Abstract
Pyrethroids are commonly used insecticides that are considered to pose little risk to human health. However, there is an increasing concern that children are more susceptible to the adverse effects of pesticides. We used the zebrafish model to test the hypothesis that developmental exposure to low doses of the pyrethroid deltamethrin results in persistent alterations in dopaminergic gene expression, neurochemistry, and locomotor activity. Zebrafish embryos were treated with deltamethrin (0.25–0.50 μg/l), at concentrations below the LOAEL, during the embryonic period [3–72 h postfertilization (hpf)], after which transferred to fresh water until the larval stage (2-weeks postfertilization). Deltamethrin exposure resulted in decreased transcript levels of the D1 dopamine (DA) receptor (drd1) and increased levels of tyrosine hydroxylase at 72 hpf. The reduction in drd1 transcripts persisted to the larval stage and was associated with decreased D2 dopamine receptor transcripts. Larval fish, exposed developmentally to deltamethrin, had increased levels of homovanillic acid, a DA metabolite. Since the DA system is involved in locomotor activity, we measured the swim activity of larval fish following a transition to darkness. Developmental exposure to deltamethrin significantly increased larval swim activity which was attenuated by concomitant knockdown of the DA transporter. Acute exposure to methylphenidate, a DA transporter inhibitor, increased swim activity in control larva, while reducing swim activity in larva developmentally exposed to deltamethrin. Developmental exposure to deltamethrin causes locomotor deficits in larval zebrafish, which is likely mediated by dopaminergic dysfunction. This highlights the need to understand the persistent effects of low-dose neurotoxicant exposure during development.
Keywords: pyrethroid, zebrafish, developmental exposure, dopamine, locomotor activity
Pyrethroids are generally considered to be a safer alternative to other insecticides because they exhibit low mammalian toxicity and low environmental persistence (Demoute, 1989). Consequently, their use and popularity is on the rise (Grube et al., 2011). Human exposure is well documented, with 70% of urine samples collected from the 1999–2002 National Health and Nutrition Examination Survey cohort containing detectable levels of the pyrethroid metabolite 3-phenoxybenzoic acid (3PBA) (Barr et al., 2010). Because it is widely recognized that children are potentially more sensitive to the adverse effects of pesticides (National Research Council (U.S.). Committee on Pesticides in the Diets of Infants and Children, 1993), a troubling statistic highlighted by this survey was the fact that levels of 3PBA were significantly higher in children than adolescents or adults (Barr et al., 2010). Other surveys reported finding pyrethroid metabolites in the urine of pregnant women (Berkowitz et al., 2003; Qi et al., 2012; Whyatt et al., 2002) and children (Babina et al., 2012; Bradman et al., 2007; Couture et al., 2009; Fortin et al., 2008; Heudorf et al., 2004; Morgan et al., 2007; Richardson et al., 2015) Thus, it is important to understand the consequences of pyrethroid exposure during development.
An increasing number of studies point to toxicant exposure during critical developmental periods as having long-term effects, including contributing to neurological disease in adulthood (Fox et al., 2012). Currently, there is limited evidence in rodents indicating that developmental exposure to pyrethroids results in persistent changes in neurochemical, behavioral, and cognitive endpoints, reviewed in Shafer et al. (2005). Increased locomotor activity was observed in adult mice that had been exposed to nontoxic doses of deltamethrin (0.7 mg/kg) between PND10-16 (Eriksson and Fredriksson, 1991). In addition to increased locomotor activity, behavioral effects such as increased impulsivity and working memory deficits have also been observed in 6-week-old male mice that had been developmentally exposed to deltamethrin at levels below the no observed adverse effect level (NOAEL) (3 mg/kg) (Richardson et al., 2015). In rats, gestational exposure to deltamethrin resulted in decreased locomotor activity at 3 and 6 weeks (Husain et al., 1992). Similar findings have been reported using even lower doses (0.08–0.5 mg/kg) of commercial deltamethrin formulations and persisting to longer timepoints (6–9 weeks) (Johri et al., 2006), including adulthood (Lazarini et al., 2001). Together, this data suggest that deltamethrin may be a developmental neurotoxicant that results in neurobehavioral deficits.
Because the dopamine (DA) system plays an essential role in mediating many of the observed behavioral and locomotor effects, it is possible that disruption of DA neurotransmission during a phase of developmental plasticity is a mode of action for pyrethroid-induced locomotor changes. A recent study in mice demonstrates that hyperactivity resulting from developmental exposure to deltamethrin at doses below the NOAEL (3 mg/kg) is associated with increased protein levels of the DA transporter and D1 dopamine receptor, and decreased levels of extracellular DA. Importantly, deltamethrin-induced hyperactivity was attenuated by treatment with D1 receptor antagonists and D2 receptor agonists, implicating the role of the DA system in mediating these effects (Richardson et al., 2015). Acutely, pyrethroid exposure influences the kinetics of DA transmission by altering DA uptake in synaptosomes prepared from pyrethroid-treated mice (Bloomquist et al., 2002; Elwan et al., 2006; Karen et al., 2001; Kirby et al., 1999). Similarly, in vivo microdialysis analysis of conscious rats demonstrates that both DA reuptake and release are affected by acute pyrethroid exposure (Hossain et al., 2006). Pyrethroid-induced changes in DA uptake has been linked to immediate (Bloomquist et al., 2002; Elwan et al., 2006; Kirby et al., 1999) and persistent (Gillette and Bloomquist, 2003; Richardson et al., 2015) changes in DA transporter expression and DA uptake activity in mice. In addition, pyrethroid exposure was associated with changes in the concentrations of striatal DA and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in rat models (Doherty et al., 1988; Hudson et al., 1986). Thus, if these disturbances occur during a critical period, such as neurodevelopment, this could cause disruption of normal development, leading to persistent changes in function.
The zebrafish has gained popularity as a model organism due to its small size, high fecundity, optical transparency, and genetic similarity to humans. In addition, the availability of an annotated genome and molecular genetic tools allows for the study of basic vertebrate biology at the molecular level (Driever et al., 1994). The zebrafish model is also being used for pharmacological and toxicological studies due to the high level of conservation of functional substrate binding domains between the zebrafish and humans (Renier et al., 2007). Together, these attributes make the zebrafish a powerful model of human disease (Dooley and Zon, 2000).
We previously characterized the developmental toxicity associated with an acute exposure to 6 different pyrethroid pesticides and found that type II pyrethroids were more toxic than type I pyrethroids, based on LC50 values. In addition, at higher concentrations, zebrafish embryos presented with tremors and spasms. Finally, it was determined that the tremors and spasms were the result of sodium channel activation since sodium channel blockade ameliorated these effects (DeMicco et al., 2010). The effects of pyrethroid exposure observed in the zebrafish embryo model are consistent with that reported in the mammalian literature, thus validating this model system. Here, the vertebrate zebrafish model was utilized to recapitulate the persistent motor deficits caused by embryonic exposure to the pyrethroid pesticide deltamethrin, and to test the hypothesis that dopaminergic dysfunction is responsible for mediating these effects. Zebrafish embryos were exposed to deltamethrin at concentrations below the lowest observed adverse effect level (LOAEL) (0.33–0.50 μg/l). At these concentrations, mortality, dysmorphogenesis, or acute signs of toxicity (tremors or spasms) do not occur. The data reveal that developmental exposure [3–72 h postfertilization (hpf)] to deltamethrin, led to increases in locomotor activity following a transition into darkness at the larval stage. Decreased DA receptor transcript levels and increased HVA levels accompanied these locomotor effects. Using methylphenidate, a DA transporter inhibitor, and a morpholino (MO) to knockdown DA transporter gene (slc6a3) expression, we demonstrated that DA dysfunction is a possible mechanism for the long-term locomotor deficits caused by developmental deltamethrin exposure.
