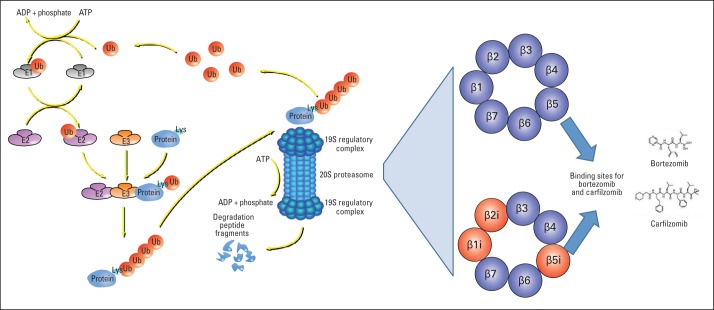
For both normal and malignant cells, the degradation of intracellular proteins must be carefully controlled to adjust the levels of important regulatory proteins and rapidly eliminate damaged or misfolded proteins before their toxic aggregates compromise the cell function or survival.1,2 The ubiquitin-proteasome pathway degrades most intracellular proteins. This complex system (Fig 1) identifies proteins intended for degradation and attaches to them chains of ubiquitin (Ub) molecules3 through a sequential system of Ub-activating enzymes, Ub-conjugating enzymes, and Ub ligases.4,5 Ubiquitinated proteins are then recognized by the 26S proteasome7 and selectively digested by its distinct (chymotrypsin-like, trypsin-like, and caspase-like) proteolytic activities.8–10
Fig 1.
Overview of the ubiquitin-proteasome pathway and its therapeutic implications. Most intracellular proteins are degraded by the 26S proteasome, a large complex (> 60 known subunits) that selectively digests proteins with covalently attached ubiquitin (Ub) chains.3 The 26S proteasome comprises two 19S regulatory complexes flanking a hollow cylindrical core particle (termed 20S proteasome). Proteins are marked for degradation by a complex enzymatic system upstream of the 26S proteasome: one of two Ub-activating enzymes (E1s) uses the energy from adenosine triphosphate (ATP) hydrolysis (to adenosine diphosphate [ADP] and inorganic phosphate) to transfer Ub to one of 40 Ub-conjugating enzymes (E2s), which interact with one of approximately 600 Ub ligases (E3s).4,5 The latter covalently attach Ub chains to specific lysine (Lys) residue(s) of different sets of protein substrates.4,6 The 19S proteasome complexes recognize (through their Rpn13 or S5a/Rpn10 subunits,7 not depicted in this figure) these ubiquitinated substrates, disassemble the Ub chains (which are then recycled), unfold the target proteins, and translocate them to the 20S proteasome chamber.8–10 The 20S proteasome chamber (shown in cross-section in the right-hand panel) comprises three types of proteolytic subunits, β5, β2, and β1: each subunit cleaves proteins preferentially after large hydrophobic, basic, or acidic residues (chymotrypsin-like, trypsin-like, and caspase-like activities, respectively). Most tissues express this canonical constitutive 20S proteasome. Cells of the immune system also express (particularly when exposed to certain proinflammatory cytokines) the immunoproteasome, a variant form with different catalytic subunits (β1i, β2i, and β5i) and often associated with 11S regulatory complexes, to optimize presentation of antigenic peptides through major histocompatibility complex class I molecules. The proteasome inhibitors bortezomib and carfilzomib both bind to and inhibit the chymotrypsin-like activity of the β5 subunit.
