Abstract
Treatment of glioblastoma (GBM), the most common primary malignant brain tumor in adults, remains a significant unmet need in oncology. Historically, cytotoxic treatments provided little durable benefit, and tumors recurred within several months. This has spurred a substantial research effort to establish more effective therapies for both newly diagnosed and recurrent GBM. In this context, antiangiogenic therapy emerged as a promising treatment strategy because GBMs are highly vascular tumors. In particular, GBMs overexpress vascular endothelial growth factor (VEGF), a proangiogenic cytokine. Indeed, many studies have demonstrated promising radiographic response rates, delayed tumor progression, and a relatively safe profile for anti-VEGF agents. However, randomized phase III trials conducted to date have failed to show an overall survival benefit for antiangiogenic agents alone or in combination with chemoradiotherapy. These results indicate that antiangiogenic agents may not be beneficial in unselected populations of patients with GBM. Unfortunately, biomarker development has lagged behind in the process of drug development, and no validated biomarker exists for patient stratification. However, hypothesis-generating data from phase II trials that reveal an association between increased perfusion and/or oxygenation (ie, consequences of vascular normalization) and survival suggest that early imaging biomarkers could help identify the subset of patients who most likely will benefit from anti-VEGF agents. In this article, we discuss the lessons learned from the trials conducted to date and how we could potentially use recent advances in GBM biology and imaging to improve outcomes of patients with GBM who receive antiangiogenic therapy.
INTRODUCTION
Glioblastoma (GBM), the most common primary malignant brain tumor in adults, has a poor prognosis with a 2-year survival rate of less than 10% and 5-year survival rate of less than 5% in unselected patients. Currently, standard treatment for newly diagnosed GBM (nGBM) consists of maximum safe resection followed by fractionated involved-field radiotherapy with concurrent temozolomide followed by 6 to 12 monthly cycles of postradiation temozolomide. With this combined approach, the prognosis still remains poor with a median overall survival (OS) of 14.7 months.1 Survival outcomes for recurrent GBM (rGBM) are dismal, with 6-month progression-free survival of approximately 10% to 25% in patients receiving standard chemotherapy.2–4 Clearly, a better understanding of glioblastoma biology and more effective therapeutic options are needed.
The Cancer Genome Atlas Research Network has provided a comprehensive genomic catalog of abnormalities in GBM. Data indicate that GBMs could be classified into four molecular subtypes: classical (driven by epidermal growth factor receptor [EGFR]), mesenchymal (driven by NF1), proneural (driven by platelet-derived growth factor receptor A [PDGFR-A or isocitrate dehydrogenase 1 [IDH1]), and neural.5 Interestingly, these subtypes were associated with specific clinical and tumor characteristics. This molecular heterogeneity may shape the GBM response to various treatments, although its utility in selecting patients for a specific therapy remains unclear.
Given the limitations of cytotoxic treatment, new approaches targeting the stroma have emerged, such as antiangiogenic therapy, which is largely based on positive results in other solid cancers.6 GBMs are highly vascular tumors, with high expression of vascular endothelial growth factor (VEGF), a proangiogenic cytokine.7 Thus, anti-VEGF and other antiangiogenic agents would seem to be attractive therapeutic strategies. Initial phase II studies demonstrated promising results with significant radiographic response rates and improved progression-free survival (PFS) in rGBM achieved with bevacizumab therapy, a humanized monoclonal antibody against VEGF.8–11 On the basis of these results, the US Food and Drug Administration granted approval for the use of bevacizumab in rGBM in 2009. However, two subsequent randomized, placebo-controlled phase III trials of bevacizumab with chemoradiotherapy in patients with nGBM (RTOG-0825/NCT00884741 [Temozolomide and Radiation Therapy With or Without Bevacizumab in Treating Patients With Newly Diagnosed Glioblastoma] and AVAglio/NCT00943826 [A Study of Avastin (Bevacizumab) in Combination With Temozolomide and Radiotherapy in Patients With Newly Diagnosed Glioblastoma]) failed to demonstrate an improvement in OS.12,13 Moreover, two other phase III trials—one with the pan-VEGF receptor (VEGFR) tyrosine kinase inhibitor (TKI) cediranib (NCT00777153 [Cediranib in Combination With Lomustine Chemotherapy in Recurrent Glioblastoma (REGAL)]) and one with enzastaurin, an inhibitor of protein kinase C beta whose activation can lead to VEGF expression (NCT00295815 [Enzastaurin Versus Lomustine in Glioblastoma])—also failed to demonstrate OS benefit in rGBM.14,15 These failures demonstrate that anti-VEGF/anti-VEGFR agents, although they are biologically active and well tolerated, do not extend survival in populations of unselected patients with GBM. Interestingly, hypothesis-generating data from single-arm phase II trials in nGBM and rGBM revealed that patients whose tumor blood perfusion, volume, and/or oxygenation increased during treatment with these agents might survive longer than those without such an increase.16–19 A retrospective study of two independent cohorts of high-grade glioma suggested lower doses of bevacizumab than the currently recommended dosage (5 mg/kg per week) may be superior.50 This could indicate that tumor vascular normalization rather than vascular pruning may be an important therapeutic mechanism in GBM. Whether this strategy could provide a means for patient stratification for anti-VEGF/anti-VEGFR therapeutics is unknown and should be tested prospectively. However, these findings support the notion that there may be patients who derive more substantial benefit than others. Here, we discuss the lessons learned from clinical trials and how we could use this knowledge to potentially improve the OS of patients with GBM who receive anti-VEGF/anti-VEGFR therapy.
ANGIOGENESIS IN GBM
Currently, six mechanisms of tumor vessel formation have been postulated: vasculogenesis, sprouting angiogenesis, vessel co-option, intussusception, vascular mimicry, and transdifferentiation of tumor cells into endothelial cells.20 These modes of new vessel formation may be regulated by VEGF but also by myriad other molecules and signal transduction pathways (Appendix, online only). Endogenous antiangiogenic factors, such as soluble fms-like tyrosine kinase-1 (FLT1 or soluble VEGFR-1 [sVEGFR-1], a blocker of VEGF and placental growth factor [PlGF]), angiostatin, endostatin, interferon-α and interferon-β, and thrombospondin-1 and -2, counterbalance the activity of proangiogenic factors to maintain homeostasis.21 A disruption of this balance results in the pathologic angiogenesis associated with tumor formation and progression.
GBM is associated with increased levels of VEGF expression that result in highly angiogenic tumors leading to abnormal vasculature. Morphologically, GBM vessels are disorganized and tortuous with decreased pericyte coverage, larger vessel diameter, and thicker basement membranes than those of normal brain vessels.22–27 Functionally, this results in increased tumor vessel permeability, which leads to nonuniform delivery of oxygen and nutrients.28 Consequently, tumors develop regions of localized hypoxia with ensuing pseudopalisading necrosis. Hypoxia leads to further increase in VEGF expression, contributing to the creation of a vicious cycle.
RATIONALE FOR USE OF ANTIANGIOGENIC AGENTS IN GBM
Although there is a strong biologic rationale for using antiangiogenic agents against GBM, the mechanisms of potential benefit remain unclear. This is a key issue for the successful implementation of this therapeutic modality in GBM. Prevention of new blood vessel formation in a growing tumor should theoretically lead to increased hypoxia and nutrient deprivation, thereby limiting growth of the tumor or even causing regression. However, it is well established that increased hypoxia fuels tumor progression by promoting angiogenesis, cancer cell invasion, genetic instability, stem-like phenotype, epithelial-to-mesenchymal transition, resistance to apoptosis/autophagy, altered metabolism, and creation of an immunosuppressive microenvironment.6 In addition, hypoxia may promote treatment resistance because radiation and some chemotherapeutics depend on oxygen to achieve antitumor effects.
More than a decade ago, we proposed an alternative use of antiangiogenic agents—normalization of abnormal tumor vessels—to increase tumor blood perfusion and decrease hypoxia.29 Indeed, several preclinical studies support this notion.24,26,30,31 More importantly, outcomes supportive of vascular normalization have been observed in human patients with a variety of solid tumors enrolled onto clinical trials of various antiangiogenic agents. As an example for GBM, cediranib induced a time window of tumor vascular normalization with vasogenic edema control in patients with rGBM or nGBM.16,32 However, the duration and extent of vascular normalization and of clinical benefit differed significantly between individual patients.32 Importantly, the patients with rGBM or nGBM whose tumor blood perfusion and oxygenation increased as a result of vascular normalization survived longer.16,17 It is conceivable that enhanced delivery of therapeutics and oxygen accounted for the OS benefit.16 Future studies will determine whether vascular normalization played a beneficial role by decreasing immunosuppression, as observed in preclinical models.33 A second benefit of anti-VEGF agents is reduction in vasogenic brain edema, a major cause of neurologic morbidity in all patients with GBM.32 Future studies should also address whether and how vascular normalization alters the cancer cell phenotype. It has been proposed that stem-like GBM cells initiate and maintain the malignant growth of GBMs.34 Stem-like GBM cells, located in the perivascular niche, appear to be regulated by surrounding endothelial cells, which may maintain them in an undifferentiated and self-renewing state.35 Application of antiangiogenic agents may thus disrupt the tumor vasculature–associated stem-like GBM cells, thereby arresting tumor growth, as seen in mouse models of brain tumor.35 This link between angiogenesis and stem-like GBM cells needs to be validated in humans, but it could provide another rationale for the use of antiangiogenic agents.36 Finally, some of the antiangiogenic agents could directly target GBM cells.37 Limited clinical evidence for this mechanism has emerged from a phase II trial with cediranib in patients with rGBM.38
SUMMARY OF CLINICAL STUDIES OF ANTIANGIOGENIC AGENTS IN GBM
Antiangiogenic strategies tested in the clinic include targeting VEGF and/or VEGFR with antibodies or small-molecule TKIs. A summary of this clinical experience is presented in Tables 1 and 2 and in the Appendix.
Table 1.
Antibodies Currently in Clinical Development for Glioblastoma
| Agent | Mechanism | Phase | Disease Type | Response Rate (%) | PFS (months) | PFS6 (%) | OS (months) | Combination | Reference | ClinicalTrials.gov No. |
|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 28.2 | 42.6 | 8.6 | — | Friedman et al9 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 37.8 | 50.3 | 8.1 | Irinotecan | Friedman et al9 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 60.9 | 30 | 9.3 | Irinotecan | Vredenburgh et al104 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 57 | 46 | 9.8 | Irinotecan | Vredenburgh et al11 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 35 | 29 | 7.2 | — | Kreisl et al10 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 33 | 46.5 | 8.3 | Irinotecan, carboplatin | Reardon et al45 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 28 | 18.8 | 8.6 | Temozolomide | Desjardins et al47 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 24.5 | 25 | 6.5 | — | Raizer et al52 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 23 | 44.4 | 10.7 | Etoposide | Reardon et al46 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 0 | 0 | 2.9 | Temozolomide | Reardon et al44 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 0 | 7.7 | 4.4 | Etoposide | Reardon et al44 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 26 | 33 | 6.7 | Irinotecan, cetuximab | Hasselbalch et al53 | ||
| Bevacizumab | VEGF-A–blocking antibody | II | rGBM | 50 | 29.2 | 10.5 | Erlotinib | Sathornsumetee et al54 | ||
| Bevacizumab | VEGF-A–blocking antibody | III | nGBM | 10.7 | 15.7 | Temozolomide, radiotherapy | Gilbert et al13 | |||
| Bevacizumab | VEGF-A–blocking antibody | III | nGBM | 10.6 | Temozolomide, radiotherapy | Chinot et al12 | ||||
| Bevacizumab | VEGF-A–blocking antibody | II | nGBM | — | 13 | 85.1 | 23 | Temozolomide, radiotherapy | Narayana et al56 | |
| Bevacizumab | VEGF-A–blocking antibody | II | nGBM | — | 14.2 | — | 21.2 | Temozolomide, radiotherapy, irinotecan | Vredenburgh et al57 | |
| Bevacizumab | VEGF-A–blocking antibody | II | nGBM | — | 13.6 | 88 | 19.6 | Temozolomide, radiotherapy | Lai et al25 | |
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 83 | 22 | 7.0 | Carboplatin, etoposide | Francesconi et al164 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 42 | 42 | 7.9 | — | Chamberlain et al165 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | — | 41* | 9.0* | Irinotecan, carboplatin, lomustine, etoposide | Nghiemphu et al166 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 67.6 | 63.7 | 10.7 | Irinotecan | Zuniga et al41 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 77 | — | 6.3 | Irinotecan | Ali et al167 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | — | 17 | 7.1 | Irinotecan | Kang et al168 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | Irinotecan | Bokstein et al169 | |||||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 40* | — | — | Carboplatin, irinotecan, etoposide | Pope et al170 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 9/21 MG | Irinotecan | Stark-Vance et al171 | ||||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 50 | 0 | 1.5 | — | Scott et al172 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 19 | 14 | 5.2 | Irinotecan, carboplatin, or temzolomide | Scott et al172 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 29* | 29* | 7.8* | Irinotecan, carboplatin | Goldlust et al173 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | — | 42* | — | Irinotecan, carboplatin, lomustine, etoposide | Norden et al174 | ||
| Bevacizumab | VEGF-A–blocking antibody | Retrospective | rGBM | 0* | — | — | Carboplatin, irinotecan, carmustine, lomustine, erlotinib, etoposide | Quant et al70 | ||
| Aflibercept | VEGF-A, VEGF-B, PlGF decoy receptor | II | rGBM | 18 | 7.7 | 9.1 | — | De Groot et al62 | ||
| Aflibercept | VEGF-A, VEGF-B, PlGF decoy receptor | I† | nGBM | Temozolomide, radiotherapy | NCT00650923 | |||||
| Olaratumab (IMC-3G3) | Antibody against PDGFR-α | II† | rGBM | — | NCT00895180 | |||||
| Ramucirumab (IMC-1121B) | Antibody against VEGFR-2 | II† | rGBM | — | NCT00895180 |
Abbreviations: MG, malignant glioma; nGBM, newly diagnosed glioblastoma; OS, overall survival; PDGFR-α, platelet-derived growth factor receptor alpha; PFS, progression-free survival; PFS6, 6-month PFS; PlGF, placental growth factor; rGBM, recurrent glioblastoma; VEGF-A, vascular endothelial growth factor A; VEGFR-2, vascular endothelial growth factor receptor 2.
For overall group.
Ongoing trial.
Table 2.