MATERIALS AND METHODS
Animal handling
The AB strain zebrafish (Zebrafish International Resource Center, Eugene, Oregon) were used for all experiments. Breeding stocks were bred and housed in Aquatic Habitats (Apopka, Florida) recirculating systems under a 14:10 h light:dark cycle. System water was obtained by carbon/sand filtration of municipal tap water and water quality was maintained at <0.05 ppm nitrite, <0.2 ppm ammonia, pH between 7.2 and 7.7, and between 26 and 28°C. All experiments were conducted in accordance with the zebrafish husbandry protocol and embryonic exposure protocol (08-025) approved by the Rutgers University Animal Care and Facilities Committee.
Chemicals
Deltamethrin [purity 99.5%] (CAS52918-63-5) was obtained from ChemService (West Chester, Pennsylvania). N,N-dimethylformamide (DMF) [purity 99.9%] and methylphenidate HCl (purity > 98%) were obtained from Sigma-Aldrich (St. Louis, Missouri).
Pesticide exposures
A deltamethrin stock solution (2 mg/ml) was prepared fresh by dissolving deltamethrin into DMF and subsequently diluted with DMF to make working solutions. 1:10,000 dilutions of the working solutions were performed into aerated egg water (60 μg/ml Instant Ocean in DI water) to obtain final nominal concentrations of 0.25, 0.33, and 0.50 μg/l deltamethrin (0.50, 0.65, and 1.0 nM, respectively, 0.01% DMF). Embryos exposed to 0.01% DMF were vehicle controls.
Fertilized embryos were staged (Kimmel et al., 1995) and exposure began at 3 hpf (512-cell stage). A total of 30–50 embryos were treated with deltamethrin in a 10 ml static nonrenewal bath exposure in 60 × 15 mm glass Petri dishes. Embryos were incubated at 25–26°C in darkness and observed daily using a dissecting microscope for mortality and to ensure that there were no developmental abnormalities or signs of acute toxicity. For larval studies, sac fry larvae (72 hpf) were removed from treatment and reared in treatment free water until the larval stage [2-weeks postfertilization (wpf)] as per normal rearing protocol. In brief, all embryos contained in 1 dish were transferred to a 600-ml glass beaker containing aerated egg water and maintained in an incubator (25–26°C) under a 14:10 h light:dark cycle. A 50% water change was performed every 4th day using aerated egg water and debris was removed from the bottom. Larvae were fed 2–3 × daily with Zeigler Larval AP50 (Aquatic Habitats, Apopka, Florida).
Reverse-transcriptase quantitative PCR
Embryos were exposed to 0.25, 0.33, or 0.50 μg/l deltamethrin and reared as previously described (Pesticide Exposures section). At 72 hpf or 2 wpf, embryos were pooled and snap frozen in liquid nitrogen. (N = 4 pooled replicates/concentration, 15 embryos per replicate.) RNA isolation, RT-qPCR, and analysis were performed as previously described (Hillegass et al. 2007). Primer sequences are provided in Supplementary Table S1. The entire experiment, from exposure to analysis, was repeated a minimum of 3 times.
High-performance liquid chromatography – electrochemical detection (HPLC-ECD)
Embryos were exposed to 0, 0.33, or 0.50 μg/l deltamethrin and reared as previously described (Pesticide Exposures section). At 2 wpf, larvae were snap-frozen in liquid nitrogen (N = 6 pooled replicates/concentration, 50 embryos per replicate). An aliquot of 50 μl of cold 0.1 N PCA solution (with 300 μM EDTA and 300 μM sodium metabisulfite) was added to each sample and homogenized 2 × on ice using a motorized pestle. Samples were spun at 16,000 × g for 10 min at 4°C and the supernatant was filtered through a 0.22 μM spin filter (EMD Millipore, Billerica, Massachusetts). The remaining pellet was dried and dissolved in 200 μl of 0.5 N NaOH for protein concentration determination using the Modified Lowry Protein Assay (Thermo Scientific, Waltham, Massachusetts). Filtered supernatant was diluted 1:10 in PCA solution and 5 μl was injected onto a Waters Alliance HPLC equipped with a Waters 2465 ECD (Waters Corporation, Milford, Massachusetts). DA, DOPAC, and HVA were separated on a MD-150 × 3.2 column (Thermo Scientific) using isocratic MDTM Mobile Phase (Thermo Scientific) with NaCl (2 mM) at a flowrate of 0.5 ml/min. The compounds identified by electrochemical detection were quantified by area under the curve with known standards (Schuh et al. 2009; Sheleg et al. 2013). The entire experiment, from exposure to analysis, was repeated twice.
Activity monitoring of 2-week-old larvae
The testing apparatus consisted of 4 infrared (IR) sensitive Ikegami ICD-49 CCD Cameras with Computar IR manual vari-focal lenses (1/2″) and IR filters mounted to the ceiling directly over 4 light boxes. Each light box consisted of a semi-transparent platform on which the plates were placed and transilluminated by an IR-Room IR illuminator and/or 3 LED lights directly underneath. The camera, lens, filters, and IR illuminators were purchased from Noldus Information Technology (Leesburg, Virginia).
Embryos were exposed to 0.25, 0.33, or 0.50 μg/l deltamethrin and reared as previously described (Pesticide Exposures section). At 2 wpf, larvae were placed into white-walled, clear-bottom 24-well plates containing 1.5 ml of room temperature (25°C) system water. Each plate contained all experimental conditions distributed in a random order. Larvae were allowed to acclimate on the testing apparatus for 1 h in light (400 lux). After 1 h, lights were turned off to stimulate activity and video was recorded using the Noldus MPEG Recorder 2.1 (Noldus Information Technology) for 30 min. Testing occurred between 13:00 and 16:30 h, reducing the time of day parameter (MacPhail et al. 2009).
EthoVision XT 8 (Noldus Information Technology) was used to track the videos and quantify distance traveled. A differencing background subtraction method was applied to detect objects that were darker than the background at a sample rate of 29.97 samples/s. Larvae that did not move (<100 mm total distance traveled in 25 min) were excluded from analysis. Studies were repeated 3 times (from 3 different breeding sets) and data collapsed such that each larvae was considered an individual and the distance traveled/time bin was an average of all individuals for that treatment group.
Morpholino injections
An antisense MO was designed to target the I2E3 splice junction of slc6a3 (ENSDART00000129303, Zv7) pre-mRNA, resulting in the deletion of exon 3 in the mature transcript. The I2E3 splice junction is conserved between the 2 splice variants of slc6a3; therefore, the MO would target both splice variants. The slc6a3 MO 5′-CCCACGGCTGATAAAACAACACACAC-3′ was 3′ fluorescein tagged and synthesized by GeneTools, LLC (Philomath, Oregon). qPCR and gel validation of the slc6a3 MO can be found in Supplementary Figure S1. A standard control MO 5′-CTCTTACCTCAGTTACAATTTATA-3′ was used as an injection control. Embryos were injected with 50 μM of the slc6a3 MO or control MO as previously described (Hillegass et al. 2007). The total injection volume was 8 nl. Embryos exhibiting even distribution of fluorescence 3 h after injections were used. The injected embryos were treated with 0.33 μg/l deltamethrin or vehicle and raise to 2 wpf as described above (Pesticide Exposures section). At 2 wpf, activity monitoring was performed as described above (Activity Monitoring of 2-week-Old Larvae section).
