase PTEN. These membrane phospholipids recruit and activate Akt, which regulates many downstream events. In HER2-driven cancer cells, it also regulates HER3 in a feedback loop shown by the arrow.
Numerous cell cultured and mouse transgenic models have confirmed that the overexpression of HER2 is tumorigenic and continues to be a driver of the tumors that it generates.1,2 It is now also apparent from several cell-based, xenograft, and transgenic mouse models that HER3 is an essential partner and codriver for HER2 in tumorigenesis.3–5 HER3 functions both upstream and downstream of HER2. It functions upstream because its own KD, although catalytically inactive, is a highly competent allosteric activator of the HER2 KD.6 It functions downstream because it is a key substrate of HER2, particularly competent at recruiting and activating PI3K, and HER2 activates this pathway through the phosphorylation of the HER3 c-tail.7,8
The 25-year endeavor to develop targeted therapies for this type of cancer has had an evolutionary course closely following the trail of scientific developments. The monoclonal antibody trastuzumab was developed in the early days following the discovery of HER2 and is now known to bind the juxta-membrane region of the HER2 ECD.9,10 Pertuzumab was designed much later to interfere with HER2 signaling and binds the dimerization interface of the HER2 ECD (Fig 1).11,12 These agents exhibit only limited activity in the monotherapy of advanced-stage HER2-amplified breast or gastric cancers.13–17 But they do enhance the efficacies of active chemotherapy regimens and have become staples of combination regimens for the management of advanced breast and gastric cancers.18–20 The efficacy enhancement afforded by trastuzumab is even more pronounced in early-stage breast cancer, with significant survival benefits,21,22 and the neoadjuvant data available thus far suggest further enhancement by the addition of pertuzumab.23
The antibody trastuzumab was developed on the basis of 1980s understanding of HER2, and it is now clear that it does not actually inhibit HER2 signaling functions very well. A mixed literature has precluded finality in this debate, because some investigators find profound trastuzumab effects on HER2 expression or signaling.24–26 But the majority of investigators, including our own group, see only partial, minimal, or no effects on HER2 expression or signaling, even at high concentrations of trastuzumab.27–41 The antibody pertuzumab, which was specifically designed to interfere with the ECD-mediated dimerization of HER2, does in fact inhibit this dimerization function in its physiologic setting of ligand-induced HER2 signaling when HER2 levels are normal.12 But it shows no such effects in the pathologic scenario of constitutive HER2 signaling seen in cancer cells with massive HER2 overexpression.27,35,36,42 The failure of these antibodies to inactivate HER2 signaling in HER2-amplified cancers reflects our naive understanding of how constitutive signaling is generated in these cancers. It is plausible that massive overexpression of HER2 leads to KD interactions and constitutive signaling without the requirement for ligand-driven ECD dimerization, and the conformation and interactions of the ECD may be irrelevant in this disease state of overexpression. If true, this would suggest that targeting the KDs directly would be a much more effective therapeutic strategy.
Advances in small-molecule discovery platforms and sophisticated structure-guided chemistries have enabled the development of potent and selective kinase inhibitors, and lapatinib is at the pinnacle of these accomplishments. Lapatinib inhibits the HER2 kinase with low nanomolar potency,43 in part because of a slow off-rate,44 near singular selectivity for the HER family,45 and excellent pharmacokinetic properties,46 making it one of the most potent and selective clinical tyrosine kinase inhibitors (TKIs) yet developed. Despite its truly remarkable chemical and pharmacologic attributes, lapatinib has only limited single-agent activity in patients with HER2-amplified breast, gastric, or gastroesophageal cancers.47–51 The irreversible HER2 TKI neratinib shows only slightly higher activity at significant cost in toxicity profile,52,53 and there is little evidence that any of the plethora of other TKIs in the pharmaceutical pipelines are able to substantially improve on these TKIs. In patients with HER2-amplified breast cancer, the incremental activity of lapatinib is more clinically useful in combination regimens with capecitabine, paclitaxel, or trastuzumab.49,54,55 In the accompanying article, Satoh et al report that lapatinib only modestly enhances the efficacy of paclitaxel in patients with HER2-amplified gastric cancer. It remains to be determined whether lapatinib can perform better when combined with more active gastric cancer chemotherapy regimens. The clinical activity of lapatinib does not compare favorably with HER2-targeting antibodies in randomized studies.56,57 However, unlike HER2-targeting antibodies, lapatinib potently inactivates constitutive HER2 signaling in HER2-amplified cancer cells.34,58,59
These developments in HER2-targeting have brought about two key conundrums: First, considering the overwhelming evidence that HER2 is a disease-driving oncogene, why do HER2-targeting agents not have much higher clinical activity in monotherapy? Second, why are the HER2-targeting antibody therapies more active than the TKIs clinically, if they are much poorer inhibitors of HER2 signaling? Hypothesis-driven experimental science has provided resolutions to these dilemmas and identified new directions for pursuit and renewed hope in the development of far more effective HER2-directed therapies.