This complex degradative network and its substrate proteins influence diverse aspects of cancer biology, thus creating opportunities for therapeutic interventions. The first agent targeting this cascade was bortezomib (formerly known as PS-341), an inhibitor of the chymotrypsin-like activity of the proteasome. Bortezomib has pronounced clinical activity in multiple myeloma (MM)11–13 and other plasma cell dyscrasias (amyloidosis14–16 and Waldenström macroglobulinemia17,18), is also active in mantle-cell lymphoma,19,20 but has limited, if any, activity in most other hematologic malignancies or solid tumors. Its complex molecular sequelae include suppression of antiapoptotic molecules, such as nuclear factor kappa B, Bcl-2 family members, and caspase inhibitors,21–24 and sensitization of MM cells to diverse established22,23 or investigational24 agents. Bortezomib thus emerged as a key component of diverse anti-MM combination regimens.25,26 Eventually, patients become resistant to bortezomib or intolerant to its main dose-limiting toxicity, namely sensory peripheral neuropathy.27 To overcome these limitations, second-generation proteasome inhibitors were developed. One of them, carfilzomib, received accelerated US Food and Drug Administration approval in 2012 for treatment of patients with MM who had relapsed from and were refractory to bortezomib and at least one thalidomide derivative.28 In the accompanying article, Papadopoulos et al29 report results of a phase I trial of carfilzomib infusion over 30 minutes. Compared with prior studies with shorter infusion time (2 to 10 minutes),30 this trial delivered higher doses (maximum-tolerated dose of 56 mg/m2) and had a higher overall response rate (50% in patients with relapsed and refractory MM, including those who were resistant to bortezomib). The safety profile for carfilzomib included thrombocytopenia (similar to bortezomib), likely because constitutive proteasome activity in platelets is required to degrade Bax and preserve their normal life span.31 In contrast to historical experience with bortezomib, but consistent with prior carfilzomib studies,28 peripheral neuropathy was not observed, but cardiopulmonary adverse effects (eg, dyspnea, hypoxemia, pulmonary hypertension) and serum creatinine elevations were noted.
Bortezomib and carfilzomib can be administered without catastrophic clinical toxicities, likely because their clinically achievable concentrations do not completely abrogate the chymotrypsin-like activity,11,32,33 and also spare other proteolytic (trypsin-like and caspase-like)34,35 activities of the proteasome. Overall protein degradation is thus only modestly (< 40%) suppressed in either normal or tumor cells. Normal cells can conceivably tolerate this perturbation, but malignant plasma cells may not be able to, because they depend on higher levels of proteasome activity for a process termed endoplasmic reticulum (ER) –associated degradation8,34,36: misfolded or unassembled proteins in the ER lumen must undergo retrograde transport to the cytoplasm to be degraded by the proteasome and prevent ER stress and apoptosis. In plasma cell dyscrasias, the proteasome capacity (availability of active proteasome particles) is apparently close to being saturated by the increased proteasome load (ie, the amount of misfolded or unassembled proteins such as immunoglobulins). Indeed, these plasma cells produce large quantities of immunoglobulins, but their assembly has an appreciable error rate (hence, the free immunoglobulin light chains detected in sera of patients with plasma cell dyscrasias). This high proteasome load for a given proteasome capacity may explain in part why proteasome inhibitors are more active in plasma cell dyscrasias, compared with most other hematologic malignancies or solid tumors, whereas differences in this relationship of proteasome load versus capacity among patients with MM have been proposed to account for the heterogeneity of clinical responses to bortezomib.33,37,38
Carfilzomib inhibits the β5 proteasome subunit by forming with it an irreversible adduct through two covalent bonds,39 conceivably allowing more sustained and more specific inhibition than the single reversible adduct formed by bortezomib. For instance, bortezomib, but not carfilzomib, is proposed to inhibit not only the β5 but also the neuroprotective molecule Htra2/Omi40,41 and other serine proteases (eg, cathepsin G, cathepsin A),42–44 some of which are proposed to contribute to renal injury.45 These differences could explain the more frequent peripheral sensory neuropathy observed with bortezomib and the increase in serum creatinine levels often observed with carfilzomib.29,46 Proteasome dysfunction and accumulation of cardiotoxic misfolded proteins47–53 have been linked with different forms of cardiac dysfunction (eg, cardiomyopathies): the cardiopulmonary adverse events that occur with carfilzomib thus merit mechanistic dissection to identify possible predisposing factors and determine whether irreversible proteasome inhibition could be one of them.