Methylphenidate challenge
Methylphenidate HCl was dissolved in water to obtain a stock concentration of 1 mg/ml. Embryos were exposed to 0 or 0.33 μg/l deltamethrin during development and reared until 2 wpf as described above (Pesticide Exposures section). The larvae were subjected to activity monitoring as described earlier (Activity Monitoring of 2-week-Old Larvae section) except that 1 h prior to video recording, 1.5 μl of the methylphenidate stock (or water) was added to the appropriate wells to obtain a final concentration of 1 mg/l (3.7 µM). The concentration used and duration of methylphenidate exposure was based on parameters described in Lange et al. (2012).
Statistical analyses
Data were analyzed using SigmaPlot v.11 (Systat Software Inc., San Jose, California). The probability level for statistical significance was (P ≤ 0.05) for all studies.
RESULTS
Developmental Deltamethrin Exposure Increases Larval Swim Activity
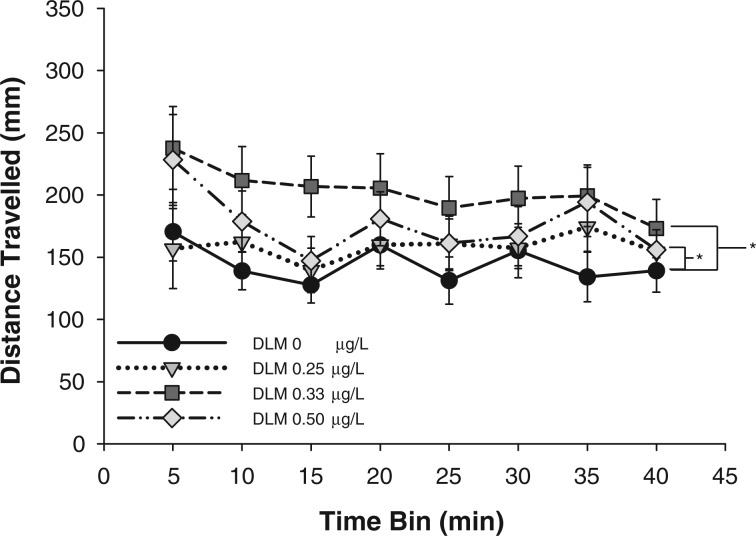
Swim activity of 2-week old fish was measured to determine the effects of developmental deltamethrin exposure on larval behavioral activity (Fig. 1). The concentrations tested (0, 0.25, 0.33, or 0.50 µg/l) were below the established LOAEL of 1 µg/l for deltamethrin in zebrafish (DeMicco et al. 2010); as such, mortality or acute toxicities (i.e. tremors, spasms, or dysmorphogenesis) were not observed. There was a significant effect of developmental deltamethrin exposure on swim activity (distance traveled) at 2 wpf (F(3,1103) = 8.807, P = 0.001). Swim activity across all time bins was significantly increased in larvae that had been developmentally exposed to 0.33 or 0.50 µg/l deltamethrin compared with vehicle control. There was no significant difference between the activities of larvae developmentally exposed to 0.25 µg/l deltamethrin or control. There was no significant effect of time on swim activity, nor was there a significant interaction between time and deltamethrin treatment. Developmental exposure to 0.33 and 0.50 µg/l deltamethrin resulted in increased swim activity across all time bins following a transition to darkness at the larval stage.
FIG. 1.
Swim activity following a transition into darkness of 2-week old larval zebrafish developmentally exposed to deltamethrin (DLM). Data points are presented as mean distance traveled (mm) ± S.E.M. in 5-min time bins during 40-min sessions (n = 31–38). A 2-way ANOVA was used to determine whether there was a significant effect of deltamethrin exposure across time on larval swim activity. * indicates that distance traveled across time in animals developmentally exposed to 0.333 and 0.5 μg/l deltamethrin were significantly different from vehicle control exposed animals as determined by Holm-Sidak multiple comparisons (P ≤ 0.05).
Developmental Deltamethrin Exposure Alters Dopaminergic Gene Expression
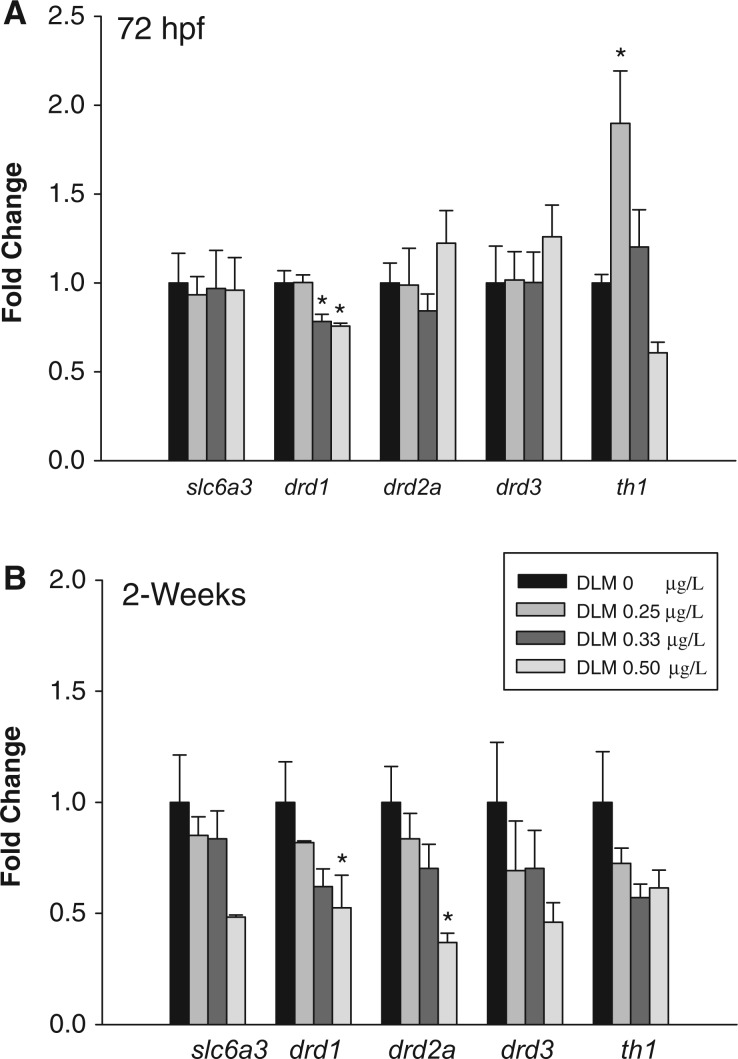
Transcript levels of several dopaminergic genes were measured at 72 hpf and 2 wpf to characterize the immediate and persistent effects of deltamethrin exposure on the DA system. At 72 hpf (Fig. 2A), there was a significant effect of developmental deltamethrin exposure on drd1 (F(3,13) = 8.02, P = 0.005) and th1 (F(3,14) = 7.83, P = 0.004) transcript levels. Developmental exposure to 0.33 and 0.50 µg/l deltamethrin resulted in a significant 1.3- and 1.3-fold decrease in drd1 transcript levels, respectively. Developmental exposure to 0.25 µg/l deltamethrin significantly increased th1 transcripts 1.9-fold. Developmental deltamethrin exposure did not significantly alter slc6a3, drd2a, or drd3 transcript levels.