Tyrosine Kinase Inhibitors Currently in Clinical Development for Glioblastoma
| Drug | Mechanism | Phase | Disease Type | Response Rate (%) | PFS (months) | PFS6 (%) | OS (months) | Combination | Reference | ClinicalTrials.gov No. |
|---|---|---|---|---|---|---|---|---|---|---|
| Cediranib (AZD2171) | VEGFR1-3, PDGFR-β, c-kit TKI | II | rGBM | 27 (Macdonald) | 25.8 | 7.5 | — | Batchelor et al69 | ||
| Cediranib (AZD2171) | VEGFR1-3, PDGFR-β, c-kit TKI | II | rGBM | 56.7 (volumetric) | 25.8 | 7.5 | — | Batchelor et al69 | ||
| Cediranib (AZD2171) | VEGFR1-3, PDGFR-β, c-kit TKI | III | rGBM | — | 16 | — | — | Batchelor et al14 | ||
| Cediranib (AZD2171) | VEGFR1-3, PDGFR-β, c-kit TKI | III | rGBM | — | 34.5 | — | Lomustine | Batchelor et al14 | ||
| Cediranib (AZD2171) | VEGFR1-3, PDGFR-β, c-kit TKI | II* | nGBM | Temozolomide, radiotherapy | NCT01062425 | |||||
| Vatalanib (PTK787) | VEGFR1-3, PDGFR-β, c-kit TKI | I/II | rGBM | — | 25 | 11.1 | Imatinib, hydroxyurea | Reardon et al72 | ||
| Vatalanib (PTK787) | VEGFR1-3, PDGFR-β, c-kit TKI | I/II | rGBM | 8.1 | Temozolomide | Reardon et al175 | ||||
| Vatalanib (PTK787) | VEGFR1-3, PDGFR-β, c-kit TKI | I/II | rGBM | 4.3 | Lomustine | Reardon et al175 | ||||
| Vatalanib (PTK787) | VEGFR1-3, PDGFR-β, c-kit TKI | I | nGBM | 15 | 7.2 | 16.2 | Temozolomide, radiotherapy | Gerstner et al73 | ||
| Vatalanib (PTK787) | VEGFR1-3, PDGFR-β, c-kit TKI | I/II | nGBM | — | 6.8 | 63.2 | 17.3 | Temozolomide, radiotherapy | Brandes et al74 | |
| Pazopanib (GW786034) | VEGFR1-3, PDGFR-α, PDGFR-β, c-Kit TKI | II | rGBM | 5.9 | 3 | 8.1 | — | Iwamoto et al75 | ||
| Pazopanib (GW786034) | VEGFR1-3, PDGFR-α, PDGFR-β, c-Kit TKI | II* | rGBM | Topotecan | NCT01931098 | |||||
| Cabozantanib (XL-184) | VEGFR-2, c-Met TKI | II | rGBM | 21 | — | — | — | — | Wen et al210 | |
| Cabozantanib (XL-184) | VEGFR-2, c-Met TKI | II | rGBM | 30 | — | — | — | — | Wen et al210 | |
| Cabozantanib (XL-184) | VEGFR-2, c-Met TKI | II* | rGBM | — | NCT00704288 | |||||
| Cabozantanib (XL-184) | VEGFR-2, c-Met TKI | II* | rGBM | — | NCT01068782 | |||||
| Cabozantanib (XL-184) | VEGFR-2, c-Met TKI | I* | nGBM | — | NCT00960492 | |||||
| Sunitinib | VEGR2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | II | rGBM | 0 | — | — | — | Neyns et al77 | ||
| Sunitinib | VEGR2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | II | rGBM | 0 | — | 16.7 | 12.6 | — | Pan et al78 | |
| Sunitinib | VEGR2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | II | rGBM | 10 | 10.4 | 9.4 | — | Kreisl et al76 | ||
| Sunitinib | VEGR2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | II | rGBM | 0 | 0 | 4.4 | — | Kreisl et al76 | ||
| Sunitinib | VEGR2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | I | rGBM | 24* | 12.2* | Irinotecan | Reardon et al90 | |||
| Sorafenib | VEGFR-2, Raf-1, PDGFR, c-Kit, Flt-3 TKI | II | rGBM | 3 | — | 9.4 | 9.7 | Temozolomide | Reardon et al177 | |
| Sorafenib | VEGR-2, PDGFR-α, PDGFR-β, c-Kit, FLT3 TKI | I/II | rGBM | 12 | — | 0 | — | Temsirolimus | Lee et al80 | |
| Sorafenib | VEGR-2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | II | rGBM | 5 | — | 14 | 5.7 | Erlotinib | Peereboom et al81 | |
| Sorafenib | VEGR-2, PDGFR-α, PDGFR-β, c-Kit, FLT-3 TKI | II | nGBM | 13 | 6 | — | 12 | Temozolomide | Hainsworth et al178 | |
| Vandetanib | VEGFR-2, EGFR | I/II | rGBM | 12.5 | — | 6.5 | 6.3 | — | Kreisl et al176 | |
| Vandetanib | VEGFR-2, EGFR | I | nGBM | 0 | 8 | 11 | Temozolomide, radiotherapy | Drappatz et al179 | ||
| Vandetanib | VEGFR-2, EGFR | I* | rGBM | Sirolimus | NCT00821080 | |||||
| AEE788 | VEGFR, EGFR | I | rGBM | 0 | — | — | — | — | Reardon et al106 | |
| Lenvatinib (E7080) * | VEGFR-2, VEGFR-3, FGFR1, c-kit, PDGFR-β | I/II* | rGBM | NCT01433991 | ||||||
| Tivozanib | VEGR-3 | II | rGBM | NCT01846871 | ||||||
| Enzastaurin | PKCβ, PI3K/AKT/mTOR | III* | rGBM | 2.9 | 11.1 | 6.6 | — | Wick et al15 |
Abbreviations: : EGFR, endothelial growth factor receptor; FGFR1, fibroblast growth factor receptor 1; FLT-3/Flt-3, fms-like tyrosine kinase 3; mTOR, mammalian target of rapamycin; nGBM, newly diagnosed glioblastoma; OS, overall survival; PDGFR, platelet-derived growth factor receptor; PFS, progression-free survival; PFS6, 6-month PFS; PI3K, phosphatidylinositol 3′-kinase; PKCβ, protein kinase C beta; rGBM, recurrent glioblastoma; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
Ongoing trial.
For overall group.
Bevacizumab is the most thoroughly studied anti-VEGF agent in GBM. Promising data from phase II studies of bevacizumab led to two randomized, placebo-controlled phase III trials of bevacizumab with standard chemoradiotherapy in patients with nGBM.12,13 These trials demonstrated improvement in PFS with the addition of bevacizumab to radiotherapy and temozolomide versus chemoradiotherapy alone but no improvement in median OS. However, the Single-Agent Bevacizumab or Lomustine Versus a Combination of Bevacizumab Plus Lomustine in Patients With Recurrent Glioblastoma Study (BELOB) showed an increased OS in the combination arm.49 That led to a randomized phase III study to test whether there is a role for bevacizumab at recurrence if not at first diagnosis. There are more than 15 active trials of bevacizumab for patients with nGBM and more than 35 active trials for patients with rGBM, some in combination with other agents in an attempt to avoid resistance to anti-VEGF therapy (Table 1 and Appendix).
Another anti-VEGF strategy, clinically validated for other solid cancers, has been the use of orally bioavailable VEGFR TKIs (Table 2). However, these agents typically lack specificity, impact other kinases, and result in undesirable off-target adverse effects. Of these, cediranib, a relatively selective pan-VEGFR TKI,68 has been most extensively evaluated in GBM. In a randomized, placebo-controlled phase III study, cediranib was assessed either as monotherapy or in combination with lomustine versus lomustine alone in patients with rGBM.14 There were no significant differences in PFS or OS between the cediranib-containing arms and the lomustine arm in this clinical trial.14 Similar studies of other anti-VEGFR TKIs (eg, vatalanib, pazopanib, cabozantinib, sorafenib, vandetanib) have shown limited efficacy in phase II studies in nGBM or rGBM (Table 1 and Appendix).
There is emerging interest in targeting other non-VEGF proangiogenic pathways. For example, inhibitors of angiopoietin-2 (Ang-2) have attracted attention as an alternative or complementary antiangiogenic strategy to VEGF inhibition.83 Preclinical studies have shown improved antitumor efficacy when VEGF and Ang-2–targeting therapies are combined.85 Moreover, it has been demonstrated that anti-VEGF therapy only transiently decreases circulating Ang-2 in patients with nGBM and rGBM and that Ang-2 overexpression can interfere with the vascular normalizing effect of anti-VEGF agents in GBM models in mice.69,84 Several anti-Ang-2 agents are currently being evaluated in clinical trials in extra-CNS cancers.
In summary, the last decade has witnessed an enormous effort to develop various agents targeting VEGF or its receptors for GBM as well as to translate exciting preclinical findings into the clinic. However, despite measurable radiographic responses, reduction in vasogenic brain edema, and delay in radiographic tumor progression observed for some of these agents, so far there has been no OS benefit observed in populations of unselected patients with GBM with the exception of preliminary data from the BELOB study. This emphasizes the critical importance of identifying biomarkers of response to allow selection of patients most likely to benefit from this expensive and potentially toxic class of antitumor treatment.
BIOMARKERS OF RESPONSE TO ANTIANGIOGENIC AGENTS IN GBM
Biomarker discovery efforts have focused on tumor, blood, and radiographic parameters (Tables 3, 4, and 5).
Table 3.
Potential Tissue Biomarkers of Antiangiogenic Therapy
| Tissue Biomarkers | Effect, Agent, and Trial Type | Challenges and Comments | Reference |
|---|---|---|---|
| ProB-GBM (mesenchymal-like genes) | Predicted survival advantage in subset of patients from phase III trials of bevacizumab in nGBM | Needs to be prospectively validated in larger studies Not predictive for response at recurrence |
Sulman et al86 |
| VEGF | Radiographic response to bevacizumab correlated with increased tissue expression in phase II trials in rGBM | No correlation was seen with survival Prospective studies are lacking |
Sathornsumetee et al87 |
| MGMT promoter methylation | Failed to predict response to bevacizumab in phase III trials in nGBM | Prognostic value validated Several recent studies in Europe are investigating bevacizumab in patients with unmethylated MGMT |
Sulman et al86; DePrimo et al88 |
| EGFR, PDGFR-α, c-KIT | Failed to correlate with outcome in phase II trials of cediranib in nGBM or bevacizumab in rGBM | Needs to be prospectively investigated | Batchelor et al16 |
Abbreviations: EGFR epidermal growth factor receptor; MGMT, O-6-methylguanine-DNA methyltransferase; nGBM, newly diagnosed glioblastoma; PDGFR-α, platelet-derived growth factor receptor alpha; rGBM, recurrent glioblastoma; VEGF, vascular endothelial growth factor.
Table 4.
Potential Biomarkers of Antiangiogenic Therapy Measured in Blood Circulation in Patients With Glioblastoma
| Plasma Biomarkers | Agent and Regimen | Effect on Biomarker | Comments |
|---|---|---|---|
| VEGF | Bevacizumab with chemoradiation (phase III study) | Not available | Baseline VEGF does not correlate with survival outcomes90 Unclear when the optimal time is for evaluation as pharmacodynamic biomarker after anti-VEGF treatment |
| VEGF | Cediranib alone and with chemotherapy in patients with rGBM (phase II and III studies) Cediranib with chemoradiation in patients with nGBM (phase II study) |
Increase in plasma VEGF | Seen only in cediranib-containing arms in phase III study16 Does not correlate with survival outcomes14,16,69 |
| VEGF | Vandetanib in patients with nGBM (phase II study) | Increase in plasma VEGF | Minor increases at some but not all time-points (weak VEGFR inhibition?)89 |
| VEGF | Vatalanib with chemoradiation in patients with nGBM (phase I study) | Increase in plasma VEGF | Agent has short half-life (weak VEGFR inhibition?)73 |
| VEGF | Aflibercept alone in patients with rGBM (phase II study) | Decrease in free plasma VEGF | Potential accumulation of bound VEGF in blood circulation91 |
| VEGF | Cabozantinib alone in patients with rGBM (phase II study) | Increase in plasma VEGF | Mature data not available93 |
| PlGF | Cediranib alone in patients with rGBM (phase II study) Cediranib with chemoradiation in patients with nGBM (phase II) |
Increase in plasma PlGF | Substantial increases (by 30% to 386% from 8 hours to 112 days) but unclear when the optimal time is for evaluation as pharmacodynamic biomarker after anti-VEGF treatment Does not correlate with survival outcomes16,69 Correlates with perfusion changes measured by MRI16 |
| PlGF | Vandetanib with chemoradiation in patients with nGBM (phase II study) | Increase in plasma PlGF | PlGF initially decreases (at 4 hours) but then increases (by 6% to 40% from day 1 to 22; weak VEGFR inhibition?)89 |
| PlGF | Vatalanib with chemoradiation in patients with nGBM (phase I study) | Increase in plasma PlGF | Sustained but minor increases (by 54% to 61% from 8 hours to 70 days). Agent has short half-life (weak VEGFR inhibition?)73 |
| PlGF | Aflibercept alone in rGBM patients (phase II study) | Increase in plasma PlGF | Dramatic increase in PlGF91; potential accumulation of bound PlGF in blood circulation? Inverse correlation with response91 |
| PlGF | Cabozantinib alone increases plasma PlGF (phase II study) | Increase in plasma PlGF | Mature data not available93 |
| sVEGFR-1 | Cediranib alone in patients with rGBM (phase II study) Cediranib with chemoradiation in patients with nGBM (phase II) |
No change in plasma sVEGFR-1 Decrease in plasma sVEGFR-1 |
An increase in sVEGFR-1 on treatment correlates with survival outcomes in nGBM and rGBM patients16,69 Correlates with perfusion changes measured by MRI69 Unclear whether sVEGFR-1 is a pharmacodynamic or predictive biomarker for anti-VEGF therapy16 |
| sVEGFR-1 | Vandetanib with chemoradiation in patients with nGBM (phase II study) | No change in plasma sVEGFR-1 | High sVEGFR-1 at baseline correlated with survival outcomes in nGBM patients89 |
| sVEGFR-1 | Vatalanib with chemoradiation in patients with nGBM (phase I study) | Increase in plasma sVEGFR-1 | No significant correlation with survival73 |
| sVEGFR-2 | Bevacizumab with chemoradiation (phase III study) | Not available | Baseline sVEGFR-2 does not correlate with survival outcomes90 Bevacizumab may not decrease the plasma VEGFR-2 levels94 |
| sVEGFR-2 | Cediranib alone and with chemotherapy in patients with rGBM (phase II and III studies) | Decrease in plasma sVEGFR-2 | Seen only in cediranib-containing arms in phase III study14 Unclear when the optimal time of evaluation is as pharmacodynamic biomarker after anti-VEGF treatment with TKIs Does not correlate with survival outcomes14,16,69 Correlates with perfusion changes measured by MRI16 |
| sVEGFR-2 | Vandetanib with chemoradiation in patients with nGBM (phase II study) | Decrease in plasma sVEGFR-2 | Change inversely correlated with overall survival89 |
| sVEGFR-2 | Vatalanib with chemoradiation in patients with nGBM (phase I study) | Decrease in plasma sVEGFR-2 | Does not correlate with survival outcomes73 |
| sVEGFR-2 | Cabozantinib alone in patients with rGBM (phase II study) | Decrease in plasma sVEGFR-2 | Mature data not available93 |
| Collagen IV | Cediranib alone in patients with rGBM (phase II study) | Decrease in plasma collagen IV | Early change (at day 1) inversely correlates with PFS92 |
| Collagen IV | Vandetanib with chemoradiation in patients with nGBM (phase II study) | No change in plasma collagen IV | Early change (at day 1) inversely correlates with response (RECIST)89 |
| Collagen IV | Vatalanib with chemoradiation in patients with nGBM (phase I study) | Decrease in plasma collagen IV | Early change (at day 1) inversely correlates with PFS73 |
| bFGF | Cediranib alone or with chemotherapy in patients with nGBM (phase III study) Cediranib with chemoradiation in patients with nGBM (phase II study) |
No consistent change in plasma bFGF Decrease in plasma bFGF |
Does not correlate with survival outcomes16,69 |
| bFGF | Vandetanib with chemoradiation in patients with nGBM (phase II study) | No change in plasma bFGF | Does not correlate with survival outcomes; baseline plasma bFGF inversely associated with increase responses (by RECIST)89 |
| bFGF | Vatalanib with chemoradiation in patients with nGBM (phase I study) | No change in plasma bFGF | Does not correlate with survival outcomes73 |
| Ang-2 | Cediranib alone in patients with rGBM (phase II study) Cediranib with chemoradiation in patients with nGBM (phase II study) |
Decrease in plasma Ang-2 Decrease in plasma Ang-2 |
Decrease is transient in patients with nGBM after cediranib alone69 but more sustained in nGBM after cediranib with chemoradiation16 Does not correlate with survival outcomes16,69 |
| sTie-2 | Cediranib alone in patients with rGBM (phase II study) | Decrease in plasma sTie-2 | Low levels associated with radiographic response; high levels associated with progression69 |
| sTie-2 | Cediranib with chemoradiation in patients with nGBM (phase II study) | No change in plasma sTie-2 | |
| sTie-2 | Vandetanib with chemoradiation in patients with nGBM (phase II study) | Increase in plasma sTie-2 | |
| sTie-2 | Vatalanib with chemoradiation in patients with nGBM (phase I study) | Transient decrease in plasma sTie-2 | Does not correlate with survival outcomes89 |
| CA-9 | Cediranib with chemoradiation in patients with nGBM (phase II study) | Increase in plasma CA-9 | |
| CA-9 | Aflibercept alone in patients with rGBM (phase II study) | Not reported | Plasma CA-9 correlated with plasma VEGF at baseline91 |
| CA-9 | Vandetanib with chemoradiation in patients with nGBM (phase II study) | No change in plasma CA-9 | A rapid decrease in CA-9 (at 4 hours) associated with response (RECIST)89 |
| MMP-9 | Cediranib alone in patients with rGBM (phase II study) | No change in plasma MMP-9 | No association with outcome69 |
| MMP-9 | Aflibercept alone in patients with rGBM (phase II study) | Not reported | An increase at day 28 associated with disease progression91 |
| MMP-2 | Cediranib alone in patients with rGBM (phase II study) | Transient decrease in plasma MMP-2 | An increase in plasma MMP-2 at 8 hours after first administration of cediranib correlated with reduced PFS and OS69 |
| MMP-10 | Cediranib alone in patients with rGBM (phase II study) | Transient decrease and then sustained increase in plasma MMP-10 | No association with outcome69 |
| SDF-1α | Cediranib alone in patients with rGBM (phase II study) Cediranib with chemoradiation in patients with nGBM (phase II study) |
Increase in plasma SDF-1α Increase in plasma SDF-1α |
No association with survival outcomes16,69 Increased levels of SDF-1α associated with radiographic progression69 |
| SDF-1α | Vandetanib with chemoradiation in patients with nGBM (phase II study) | Transient decrease followed by increase in plasma SDF-1α | A subtle drop at 4 hours and a small increase at day 2289 No association with survival outcomes89 |
| SDF-1α | Vatalanib with chemoradiation in patients with nGBM (phase I study) | No change in plasma SDF-1α | |
| IL-8 | Cediranib alone in patients with rGBM (phase II study) Cediranib with chemoradiation in patients with nGBM (phase II study) |
No change in plasma IL-8 Transient increase in plasma IL-8 |
High levels associated with radiographic response after cediranib alone69 Late increase in IL-8 (at day 43) after cediranib and chemoradiation correlated with poor PFS and showed a nonsignificant trend for association with poor OS16 |
| IL-8 | Vatalanib with chemoradiation in patients with nGBM (phase I study) | No change in plasma IL-8 | No association with survival outcomes73 |
| MCP3/CCL7 | Aflibercept in patients with rGBM (phase II study) | Not reported | High baseline levels of CCL7 were associated with improved response91 |
| MIF | Aflibercept in patients with rGBM (phase II study) | Transient decrease in plasma MIF | High baseline levels of MIF were associated with improved response91 |
| CTACK/CCL27 | Aflibercept in patients with rGBM (phase II study) | Not reported | High baseline levels of CCL27 were associated with improved response91 |
| IP-10/CXCL10 | Aflibercept in patients with rGBM (phase II study) | Not reported | High baseline levels of CXCL10 were associated with improved response91 |
Abbreviations: Ang-2, angiopoietin 2; bFGF, basic fibroblast growth factor; CA-9, carbonic anhydrase 9; IL-8, interleukin-8; MMP-2, matrix metalloproteinase 2; MRI, magnetic resonance imaging; nGBM, newly diagnosed glioblastoma; OS, overall survival; PFS, progression-free survival; PlGF, placental-derived growth factor; rGBM, recurrent glioblastoma; SDF-1α, stromal-derived factor 1 alpha; sVEGFR-1, soluble vascular endothelial growth factor receptor 1; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Table 5.