The irreversible β5 inhibition in carfilzomib-treated cells means that proteasome capacity cannot be restored before new proteasomes are synthesized.28,54,55 Such delayed recovery is proposed to account for observations that carfilzomib can be active in some cases in which bortezomib is not (eg, 18.6% response rate with carfilzomib in a phase II study30 of patients with MM who had progressed from a bortezomib-containing last line of therapy). This observation is concordant with preclinical data that some MM cells can be resistant to one of these two proteasome inhibitors, but sensitive to the other (eg, bortezomib-resistant, carfilzomib-sensitive MM cells).56,57 Interestingly, another second-generation proteasome inhibitor MLN2238 and its clinically administered prodrug ixazomib (MLN9708) are also active preclinically in MM cells58 and clinically in patients who were bortezomib-resistant,59 although MLN2238 binds the β5 subunit reversibly and with faster kinetics of release than bortezomib itself.60 Therefore, the relationship of clinical activity with reversible versus irreversible inhibition of chymotrypsin-like activity is likely to be more complex and influenced by other pharmacodynamic and pharmacokinetic parameters. Still, the response rates reported by Papadopoulos et al29 can be considered promising, compared with historical data from single-agent trials of bortezomib,13,61 lenalidomide,62 and pomalidomide63,64 in relapsed and refractory MM. Ultimately, results from randomized trials of carfizomib-containing versus bortezomib-containing regimens (eg, ENDEAVOR [NCT01568866]; Phase 3 Study With Carfilzomib and Dexamethasone Versus Velcade and Dexamethasone for Relapsed Multiple Myeloma Patients [ENDEAVOR]) should shed light on the differential safety and efficacy profiles of these agents. It will also be important to determine the depth and durability of responses to bortezomib in patients with carfilzomib-resistant, bortezomib-naive MM.
Recent studies of second-generation proteasome inhibitors, including the report by Papadopoulos et al29 and other carfilzomib trials, highlight the promising clinical activity of these agents and the potential to improve their efficacy and hopefully their safety through modified infusion rates (eg, in the study by Papadopoulos et al29) and more broadly, through optimized dosing, schedules, and combinations with other established or investigational anti-MM agents (eg, Berenson et al,65 Niesvizky et al,66 Moreau et al,67 and Wang et al,68 and Stewart et al68a). Recent reports indicate that thalidomide derivatives endow the E3 ligase Cereblon with the ability to ubiquitinate IKZF1 and IKZF3, two important transcription factors for MM cells.69,70 Active research is also exploring the selective therapeutic targeting of other enzymes that regulate the ubiquitination state of substrate proteins, including other E3 ubiquitin ligases (eg, Ooi et al71) or deubiquitinases (eg, Wang et al72 and Tian et al73), and more specific inhibitors of immunoproteasome74,75 are being evaluated in lymphoid neoplasias. These studies and the ongoing progress in the clinical development of second-generation proteasome inhibitors have further validated the overall concept that the regulation of protein degradation provides promising targets for therapeutic interventions in MM and beyond.
Acknowledgment
Supported by Grants No. R01 CA127435, R01 CA179483, and P01 CA155258 (C.S.M.) from the National Institutes of Health, by the Shawna Ashlee Corman Investigatorship in Multiple Myeloma Research, the de Gunzburg Myeloma Research Fund, the Cobb Family Myeloma Research Fund, the Chambers Family Advanced Myeloma Research Fund, the Leukemia and Lymphoma Society Translational Research Program and Quest for Cure Program, the Elsa U. Pardee Foundation, and the Multiple Myeloma Research Foundation.
Footnotes
See accompanying article on page 732
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Therapeutic Landscape of Carfilzomib and Other Modulators of the Ubiquitin-Proteasome Pathway
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Constantine S. Mitsiades
Honoraria: Millenium, Celgene
Research Funding: Johnson and Johnson, Amgen
Patents, Royalties, Other Intellectual Property: Previously submitted patent application on methods for treating cancer using proteasome inhibitors
REFERENCES
- 1.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL, Dice JF. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43:835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- 3.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger MB, Pruneda JN, Klevit RE, et al. RING-type E3 ligases: Master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim Biophys Acta. 2014;1843:47–60. doi: 10.1016/j.bbamcr.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metzger MB, Hristova VA, Weissman AM. HECT and RING finger families of E3 ubiquitin ligases at a glance. J Cell Sci. 2012;125:531–537. doi: 10.1242/jcs.