It is now recognized that the HER2-HER3 complex, which is the functionally relevant tumor driver in HER2-amplified cancers, is much more resilient to inhibition than had been anticipated. This is because tumor cells will not tolerate the loss of Akt activity, and negative feedback signaling loops induced by the loss of Akt activity can execute a marked increase in HER3 signaling output to preserve this critical signaling throughput60,61 (Fig 1). The highly dynamic nature of HER3 signaling endows the HER2-HER3 complex with the ability to increase its signaling output approximately 100-fold in response to pharmaceutical inhibitors, overpowering and undermining the activity of all such HER2 or HER3 inhibitors.62 Although inhibitors such as lapatinib can inactivate HER2-HER3 signaling at clinically relevant concentrations, the inhibition lasts less than 24 hours and is ultimately overpowered by the compensatory mechanisms. These findings have redefined the HER2-HER3 complex as the true driver of HER2-amplified cancer and the effective inactivation of this complex is the new bar for pharmaceutical drug development. None of the current agents rise to this bar, entirely consistent with their limited activities as monotherapy.
The fact that HER2-targeting antibodies are far weaker inhibitors of oncogenic HER2 signaling than TKIs, even though they exhibit greater clinical efficacy than TKIs in HER2-amplified cancers, seems paradoxical at first glance. But the key to the paradox lies in the immunologic dimension encompassed by antibody therapies that are completely lacking in TKIs. The massive cell-surface expression of HER2 in HER2-amplified cancer cells enables abundant binding of engineered HER2-targeting antibodies, thus providing a therapeutic index for endogenous immunologic responses that would ordinarily be lacking against self-antigens. Trastuzumab and pertuzumab are both fully competent human antibodies capable of mediating immune functions such as antibody-dependent cell-mediated cytotoxicity as well as potentially other immune effector functions, and there is now considerable evidence in support of this mode of action. The in vivo antitumor efficacy of trastuzumab or its murine predecessor in a xenograft model is almost entirely lost by a single mutation or a proteolytic disruption in the Fc region of trastuzumab or elimination of the mouse Fc receptor gamma, all of which impair the ability of the mouse to mount an immunologic response to the trastuzumab-coated xenograft tumor.63,64 Immunocompetent models of neu-driven mammary tumorigenesis further elucidate the roles of both innate and adaptive immunities underlying the antitumor activities of HER2/neu-targeting antibodies.65 There is an abundance of clinical evidence in patients treated with trastuzumab that further supports an immunologic mode of action, including the induction of antibody-dependent cellular cytotoxicity,66 endogenous humoral and enhanced T-cell–mediated immune responses,67 suppression of regulatory T cells and induction of Th17 cells,68,69 and increased tumor infiltration with immune effectors including natural killer cells.70–72 The endogenous immunity induced by trastuzumab therapy appears to last beyond the cessation of therapy and has been detected in patients enrolling onto subsequent vaccine studies.73 It is widely believed that the greatest potential in the immunologic approach to cancer therapy lies in the treatment of microscopic residual disease. Consistent with this, the greatest impact of trastuzumab therapy has been seen in the adjuvant setting with improvements in outcomes surpassing other systemic modalities.21,22
The hypothesis that HER2-amplified cancers can be effectively treated through the inactivation of their HER2-HER3 drivers remains a solid treatment hypothesis vigorously being pursued through numerous innovative pharmaceutical approaches. The fact that massive HER2 expression adorns the outside surface of HER2-amplified cancer cells, forsaking their ability to camouflage themselves, creates the opportunity for an entirely separate dimension of pharmaceutical immunotherapy and immunodelivery approaches. Although much of the promise of the HER2-inhibiting dimension remains in its future, HER2 immunotherapy approaches have made a seismic impact already, even without their appropriate label and accolades as the true pioneers of the cancer immunotherapy era.