Potential Imaging Biomarkers of Antiangiogenic Therapy in Glioblastoma
| Drug | Combination | Disease Type | No. of Patients | Imaging Modality | Technique | Response Biomarker* | Day(s) of Imaging | Reference |
|---|---|---|---|---|---|---|---|---|
| Bevacizumab | — | rGBM | 16 | MRI | DSC | ↓CBVHPV† | 42 | Sawlani et al94 |
| Bevacizumab | — | rGBM | 9 | MRI | DWI | ↓ADC ↓RSI |
16-112 | Kothari et al156 |
| Bevacizumab | Irinotecan | rGBM | 14 | MRI | DSC | ↓CBV | 56 | Reiger et al181 |
| Bevacizumab | Irinotecan | rGBM | 42 | MRI | DSC | ↑CBV† | 56 | Eoli et al19 |
| Bevacizumab | Irinotecan | rGBM | 20 | MRI | DCE | ↓Ktrans ↓Ve |
1-14 1 |
Ferl et al182 |
| Bevacizumab | Irinotecan | rGBM | 20 | MRI | DCE | ↓Ktrans ↓Ve |
1-14 1 |
Hsu et al187 |
| Bevacizumab | Irinotecan | rGBM | 13 | MRI | DCE | ↓Ktrans | 1-14 | Desjardins et al188 |
| Bevacizumab | Irinotecan | rGBM | 41§ | MRI | DWI | ↑ADCL† | baseline | Pope et al189 |
| Bevacizumab | Irinotecan | rGBM | 14 | MRI | DWI | ↓ADC | 56 | Rieger et al181 |
| Bevacizumab | Irinotecan | rGBM | 16‡ | MRI | DWI | ↑ADCNEL† | 42 | Jain et al183 |
| Bevacizumab | Irinotecan | rGBM | 6 | MRI | DWI | ↑ADChist† | 56-84 | Nowosielski et al184 |
| Bevacizumab | Irinotecan | rGBM | 16 | MRI | DWI | ↓fDM† | 30-90 | Ellingson et al189a,189b |
| 70‡ | ||||||||
| Bevacizumab | Irinotecan | rGBM | 22 | MRI | DWI | ↓LADC† | 40 | Hwang et al189c |
| Bevacizumab | Irinotecan | rGBM | 13‡ | MRS | ↑NAA/Cho† ↓Cho/Cr† ↑NAA/Cr† |
56-168 | Ratai et al186 | |
| 112 | ||||||||
| 112 | ||||||||
| Bevacizumab | Irinotecan | rGBM | 41 | MRI | DWI | ↑ADCL† | baseline | Pope et al155 |
| Bevacizumab | Irinotecan | rGBM | 36‡§ | CT | Cal | → Not present† | 56 | Bähr et al122 |
| Bevacizumab | Irinotecan | rGBM | 17 | PET | [18F]-FLT | ↓SUV† | 7-49 | Chen et al et al190 |
| Bevacizumab | Irinotecan | rGBM | 11 | PET | [18F]-FLT | ↓SUV† | 7-49 | Schiepers et al191 |
| Bevacizumab | Irinotecan | rGBM | 16 | PET | [18F]-FLT | ↓SUV† | 7-49 | Wardak et al192 |
| Bevacizumab | Irinotecan | rGBM | 24‡ | PET | [18F]-FLT | ↓SUV† | 7-49 | Schwarzenberg et al134 |
| Bevacizumab | Irinotecan | rGBM | 18‡ | PET | [18F]-FLT | ↓SUV† | 7-49 | Harris et al135 |
| Bevacizumab | Irinotecan | rGBM | 5 | PET | [18F]-FET | ↓SUV† | 56-96 | Hutterer et al193 |
| Bevacizumab | Irinotecan | rGBM | 5 | PET | [18F]-FET | ↓SUVvol† | 20 | Galldiks et al195 |
| Bevacizumab | Irinotecan | rGBM | 20 | PET | [18F]-FDG | ↓SUVmax† | baseline | Colavolpe et al194 |
| Bevacizumab | Irinotecan | rGBM | 18‡ | PET | [18F]-FDOPA | ↓SUV† | 7-49 | Harris et al135 |
| Bevacizumab | Fotemustine | rGBM | 9‡ | CT | PCT | ↓CBV | 21 | Vidiri et al196 |
| Bevacizumab | Carboplatin | rGBM | 26‡ | MRI | DQT2 | ↓ΔT2† | 28-42 | Ellingson et al196a |
| Bevacizumab | Temozolomide | rGBM | 27‡ | MRI | DSC | ↓CBV | 60 | Gupta et al197 |
| Bevacizumab | Temozolomide | rGBM | 23§ | MRI | DSC | ↓ΔAVOL† | 38 | LaViolette et al199 |
| Bevacizumab | Temozolomide | rGBM | 14‡ | MRI | DWI | →RDL (yes)† | baseline, control | Mong et al198 |
| Bevacizumab | Temozolomide | nGBM | 40§ | MRI | DSC | ↓CBV | 42-120 | Grommes et al200 |
| Bevacizumab | Temozolomide | nGBM | 56§ | MRI | DWI | ↓ADCL† | baseline, control | Pope et al200a |
| Bevacizumab | Temozolomide | nGBM | 40§ | MRI | [18F]-FDG | ↓SUV† | 180 | Grommes et al200 |
| Cediranib | — | rGBM | 16 | MRI | DSC | ↓CBV | 1-28 | Batchelor et al32 |
| Cediranib | — | rGBM | 30 | MRI | DSC | ↑CBV† ↑CBF† |
1 | Sorensen et al17 |
| 1-56 | ||||||||
| Cediranib | — | rGBM | 16 | MRI | DSC | ↓VCI† | 1 | Batchelor et al32 |
| Cediranib | — | rGBM | 30 | MRI | DSC | ↓VCI† ↑VNI† |
1 | Sorensen et al92 |
| Cediranib | — | rGBM | 30 | MRI | DSC | ↓VCI† ↑VNI† ↑A/V† ↓ΔSO2† |
1 | Emblem et al151,200b |
| 1 | ||||||||
| 1-56 | ||||||||
| 1-56 | ||||||||
| Cediranib | — | rGBM | 30 | MRI | DCE | ↓Ktrans† | 1-112 | Sorensen et al92 |
| Cediranib | — | rGBM | 16 | MRI | DCE | ↓Ktrans† ↓Ve |
1-112 1-56 |
Batchelor et al32 |
| Cediranib | — | rGBM | 30 | MRI | DCE | ↓Ktrans† | 1-112 | Gerstner et al159 |
| Cediranib | — | rGBM | 30 | MRI | ASL | ↑CBF† | 1-56 | Sorensen et al17 |
| Cediranib | — | rGBM | 30 | MRI | MRS | ↑NAA/Cho† | 1-56 | Kim et al38 |
| Cediranib | — | rGBM | 30 | MRI | DWI | ↓ADC | 1-112 | Batchelor et al32 |
| Cediranib | — | rGBM | 30 | MRI | DWI | ↓ADC | 1-112 | Gerstner et al159 |
| Cediranib | — | rGBM | 30 | MRI | DWI | ↑ADCsub† | 1-112 | Gerstner et al159 |
| Cediranib | Temozolomide | nGBM | 40§ | MRI | DSC | ↑CBF† ↓VCI ↓ΔSO2† |
1-50 | Batchelor et al16 |
| Cediranib | Temozolomide | nGBM | 40§ | MRI | DSC | ↓ΔSO2† ↓A/V† |
1-50 | Emblem et al200c |
| 1-50, control | ||||||||
| Cediranib | Temozolomide | nGBM | 40§ | MRI | DCE | ↓Ktrans | 1-50 | Batchelor et al16 |
| Cediranib | Temozolomide | nGBM | 40§ | MRI | DWI | ↓ADC | 1-50 | Batchelor et al16 |
| Vatalanib | — | rGBM | 47 | MRI | DSC | ↓CBV | 2-30 | Conrad et al18 |
| Vatalanib | — | rGBM | 47 | MRI | DCE | ↓Ktrans | 2-30 | Conrad et al18 |
| Ramucirumab | — | rGBM | MRI | DSC | ↓CBV | 1 | O'Neill Blakeley et al202 | |
| Ramucirumab | — | rGBM | MRI | DWI | ↓ADC | 28 | O'Neill Blakeley et al202 | |
| Cabozantinib | — | rGBM | 38 | MRI | DCE | ↓Ktrans | 28 | Sorensen et al202a |
| Cabozantinib | — | rGBM | 38 | MRI | MRS | ↑NAA/Cho ↓Lipids |
28 | Sorensen et al202a |
| Pazopanib | — | rGBM | 11 | MRI | DSC | ↓CBV† | 28-56 | Iwamoto et al75 |
| Pazopanib | — | rGBM | 11 | MRI | DCE | ↓Ktrans† | 28-56 | Iwamoto et al75 |
| Enzastaurin | Temozolomide | nGBM | 35§ | MRI | DSC | ↓PH† ↑PR† |
60 | Essok-Burns et al204 |
| Enzastaurin | Temozolomide | nGBM | 25§ | MRI | SWI | ↑%SWI-h† | baseline | Lupo et al203 |
| Thalidomide | Carboplatin | rGBM | 15 | MRI | DSC | ↓CBV | 60 | Cha et al205 |
| Cilengitide | — | rGBM | 24 | MRI | DSC | ↓CBF | 56-280 | Akella et al206 |
| Cilengitide | — | rGBM | 37 | MRI | DSC | ↓CBF | 56-280 | Nabors et al207 |
| Olaratumab | — | rGBM | 17 | MRI | DSC | ↓CBV | 28 | O'Neill Blakeley et al202 |
| Sunitinib | — | rGBM | 7 | MRI | DSC | ↓CBF | 28 | Chaskis et al134 |
| Sunitinib | — | rGBM | 14 | MRI | DSC | ↓CBV ↓CBF |
28 28 |
Neyns et al209 |
| Aflibercept | — | rGBM | 14 | MRI | DCE | ↓Ktrans | 1 | De Groot et al62 |
NOTE. ↑, increase; →, no change/presence; ↓, decrease.
Abbreviations: A/V, arteriovenous ratio; ADC, apparent diffusion coefficient; ADChist, ADC histogram features; ADCL, lower curve mean of two-peak ADC histogram; ADCNEL, ADC in nonenhancing lesion; ADCsub, volume of subthreshold ADC in tumor; ASL, arterial spin labeling; Cal, calcifications; CBF, cerebral blood flow; CBV, cerebral blood volume; CBVHPV, CBV hyperperfusion volume; Cho, choline; Cr, creatinine; CT, computed tomography; DCE, dynamic contrast-enhanced [MRI]; DQT2, differential quantitative T2 relaxometry mapping; DSC, dynamic susceptibility contrast [MRI]; DWI, diffusion weighted imaging; fDM, functional diffusion map; [18F]-FLT, [18F]fluorothymidine; [18F]-FET, O-(2-18F-fluoroethyl)-l-tyrosine; [18F]-FDG, [18F]fluorodeoxyglucose; [18F]-FDOPA, 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine; Ktrans, capillary permeability transfer constant; LADC, tumor ADC lower-than-normal cortex; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate; nGBM, newly diagnosed glioblastoma; PCT, perfusion computed tomography; PET, positron emission tomography; PH, peak height of tissue relaxivity (a pseudoestimate of vascular density); PR, percent recovery of tissue relaxivity (a pseudoestimate of leakage); RDL, restricted-diffusion lesions with well-demarcated high signal intensity on DWI; rGBM, recurrent glioblastoma; RSI, restriction spectrum imaging; SUV, standardized uptake value (g/mL); SUVvol, tumor volume by SUV; SWI, susceptibility-weighted imaging; SWI-h, fraction of SWI hypointensity in total contrast-enhanced volume; T2, transverse (proton spin-spin) magnetic relaxation; VCI, vessel caliber imaging; Ve, extravascular extracellular space volume; VNI, vascular normalization index; ΔAVOL, change in arteriovenous overlap; ΔSO2, change in relative oxygen saturation.
Limited to antiangiogenic studies reporting significant patient group effects from univariable advanced imaging parameters (beyond Macdonald's/RANO criteria) and compared with pretherapy baseline or controls.
Response in a subgroup of patients with favorable outcome (radiologic response, progression-free survival, or overall survival).
Other combination drugs used in some patients.
Radiotherapy.