FIG. 2.
RNA transcript levels of genes related to dopamine transport (slc6a3), reception (drd1, drd2a, drd3), and synthesis (th1) in zebrafish exposed to deltamethrin (DLM) during development. Transcript levels were assayed at (A) 72 hpf and (B) 2 wpf. The graphs represent the mean fold change in transcript copy number ± S.E.M of one representative experimental replicate (n = 4). A 1-way ANOVA or a Kruskal-Wallis ANOVA on Ranks was used to determine if there was a significant effect of deltamethrin exposure on transcript levels where appropriate. * indicates a significant difference versus control as determined by Holm-Sidak or Dunn’s post hoc analyses where appropriate (P ≤ 0.05).
At the larval stage (Fig. 2B), there was a significant effect of developmental deltamethrin exposure on drd1 (χ2(3) = 8.75, P = 0.03) and drd2a (F(3,15) = 5.51, P = 0.01) transcript levels. Developmental exposure to 0.50 µg/l deltamethrin decreased drd1 transcript levels by 1.90-fold and drd2a transcript levels by 2.72-fold. The transcript levels of slc6a3, drd3, or th1 at the larval stage were not significantly changed by developmental deltamethrin exposure. The immediate effects of developmental deltamethrin exposure included decreased drd1 transcript levels and increased th1 transcript levels (Fig. 2A). drd1 downregulation persisted to the larval stage and was accompanied by a decrease in drd2a transcripts (Fig. 2B). th1 transcript levels returned to baseline at the 2-week time point (Fig. 2B).
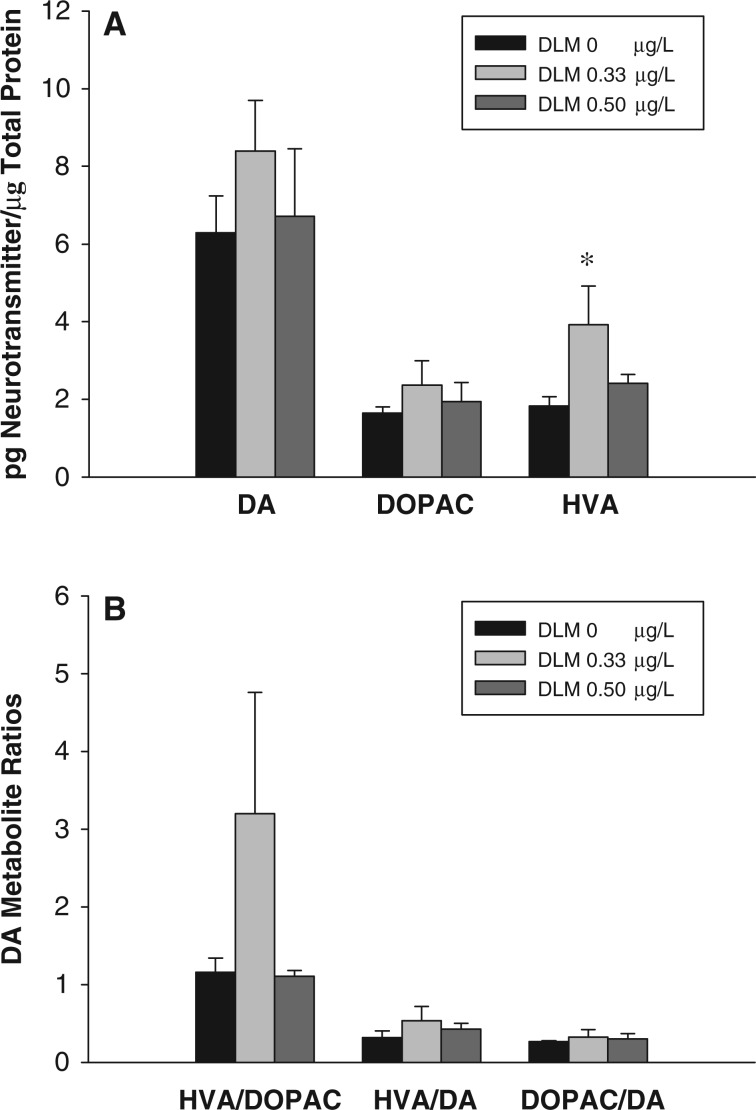
The Effect of Developmental Deltamethrin Exposure on Dopaminergic Neurotransmitter Levels
To determine if developmental deltamethrin exposure results in persistent changes in DA neurochemistry, the levels of DA and its metabolites, HVA, and DOPAC, were measured at 2 wpf (Fig. 3A), a time point where deltamethrin induced hyperactivity was observed (Fig. 1). At the larval stage, there was a significant effect of deltamethrin exposure on HVA levels (χ2(2) = 6.25, P = 0.03). Developmental exposure to 0.33 µg/l deltamethrin resulted in a 2.14-fold increase in HVA levels. There were no significant differences in the levels of DA or DOPAC between deltamethrin treated and control embryos.
FIG. 3.
The effects of developmental deltamethrin (DLM) exposure on DA neurotransmitter levels at 2-weeks of age. (A) Whole body concentrations of DA, HVA, and DOPAC, and (B) metabolite ratios. Values represent the mean ± SEM (n = 5) of one representative experimental replicate. A Kruskal-Wallis ANOVA on Ranks was performed to determine if there was a significant effect of developmental deltamethrin exposure. * indicates a significant difference versus control animals as determined by Dunn's multiple comparisons (P ≤ 0.05).
Metabolite/DA ratios were calculated to examine changes in the rate of DA metabolism (Fig. 3B). There were no significant differences in DOPAC/DA, HVA/DA, or HVA/DOPAC ratios between control and deltamethrin treated embryos.
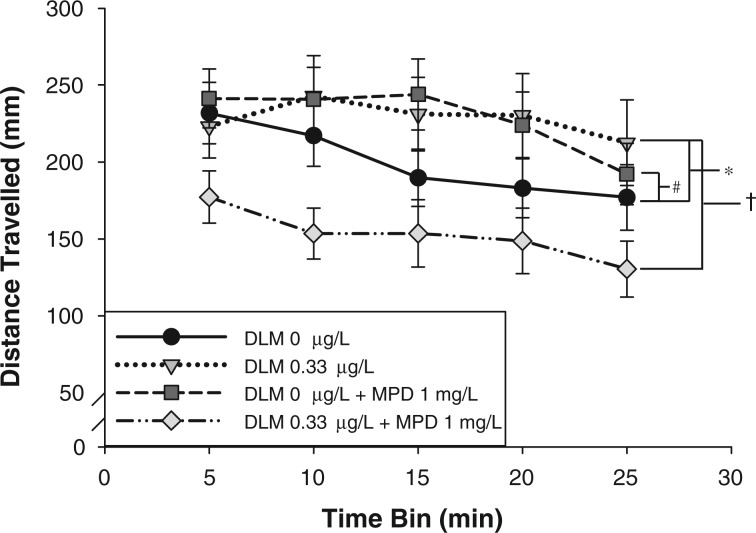
Effect of Methylphenidate Exposure on Swim Activity in Larvae Developmentally Exposed to Deltamethrin
2-week-old larvae were treated with methylphenidate, an indirect DA receptor agonist (Fig. 4). It was hypothesized that stimulation of the downregulated DA receptors (Fig. 2B) would rescue deltamethrin induced swim activity (Fig. 1). There was a significant effect of developmental deltamethrin exposure (F(1,1754) = 5.83, P = 0.02) and methylphenidate exposure (F(1,1754) = 5.59, P = 0.02) on larval swim activity. Compared with vehicle-treated embryos, developmental exposure to 0.33 µg/l deltamethrin resulted in a significant increase in swim activity across all time bins within the non-methylphenidate-treated group, similar to the results in Figure 1. There was also a significant interaction between deltamethrin exposure and methylphenidate treatment (F(1,1754) = 28.33, P = 0.001). In larvae that had been developmentally exposed to vehicle control, subsequent methylphenidate exposure resulted in a significant increase in swim activity across all time bins. Whereas, in larvae that had been developmentally exposed to deltamethrin, subsequent methylphenidate exposure resulted in a significant decrease in swim activity across all time bins, to levels below that of control larvae. No other significant interactions were detected (deltamethrin × time, methylphenidate × time, deltamethrin × methylphenidate × time). The effect of methylphenidate on swim activity depended on whether the larvae had previously been exposed to deltamethrin during development. Methylphenidate treatment at 2 wpf resulted in a significant reduction in swim activity in deltamethrin-treated larvae instead of the expected increase in swim activity observed in vehicle control larvae.