Acknowledgment
M.M.M. is supported by National Institutes of Health Grants No. CA122216 and CA112970, the California Breast Cancer Research Program 16OB-0150, and the American Association for Cancer Research Breast Cancer Research Fund.
Footnotes
See accompanying article on page 2039
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author indicated no potential conflicts of interest.
REFERENCES
- 1.Moasser MM. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ursini-Siegel J, Schade B, Cardiff RD, et al. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7:389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 3.Holbro T, Beerli RR, Maurer F, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 5.Vaught DB, Stanford JC, Young C, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jura N, Shan Y, Cao X, et al. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor. Proc Natl Acad Sci U S A. 2009;106:21608–21613. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltoff SP, Carraway KL, 3rd, Prigent SA, et al. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shepard HM, Lewis GD, Sarup JC, et al. Monoclonal antibody therapy of human cancer: Taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117–127. doi: 10.1007/BF00918679. [DOI] [PubMed] [Google Scholar]
- 10.Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 11.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 12.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26:78–83. [PubMed] [Google Scholar]
- 15.Cortés J, Baselga J, Petrella T, et al. Pertuzumab monotherapy following trastuzumab-based treatment: Activity and tolerability in patients with advanced HER2- positive breast cancer. J Clin Oncol. 2009;27(suppl):46s. doi: 10.1200/JCO.2011.37.4207. abstr 1022. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Cameron D, Miles D, et al. Objective response rate in a phase II multicenter trial of pertuzumab (P), a HER2 dimerization inhibiting monoclonal antibody, in combination with trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) which has progressed during treatment with T. J Clin Oncol. 2007;25(suppl):33s. abstr 1004. [Google Scholar]
- 17.Rech J, Arnold D, Folprecht G, et al. A pilot study of trastuzumab (Herceptin) monotherapy in patients who progressed while on chemotherapy for metastatic or locally advanced HER2-positive gastric cancer. Ann Oncol. 2006:17. (abstr 1096P) [Google Scholar]
- 18.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 19.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 21.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 22.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 23.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 24.Cuello M, Ettenberg SA, Clark AS, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61:4892–4900. [PubMed] [Google Scholar]
- 25.Wang SE, Xiang B, Guix M, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605–5620. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yakes FM, Chinratanalab W, Ritter CA, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 27.Cai Z, Zhang G, Zhou Z, et al. Differential binding patterns of monoclonal antibody 2C4 to the ErbB3-p185her2/neu and the EGFR-p185her2/neu complexes. Oncogene. 2008;27:3870–3874. doi: 10.1038/onc.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuino L, Bali P, Wittmann S, et al. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003;2:971–984. [PubMed] [Google Scholar]
- 29.Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 30.Marches R, Uhr JW. Enhancement of the p27Kip1-mediated antiproliferative effect of trastuzumab (Herceptin) on HER2-overexpressing tumor cells. Int J Cancer. 2004;112:492–501. doi: 10.1002/ijc.20378. [DOI] [PubMed] [Google Scholar]
- 31.Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009;28:461–468. doi: 10.1038/onc.2008.394. [DOI] [PubMed] [Google Scholar]
- 32.Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 33.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Liu LH, Ho P, et al. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 35.Yao E, Zhou W, Lee-Hoeflich ST, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–4156. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Meng Y, Zheng L, et al. Bispecific antibody to ErbB2 overcomes trastuzumab resistance through comprehensive blockade of ErbB2 heterodimerization. Cancer Res. 2013;73:6471–6483. doi: 10.1158/0008-5472.CAN-13-0657. [DOI] [PubMed] [Google Scholar]