Tumor Tissue Biomarkers
Several studies have prospectively evaluated tumor tissue biomarkers of response to antiangiogenic therapy in GBM (Table 3). Most of these studies were performed with bevacizumab. Thus, whether the data are relevant for anti-VEGFR agents remains to be demonstrated. In the Radiation Therapy Oncology Group 0825 (RTOG-0825) trial, the patients with nGBM who have O-6-methylguanine-DNA methyltransferase (MGMT) methylation in their tumors had superior OS (23.2 v 14.3 months; P < .001) and PFS (14.1 v 8.2 months; P < .001), confirming the prognostic utility of this epigenetic marker. However, results showed that neither a prespecified nine-gene signature nor MGMT methylation status predicted selective benefit for bevacizumab treatment.86 Unpublished data suggest that another 10-gene expression signature termed Pro-GBM may identify a subset of patients with nGBM in whom bevacizumab may be detrimental; however, these data will require prospective validation.86
It has been observed in tissue studies conducted in patients with recurrent high-grade glioma treated with bevacizumab and irinotecan that high expression of VEGF correlates with a higher likelihood of achieving a radiographic response but not increased survival.87 It was also observed in this same study that elevated levels of carbonic anhydrase 9, a marker of hypoxia, were significantly associated with poor 1-year survival.87 In another tumor tissue study in patients with malignant glioma, it was observed that low carbonic anhydrase 9 expression and increased VEGF expression were associated with better PFS among patients with GBM treated with metronomic etoposide and bevacizumab.46 In contrast, in a study of patients with GBM treated with bevacizumab and irinotecan with or without cetuximab (an EGFR inhibitor), no biomarker was predictive of response or prolongation of PFS.136 Finally, a retrospective autopsy study of patients with rGBM treated with various anti-VEGF agents including bevacizumab showed that elevated numbers of CD68+ and CD11+ tumor-associated macrophages (TAMs) were associated with poor survival, indicating a potential biomarker of escape.137
In other retrospective studies, the established prognostic markers—MGMT promoter methylation and IDH1—did not correlate with response to antiangiogenic therapy in rGBM.138,139 EGFR, a tyrosine kinase frequently amplified in GBM, also did not correlate with response to bevacizumab in rGBM on the basis of retrospective studies.138 In a prospective phase II study of patients with nGBM treated with cediranib and chemoradiotherapy, no association was observed between amplifications of the common tyrosine kinase receptors (EGFR, PDGFR-α, and c-KIT) and outcome.16
Several single nucleotide polymorphisms in the VEGF and VEGFR-2 promoters correlated with improved 6-month progression-free survival in a phase II study of bevacizumab and sorafenib for rGBM. Single nucleotide polymorphisms in the VEGF promoter also correlated with more severe toxicities.140
In summary, several studies have identified different tumor tissue markers that may serve as biomarkers for response. However, larger prospective studies are required to validate these preliminary results.
Circulating Blood Biomarkers
Similar efforts have been conducted for circulating (blood) biomarkers (Table 4). Several studies with various anti-VEGF/anti-VEGFR agents have failed to identify a correlation between baseline or pretreatment VEGF or sVEGFR-2 levels with outcomes.14,32,69,73,89 The AVAglio study, which included evaluation of pretreatment plasma VEGF and sVEGFR-2, found no association with PFS.90 Similar lack of associations between pretreatment biomarkers, including VEGF and sVEGFR-2, and treatment outcome in patients with GBM were reported with cediranib, vatalanib, and vandetanib.32,69,73,89
However, akin to the experience with anti-VEGF agents in extra-CNS tumors, the actual change in levels of various soluble factors may function as pharmacodynamic biomarkers, reflecting the actual biologic activity of the agents. For instance, increased levels of VEGF, stromal-derived factor 1α (SDF-1α), and PlGF and decreased levels of sVEGFR-2 after treatment with cediranib, vatalanib, and vandetanib were consistently observed in patients with either nGBM or rGBM.32,69,73,89 These changes seem to be specific to anti-VEGFR treatment because the changes in SDF-1α, PlGF, and sVEGFR-2 were significantly different in patients with nGBM treated with cediranib and chemoradiotherapy compared with a contemporary control group of patients with nGBM treated with chemoradiotherapy alone.16 Whether similar findings from these trials of anti-VEGFR TKIs can be translated to patients treated with bevacizumab is unknown.
More importantly, some studies have reported an association between the biomarker changes and treatment outcome, suggesting that these dynamic changes should be pursued as potential response or resistance biomarkers. For example, increases in sVEGFR-1 have been associated with poor survival in patients treated with cediranib.69 We previously proposed that sVEGFR-1, a negative regulator of the VEGF pathway, is a potential resistance biomarker to anti-VEGF therapy.141 A phase II trial of cediranib in patients with rGBM found that elevated SDF-1α was associated with tumor progression; however, this was not consistently observed across trials.69 The same trial also reported that an increase in plasma matrix metalloproteinase 2 (MMP-2), a key enzyme in angiogenesis, was associated with decreased PFS and OS after cediranib treatment. However, a recent study of 26 patients found that elevated plasma levels of MMP-2 after bevacizumab administration were associated with prolonged tumor control and survival in recurrent high-grade glioma.142 These differences may be attributable to an anti-VEGF agent versus an anti-VEGFR agent or to the unknown enzyme activation level of the measured MMP-2. Further prospective studies are needed to clarify these conflicting findings. Finally, increased levels of MMP-9 at 28 days compared with baseline were associated with tumor progression in patients with GBM treated with aflibercept.91
Exploratory studies also identified potential biomarkers of response. A phase I study reported an association between changes in plasma collagen IV and circulating progenitor cells with response after treatment with vatalanib and chemoradiotherapy.73 Similarly, greater increases in collagen IV levels were associated with extended PFS in patients with rGBM treated with cediranib.92 Surprisingly, aflibercept, which rapidly sequesters VEGF and PlGF with significant and rapid decrease in circulating levels, found no association between the decrease in plasma VEGF and radiographic responses.91 Instead, high baseline expression of monocyte-associated factors such as cutaneous T-cell–attracting chemokine (CCL27), macrophage chemotactic protein-3 (CCL7), macrophage migratory inhibitory factor, and interferon gamma–inducible protein 10 (IP-10) were associated with radiographic response. Finally, greater decreases in VEGFR-1–expressing monocytes at day 1 from baseline were correlated with response to aflibercept.91
These hypothesis-generating studies suggest that there may be a role for the use of circulating biomarkers as biomarkers of response to therapy, and there are several potential candidates. The limitation is that most of these candidates have resulted from single-arm studies and from studies that did not meet their prespecified end points. Placebo-controlled prospective studies are required to validate these candidates as predictive biomarkers. Lack of these studies remains a major unmet need in antiangiogenic drug development in GBM.
Imaging Biomarkers
Imaging parameters are particularly promising as potential predictive biomarkers of response to antiangiogenic therapy in GBM.143–145 Conventional magnetic resonance imaging (MRI), the preferred imaging modality of choice in brain tumors, provides important in vivo information regarding the anatomy of the tumor and surrounding brain but reveals little information on metabolic and hemodynamic status and function.146 However, dynamic contrast-enhanced and dynamic susceptibility contrast MRI techniques may shed light on baseline and dynamic features of GBM vasculature. Positron emission tomography (PET) techniques such as 2-[18F]-fluoro-2-deoxy-d-glucose PET, [18F]-fluorothymidine PET, [18F]-fluoromisonidazole PET, or O-(2-[18F]-fluoroethyl)-l-tyrosine PET are being studied as biomarkers of response (Appendix).
Historically, the preferred method for assessing radiographic response in high-grade gliomas was based on the Macdonald criteria, which provided an objective measure of tumor response based on the product of the maximal cross-sectional diameters of the contrast-enhanced tumor margins from a disrupted blood-brain barrier.124 However, Macdonald criteria have several limitations125,126 (Appendix). To address some of these limitations, a Response Assessment in Neuro-Oncology Working Group proposed revised response criteria that are more useful for the assessment of antiangiogenic agents147 (Appendix).
With the advent of antiangiogenic therapies in clinical trials of GBMs, conventional imaging techniques are limited in their ability to detect antitumor activity.126 Blockade of VEGF results in decreased vascular permeability and thus reduced tumor contrast enhancement as early as 1 day after the start of therapy.92 These pseudoresponses do not translate into prolonged OS.148,149 To this end, advanced imaging techniques beyond traditional structural imaging have been introduced. Perfusion and diffusion MRI, as well as magnetic resonance spectroscopy and PET better reveal the functional and hemodynamic status of the tumor and may identify patients with GBM who are likely to benefit from antiangiogenic therapy (Table 5).
Measures of vascular permeability from dynamic contrast-enhanced MRI complements traditional imaging by estimating the restoration of the blood-brain barrier whereas tumor perfusion by dynamic susceptibility contrast MRI is sensitive to perfused regions outside a disrupted blood-brain barrier and can therefore assess blood volume, blood flow, and vessel calibers in both tumor and surrounding tissue.143,146,150 In a phase II study of cediranib in patients with rGBM, the decrease in vascular permeability (Ktrans) and increase in microvessel volume correlated with OS.92 Combining these imaging parameters with circulating levels of collagen IV, a composite vascular normalization index correlated with OS and PFS. In addition, increase in tumor blood perfusion on MRI in patients with rGBM treated with cediranib was associated with a 6-month increase in OS when compared with patients whose tumor blood perfusion did not increase.17 A similar correlation was found in patients with nGBM treated with cediranib and chemoradiotherapy.16 These studies suggest that it might be possible to select patients with nGBM or rGBM who are likely to optimally benefit from anti-VEGF therapy on the basis of early changes in tumor perfusion after treatment with cediranib.6 This work has been augmented by vessel architectural imaging, which represents a noninvasive MRI technique for the estimation of brain and brain tumor oxygenation status.151 Application of the vessel architectural imaging technique to patients with rGBM or nGBM treated with cediranib demonstrated that patients with the longest survival had reduction of abnormal vessel calibers, normalization of the microvascular architecture, and improved oxygen saturation levels.16,151
Diffusion MRI monitors the Brownian movement of water in tissue without the use of a contrast agent and provides information on tissue cellularity, which is a useful indicator of tumor grade and response to chemoradiotherapy.152 Before the advent of antiangiogenic agents, functional diffusion imaging was examined as a potential predictor of survival,153,154 but this approach may be unreliable in the setting of antiangiogenic therapy because of the antipermeability properties of anti-VEGF therapeutics, which reduce water content in the brain. However, this hurdle can be addressed by using distribution analysis of the apparent diffusion coefficient signature155 or an alternative method known as “restriction spectrum imaging” that is also less sensitive to reductions in vasogenic edema and pseudoprogression.156 An overview of the advantages and limitations of various imaging modalities used for in vivo monitoring of antiangiogenic therapy response in patients with GBM is provided in Appendix Table A1 (online only). Collectively, these advanced imaging biomarkers may help shed light on how antiangiogenic therapy arrests tumor development in vivo and distinguish which patients' tumors are more likely to respond to antiangiogenic agents.
POTENTIAL MECHANISMS OF RESISTANCE
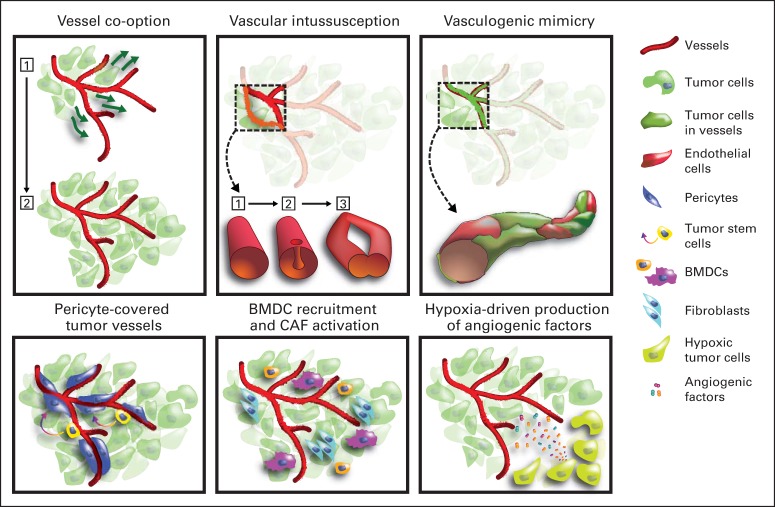
Despite improvements in PFS, patients with GBM treated with antiangiogenic therapy eventually develop tumor progression. Two main types of resistance to antiangiogenic therapy have been proposed: adaptive (evasive), in which the tumor acquires the ability to functionally evade the effects of angiogenic blockade, and inherent (intrinsic), which describes primary resistance to antiangiogenic therapy.157 Potential mechanisms of resistance include upregulation of alternative proangiogenic pathways, leading to revascularization; recruitment of bone marrow–derived proangiogenic cells, thereby precluding the need for VEGF signaling; increased fibrosis and pericyte coverage to provide stabilization to the vessels; and change to an invasive phenotype to co-opt host vasculature20,157 (Fig 1).
Fig 1.
Resistance to anti–vascular endothelial growth factor (VEGF)/anti–VEGF receptor (VEGFR) strategies in glioblastoma (GBM): (1) Angiogenesis is a critical process in GBM progression, which is accompanied by endothelial cell hyperproliferation and abnormal vascular structure and function. (2) VEGF is overexpressed in GBM and is a validated target for antiangiogenic therapy. (3) Anti-VEGF/anti-VEGFR therapy induces high rates of radiographic response and reduces vasogenic edema in GBM, but these benefits do not translate into increased overall survival in all patients. (4) An increase in survival will likely require patient stratification based on biomarkers, and promising circulating and imaging biomarkers have emerged from small phase II studies. (5) Identifying escape biomarkers may help in designing trials that combine antiangiogenic agents with agents targeting these evasion pathways. (6) These biomarkers should be prospectively tested in large clinical studies. Potential mechanisms of resistance to targeted VEGF therapy in cancer. Different mechanisms underlie the resistance to VEGF blockade seen in some patients with cancer. These mechanisms are not exclusive, and it is likely that several occur simultaneously in a single tumor: (1) vessel co-option: tumor cell migration and growth along the existing vasculature without generation of new vessels; (2) vascular intussusception: vascular network expansion through enlargement of existing vessels; (3) vasculogenic mimicry: incorporation of tumor cells into the endothelial lining of the vasculature, potentially via endothelial differentiation from putative tumor stem cells; (4) pericyte-covered vessels: persistence of more mature vessels characterized by coverage by pericytes of mesenchymal origin or differentiated from putative tumor stem cells; (5) bone marrow–derived cell (BMDCs) and cancer associated fibroblast (CAF) activation: paracrine support of tumor vascularization through increased recruitment of angiogenic BMDCs and CAFs; and (6) hypoxia-driven production of angiogenic factors: in established tumors, VEGF blockade aggravates hypoxia, which upregulates the production of other angiogenic factors. Figure courtesy of Giorgio Seano, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Clinical Evidence
A clinical study of cediranib in patients with rGBM observed that elevated levels of fibroblast growth factor correlated with tumor progression.32 But the relevance of this pathway in escape after anti-VEGF/anti-VEGFR treatment in patients with GBM is unknown. In addition to growth factors, chemokines and cytokines may be elevated after VEGF pathway inhibition. Among these, in line with preclinical evidence (Appendix), we have shown that elevated levels of SDF-1α correlated with tumor progression.32,69 In addition, an autopsy study in patients with rGBM treated with cediranib also demonstrated an increase in TAMs and CD11b+ myeloid cells in both the tumor bulk and infiltrative edge when compared with control autopsy specimens from patients who did not receive cediranib, suggesting that TAMs mediate resistance to antiangiogenic therapy.137 Moreover, studies in autopsy GBM specimens have begun to shed some light on the role of vascular co-option as an evasion mechanism. Tissue studies from patients with rGBM who were treated with cediranib demonstrated a change in growth pattern with persistent normalized vasculature, suggestive of increased infiltration rather than rebound revascularization from a second wave of angiogenesis, even after cessation of treatment.158 Radiographic and tissue studies in patients with GBM treated with bevacizumab or cediranib demonstrate (in a subset of patients) that there is a shift to a predominantly infiltrative phenotype as evidenced by an increase in T2-weighted hyperintensity on MRI with concurrent infiltrative growth on histology.159–161 Unfortunately, a phase II trial of cediranib and cilengitide (an anti-invasive agent) conducted in patients with rGBM had disappointing results.162 Possible explanations include ineffective targeting of invasion, excessive vascular pruning, or limited CNS penetration of cilengitide.