FIG. 4.
Effects of methylphenidate (MPD) on larval swim activity following a transition into darkness in zebrafish developmentally exposed to deltamethrin (DLM). Data points are presented as mean distance traveled (mm) ± S.E.M. in 5-min time bins during 25-min sessions (n = 76–97). A 3-way ANOVA was used to determine if there was a significant effect of deltamethrin, methylphenidate, and time on larval swim activity as well as an interaction (deltamethrin ×methylphenidate × time). As determined by Holm-Sidak multiple comparisons, * indicates that distance traveled across time in deltamethrin exposed animals are significantly different from vehicle control exposed animals (P ≤ 0.05). # indicates that there is a significant effect of methylphenidate on the distance traveled across time within vehicle control exposed animals (P ≤ 0.05). † indicates that there is a significant effect of methylphenidate on the distance traveled across time within deltamethrin exposed animals (P ≤ 0.05).
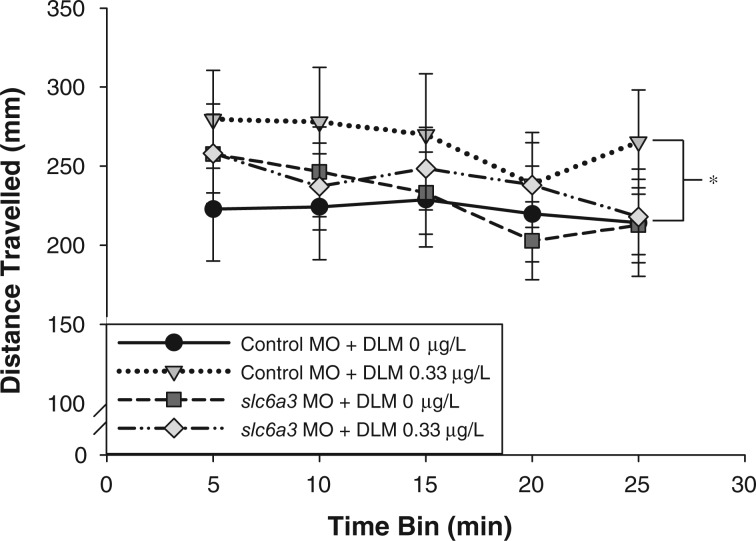
slc6a3 MO Attenuation of Deltamethrin-Induced Swim Activity
An antisense MO was used to specifically reduce dopamine transporter expression (Fig. 5) in an attempt to saturate the DA receptors and attenuate deltamethrin-induced hyperactivity. Transient dopamine transporter knockdown during development, via the slc6a3 MO (slc6a3-MO) (50 μM), did not result in MO toxicity (data not shown) or alter larval swim activity (F(1,1044) = 0.45 P = 0.50). There was a significant effect of deltamethrin exposure on swim activity (F(1,1044) = 4.07, P = 0.04). Within the control MO (CO-MO) group, treatment with 0.33 µg/l deltamethrin during development resulted in increased swim activity across all time bins compared with control animals, similar to Figure 1. However, within the slc6a3-MO group, there was no significant difference in activity levels of deltamethrin-treated and control larvae. slc6a3-MO knockdown returned deltamethrin induced increases in swim activity to control levels.
FIG. 5.
Effects of developmental DA transporter (slc6a3) knockdown and deltamethrin (DLM) exposure on larval swim activity following a transition into darkness. Data points are presented as mean distance traveled (mm) ± S.E.M. in 5-min time bins during a 25-min session (n = 40–62). A 3-way ANOVA was used to determine if there was a significant effect of deltamethrin exposure, slc6a3 MO (slc6a3-MO), and time on larval swim activity as well as an interaction (deltamethrin × slc6a3 MO × time). As determined by Holm-Sidak multiple comparisons, * indicates that distance traveled in deltamethrin exposed animals is significantly different from vehicle control exposed animals within the control MO group (P ≤ 0.05). There is no significant difference in distance traveled between deltamethrin and vehicle control exposed animals within the slc6a3 MO group (P ≥ 0.05).
DISCUSSION
Zebrafish embryos were exposed to concentrations of deltamethrin that were below the established LOAEL. At these concentrations mortality, dysmorphogenesis, or acute toxicity (tremors or spasms) were not present. However, developmental exposure to deltamethrin resulted in persistent changes in larval swim activity, dopaminergic gene expression, and neurochemistry. Our studies indicate that dopaminergic dysfunction is a likely mode of action for deltamethrin induced increases in locomotion.
The increases in larval swim activity following developmental deltamethrin exposure, even after being reared in treatment free water since 72 hpf, are consistent with findings in rodents. Daily exposure to nontoxic doses of deltamethrin between PND10-16 resulted in increased locomotion in adult mice (Eriksson and Fredriksson 1991). We previously characterized the developmental toxicity associated with an acute exposure to 6 different pyrethroids, and determined that the observed toxicities were consistent with that reported in the mammalian literature (DeMicco et al., 2010). Together, this demonstrates that the zebrafish is an appropriate vertebrate model for studying the long-term behavioral effects of neurotoxicant insult during development.
Given the role of the DA system in mediating locomotion and behavior in zebrafish (Levin et al., 2011), the effects of developmental deltamethrin exposure on the dopaminergic system was examined at the gene expression level. The expression of th1 expression was measured to determine if developmental deltamethrin exposure affected DA metabolism. While a second tyrosine hydroxylase (th2) has been identified in the zebrafish based on sequence homology, recent characterization of this possible homologue indicates that the zebrafish th2 encodes for a protein with tryptophan hydroxylase activity and not tyrosine hydroxylase activity and is instead a marker for serotonergic neurons (Ren et al., 2013). Exposure to the lowest concentration of deltamethrin resulted in increased th1 expression immediately after exposure; however, this change was transient and returned to basal levels by the 2-week time point. Repeated exposure to deltamethrin has been shown to decrease tyrosine hydroxylase mRNA and protein expression and hydroxylase activity in adult male rats (Liu et al., 2006). The difference in the directionality of th1 expression could be due to inherent differences between the rat and zebrafish models, as well as, to the differential responses of developing and adult organisms to toxicant exposure. However, both studies indicate the potential for deltamethrin to modulate tyrosine hydroxylase expression, thereby altering DA biosynthesis.