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulathu Y, Komander D. Atypical ubiquitylation: The unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Biol. 2012;13:508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- 7.Besche HC, Sha Z, Kukushkin NV, et al. Autoubiquitination of the 26S proteasome on Rpn13 regulates breakdown of ubiquitin conjugates. EMBO J. 2014;33:1159–1176. doi: 10.1002/embj.201386906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meusser B, Hirsch C, Jarosch E, et al. ERAD: The long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 9.Matyskiela ME, Martin A. Design principles of a universal protein degradation machine. J Mol Biol. 2013;425:199–213. doi: 10.1016/j.jmb.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lander GC, Estrin E, Matyskiela ME, et al. Complete subunit architecture of the proteasome regulatory particle. Nature. 2012;482:186–191. doi: 10.1038/nature10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 13.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 14.Reece DE, Sanchorawala V, Hegenbart U, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: Results of a phase 1 dose-escalation study. Blood. 2009;114:1489–1497. doi: 10.1182/blood-2009-02-203398. [DOI] [PubMed] [Google Scholar]
- 15.Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: Targeted therapy? Haematologica. 2007;92:1302–1307. doi: 10.3324/haematol.12136. [DOI] [PubMed] [Google Scholar]
- 16.Kastritis E, Wechalekar AD, Dimopoulos MA, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]
- 17.Chen CI, Kouroukis CT, White D, et al. Bortezomib is active in patients with untreated or relapsed Waldenstrom's macroglobulinemia: A phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1570–1575. doi: 10.1200/JCO.2006.07.8659. [DOI] [PubMed] [Google Scholar]
- 18.Treon SP, Hunter ZR, Matous J, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenstrom's macroglobulinemia: Results of WMCTG Trial 03-248. Clin Cancer Res. 2007;13:3320–3325. doi: 10.1158/1078-0432.CCR-06-2511. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 21.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: Therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 23.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: Therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc Natl Acad Sci U S A. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson PG, Weller E, Jagannath S, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27:5713–5719. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 28.Herndon TM, Deisseroth A, Kaminskas E, et al. U.S. Food and Drug Administration approval: Carfilzomib for the treatment of multiple myeloma. Clin Cancer Res. 2013;19:4559–4563. doi: 10.1158/1078-0432.CCR-13-0755. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos KP, Siegel DS, Vesole DH, et al. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33:732–739. doi: 10.1200/JCO.2013.52.3522. [DOI] [PubMed] [Google Scholar]
- 30.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayak MK, Kulkarni PP, Dash D. Regulatory role of proteasome in determination of platelet life span. J Biol Chem. 2013;288:6826–6834. doi: 10.1074/jbc.M112.403154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 33.Shabaneh TB, Downey SL, Goddard AL, et al. Molecular basis of differential sensitivity of myeloma cells to clinically relevant bolus treatment with bortezomib. PLoS One. 2013;8:e56132. doi: 10.1371/journal.pone.0056132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg AL. Functions of the proteasome: From protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35:12–17. doi: 10.1042/BST0350012. [DOI] [PubMed] [Google Scholar]
- 35.Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 36.Kostova Z, Wolf DH. For whom the bell tolls: Protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 2003;22:2309–2317. doi: 10.1093/emboj/cdg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meister S, Schubert U, Neubert K, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 38.Gu JL, Li J, Zhou ZH, et al. Differentiation induction enhances bortezomib efficacy and overcomes drug resistance in multiple myeloma. Biochem Biophys Res Commun. 2012;420:644–650. doi: 10.1016/j.bbrc.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 39.Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 40.Martins LM, Morrison A, Klupsch K, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arastu-Kapur S, Anderl JL, Kraus M, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clin Cancer Res. 2011;17:2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- 42.Groll M, Berkers CR, Ploegh HL, et al. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure. 2006;14:451–456. doi: 10.1016/j.str.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Adams J, Behnke M, Chen S, et al. Potent and selective inhibitors of the proteasome: Dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8:333–338. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 44.Dorsey BD, Iqbal M, Chatterjee S, et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem. 2008;51:1068–1072. doi: 10.1021/jm7010589. [DOI] [PubMed] [Google Scholar]