In conclusion, angiogenesis is a highly complex process consisting of redundant proangiogenic pathways that are both VEGF dependent and VEGF independent. Thus far, with the exception of the BELOB phase II study, the results of anti-VEGF/anti-VEGFR trials have been modest, with improvements in radiographic responses, tumor-associated brain edema, and PFS without an increase in OS.163 Decreased perfusion after excessive tumor vessel pruning could block the clearance of chemotherapuetic drugs, and this may enhance the efficacy of drugs that are more toxic under hypoxic and/or acidic conditions such as lomustine.180 Studies are now incorporating biomarkers as an end point in an effort to identify those patients who may respond to particular treatments. Circulating and imaging biomarkers have shown promising potential as biomarkers of response. In uncontrolled studies of anti-VEGF/anti-VEGFR agents, those patients in whom tumor perfusion increased survived longer. Further trials are warranted to validate this counterintuitive finding because it could represent an opportunity to define patients most likely to benefit from anti-VEGF/anti-VEGFR therapy. It is clear that complex acquired or intrinsic mechanisms might underlie the resumption of tumor growth and progression after the temporary delay induced by antiangiogenic therapies. Future studies should determine whether these phenomena are specific to anti-VEGF/anti-VEGFR versus other treatments or whether they reflect the natural history of GBM. Clearly, only a better understanding of how tumors escape from anti-VEGF therapy will allow the development of more effective strategies to improve patient outcomes.
Appendix
ANGIOGENESIS IN GLIOBLASTOMA
Mechanisms of Tumor Vessel Formation
Currently, six mechanisms of tumor vessel formation have been postulated: (1) vasculogenesis, (2) sprouting angiogenesis, (3) vessel co-option, (4) intussusception, (5) vascular mimicry, and (6) transdifferentiation of tumor cells into endothelial cells.20 Vasculogenesis occurs in the developing embryo when endothelial precursor cells (EPCs [angioblasts]) differentiate into endothelial cells and form a de novo vasculature (Coultas L, et al: Nature 438:937-945, 2005). Subsequent vessel sprouting from existing vessels (angiogenesis) expands the vascular network, which, in normal physiologic conditions, contributes to organ development, wound healing, and other specific processes such as placenta development (Carmeliet P: Nature 438:932-936, 2005). In pathologic conditions such as tumors, this angiogenic switch confers the malignant phenotype of unrestricted growth.20 There are three main steps involved in angiogenesis: quiescence, activation, and resolution.20 Normally, endothelial cells remain quiescent, covered by pericytes, which suppress endothelial cell proliferation and maintain cell survival. In response to a proangiogenic signal, the pericytes detach from the vessels, and endothelial cell tight junctions become loose, resulting in increased permeability. Extravasation of proteins creates a provisional extracellular matrix scaffold to which endothelial cells, led by a specific endothelial cell—the tip cell—migrate. Together with neighboring endothelial cells (the stalk cells), these endothelial cells migrate and elongate the vessel. Phalanx cells, the most quiescent of the endothelial cells, line the vessel as a smooth monolayer, re-establish tight junctions and full pericyte coverage, and render the vessel mature and functional. The role of vasculogenesis in tumors and in the origin of EPCs is not as well characterized.
In addition to growing by angiogenesis, glioblastomas (GBMs) can grow through vessel co-option by which tumor cells migrate along existing blood vessels, thereby compressing and destabilizing them (Holash J, et al: Science 284:1994-1998, 1999; Leenders WP, et al: Endothelium 9:83-87, 2002; De Spiegelaere W, et al: J Vasc Res 49:390-404, 2012). Vessel regression, decreased perfusion, cell death, and increased hypoxia ensue, triggering the secretion of proangiogenic factors (Carmeliet P: Nature 438:932-936, 2005).20,27 Intussusception is another mode of neovascularization that is not well understood but is thought to represent vessel formation through the split of pre-existing vessels into daughter vessels (De Spiegelaere W, et al: J Vasc Res 49:390-404, 2012; Kurz H, et al: News Physiol Sci 18:65-70, 2003).20 Vascular mimicry describes the formation of fluid-conducting channels lined by tumor cells. These “vessels” may or may not resemble true endothelial-lined blood vessels.39 Finally, stem-like GBM cells are able to transdifferentiate into endothelial cells, generating tumor-derived vessels, which may be less sensitive to anti-vascular endothelial growth factor (anti-VEGF) therapies.40,42,51 Of note, anti-VEGF therapy seems to promote a change in GBM neovascularization that is more consistent with brain vessel co-option than with abnormal angiogenesis.50
Molecular Mechanisms of Angiogenesis: Potential Targets for Therapy
Several proangiogenic molecules such as VEGF, hepatocyte growth factor/scatter factor, basic fibroblast growth factor (bFGF), and angiopoietin 2 (Ang-2) have been implicated in the angiogenic switch.27,67,82,95–97 VEGF and its tyrosine kinase receptors (VEGFRs) are the most extensively studied by virtue of being the targets of various antiangiogenic agents in GBM. Although VEGF binds with a higher affinity to VEGFR-1 (FLT-1), it is widely believed that the main driver of tumor neovascularization is the interaction between VEGF and VEGFR-2 (KDR). Binding of VEGF to VEGFR-2 results in dimerization of the receptor, activating a variety of different pathways, including the phosphatidylinositol 3′-kinase (PI3K)/AKT and Ras/mitogen-activated protein kinase (MAPK) pathways (Maity A, et al: Cancer Res 60:5879-5886, 2000; Pore N, et al: Cancer Res 63:236-241, 2003; Yoshino Y, et al: Int J Oncol 29:981-987, 2006).98
This in turn results in endothelial proliferation, migration, formation of vascular networks, and survival. Interactions of VEGF with VEGFRs can be modulated by coreceptors such as neuropilin 1 (Klagsbrun M, et al: Adv Exp Med Biol 515:33-48, 2002). Another pathway upregulated by VEGF is the Delta-like ligand 4 (DLL4)-Notch pathway, a pathway important in many biologic processes including angiogenesis (Li JL, et al: Front Biosci 14:3094-3110, 2009). Under physiologic conditions, DLL4/Notch signaling inhibits tip cell formation, resulting in decreased angiogenic sprouting (Hellström M, et al: Nature 445:776-780, 2007; Siekmann AF, et al: Nature 445:781-784, 2007). In tumors, DLL4/Notch signaling affects tumor growth and improves vascular function by stabilizing the vasculature and decreasing angiogenesis (Li JL, et al: Cancer Res 67:11244-11253, 2007). Paradoxically, blockade of this pathway results in decreased tumor growth by stimulation of abnormal (inefficient) angiogenesis (Noguera-Troise I, et al: Nature 444:1032-1037, 2006; Ridgway J, et al: Nature 444:1083-1087, 2006).
Several molecules either act in concert with VEGF to stimulate angiogenesis or upregulate VEGF itself. For instance, in the presence of VEGF, Ang-2 promotes the detachment of pericytes from the vasculature, resulting in vessel instability (Augustin HG, et al: Nat Rev Mol Cell Biol 10:165-177, 2009). Platelet-derived growth factor B (PDGF-B), epidermal growth factor, tumor necrosis factor α, and bFGF have the ability to upregulate VEGF expression in gliomas (Goldman CK, et al: Mol Biol Cell 4:121-133, 1993; Ryuto M, et al: J Biol Chem 271:28220-28228, 1996; Tsai JC, et al: J Neurosurg 82:864-873, 1995).99 Placental growth factor (PlGF) is a member of the VEGF family that binds to VEGFR-1 and neuropilin 1 and is thought to affect tumor angiogenesis directly by amplifying overall responsiveness to VEGF through a synergism between PlGF and VEGF (Maglione D, et al: Proc Natl Acad Sci U S A 88:9267-9271, 1991; Carmeliet P, et al: Nat Med 7:575-583, 2001) and indirectly by recruitment of type 2 (tumor-promoting) tumor-associated macrophages (TAMs; Loges S, et al: Clin Cancer Res 15:3648-3653, 2009). The relevance of this mechanism remains unclear because agents such as aflibercept (a dual VEGF/PlGF blocker) and VEGFR-1 tyrosine kinase inhibitors (TKIs) have yet to show efficacy in clinical trials.100 Chemokines, including interleukin-8 (IL-8) and stromal-derived factor 1α (SDF-1α)/CXC chemokine ligand 12 (CXCL12) are also implicated in angiogenesis. IL-8, which is highly expressed and secreted by gliomas, is known to have proangiogenic properties and has been implicated in the invasiveness of glioma cells (Raychaudhuri B, et al: J Neurooncol 101:227-235, 2011).48 SDF-1α, through its interactions with its receptors—CXC chemokine receptor 4 (CXCR4) and CXCR7—seems to promote tumor cell survival and invasion and facilitate angiogenesis by recruiting immunosuppressive and proangiogenic myeloid cells to support tumor growth and spread (Duda DG, et al: Clin Cancer Res 17:2074-2080, 2011).
In addition to local vasculature and various stromal cells (local stroma), solid tumors recruit bone marrow–derived cells (BMDCs) (distal stroma) to sustain their growth. BMDCs may include EPCs, pericyte progenitor cells, and vascular modulatory myeloid cells, such as TAMs, monocytes and neutrophils, VEGFR-1+ hemangiocytes, or Tie-2+–expressing monocytes (TEMs; Aghi M, et al: Mol Ther 12:994-1005, 2005; De Palma M, et al: Cancer Cell 8:211-226, 2005; Lin EY, et al: Cancer Res 67:5064-5066, 2007; Yang L, et al: Cancer Cell 6:409-421, 2004; Hattori K, et al: Nat Med 8:841-849, 2002).43,66 EPCs and pericyte progenitor cells are thought to incorporate into the vasculature as endothelial cells and pericytes or vascular smooth muscle cells, respectively, but this issue remains controversial. Vascular modulatory myeloid cells are not physically part of the vascular structure, but they seem to be recruited from circulation to promote neovascularization in tumor tissue. For example, it is believed that they serve as one of the main sources of matrix metalloproteinase 9 (MMP-9), a crucial component in neovascularization and the angiogenic switch (Du R, et al: Cancer Cell 13:206-220, 2008). Cytokines involved in chemoattraction of these cells to the tumor site include VEGF, granulocyte-macrophage colony-stimulating factor, Bv8, IL-17, and SDF-1α (Rempel SA, et al: Clin Cancer Res 6:102-111, 2000; Santarelli JG, et al: Neurosurgery 59:374-382, 2006; Kozin SV, et al: Cancer Res 70:5679-5685, 2010; Chung AS, et al: Nat Med 19:1114-1123, 2013; Shojaei F, et al: Proc Natl Acad Sci U S A 106:6742-6747, 2009; Shojaei F, et al: Nature 450:825-831, 2007; Pyonteck SM, et al: Nat Med 19:1264-1272, 2013). Recruitment of BMDCs intensifies with increased hypoxia in part through upregulation of SDF-1α by hypoxia-inducible factor 1α, a transcription factor critical for hypoxia-induced angiogenesis (Du R, et al: Cancer Cell 13:206-220, 2008; Giaccia AJ, et al: Genes Dev 18:2183-2194, 2004; Chen Y, et al: Hepatology 59:1435-1447, 2014).
To expand and create new vascular networks, tumors need to actively remodel their extracellular matrix to allow for endothelial migration during angiogenesis. Endothelial migration factors include proteinases such as MMPs, plasminogen-activator factor 1, cathepsin B1, and urokinase type plasminogen activator (Lakka SS, et al: J Biol Chem 280:21882-21892, 2005; Lakka SS, et al: Brain Pathol 15:327-341, 2005; Wang D, et al: Brain Pathol 15:318-326, 2005).101
In addition, MMPs are involved in recruitment of progenitor cells from the bone marrow through the release of various cytokines (Heissig B, et al: Cell 109:625-637, 2002). Integrins mediate the ability of vascular cells to adhere to the extracellular matrix proteins, providing local survival cues and a path for the invading endothelial cells.20 They influence the behavior of endothelial cells and pericytes by binding to growth factors and/or their receptors, upregulating proteases, regulating interactions between the structural components of vessel walls, and binding BMDCs to vascular endothelium.20 Integrins are also implicated in the activation of transforming growth factor beta (TGF-β), a key molecule that controls migration, invasion, angiogenesis, and maintenance of glioma-initiating cells in GBM (Anido J, et al: Cancer Cell 18:655-668, 2010; Peñuelas S, et al: Cancer Cell 15:315-327, 2009; Wick W, et al: Curr Pharm Des 12:341-349, 2006). Inhibition of the TGF-β pathway with a TGF-β receptor 1 inhibitor resulted in decreased neurosphere formation potential by decreasing expression of Id1 and Id3, transcription regulators involved in the self-renewal capacity of stem cells (Anido J, et al: Cancer Cell 18:655-668, 2010; Nam HS, et al: Cell Stem Cell 5:515-526, 2009). Blockade of the TGF-β pathway downregulated insulin-like growth factor-binding protein 7–dependent proangiogenic pathways in GBM-U87 cells (Pen A, et al: Oncogene 27:6834-6844, 2008). In addition, knockdown of TGF-β receptor 2 with short hairpin RNA diminished the invasiveness of glioma cells.102 Finally, the immunosuppressive effects of TGF-β have been effectively neutralized with TGF-β receptor 1 inhibitors, which resulted in increased tumor infiltration by natural killer cells, CD8 T cells, and TAMs with concurrent enhanced release of proinflammatory cytokines and improved median survival (Uhl M, et al: Cancer Res 64:7954-7961, 2004). Taken together, the preclinical data suggest that TGF-β targeted therapy is an attractive option in the treatment of malignant gliomas. Several clinical trials of TGF-β inhibitors have been conducted. Two studies evaluated trabedersen (AP-12009), a TGF-β–specific antisense oligodeoxynucleotide, in malignant gliomas (Hau P, et al: Oligonucleotides 17:201-212, 2007).103 Although neither study demonstrated a significant effect on median survival, there was evidence of increased response rates in patients with recurrent GBM (rGBM). Ongoing studies involve a TGF-β receptor kinase inhibitor (NCT01582269 [A Study in Recurrent Glioblastoma (GB)] and NCT01220271 [A Study Combining LY2157299 With Temozolomide-based Radiochemotherapy in Patients With Newly Diagnosed Malignant Glioma]) and a neutralizing antibody against TGF-β (NCT 01472731 [Safety and Imaging Study of GC1008 in Glioma]).
LESSONS FROM CLINICAL STUDIES OF ANTIANGIOGENIC AGENTS IN GLIOBLASTOMA
Antibodies
Bevacizumab.
Bevacizumab, a recombinant humanized monoclonal antibody against VEGF, has been studied extensively in patients with GBM (Table 1).8 A pilot retrospective study of 21 patients with malignant glioma (11 rGBM) had one patient with a complete response (CR), eight with partial responses (PRs), and 11 with stable disease (SD) after treatment with bevacizumab and irinotecan.171 Furthermore, this study demonstrated an acceptable toxicity profile associated with this regimen.