This study is the first to report deltamethrin-induced changes in DA receptor transcript levels. Embryos exposed developmentally to deltamethrin exhibited a decrease in drd1 transcript levels which persisted to the larval stage. Persistent effects on drd1 levels were also observed in male mice that had been exposed to deltamethrin during development. Based on radiolabeled binding studies, developmental deltamethrin exposure increased striatal drd1 levels in male mice (Richardson et al., 2015). Similar to what was observed in tyrosine hydroxylase expression, the difference in the directionality in D1 DA receptor expression is likely due to differences between the rodent and zebrafish models. However, both studies demonstrate that developmental deltamethrin exposure can cause persistent changes in drd1 levels. Both the dopamine D1 and D2 receptors undergo heterologous and/or homologous desensitization if subject to prolonged activation (Balmforth et al., 1990; Barton and Sibley, 1990; Memo et al., 1982). Given that deltamethrin exposure can potentiate DA release (Bloomquist et al., 2002; Eells and Dubocovich, 1988; Hossain et al., 2006) or decrease DA uptake (Elwan et al., 2006), these actions may result in increased levels of DA in the synapse and promote DA receptor activation. Accordingly, prolonged exposure to DA downregulated DRD1 expression in SK-N-MC neuroblastoma cells (Sidhu et al., 1999), suggesting that transcriptional regulation may be a mechanism of DA receptor desensitization. At the larval stage, drd1 downregulation was accompanied by a decrease in drd2a transcript levels. Despite the fact that the D1 receptor and D2 autoreceptor have opposing actions on cAMP signal transduction, they act in a synergistic manner to modulate locomotor activity (Robertson, 1992). Altering D1 receptor signal transduction often results in corresponding changes in D2 receptor-mediated responses; D1 regulates D2 sensitivity (Braun et al., 1997; Hasbi et al., 2011; Hu et al., 1992; Walters et al., 1987). Therefore, it is plausible that a sustained decrease in drd1 transcript could promote a subsequent downregulation of drd2a at the transcript level. Since Drd1 and Drd2 knockout mice exhibited altered locomotion (Holmes et al., 2004), our data suggest that reduced D1 and/or D2 receptor levels following developmental deltamethrin exposure likely influences locomotor activity.
Developmental exposure to deltamethrin increased levels of HVA at the larval stage. In male mice, developmental exposure to levels of deltamethrin below the NOAEL resulted in decreased levels of extracellular DA in the nucleus accumbens (Richardson et al., 2015). Similarly, in adult male rats, developmental exposure to nontoxic doses of deltamethrin resulted in increased levels of striatal DOPAC and DOPAC/DA ratios (Lazarini et al., 2001). Neonatal permethrin or cypermethrin exposure resulted in decreased DA and increased HVA levels in the striatum of male rats 3 weeks after exposure (Nasuti et al., 2007). In all of these studies, the affected animals had indications of increased DA metabolism, which were associated with locomotor deficits.
In our studies, there were no significant differences in levels of DA, DOPAC, or the metabolite/DA ratios between control and deltamethrin-treated embryos at the larval stage. This could be due to the fact that our data represent neurotransmitter levels from whole body preparations as opposed to very specific brain regions, such as the striatum. Therefore, the assay was in all likelihood not sensitive enough to detect differences over the background contributions of DA or DOPAC from other brain regions or from peripheral sources. In addition, since little is known about DA metabolism in the zebrafish, more research would be necessary to determine the preferred pathway of DA metabolism. However, since CSF, plasma, and/or urinary HVA levels are often used as a biomarker of CNS dopaminergic dysfunction in humans, the increased HVA levels in deltamethrin-treated fish could be indicative of an altered DA system.
Because developmental deltamethrin exposure results in decreased transcript levels of drd1, drd2a, and increased swim activity during the larval stage, we hypothesized that stimulating the DA receptors would attenuate this behavioral phenotype. Methylphenidate, an indirect DA receptor agonist, was used to increase extracellular levels of DA in the synapse in an attempt to saturate the DA receptors. Methylphenidate was chosen because it rescues hyperactive phenotypes in zebrafish (Lange et al., 2012) and rodents (Richardson et al., 2015; Sagvolden et al., 2005). This study demonstrates that developmental exposure to deltamethrin modifies the susceptibility of the dopaminergic system in larval animals. In control animals, methylphenidate exposure increased activity. At higher doses, methylphenidate is known to increase locomotor activity in rodents (McNamara et al., 1993; Solanto, 1998) and zebrafish (Lange et al., 2012). However, in larvae that had been developmentally exposed to deltamethrin, methylphenidate reduced swim activity to below the basal level of control animals. This is consistent with methylphenidate's effects on locomotion and behavior in rodents exhibiting altered DA states (Del’Guidice et al., 2014; Shaywitz et al., 1978; Tilley and Gu, 2008). Taken together, developmental deltamethrin exposure alters the sensitivity of larval fish to methylphenidate and potentially other pharmaceuticals that target the dopaminergic system.
Because methylphenidate also functions as a norepinephrine reuptake inhibitor, a splice MO specifically targeting the DA transporter (slc6a3) pre-mRNA was employed to knockdown slc6a3 expression. Since MOs are penetrant through 72 hpf (Nasevicius and Ekker, 2000), slc6a3 knockdown would coincide exactly within the deltamethrin exposure window. In control animals, disruption of DA transmission during development by the slc6a3 MO did not reproduce the effects of developmental deltamethrin exposure in terms of persistent activity changes. This could indicate that the effects of deltamethrin are broad and not limited to DA receptor activation. Because pyrethroids primarily interact with sodium channels (DeMicco et al., 2010; Hossain and Richardson, 2011; Soderlund and Bloomquist, 1989, Soderlund et al., 2002), exposure likely influences multiple components of the DA system and other neurotransmitter systems as well. However, because Slc6a3 KO mice exhibit hyperactivity (Giros et al., 1996; Jones et al., 1998) and developmental methylphenidate exposure results in persistent behavioral changes in the zebrafish (Levin et al., 2011), the lack of effect of slc6a3 MO knockdown is likely due to the fact that the zebrafish embryos were able to overcome the transitory effects elicited by the concentration of slc6a3 MO used. Despite this, in deltamethrin exposed animals, simultaneous slc6a3 MO knockdown was sufficient to attenuate deltamethrin-induced increases in swim activity at the larval stage.
In conclusion, developmental exposure to deltamethrin resulted in increased swim activity following a transition to darkness in larval zebrafish. This was associated with changes in DA neurochemistry and gene expression as evidenced by increased levels of HVA, and decreased levels of the drd1 and drd2a transcripts. slc6a3 knockdown during development attenuated deltamethrin induced hyperactivity and methylphenidate exposure resulted in a paradoxical reduction of activity in deltamethrin-treated fish. Together, these data indicate that dopaminergic dysfunction is a likely mode of action for the persistent hyperactivity observed in the zebrafish exposed developmentally to deltamethrin. These results highlight the risk of low-dose neurotoxicant exposure during the critical developmental period on more subtle endpoints such as locomotor activity and drug response.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENT
We are grateful for the assistance from Dr. Muhammed Hossain with HPLC-ECD analysis.