- 45.Shimoda N, Fukazawa N, Nonomura K, et al. Cathepsin G is required for sustained inflammation and tissue injury after reperfusion of ischemic kidneys. Am J Pathol. 2007;170:930–940. doi: 10.2353/ajpath.2007.060486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: Experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98:1753–1761. doi: 10.3324/haematol.2013.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlossarek S, Frey N, Carrier L. Ubiquitin-proteasome system and hereditary cardiomyopathies. J Mol Cell Cardiol. 2014;71:25–31. doi: 10.1016/j.yjmcc.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann J, Wohlert C, Saguner AM, et al. Primary proteasome inhibition results in cardiac dysfunction. Eur J Heart Fail. 2013;15:614–623. doi: 10.1093/eurjhf/hft034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Day SM. The ubiquitin proteasome system in human cardiomyopathies and heart failure. Am J Physiol Heart Circ Physiol. 2013;304:H1283–H1293. doi: 10.1152/ajpheart.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glembotski CC. Clarifying the cardiac proteasome paradox: Protein quality control. Circ Res. 2012;111:509–512. doi: 10.1161/CIRCRESAHA.112.275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlossarek S, Carrier L. The ubiquitin-proteasome system in cardiomyopathies. Curr Opin Cardiol. 2011;26:190–195. doi: 10.1097/HCO.0b013e32834598fe. [DOI] [PubMed] [Google Scholar]
- 52.Su H, Wang X. The ubiquitin-proteasome system in cardiac proteinopathy: A quality control perspective. Cardiovasc Res. 2010;85:253–262. doi: 10.1093/cvr/cvp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Predmore JM, Wang P, Davis F, et al. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kisselev AF, van der Linden WA, Overkleeft HS. Proteasome inhibitors: An expanding army attacking a unique target. Chem Biol. 2012;19:99–115. doi: 10.1016/j.chembiol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borissenko L, Groll M. 20S proteasome and its inhibitors: Crystallographic knowledge for drug development. Chem Rev. 2007;107:687–717. doi: 10.1021/cr0502504. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn DJ, Orlowski RZ, Bjorklund CC. Second generation proteasome inhibitors: Carfilzomib and immunoproteasome-specific inhibitors (IPSIs) Curr Cancer Drug Targets. 2011;11:285–295. doi: 10.2174/156800911794519725. [DOI] [PubMed] [Google Scholar]
- 58.Chauhan D, Tian Z, Zhou B, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 2011;17:5311–5321. doi: 10.1158/1078-0432.CCR-11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson PG, Baz R, Wang W, et al. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124:1038–1046. doi: 10.1182/blood-2014-01-548826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kupperman E, Lee EC, Cao Y, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 61.Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson P, Jagannath S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772–778. doi: 10.1182/blood-2008-12-196238. [DOI] [PubMed] [Google Scholar]
- 63.Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood. 2014;123:1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–1967. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berenson JR, Hilger JD, Yellin O, et al. Replacement of bortezomib with carfilzomib for multiple myeloma patients progressing from bortezomib combination therapy. Leukemia. 2014;28:1529–1536. doi: 10.1038/leu.2014.27. [DOI] [PubMed] [Google Scholar]
- 66.Niesvizky R, Martin TG, 3rd, Bensinger WI, et al. Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. 2013;19:2248–2256. doi: 10.1158/1078-0432.CCR-12-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreau P, Kolb B, Hulin C, et al. Carfilzomib plus melphalan and prednisone (CMP) is a promising combination therapy for the treatment of elderly patients with newly diagnosed multiple myeloma: Results of a phase I/II trial in 68 cases. Blood. 2013;122 (abstr 1933) [Google Scholar]
- 68.Wang M, Martin T, Bensinger W, et al. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122:3122–3128. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68a.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. doi: 10.1056/NEJMoa1411321. [epub ahead of print on December 6, 2014] [DOI] [PubMed] [Google Scholar]
- 69.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ooi MG, Hayden PJ, Kotoula V, et al. Interactions of the Hdm2/p53 and proteasome pathways may enhance the antitumor activity of bortezomib. Clin Cancer Res. 2009;15:7153–7160. doi: 10.1158/1078-0432.CCR-09-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Stafford W, Mazurkiewicz M, et al. The 19S deubiquitinase inhibitor b-AP15 is enriched in cells and elicits rapid commitment to cell death. Mol Pharmacol. 2014;85:932–945. doi: 10.1124/mol.113.091322. [DOI] [PubMed] [Google Scholar]
- 73.Tian Z, D'Arcy P, Wang X, et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014;123:706–716. doi: 10.1182/blood-2013-05-500033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson KC. The 39th David A. Karnofsky Lecture: Bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30:445–452. doi: 10.1200/JCO.2011.37.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuhn DJ, Orlowski RZ. The immunoproteasome as a target in hematologic malignancies. Semin Hematol. 2012;49:258–262. doi: 10.1053/j.seminhematol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]