Several prospective phase II studies were subsequently conducted. In one phase II study, 35 patients with rGBM were treated with bevacizumab and irinotecan with a radiographic response rate of 57%.11 In another study of 32 patients with malignant gliomas (23 rGBM), a response rate of 60.9% was achieved in the rGBM subpopulation.104 Together, these two studies showed a 6-month progression-free survival (PFS6) rate of 30% to 46% and median overall survival (OS) of 9 to 10 months. These results demonstrated improvement over the historical radiographic response rates of 5% to 10%, PFS6 rates of 9% to 25%, and median OS of 5 to 6 months, respectively, in patients with rGBM after salvage therapy.2–4
Two additional phase II prospective studies eventually resulted in the accelerated approval of bevacizumab for patients with rGBM. The first study was a single-arm phase II trial of single-agent bevacizumab in 48 patients with rGBM.10 Study results showed a radiographic response rate of 35%, PFS6 rate of 29%, and median OS of 7.2 months. The second was the multicenter BRAIN study (A Study to Evaluate Bevacizumab Alone or in Combination With Irinotecan for Treatment of Glioblastoma Multiforme [BRAIN]), in which 167 patients with rGBM were randomly assigned to either bevacizumab combined with irinotecan (n = 85) or bevacizumab monotherapy (n = 82).9 Radiographic response rates were 37.8% and 28.2% for the combination group and monotherapy arm, respectively. The primary end point was PFS6, which compared favorably with historical controls and was comparable between the groups at 50.3% and 42.6%. Of note, this study was not originally designed to detect superiority between the two arms, and patients were allowed to cross over to the combination arm on progression during bevacizumab monotherapy. In both studies, the toxicity profiles were similar and consistent with previously reported studies.9,10,104 Several other retrospective studies of bevacizumab and irinotecan reported largely similar data.41,167–169
Subsequent studies have evaluated bevacizumab in combination with other chemotherapies or with different dosing schedules. Four phase II studies evaluated bevacizumab with other chemotherapeutic agents such as irinotecan combined with carboplatin, etoposide, or temozolomide.44–47 No additional benefit was conferred with the addition of metronomic etoposide to bevacizumab when compared with bevacizumab alone or bevacizumab combined with irinotecan in bevacizumab-naive patients with rGBM, but toxicity was increased.46 Similarly, the addition of etoposide or temozolomide to bevacizumab in patients with rGBM who progressed on prior bevacizumab therapy was also ineffective.44 The combination of bevacizumab with carboplatin and irinotecan resulted only in increased toxicity without any additional antitumor effect when compared with bevacizumab alone.45 More recently, the BELOB study reported promising results with bevacizumab combined with lomustine.49 Improved OS at 9 months (59% v 43% v 38%) and PFS6 (41% v 13% v 16%) were seen with the combination arm compared with single-agent lomustine and single-agent bevacizumab, respectively. Several studies have tested various schedules of bevacizumab dosing. Thus far, doses of 5 mg/kg or 10 mg/kg once every 2 weeks or 15 mg/kg once every 3 weeks have been reported (Kozin SV, et al: Cancer Res 70:5679-5685, 2010; Bergers G, et al: J Clin Invest 111:1287-1295, 2003).39,62 Dose adjustments are made on the basis of toxicity and duration of therapy, but the optimal dose remains unclear.
Bevacizumab has also been used in conjunction with other biologic drugs. In one study, 43 patients with rGBM were treated with bevacizumab, irinotecan, and cetuximab, an antibody against epidermal growth factor receptor (EGFR).53 Of the 32 evaluable patients, 34% had a radiographic response with a PFS6 of 30% and median OS of 6.7 months. Although the combination was generally well tolerated, it was not deemed superior to bevacizumab monotherapy. Another phase II study used erlotinib, an EGFR TKI, with bevacizumab in 57 patients with malignant glioma, 25 of whom had rGBM.54 Once again, although the regimen was generally well tolerated, response rates and PFS6 were similar to those of historical bevacizumab-containing regimens.
Although the majority of studies of bevacizumab in GBM have been conducted in patients with rGBM, a growing number of clinical trials in patients with newly diagnosed GBM (nGBM) have evaluated bevacizumab in combination with standard radiation and temozolomide55,56 (Table 1). Results from two such studies were similar with a PFS6 of 85% to 88% and median OS of 20 to 23 months. In a third study, 75 patients with nGBM were treated with bevacizumab, temozolomide, and radiation followed by postradiation temozolomide, bevacizumab, and irinotecan.57 Results showed moderate toxicity and a median OS of 21.2 months, suggestive of possible benefit over that of standard chemoradiotherapy.1
These nonrandomized studies were followed by two phase III trials of bevacizumab or placebo in combination with radiation and temozolomide in patients with nGBM.12,13,58 The results from the phase III AVAglio study (A Study of Avastin [Bevacizumab] in Combination With Temozolomide and Radiotherapy in Patients With Newly Diagnosed Glioblastoma) showed a significant improvement in PFS with the addition of bevacizumab to radiotherapy and temozolomide chemotherapy versus chemoradiotherapy alone (hazard ratio, 0.64; P < .001), but median OS was not significantly improved (16.8 v 16.7 months). A similar phase III trial (RTOG 0825) was conducted by the Radiation Therapy Oncology Group (RTOG), North Central Cancer Treatment Group, and Eastern Cooperative Oncology Group to test bevacizumab with standard chemoradiotherapy versus chemoradiotherapy alone for nGBM. Once again, the addition of bevacizumab to chemoradiotherapy improved PFS (hazard ratio, 0.79; P = .007) but did not meet the prespecified threshold of a 30% reduction in the hazard of failure. In addition, there was no significant difference in median OS (16.1 v 15.7 months).
Despite these disappointing data, more than 50 trials of bevacizumab are still ongoing in patients with nGBM and rGBM. Some of these trials are testing bevacizumab in combination with other agents in an attempt to avoid resistance to anti-VEGF therapy (Table 1). These include a phase II study (NCT01339039 [Plerixafor (AMD3100) and Bevacizumab for Recurrent High-Grade Glioma]) of bevacizumab with plerixafor (a CXCR4 inhibitor approved for liquid malignancies).
Aflibercept.
Aflibercept (or VEGF Trap) is a chimeric soluble decoy receptor for VEGF, VEGF-B, and PlGF, with a higher affinity for VEGF than bevacizumab.59,60 On the basis of promising efficacy data in orthotopic GBM mouse models, a phase I study in advanced solid tumors (including GBM) was conducted.61,100 Dose-limiting toxicities were rectal ulcerations and proteinuria and mechanistic toxicities included dysphonia, hypertension, and proteinuria.100 Three of the 47 patients (none with glioma) demonstrated a PR suggestive of an antitumor effect. A phase II study in patients with malignant glioma (42 rGBM) reported a response rate of 18% and PFS6 of 7.7% in patients with rGBM.62 Moreover, 14% of the patients with GBM had to discontinue the drug secondary to toxicity. A phase I trial for patients with nGBM that evaluated the maximum-tolerated dose for the drug when given in conjunction with chemoradiotherapy (NCT00650923 [Aflibercept, Radiation Therapy, and Temozolomide in Treating Patients With Newly Diagnosed or Recurrent Glioblastoma Multiforme, Gliosarcoma, or Other Malignant Glioma]) recently completed accrual (Table 1).
Olaratumab (IMC-3G3) and ramucirumab (IMC-1121B).
Olaratumab is a human immunoglobulin G monoclonal antibody against PDGF receptor α (PDGFR-α).63 Preliminary data from a phase I study in patients with advanced solid tumors (none with glioma) suggested that this agent is well tolerated.64 Ramucirumab is a human monoclonal antibody that specifically blocks the interaction of VEGFR-2 with its ligands.65 A phase II study of these two antibodies has been completed in patients with rGBM, and the results are pending (NCT00895180 [Ramucirumab or Anti-PDGFR Alpha Monoclonal Antibody IMC-3G3 in Treating Patients With Recurrent Glioblastoma Multiforme]; Table 1).
VEGFR TKIs
Cediranib.
Cediranib (AZD2171) is a relatively selective pan-VEGFR TKI, with additional activity against PDGF receptor β (PDGFR-β) and c-KIT.68 A phase II study of cediranib monotherapy (45 mg per day) in 31 patients with rGBM reported a radiographic response rate of 27% and PFS6 of 25.8%.69 In addition, the agent reduced or eliminated steroid requirements in these patients. Mechanistically, cediranib rapidly normalizes the vasculature by decreasing microvessel diameter and permeability thereby reducing edema.32 Associated grade 3 or 4 drug toxicities included hypertension, diarrhea, and fatigue.69
These encouraging results prompted a randomized phase III study comparing cediranib monotherapy (30 mg per day), cediranib (20 mg per day) combined with lomustine, and lomustine monotherapy in patients with rGBM.14 There was no significant difference in median PFS between the cediranib monotherapy arm (92 days) and cediranib combined with lomustine arm (125 days) when compared with lomustine monotherapy (82 days).14 It is unclear whether the lower dose of cediranib used in this trial, the interaction between cediranib and lomustine, or the steep dose reduction in lomustine over the course of the trial played any role in this lack of benefit. We recently reported on a phase I/II trial of cediranib with standard chemoradiotherapy in patients with nGBM (NCT00662506 [Cediranib, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma]). The median PFS was 15.6 months and median OS was 20.2 months for all 46 study patients. These results in the population of patients with nGBM who were undergoing biopsy only or subtotal resection compared favorably with the results from historical controls treated with radiation and temozolomide alone or with bevacizumab.1,13 However, as observed in other trials of antiangiogenic therapies in GBM, the beneficial clinical impact is primarily on PFS, and disease progression is typically rapid after conventional radiographic progression, which would account for the lack of OS improvement.13,55,70 Currently, there are ongoing trials evaluating the efficacy of cediranib alone or in combination with other therapies in both rGBM and nGBM, including a randomized, placebo-controlled phase II trial of cediranib in combination with chemoradiotherapy in patients with nGBM (NCT01062425 [Temozolomide and Radiation Therapy With or Without Cediranib Maleate in Treating Patients With Newly Diagnosed Glioblastoma]).
Vatalanib.
Similar to cediranib, vatalanib (PTK787) is a pan-VEGFR, c-KIT, and PDGFR TKI that showed promising antitumor activity in preclinical models.71 A phase I/II study in patients with rGBM of vatalanib combined with either temozolomide or lomustine showed radiographic response of 8% with temozolomide and 4% with lomustine.72 Median time to progression was 15.7 weeks in the vatalanib plus temozolomide arm and 10.4 weeks in the vatalanib plus lomustine arm.
Two studies have been performed in the nGBM population. A phase I trial of vatalanib with radiation, temozolomide, and an enzyme-inducing antiepileptic drug in 19 patients reported a radiographic response rate of 15% with a median PFS of 7.2 months and median OS of 16.2 months.73 The drug was well tolerated with dose-limiting toxicities of thrombocytopenia and transaminitis. The European Organisation for Research and Treatment of Cancer phase I/II trial of vatalanib with standard concomitant and adjuvant therapy also showed that vatalanib in combination with radiation and temozolomide was safe and feasible.74 Median PFS was 6.8 months and median OS was 17 months. However, the development of this agent was halted because of an industry decision.
Pazopanib.
A phase II study of pazopanib, another pan-VEGFR, c-KIT, and PDGFR-α and PDGFR-β TKI, by the North American Brain Tumor Consortium in patients with rGBM demonstrated a radiographic response rate of 5.9%, PFS6 of 3 months, and median OS of 8.1 months.75 Toxicities were consistent with those associated with other anti-VEGF agents and included fatigue, leukopenia, lymphopenia, transaminitis, hemorrhage in the CNS, and thromboembolic events. A phase II trial of pazopanib with topotecan for rGBM is ongoing (NCT01931098 [Phase II Pazopanib Plus Topotecan for Recurrent Glioblastoma Multiforme (GBM)]).
Cabozantinib (XL-184).
Cabozantinib, a VEGFR-2 and MET TKI, was recently evaluated in a phase II study of 105 patients with rGBM.76 This drug is of particular interest because it may target both angiogenesis and invasion. Forty-six patients were treated at 175 mg per day, and 59 patients were treated at 125 mg per day.76 Response rates were comparable at 21% and 30%, respectively, in antiangiogenic-naive patients. There was less toxicity at the lower dose without significantly compromising efficacy. Finally, there was a suggestion of modest activity of the drug in patients treated with prior antiangiogenic therapy. Active trials of cabozantinib include phase II trials in rGBM and grade 4 astrocytic tumors (NCT00704288 [Study of XL184 (Cabozantinib) in Adults With Glioblastoma Multiforme] and NCT01068782 [Study of Multiple Doses and Regimens of XL184 (Cabozantinib) in Subjects With Grade IV Astrocytic Tumors in First or Second Relapse]) and a phase I trial in nGBM with chemoradiotherapy (NCT00960492 [Safety Study of XL184 (Cabozantinib) in Combination With Temozolomide and Radiation Therapy in the Initial Treatment of Adults With Glioblastoma]).
Sunitinib.
Sunitinib is a multitargeted TKI with activity against VEGFRs, PDGFR-α and PDGFR-β, c-KIT, and FLT-3. Two phase II studies of the drug in patients with recurrent malignant glioma did not show any objective radiographic responses.77,78 Authors of both studies concluded that sunitinib did not demonstrate significant activity in this setting. Similarly, a phase I study of sunitinib with irinotecan showed that the combination was associated with moderate toxicity but limited antitumor activity.79
Sorafenib.
Sorafenib is another multitargeted TKI with a profile similar to that of sunitinib but with additional activity against RAF kinases. The North American Brain Tumor Consortium phase I/II study of sorafenib with temsirolimus, an inhibitor of mammalian target of rapamycin (mTOR), in patients with rGBM reported that 12% of patients had a partial radiographic response but no patients remained progression free at 6 months, and median PFS was 8 weeks.80 The NABTT 0502 (Erlotinib and Sorafenib in Treating Patients With Progressive or Recurrent Glioblastoma Multiforme) phase II study evaluated sorafenib and erlotinib in patients with rGBM.81 Study data showed a median OS of 5.7 months with a PFS6 of 14%, which failed to meet the prespecified objective of a 30% increase in OS compared with historical controls. A third phase II study of 32 patients with rGBM treated with sorafenib and daily temozolomide reported a PFS6 of 9.4% with only one patient achieving a PR, suggesting limited activity of this regimen.178 Finally, a phase II study of adjuvant sorafenib and temozolomide in patients with nGBM reported a median OS of 12 months with a median PFS of 6 months.179 This suggested that the addition of sorafenib did not improve treatment efficacy when compared with standard therapy.
Vandetanib.
Vandetanib is a dual TKI of VEGFR-2 and EGFR. The latter is frequently amplified in GBMs. Four of 32 patients demonstrated a radiographic response after vandetanib in a phase II study in rGBM; PFS6 was 6.5% and median OS was 6.3 months.177 Interestingly, the study reported seizures as an unexpected toxicity of the drug. At this time, an open-label phase I study of vandetanib and sirolimus (mTOR inhibitor) is recruiting patients with rGBM (NCT00821080 [Vandetanib and Sirolimus in Patients With Recurrent Glioblastoma]). A phase I study of vandetanib in patients with nGBM established that the agent could be safely combined with radiation and temozolomide.105 This led to a randomized phase II study of standard chemoradiotherapy with or without vandetanib in patients with nGBM or gliosarcoma (NCT00441142 [Zactima With Temodar During Radiation Treatment for Newly Diagnosed Stage IV Brain Tumors]). The study was terminated early for futility based on the results of an unplanned interim analysis. Median OS and PFS were 15.9 and 6.2 months, respectively, in the control treatment arm and 16.6 and 7.7 months, respectively, in the combination arm.89 Study results suggested that addition of vandetanib to standard chemoradiotherapy was reasonably well tolerated but lacked efficacy in nGBM.
Other TKIs
AEE788 is another dual VEGFR and EGFR TKI. A phase I dose-escalation study of AEE788 in rGBM enrolled 64 patients; the best overall response was SD in 17% of the patients.106 Because the drug was associated with unacceptable toxicity and minimal antitumor activity, the study was discontinued prematurely. Lenvatinib (E7080) is a multitargeted TKI of VEGFR-2 and VEGFR-3, fibroblast growth factor receptor 1, c-KIT, and PDGFR-β.107 A phase Ib/II study of this agent in patients with rGBM is ongoing (NCT01433991 [E7050 in Combination With E7080 in Subjects With Advanced Solid Tumors (Dose Escalation) and in Subjects With Recurrent Glioblastoma or Unresectable Stage III or Stage IV Melanoma After Prior Systemic Therapy (Expansion Cohort and Phase 2)]). Tivozanib, a more selective pan-VEGFR TKI is also being evaluated in a phase II trial in patients with rGBM (NCT01846871 [Tivozanib for Recurrent Glioblastoma]).
Enzastaurin was developed as an ATP-competitive inhibitor of protein kinase C beta. At concentrations reached in the plasma of patients in clinical trials (1 to 4 μmol/L), enzastaurin also suppresses signaling through the PI3K/AKT/mTOR/p70S6K pathway.108 The protein kinase C beta and PI3K/AKT pathways have previously been implicated in tumor angiogenesis through promotion of VEGF expression.109,110 An open-label phase III study compared the efficacy and safety of enzastaurin versus lomustine in patients with rGBM.15 Enrollment was terminated at 266 patients (enzastaurin, n = 174; lomustine, n = 92) after a planned interim analysis for futility. Median PFS (1.5 v 1.6 months) and OS (6.6 v 7.1 months) did not significantly differ between enzastaurin and lomustine, respectively. SD rates were 38.5% and 35.9%, and objective response rates were 2.9% and 4.3%, respectively. The study concluded that enzastaurin, although it was well tolerated, did not show superior efficacy compared with lomustine in rGBM.