FUNDING
National Institute of Environmental Health Sciences (R01ES015991, R21ES013828, P30ES005022, R56ES018863) and New Jersey Agricultural Experiment Station (Project NJ01201).
References
- Babina K., Dollard M., Pilotto L., Edwards J. W. (2012). Environmental exposure to organophosphorus and pyrethroid pesticides in South Australian preschool children: A cross sectional study. Environ. Int. 48C, 109–120. [DOI] [PubMed] [Google Scholar]
- Balmforth A. J., Warburton P., Ball S. G. (1990). Homologous desensitization of the D1 dopamine receptor. J. Neurochem. 55, 2111–2116. [DOI] [PubMed] [Google Scholar]
- Barr D. B., Olsson A. O., Wong L. Y., Udunka S., Baker S. E., Whitehead R. D., Magsumbol M. S., Williams B. L., Needham L. L. (2010). Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect. 118, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton A. C., Sibley D. R. (1990). Agonist-induced desensitization of D1-dopamine receptors linked to adenylyl cyclase activity in cultured NS20Y neuroblastoma cells. Mol. Pharmacol. 38, 531–541. [PubMed] [Google Scholar]
- Berkowitz G. S., Obel J., Deych E., Lapinski R., Godbold J., Liu Z., Landrigan P. J., Wolff M. S. (2003). Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Perspect. 111, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist J. R., Barlow R. L., Gillette J. S., Li W., Kirby M. L. (2002). Selective effects of insecticides on nigrostriatal dopaminergic nerve pathways. Neurotoxicology 23, 537–544. [DOI] [PubMed] [Google Scholar]
- Bradman A., Whitaker D., Quiros L., Castorina R., Claus Henn B., Nishioka M., Morgan J., Barr D. B., Harnly M., Brisbin J. A., et al. (2007). Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA. J. Expo. Sci. Environ. Epidemiol. 17, 331–349. [DOI] [PubMed] [Google Scholar]
- Braun A. R., Laruelle M., Mouradian M. M. (1997). Interactions between D1 and D2 dopamine receptor family agonists and antagonists: The effects of chronic exposure on behavior and receptor binding in rats and their clinical implications. J. Neural Transm. 104, 341–362. [DOI] [PubMed] [Google Scholar]
- Couture C., Fortin M. C., Carrier G., Dumas P., Tremblay C., Bouchard M. (2009). Assessment of exposure to pyrethroids and pyrethrins in a rural population of the Monteregie area, Quebec, Canada. J. Occup. Environ. Hyg. 6, 341–352. [DOI] [PubMed] [Google Scholar]
- Del’Guidice T., Lemasson M., Etievant A., Manta S., Magno L. A., Escoffier G., Roman F. S., Beaulieu J. M. (2014). Dissociations between cognitive and motor effects of psychostimulants and atomoxetine in hyperactive DAT-KO mice. Psychopharmacology 231, 109–122. [DOI] [PubMed] [Google Scholar]
- DeMicco A., Cooper K. R., Richardson J. R., White L. A. (2010). Developmental neurotoxicity of pyrethroid insecticides in zebrafish embryos. Toxicol. Sci. 113, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoute J. P. (1989). A brief review of the environmental fate and metabolism of pyrethroids. Pestic. Sci. 27, 375–385. [Google Scholar]
- Doherty J. D., Morii N., Hiromori T., Ohnishi J. (1988). Pyrethroids and the striatal dopaminergic system in vivo. Comp. Biochem. Physiol.. 91, 371–375. [DOI] [PubMed] [Google Scholar]
- Dooley K., Zon L. I. (2000). Zebrafish: a model system for the study of human disease. Curr. Opin. Genet. Dev. 10, 252–256. [DOI] [PubMed] [Google Scholar]
- Driever W., Stemple D., Schier A., Solnica-Krezel L. (1994). Zebrafish: Genetic tools for studying vertebrate development. Trends Genet. 10, 152–159. [DOI] [PubMed] [Google Scholar]
- Eells J. T., Dubocovich M. L. (1988). Pyrethroid insecticides evoke neurotransmitter release from rabbit striatal slices. J. Pharmacol. Exp. Ther. 246, 514–521. [PubMed] [Google Scholar]
- Elwan M. A., Richardson J. R., Guillot T. S., Caudle W. M., Miller G. W. (2006). Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol. Appl. Pharmacol. 211, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P., Fredriksson A. (1991). Neurotoxic effects of two different pyrethroids, bioallethrin and deltamethrin, on immature and adult mice: Changes in behavioral and muscarinic receptor variables. Toxicol. Appl. Pharmacol. 108, 78–85. [DOI] [PubMed] [Google Scholar]
- Fortin M. C., Bouchard M., Carrier G., Dumas P. (2008). Biological monitoring of exposure to pyrethrins and pyrethroids in a metropolitan population of the Province of Quebec, Canada. Environ. Res. 107, 343–350. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Grandjean P., de Groot D., Paule M. G. (2012). Developmental origins of adult diseases and neurotoxicity: Epidemiological and experimental studies. Neurotoxicology 33, 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette J. S., Bloomquist J. R. (2003). Differential up-regulation of striatal dopamine transporter and alpha-synuclein by the pyrethroid insecticide permethrin. Toxicol. Appl. Pharmacol. 192, 287–293. [DOI] [PubMed] [Google Scholar]
- Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. [DOI] [PubMed] [Google Scholar]
- Grube A., David D., Timothy K., Wu L. (2011). Pesticides industry sales and usage: 2006 and 2007 market estimates. U. S. E. P. A. O. o. P. Programs. Washington, DC. [Google Scholar]
- Hasbi A., O’Dowd B. F., George S. R. (2011). Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol. Brain 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudorf U., Angerer J., Drexler H. (2004). Current internal exposure to pesticides in children and adolescents in Germany: Urinary levels of metabolites of pyrethroid and organophosphorus insecticides. Int. Arch. Occup. Environ. Health 77, 67–72. [DOI] [PubMed] [Google Scholar]
- Hillegass J. M., Villano C. M., Cooper K. R., White L. A. (2007). Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 100, 168–179. [DOI] [PubMed] [Google Scholar]
- Holmes A., Lachowicz J. E., Sibley D. R. (2004). Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology 47, 1117–1134. [DOI] [PubMed] [Google Scholar]
- Hossain M. M., Richardson J. R. (2011). Mechanism of pyrethroid pesticide-induced apoptosis: Role of calpain and the ER stress pathway. Toxicol. Sci. 122, 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. M., Suzuki T., Sato N., Sato I., Takewaki T., Suzuki K., Tachikawa E., Kobayashi H. (2006). Differential effects of pyrethroid insecticides on extracellular dopamine in the striatum of freely moving rats. Toxicol. Appl. Pharmacol. 217, 25–34. [DOI] [PubMed] [Google Scholar]
- Hu X. T., Brooderson R. J., White F. J. (1992). Repeated stimulation of D1 dopamine receptors causes time-dependent alterations in the sensitivity of both D1 and D2 dopamine receptors within the rat striatum. Neuroscience 50, 137–147. [DOI] [PubMed] [Google Scholar]
- Hudson P. M., Tilson H. A., Chen P. H., Hong J. S. (1986). Neurobehavioral effects of permethrin are associated with alterations in regional levels of biogenic amine metabolites and amino acid neurotransmitters. Neurotoxicology 7, 143–153. [PubMed] [Google Scholar]