- 37.Minami T, Kijima T, Kohmo S, et al. Overcoming chemoresistance of small-cell lung cancer through stepwise HER2-targeted antibody-dependent cell-mediated cytotoxicity and VEGF-targeted antiangiogenesis. Sci Rep. 2013;3:2669. doi: 10.1038/srep02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaltriti M, Eichhorn PJ, Cortés J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A. 2011;108:3761–3766. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomioka H, Mukohara T, Kataoka Y, et al. Inhibition of the mTOR/S6K signal is necessary to enhance fluorouracil-induced apoptosis in gastric cancer cells with HER2 amplification. Int J Oncol. 2012;41:551–558. doi: 10.3892/ijo.2012.1485. [DOI] [PubMed] [Google Scholar]
- 40.Wang YC, Morrison G, Gillihan R, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers: Role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13:R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weigelt B, Lo AT, Park CC, et al. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips GD, Fields CT, Li G, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: Critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014;20:456–468. doi: 10.1158/1078-0432.CCR-13-0358. [DOI] [PubMed] [Google Scholar]
- 43.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 44.Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): Relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 45.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 46.Burris HA, 3rd, Taylor CW, Jones SF, et al. A phase I and pharmacokinetic study of oral lapatinib administered once or twice daily in patients with solid malignancies. Clin Cancer Res. 2009;15:6702–6708. doi: 10.1158/1078-0432.CCR-09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 48.Toi M, Iwata H, Fujiwara Y, et al. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: Efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009;101:1676–1682. doi: 10.1038/sj.bjc.6605343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: A phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610–2615. doi: 10.1093/annonc/mdr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hecht JR, Urba SG, Koehler M. Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: Phase II efficacy and biomarker analyses. Gastrointestinal Cancers Symposium; January 25-27, 2008; Orlando, FL. (abstr 43) [Google Scholar]
- 52.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 53.Swaby R, Blackwell K, Jiang Z, et al. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: A phase I/II study. J Clin Oncol. 2009;27(suppl):42s. abstr 1004. [Google Scholar]
- 54.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 55.Guan Z, Xu B, DeSilvio ML, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J Clin Oncol. 2013;31:1947–1953. doi: 10.1200/JCO.2011.40.5241. [DOI] [PubMed] [Google Scholar]
- 56.GlaxoSmithKline. Lapatinib clinical trial update. Press release, September 9, 2011, London, United Kingdom. http://us.gsk.com/html/media-news/pressreleases/2011/2011-pressrelease-614856.htm.
- 57.Goss PE, Smith IE, O'Shaughnessy J, et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: A randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:88–96. doi: 10.1016/S1470-2045(12)70508-9. [DOI] [PubMed] [Google Scholar]
- 58.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 59.Zhang D, Pal A, Bornmann WG, et al. Activity of lapatinib is independent of EGFR expression level in HER2-overexpressing breast cancer cells. Mol Cancer Ther. 2008;7:1846–1850. doi: 10.1158/1535-7163.MCT-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garrett JT, Olivares MG, Rinehart C, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amin DN, Sergina N, Ahuja D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2010;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 64.Fan X, Brezski RJ, Fa M, et al. A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast Cancer Res. 2012;14:R116. doi: 10.1186/bcr3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beano A, Signorino E, Evangelista A, et al. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 68.Horlock C, Stott B, Dyson PJ, et al. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100:1061–1067. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez SA, Karamouzis MV, Skarlos DV, et al. CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 2007;13:2714–2721. doi: 10.1158/1078-0432.CCR-06-2347. [DOI] [PubMed] [Google Scholar]
- 70.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: An antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Capietto AH, Martinet L, Fournié JJ. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol. 2011;187:1031–1038. doi: 10.4049/jimmunol.1100681. [DOI] [PubMed] [Google Scholar]
- 72.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 73.Benavides LC, Gates JD, Carmichael MG, et al. The impact of HER2/neu expression level on response to the E75 vaccine: From U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15:2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]