Other Antiangiogenic Agents
Thalidomide and lenalidomide may have antiangiogenic properties via inhibition of VEGF, integrins, and bFGF expression. Several studies have evaluated their effects in combination with various cytotoxic chemotherapeutics in both nGBM and rGBM.111–118 Unfortunately, the outcomes of these studies suggested that their efficacy against GBM is limited, especially in light of the advent of chemoradiotherapy with temozolomide in nGBM. Rofecoxib, a cyclooxygenase 2, bFGF, and VEGF inhibitor, was studied in combination with various doses of temozolomide in patients with nGBM treated with surgery and radiotherapy.119 PFS and OS were 8 months and 16 months, respectively. These outcomes were comparable to those seen after standard chemoradiotherapy.1 ABT-510 is a thrombospondin 1 (ie, an endogenous inhibitor of angiogenesis) mimetic.120 ABT-510 was tested with standard chemoradiotherapy in a study in 23 patients with nGBM. There was no significant improvement in survival with an OS of 64.4 weeks when compared with standard therapy.
BIOMARKERS OF RESPONSE TO ANTIANGIOGENIC AGENTS IN GBM
Conventional imaging with magnetic resonance imaging (MRI) is currently the preferred modality of choice in brain tumors. Computed tomography (CT) can no longer be accepted as a sufficient imaging standard for monitoring of tumor status and response to treatment. Its use in GBM is limited to emergency situations or in aiding surgical interventions. At some institutions, CT is also used to detect or exclude the existence of bevacizumab-induced intracranial hemorrhage or calcifications.121,122
The traditional method for assessing radiographic response to treatment in cancers is using RECIST criteria.123 In high-grade gliomas, the most often used are the Macdonald criteria, which provide an objective measure of tumor response based on the product of the maximal cross-sectional diameters of the contrast-enhanced tumor margins from a disrupted blood-brain barrier.124 Developed in the late 1980s, the Macdonald criteria were originally designed for CT images, but the method is now best performed using cocalled T1-weighted MRI. The criteria consist of a set of rules based on radiologic findings that define whether a tumor has completely or partially responded, stabilized, or progressed during therapy. A CR requires disappearance of all contrast-enhanced tumor for a minimum of 4 weeks, including no corticosteroid use and a stable or improved clinical status. A PR requires a ≥ 50% reduction in contrast-enhanced tumor size compared with baseline for at least 4 weeks, no new lesions, and improved clinical status. Progressive disease requires a 35% increase in the sum of the products of perpendicular diameters of enhanced lesions, the appearance of new lesions, or clinical deterioration. An SD response is appropriate for patients who do not qualify for CR, PR, or progressive disease. In addition, to best compare response rates between clinical trials, the Macdonald report proposed a set of guidelines for patient selection based on neurologic status and steroid use.
The original Macdonald criteria have several limitations.125,126 These relate to operator variability, multifocal tumors, surgical cavities and recurrence, irregular tumor shapes, and nonspecific changes in contrast enhancement, all of which have severely limited the usefulness of the method.127–129 In particular, the level and appearance of tumor enhancement are determined by the image acquisition technique and type of contrast agent used, as well as surgery and changes in steroid dosage. Furthermore, postsurgical and therapy changes may induce non–tumor-related changes in enhancement, of which the concept of pseudoprogression versus true tumor progression after radiotherapy has received much attention.130,131 Up to 30% of patients show signs of increased tumor enhancement from irradiation-damaged vasculature after radiotherapy that can be mistaken for real tumor progression. Contrast enhancement from pseudoprogression, however, typically subsides without intervention within a few weeks or months of radiation.
The Response Assessment in Neuro-Oncology Working Group proposed updated standards for imaging definitions and, most importantly, revised the response criteria by including measures of nonenhanced lesions and vasogenic edema estimated by hyperintensities on T2-weighted and fluid-attenuated inversion recovery MRI scans. CR and PR are defined as stable or reduced nonenhanced lesions (on the same or lower dose of corticosteroids) compared with baseline scans. The simplicity of the Macdonald criteria combined with the added value of the updated response assessment by the Response Assessment in Neuro-Oncology Working Group have made this radiographic method widely accepted by the oncologic community; therefore, the Macdonald criteria are used extensively to report radiographic response in clinical trials (Table 2).
A completely different approach to functional imaging of GBMs is use of the radionuclide positron emission tomography (PET) technique, which provides highly sensitive molecular information on metabolic status and ligand interactions from radiolabeled tracers.132,133 Imaging studies using 2-[18F]-fluoro-2-deoxy-d-glucose—a direct measure of the glucose metabolic activity of tumor cells—have been largely inconclusive in bevacizumab-treated GBMs.10,46 However, other studies using [18F]fluorothymidine PET, or O-(2-[18F]-fluoroethyl)-l-tyrosine have shown that PET is indeed sensitive to cell proliferation and is therefore predictive of response and OS after therapy with bevacizumab plus irinotecan.134,135,196 Furthermore, several studies indicate that PET may predict survival and detect treatment failure earlier than MRI.191,194,196
Potential Mechanisms of Resistance
Several of these mechanisms play a role in tumor recurrence after anti-VEGF therapy, whether targeting VEGF or its receptors. Alternative proangiogenic pathways may allow maintenance of a functional tumor vascularization (Lucio-Eterovic AK, et al: Clin Cancer Res 15:4589-4599, 2009; Relf M, et al: Cancer Res 57:963-969, 1997). For instance, mouse xenograft studies demonstrate that bFGF promotes tumor growth and angiogenesis even after loss of VEGF (Yoshiji H, et al: Cancer Res 57:3924-3928, 1997). Other preclinical studies of islet pancreatic tumors reported an upregulation of members of the FGF family as a means of evading VEGF blockade and promoting tumor growth (Casanovas O, et al: Cancer Cell 8:299-309, 2005). In addition, activation of the chemokine SDF-1α pathway has been implicated in promotion of cancer cell survival, invasion, and stem/tumor initiation cell phenotype; promotion of angiogenesis; and recruitment of myeloid BMDCs to indirectly facilitate tumor growth and metastasis (Duda DG, et al: Clin Cancer Res 17:2074-2080, 2011). Recruitment of BMDCs, especially TAMs, has emerged as a potential key mediator of tumor growth and progression through antiangiogenic therapy. Subpopulations of TAMs, traditionally classified as M1 (classically activated) and M2 (alternatively activated), are implicated in multiple functions such as inflammation, immune regulation, angiogenesis, metastasis, intravasation, and invasion (Qian BZ, et al: Cell 141:39-51, 2010). A subset of these TAMs is the TEMs. These monocytes express the cognate angiopoietin receptor TIE2, which is typically expressed by endothelial cells. Activation of the Ang-2/TIE2 pathway results in vascular regrowth following treatment-induced vascular damage (Kozin SV, et al: Cancer Res 70:5679-5685, 2010). It is thought that TEMs perform their proangiogenic function by effecting tumor angiogenesis downstream of the VEGF activation pathway. Several preclinical murine studies have supported the role of TAMs and TEMs in tumor progression after antiangiogenic therapy. In vivo experiments with mice have shown that TAMs promote gliomagenesis and that tumor infiltration by CD11b+Gr1+ myeloid cells was associated with resistance to anti-VEGF therapy (Pyonteck SM, et al: Nat Med 19:1264-1272, 2013; Shojaei F, et al: Nat Biotechnol 25:911-920, 2007; Piao Y, et al: Neuro Oncol 14:1379-1392, 2012). In another preclinical study, blockade of Ang-2 disabled proangiogenic functions of TEMs, thereby preventing tumor growth in murine models that traditionally develop resistance to antiangiogenic therapy (Mazzieri R, et al: Cancer Cell 19:512-526, 2011).
Increased pericyte coverage of tumor vessels and increased vessel invasion and co-option are other postulated tumor escape mechanisms (Fig 1). Anti-VEGF therapy may selectively cause the regression of immature and/or abnormal vessels such that the remaining vessels may be more mature and tightly covered by pericytes (Bergers G, et al: J Clin Invest 111:1287-1295, 2003).43 Pericytes secrete factors that support endothelial cell survival and can attenuate the proliferation rate of endothelial cells, important for vessel maturation and stabilization (Darland DC, et al: Dev Biol 264:275-288, 2003; Hirschi KK, et al: Cardiovasc Res 32:687-698, 1996).43 By maintaining quiescence, it is conceivable that the pericytes render the vasculature less susceptible to the effects of anti-VEGF therapy, but this hypothesis remains to be confirmed clinically.
Vessel co-option with subsequent tumor invasion was first documented as a mechanism of escape from the inhibitory effects of anti-VEGF therapy in orthotopic mouse GBM models that were genetically deficient in hypoxia-inducible factor 1α and VEGF or in which VEGF was pharmacologically inhibited (Blouw B, et al: Oncogene 26:4531-4540, 2007; Rubenstein JL, et al: Neoplasia 2:306-314, 2000). Despite blockade of neovascularization, the tumors were still able to grow by invading along the existing brain vasculature, resulting in a more invasive phenotype and vascular sufficiency. Factors that have been proposed as mediators of the increased invasiveness after anti-VEGF therapy include MMP-2, MMP-9, MMP-12, secreted protein acidic and rich in cysteine, and tissue inhibitor of metalloproteinases (TIMPs; Lucio-Eterovic AK, et al: Clin Cancer Res 15:4589-4599, 2009). The relevance of vessel co-option remains unclear and difficult to explore given the lack of access to tumor tissue during anti-VEGF therapy.
Table A1.
Comparison of Imaging Modalities for Human Glioblastoma Studies
| Imaging Modality | Sensitivity | Spatial Resolution | Temporal Resolution | Speed of Examination | Radiation Hazard | Complexity* |
|---|---|---|---|---|---|---|
| CT | Low, unfavorable | Intermediate, acceptable | High, favorable | High, favorable | Intermediate, acceptable | Low, favorable |
| MRI | Intermediate, acceptable | High, favorable | Intermediate, acceptable | Intermediate, acceptable | Low, favorable | Intermediate, acceptable |
| PET | High, favorable | Low, unfavorable | Low, unfavorable | Low, unfavorable | High, unfavorable | Intermediate, acceptable |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Sum of image acquisition and image analysis.
Footnotes
Supported by Grants No. R01CA129371 and K24CA125440A (T.T.B.), P01CA080124 and R01CA163815 (R.K.J.), and R01CA159258 (D.G.D.) from the National Institutes of Health (NIH); the Proton Beam/Federal Share Program (R.K.J and D.G.D.); the National Foundation for Cancer Research (R.K.J.); funding from Merck (E.R.G.); Grant No. 191088/V50 from the Norwegian Research Council; Grant No. 2013069 from the South-Eastern Norway Regional Health Authority (K.E.E.); NIH Award No. 8UL1TR000170-05 from the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences), and by Harvard University and its affiliated academic health care centers.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Rakesh K. Jain, XTuit Pharmaceuticals (C), H&Q Healthcare Investors (C), H&Q Life Sciences Investors (C), Tekla Healthcare Opportunities Fund (C) Consultant or Advisory Role: Tracy T. Batchelor, Merck (C), Roche (C), Kyowa Hakko Kirin Pharma (C), Spectrum Pharmaceuticals (C), Amgen (C), Novartis (C), Champions Biotechnology (C), Advance Medical (C); Rakesh K. Jain, Enlight Biosciences (C), Ophthotech (C), SynDevRx (C) Stock Ownership: Rakesh K. Jain, Enlight Biosciences, SynDevRx, XTuit Pharmaceuticals, Ophthotech Honoraria: None Research Funding: Tracy T. Batchelor, Pfizer, AstraZeneca, Millennium Pharmaceuticals; Rakesh K. Jain, MedImmune, Roche Expert Testimony: None Patents, Royalties, and Licenses: Kyrre E. Emblem, US8233965, US8428327, US8718747 Other Remuneration: None
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 5.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 9.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 10.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 12.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31:3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad C, Friedman H, Reardon D, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol. 2004;22(suppl):15s. abstr 1512. [Google Scholar]
- 19.Aquino D, Di Stefano AL, Scotti A, et al. Parametric response maps of perfusion MRI may identify recurrent glioblastomas responsive to bevacizumab and irinotecan. PLoS One. 2014;9:e90535. doi: 10.1371/journal.pone.0090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rege TA, Fears CY, Gladson CL. Endogenous inhibitors of angiogenesis in malignant gliomas: Nature's antiangiogenic therapy. Neuro Oncol. 2005;7:106–121. doi: 10.1215/S115285170400119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 24.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 25.Plate KH, Mennel HD. Vascular morphology and angiogenesis in glial tumors. Exp Toxicol Pathol. 1995;47:89–94. doi: 10.1016/S0940-2993(11)80292-7. [DOI] [PubMed] [Google Scholar]
- 26.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 28.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 30.Tong RT, Boucher Y, Kozin SV, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 31.Yuan F, Chen Y, Dellian M, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Catana C, Ratai EM, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71:3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 41.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 42.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song S, Ewald AJ, Stallcup W, et al. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reardon DA, Desjardins A, Peters K, et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2011;103:371–379. doi: 10.1007/s11060-010-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J Neurooncol. 2012;107:155–164. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br J Cancer. 2009;101:1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desjardins A, Reardon DA, Coan A, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118:1302–1312. doi: 10.1002/cncr.26381. [DOI] [PubMed] [Google Scholar]
- 48.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 50.Lorgis V, Maura G, Coppa G, et al. Relation between bevacizumab dose intensity and high-grade glioma survival: A retrospective study in two large cohorts. J Neurooncol. 2012;107:351–358. doi: 10.1007/s11060-011-0748-5. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumor endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 52.Raizer JJ, Grimm S, Chamberlain MC, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116:5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 53.Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro Oncol. 2010;12:508–516. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12:1300–1310. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayana A, Gruber D, Kunnakkat S, et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg. 2012;116:341–345. doi: 10.3171/2011.9.JNS11656. [DOI] [PubMed] [Google Scholar]
- 57.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wick W, Cloughesy TF, Nishikawa R, et al. Tumor response based on adapted Macdonald criteria and assessment of pseudoprogression (PsPD) in the phase III AVAglio trial of bevacizumab (Bv) plus temozolomide (T) plus radiotherapy (RT) in newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(suppl 15s):114s. abstr 2002. [Google Scholar]
- 59.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF Trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer. 2004;4:S81–S85. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- 61.Gomez-Manzano C, Holash J, Fueyo J, et al. VEGF trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–945. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Groot JF, Lamborn KR, Chang SM, et al. Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium study. J Clin Oncol. 2011;29:2689–2695. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah GD, Loizos N, Youssoufian H, et al. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116:1018–1026. doi: 10.1002/cncr.24788. [DOI] [PubMed] [Google Scholar]
- 64.Youssoufian H, Amato RJ, Sweeney CJ, et al. Phase 1 study of IMC-3G3, an IgG1 monoclonal antibody targeting PDGFR {alpha} in patients with advanced solid malignancies. J Clin Oncol. 2008;26(suppl):636s. abstr 14617. [Google Scholar]
- 65.Spratlin J. Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2. Curr Oncol Rep. 2011;13:97–102. doi: 10.1007/s11912-010-0149-5. [DOI] [PubMed] [Google Scholar]
- 66.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–181. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7:436–451. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 69.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11:550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldbrunner RH, Bendszus M, Wood J, et al. PTK787/ZK222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases, decreases glioma growth and vascularization. Neurosurgery. 2004;55:426–432. doi: 10.1227/01.neu.0000129551.64651.74. [DOI] [PubMed] [Google Scholar]
- 72.Reardon DA, Egorin MJ, Desjardins A, et al. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115:2188–2198. doi: 10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerstner ER, Eichler AF, Plotkin SR, et al. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J Neurooncol. 2010;103:325–332. doi: 10.1007/s11060-010-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandes AA, Stupp R, Hau P, et al. EORTC study 26041-22041: Phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2010;46:348–354. doi: 10.1016/j.ejca.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 75.Iwamoto FM, Lamborn KR, Robins HI, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02) Neuro Oncol. 2010;12:855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kreisl TN, Smith P, Sul J, et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111:41–48. doi: 10.1007/s11060-012-0988-z. [DOI] [PubMed] [Google Scholar]