- Husain R., Malaviya M., Seth P. K. (1992). Differential responses of regional brain polyamines following in utero exposure to synthetic pyrethroid insecticides: A preliminary report. Bull. Environ. Contam. Toxicol. 49, 402–409. [DOI] [PubMed] [Google Scholar]
- Johri A., Yadav S., Singh R. L., Dhawan A., Ali M., Parmar D. (2006). Long lasting effects of prenatal exposure to deltamethrin on cerebral and hepatic cytochrome P450s and behavioral activity in rat offspring. Eur. J. Pharmacol. 544, 58–68. [DOI] [PubMed] [Google Scholar]
- Jones S. R., Gainetdinov R. R., Wightman R. M., Caron M. G. (1998). Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 18, 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen D. J., Li W., Harp P. R., Gillette J. S., Bloomquist J. R. (2001). Striatal dopaminergic pathways as a target for the insecticides permethrin and chlorpyrifos. Neurotoxicology 22, 811–817. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Castagnoli K., Bloomquist J. R. (1999). In vivo effects of deltamethrin on dopamine neurochemistry and the role of augmented neurotransmitter release. Pestic. Biochem. Physiol. 65, 160–168. [Google Scholar]
- Lange M., Norton W., Coolen M., Chaminade M., Merker S., Proft F., Schmitt A., Vernier P., Lesch K. P., Bally-Cuif L. (2012). The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol. Psychiat. 17, 946–954. [DOI] [PubMed] [Google Scholar]
- Lazarini C. A., Florio J. C., Lemonica I. P., Bernardi M. M. (2001). Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. Neurotoxicol. Teratol. 23, 665–673. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Sledge D., Roach S., Petro A., Donerly S., Linney E. (2011). Persistent behavioral impairment caused by embryonic methylphenidate exposure in zebrafish. Neurotoxicol. Teratol. 33, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. P., Ma Q., Shi N. (2006). Tyrosine hydroxylase as a target for deltamethrin in the nigrostriatal dopaminergic pathway. Biomed. Environ. Sci. 19, 27–34. [PubMed] [Google Scholar]
- MacPhail R. C., Brooks J., Hunter D. L., Padnos B., Irons T. D., Padilla S. (2009). Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30, 52–58. [DOI] [PubMed] [Google Scholar]
- McNamara C. G., Davidson E. S., Schenk S. (1993). A comparison of the motor-activating effects of acute and chronic exposure to amphetamine and methylphenidate. Pharmacol. Biochem. Behav. 45, 729–732. [DOI] [PubMed] [Google Scholar]
- Memo M., Lovenberg W., Hanbauer I. (1982). Agonist-induced subsensitivity of adenylate cyclase coupled with a dopamine receptor in slices from rat corpus striatum. Proc. Natl Acad. Sci. U. S. A. 79, 4456–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. K., Sheldon L. S., Croghan C. W., Jones P. A., Chuang J. C., Wilson N. K. (2007). An observational study of 127 preschool children at their homes and daycare centers in Ohio: Environmental pathways to cis- and trans-permethrin exposure. Environ. Res. 104, 266–274. [DOI] [PubMed] [Google Scholar]
- Nasevicius A., Ekker S. C. (2000). Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220. [DOI] [PubMed] [Google Scholar]
- Nasuti C., Gabbianelli R., Falcioni M. L., Di Stefano A., Sozio P., Cantalamessa F. (2007). Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 229, 194–205. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.) Committee on Pesticides in the Diets of Infants and Children. (1993). Pesticides in the diets of infants and children. National Academy Press, Washington, D.C. [Google Scholar]
- Qi X., Zheng M., Wu C., Wang G., Feng C., Zhou Z. (2012). Urinary pyrethroid metabolites among pregnant women in an agricultural area of the Province of Jiangsu, China. Int. J. Hyg. Environ. Health 215, 487–495. [DOI] [PubMed] [Google Scholar]
- Ren G., Li S., Zhong H., Lin S. (2013). Zebrafish tyrosine hydroxylase 2 gene encodes tryptophan hydroxylase. J. Biol. Chem. 288, 22451–22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier C., Faraco J. H., Bourgin P., Motley T., Bonaventure P., Rosa F., Mignot E. (2007). Genomic and functional conservation of sedative-hypnotic targets in the zebrafish. Pharmacogenet. Genomics 17, 237–253. [DOI] [PubMed] [Google Scholar]
- Richardson J. R., Taylor M. M., Shalat S. L., Guillot T. S., 3rd, Caudle W. M., Hossain M. M., Mathews T. A., Jones S. R., Cory-Slechta D. A., Miller G. W. (2015). Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J 29, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. A. (1992). Synergistic interactions of D1- and D2-selective dopamine agonists in animal models for Parkinson’s disease: sites of action and implications for the pathogenesis of dyskinesias. Can. J. Neurol. Sci.(Le journal canadien des sciences neurologiques) 19, 147–152. [PubMed] [Google Scholar]
- Sagvolden T., Russell V. A., Aase H., Johansen E. B., Farshbaf M. (2005). Rodent models of attention-deficit/hyperactivity disorder. Biol. Psychiat. 57, 1239–1247. [DOI] [PubMed] [Google Scholar]
- Schuh R. A., Richardson J. R., Gupta R. K., Flaws J. A., Fiskum G. (2009). Effects of the organochlorine pesticide methoxychlor on dopamine metabolites and transporters in the mouse brain. Neurotoxicology 30, 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer T. J., Meyer D. A., Crofton K. M. (2005). Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environ. Health Perspect. 113, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B. A., Klopper J. H., Gordon J. W. (1978). Methylphenidate in 6-hydroxydopamine-treated developing rat pups. Effects on activity and maze performance. Arch. Neurol. 35, 463–469. [DOI] [PubMed] [Google Scholar]
- Sheleg M., Yochum C. L., Wagner G. C., Zhou R., Richardson J. R. (2013). Ephrin-A5 deficiency alters sensorimotor and monoaminergic development. Behav. Brain Res. 236, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A., Olde B., Humblot N., Kimura K., Gardner N. (1999). Regulation of human D1 dopamine receptor function and gene expression in SK-N-MC neuroblastoma cells. Neuroscience 91, 537–547. [DOI] [PubMed] [Google Scholar]
- Soderlund D. M., Bloomquist J. R. (1989). Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 34, 77–96. [DOI] [PubMed] [Google Scholar]
- Soderlund D. M., Clark J. M., Sheets L. P., Mullin L. S., Piccirillo V. J., Sargent D., Stevens J. T., Weiner M. L. (2002). Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 171, 3–59. [DOI] [PubMed] [Google Scholar]
- Solanto M. V. (1998). Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: A review and integration. Behav. Brain Res. 94, 127–152. [DOI] [PubMed] [Google Scholar]
- Tilley M. R., Gu H. H. (2008). The effects of methylphenidate on knockin mice with a methylphenidate-resistant dopamine transporter. J. Pharmacol. Exp. Ther. 327, 554–560. [DOI] [PubMed] [Google Scholar]
- Walters J. R., Bergstrom D. A., Carlson J. H., Chase T. N., Braun A. R. (1987). D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science 236, 719–722. [DOI] [PubMed] [Google Scholar]
- Whyatt R. M., Camann D. E., Kinney P. L., Reyes A., Ramirez J., Dietrich J., Diaz D., Holmes D., Perera F. P. (2002). Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect. 110, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.