- 77.Neyns B, Sadones J, Chaskis C, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103:491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 78.Pan E, Yu D, Yue B, et al. A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J Neurooncol. 2012;110:111–118. doi: 10.1007/s11060-012-0943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reardon DA, Vredenburgh JJ, Coan A, et al. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105:621–627. doi: 10.1007/s11060-011-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol. 2012;14:1511–1518. doi: 10.1093/neuonc/nos264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peereboom DM, Ahluwalia MS, Ye X, et al. NABTT 0502: A phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2013;15:490–496. doi: 10.1093/neuonc/nos322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–317. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerald D, Chintharlapalli S, Augustin HG, et al. Angiopoietin-2: An attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73:1649–1657. doi: 10.1158/0008-5472.CAN-12-4697. [DOI] [PubMed] [Google Scholar]
- 84.Chae SS, Kamoun WS, Farrar CT, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16:3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashizume H, Falcón BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sulman EP, Won M, Blumenthal DT, et al. Molecular predictors of outcome and response to bevacizumab (BEV) based on analysis of RTOG 0825, a phase III trial comparing chemoradiation (CRT) with and without BEV in patients with newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(suppl 15s):116s. abstr LBA2010. [Google Scholar]
- 87.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DePrimo S, Wu B, Huang S, et al. Correlative tumor molecular profiling and plasma biomarker analysis in a phase II study of XL184 in patients with progressive or recurrent glioblastoma multiforme. J Clin Oncol. 2009;27(suppl):99s. abstr 2049. [Google Scholar]
- 89.Quant EC, Batchelor T, Lassman AB, et al. Preliminary results from a multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma. J Clin Oncol. 2011;29(suppl):157s. doi: 10.1158/1078-0432.CCR-14-3220. abstr 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishikawa R, Saran F, Mason W, et al. Biomarker (BM) evaluations in the phase III AVAglio study of bevacizumab (Bv) plus standard radiotherapy (RT) and temozolomide (T) for newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(suppl 15s):119s. abstr 2023. [Google Scholar]
- 91.de Groot JF, Piao Y, Tran H, et al. Myeloid biomarkers associated with glioblastoma response to anti-VEGF therapy with aflibercept. Clin Cancer Res. 2011;17:4872–4881. doi: 10.1158/1078-0432.CCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sawlani RN, Raizer J, Horowitz SW, et al. Glioblastoma: A method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging—Pilot study. Radiology. 2010;255:622–628. doi: 10.1148/radiol.10091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 96.Stefanik DF, Rizkalla LR, Soi A, et al. Acidic and basic fibroblast growth factors are present in glioblastoma multiforme and normal brain. Ann N Y Acad Sci. 1991;638:477–480. doi: 10.1111/j.1749-6632.1991.tb49074.x. [DOI] [PubMed] [Google Scholar]
- 97.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 98.Gomez-Manzano C, Fueyo J, Jiang H, et al. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann Neurol. 2003;53:109–117. doi: 10.1002/ana.10396. [DOI] [PubMed] [Google Scholar]
- 99.Morrison RS, Gross JL, Herblin WF, et al. Basic fibroblast growth factor-like activity and receptors are expressed in a human glioma cell line. Cancer Res. 1990;50:2524–2529. [PubMed] [Google Scholar]
- 100.Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: Contrasting, overlapping and compensatory functions. Biochim Biophys Acta. 2010;1803:103–120. doi: 10.1016/j.bbamcr.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wesolowska A, Kwiatkowska A, Slomnicki L, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion: An inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–930. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 103.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13:132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 105.Drappatz J, Norden AD, Wong ET, et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78:85–90. doi: 10.1016/j.ijrobp.2009.07.1741. [DOI] [PubMed] [Google Scholar]
- 106.Reardon DA, Conrad CA, Cloughesy T, et al. Phase I study of AEE788, a novel multitarget inhibitor of ErbB- and VEGF-receptor-family tyrosine kinases, in recurrent glioblastoma patients. Cancer Chemother Pharmacol. 2012;69:1507–1518. doi: 10.1007/s00280-012-1854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 108.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 109.Jiang BH, Zheng JZ, Aoki M, et al. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoshiji H, Kuriyama S, Ways DK, et al. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999;59:4413–4418. [PubMed] [Google Scholar]
- 111.Drappatz J, Wong ET, Schiff D, et al. A pilot safety study of lenalidomide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:222–227. doi: 10.1016/j.ijrobp.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 112.Fadul C, Kingman L, Meyer L, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;90:229–235. doi: 10.1007/s11060-008-9655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18:708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 114.Fine HA, Wen PY, Maher EA, et al. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J Clin Oncol. 2003;21:2299–2304. doi: 10.1200/JCO.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 115.Zustovich F, Cartei G, Ceravolo R, et al. A phase I study of cisplatin, temozolomide and thalidomide in patients with malignant brain tumors. Anticancer Res. 2007;27:1019–1024. [PubMed] [Google Scholar]
- 116.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60:353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 117.Gilbert MR, Gonzalez J, Hunter K, et al. A phase I factorial design study of dose-dense temozolomide alone and in combination with thalidomide, isotretinoin, and/or celecoxib as postchemoradiation adjuvant therapy for newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1167–1172. doi: 10.1093/neuonc/noq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kesari S, Schiff D, Henson JW, et al. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro Oncol. 2008;10:300–308. doi: 10.1215/15228517-2008-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tuettenberg J, Grobholz R, Korn T, et al. Continuous low-dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme. J Cancer Res Clin Oncol. 2005;131:31–40. doi: 10.1007/s00432-004-0620-5. [DOI] [PubMed] [Google Scholar]
- 120.Nabors LB, Fiveash JB, Markert JM, et al. A phase 1 trial of ABT-510 concurrent with standard chemoradiotherapy for patients with newly diagnosed glioblastoma. Arch Neurol. 2010;67:313–319. doi: 10.1001/archneurol.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khasraw M, Holodny A, Goldlust SA, et al. Intracranial hemorrhage in patients with cancer treated with bevacizumab: The Memorial Sloan-Kettering experience. Ann Oncol. 2012;23:458–463. doi: 10.1093/annonc/mdr148. [DOI] [PubMed] [Google Scholar]
- 122.Bähr O, Hattingen E, Rieger J, et al. Bevacizumab-induced tumor calcifications as a surrogate marker of outcome in patients with glioblastoma. Neuro Oncol. 2011;13:1020–1029. doi: 10.1093/neuonc/nor099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 124.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 125.Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van den Bent MJ, Vogelbaum MA, Wen PY, et al. End point assessment in gliomas: Novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol. 2009;27:2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sorensen AG, Patel S, Harmath C, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19:551–557. doi: 10.1200/JCO.2001.19.2.551. [DOI] [PubMed] [Google Scholar]
- 128.Pichler J, Pachinger C, Pelz M, et al. MRI assessment of relapsed glioblastoma during treatment with bevacizumab: Volumetric measurement of enhanced and FLAIR lesions for evaluation of response and progression—A pilot study. Eur J Radiol. 2013;82:e240–e245. doi: 10.1016/j.ejrad.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 129.Liberman G, Louzoun Y, Aizenstein O, et al. Automatic multi-modal MR tissue classification for the assessment of response to bevacizumab in patients with glioblastoma. Eur J Radiol. 2013;82:e87–e94. doi: 10.1016/j.ejrad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 130.de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 131.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 132.Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002;12:615–626. doi: 10.1016/s1052-5149(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 133.Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48:1468–1481. doi: 10.2967/jnumed.106.037689. [DOI] [PubMed] [Google Scholar]
- 134.Schwarzenberg J, Czernin J, Cloughesy TF, et al. 3′-deoxy-3′-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med. 2012;53:29–36. doi: 10.2967/jnumed.111.092387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harris RJ, Cloughesy TF, Pope WB, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14:1079–1089. doi: 10.1093/neuonc/nos141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hasselbalch B, Eriksen JG, Broholm H, et al. Prospective evaluation of angiogenic, hypoxic and EGFR-related biomarkers in recurrent glioblastoma multiforme treated with cetuximab, bevacizumab and irinotecan. APMIS. 2010;118:585–594. doi: 10.1111/j.1600-0463.2010.02631.x. [DOI] [PubMed] [Google Scholar]
- 137.Lu-Emerson C, Snuderl M, Kirkpatrick ND, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15:1079–1087. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen C, Huang R, MacLean A, et al. Recurrent high-grade glioma treated with bevacizumab: Prognostic value of MGMT methylation, EGFR status and pretreatment MRI in determining response and survival. J Neurooncol. 2013;115:267–276. doi: 10.1007/s11060-013-1225-0. [DOI] [PubMed] [Google Scholar]
- 139.Lv S, Teugels E, Sadones J, et al. Correlation between IDH1 gene mutation status and survival of patients treated for recurrent glioma. Anticancer Res. 2011;31:4457–4463. [PubMed] [Google Scholar]
- 140.Galanis E, Anderson SK, Lafky JM, et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): A North Central Cancer Treatment Group trial. Clin Cancer Res. 2013;19:4816–4823. doi: 10.1158/1078-0432.CCR-13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duda DG, Willett CG, Ancukiewicz M, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tabouret E, Boudouresque F, Barrie M, et al. Association of matrix metalloproteinase 2 plasma level with response and survival in patients treated with bevacizumab for recurrent high-grade glioma. Neuro Oncol. 2014;16:392–399. doi: 10.1093/neuonc/not226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Waldman AD, Jackson A, Price SJ, et al. Quantitative imaging biomarkers in neuro-oncology. Nat Rev Clin Oncol. 2009;6:445–454. doi: 10.1038/nrclinonc.2009.92. [DOI] [PubMed] [Google Scholar]
- 144.Gerstner ER, Batchelor TT. Imaging and response criteria in gliomas. Curr Opin Oncol. 2010;22:598–603. doi: 10.1097/CCO.0b013e32833de96e. [DOI] [PubMed] [Google Scholar]
- 145.Jarzabek MA, Sweeney KJ, Evans RL, et al. Molecular imaging in the development of a novel treatment paradigm for glioblastoma (GBM): An integrated multidisciplinary commentary. Drug Discov Today. 2013;18:1052–1066. doi: 10.1016/j.drudis.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 146.Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004;9:528–537. doi: 10.1634/theoncologist.9-5-528. [DOI] [PubMed] [Google Scholar]
- 147.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 148.Ellingson BM, Cloughesy TF, Lai A, et al. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13:401–409. doi: 10.1093/neuonc/noq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: A pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48:1176–1184. doi: 10.1016/j.ejca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 150.O'Connor JP, Jackson A, Parker GJ, et al. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167–177. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 151.Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19:1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gerstner ER, Sorensen AG. Diffusion and diffusion tensor imaging in brain cancer. Semin Radiat Oncol. 2011;21:141–146. doi: 10.1016/j.semradonc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 153.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: A noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005;102:5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: Correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26:3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pope WB, Qiao XJ, Kim HJ, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: A multi-center study. J Neurooncol. 2012;108:491–498. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kothari PD, White NS, Farid N, et al. Longitudinal restriction spectrum imaging is resistant to pseudoresponse in patients with high-grade gliomas treated with bevacizumab. AJNR Am J Neuroradiol. 2013;34:1752–1757. doi: 10.3174/ajnr.A3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.di Tomaso E, Snuderl M, Kamoun WS, et al. Glioblastoma recurrence after cediranib therapy in patients: Lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gerstner ER, Chen PJ, Wen PY, et al. Infiltrative patterns of glioblastoma spread detected via diffusion MRI after treatment with cediranib. Neuro Oncol. 2010;12:466–472. doi: 10.1093/neuonc/nop051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gerstner ER, Frosch MP, Batchelor TT. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol. 2010;28:e91–e93. doi: 10.1200/JCO.2009.25.0233. [DOI] [PubMed] [Google Scholar]
- 161.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: Radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gerstner ER, Levine M, Ye X, et al. A phase I study of cediranib in combination with cilengitide in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(suppl 15s):127s. doi: 10.1093/neuonc/nov085. abstr 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: A review. J Neurooncol. 2011;104:639–646. doi: 10.1007/s11060-011-0565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Francesconi AB, Dupre S, Matos M, et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17:970–974. doi: 10.1016/j.jocn.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 165.Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96:259–269. doi: 10.1007/s11060-009-9957-6. [DOI] [PubMed] [Google Scholar]
- 166.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: A single-institution experience. Neurology. 2009;72:1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ali SA, McHayleh WM, Ahmad A, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: A series of 13 cases. J Neurosurg. 2008;109:268–272. doi: 10.3171/JNS/2008/109/8/0268. [DOI] [PubMed] [Google Scholar]
- 168.Kang TY, Jin T, Elinzano H, et al. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008;89:113–118. doi: 10.1007/s11060-008-9599-0. [DOI] [PubMed] [Google Scholar]
- 169.Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008;112:2267–2273. doi: 10.1002/cncr.23401. [DOI] [PubMed] [Google Scholar]
- 170.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 171.Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Neuro-Oncol. 2005;7:369. (abstr 342) [Google Scholar]
- 172.Scott BJ, Quant EC, McNamara MB, et al. Bevacizumab salvage therapy following progression in high-grade glioma patients treated with VEGF receptor tyrosine kinase inhibitors. Neuro Oncol. 2010;12:603–607. doi: 10.1093/neuonc/nop073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Goldlust SA, Cavaliere R, Newton HB, et al. Bevacizumab for glioblastoma refractory to vascular endothelial growth factor receptor inhibitors. J Neurooncol. 2012;107:407–411. doi: 10.1007/s11060-011-0768-1. [DOI] [PubMed] [Google Scholar]
- 174.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 175.Reardon D, Friedman H, Yung WK, et al. A phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol. 2004;22(suppl):28s. abstr 1513. [Google Scholar]
- 176.Kreisl TN, McNeill KA, Sul J, et al. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neuro Oncol. 2012;14:1519–1526. doi: 10.1093/neuonc/nos265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reardon DA, Vredenburgh JJ, Desjardins A, et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: Results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101:57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 179.Drappatz J, Brenner AJ, Rosenfeld S, et al. ANG1005: Results of a phase I study in patients with recurrent malignant glioma. J Clin Oncol. 2010;28(suppl):182s. abstr 2009. [Google Scholar]
- 180.Jain RK. Antiangiogenesis strategies revisted: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rieger J, Bähr O, Müller K, et al. Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol. 2010;99:49–56. doi: 10.1007/s11060-009-0098-8. [DOI] [PubMed] [Google Scholar]
- 182.Ferl GZ, Xu L, Friesenhahn M, et al. An automated method for nonparametric kinetic analysis of clinical DCE-MRI data: Application to glioblastoma treated with bevacizumab. Magn Reson Med. 2010;63:1366–1375. doi: 10.1002/mrm.22335. [DOI] [PubMed] [Google Scholar]
- 183.Jain R, Scarpace LM, Ellika S, et al. Imaging response criteria for recurrent gliomas treated with bevacizumab: Role of diffusion weighted imaging as an imaging biomarker. J Neurooncol. 2010;96:423–431. doi: 10.1007/s11060-009-9981-6. [DOI] [PubMed] [Google Scholar]
- 184.Nowosielski M, Recheis W, Goebel G, et al. ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology. 2011;53:291–302. doi: 10.1007/s00234-010-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hwang EI, Jakacki RI, Fisher MJ, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer. 2013;60:776–782. doi: 10.1002/pbc.24297. [DOI] [PubMed] [Google Scholar]
- 186.Ratai EM, Zhang Z, Snyder BS, et al. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro Oncol. 2013;15:936–944. doi: 10.1093/neuonc/not044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hsu YH, Ferl GZ, Ng CM. GPU-accelerated nonparametric kinetic analysis of DCE-MRI data from glioblastoma patients treated with bevacizumab. Magn Reson Imaging. 2013;31:618–623. doi: 10.1016/j.mri.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 188.Desjardins A, Barboriak DP, Herndon JE, II, et al. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) evaluation in glioblastoma (GBM) patients treated with bevacizumab (BEV) and irinotecan (CPT-11) J Clin Oncol. 2007;25(suppl):82s. abstr 2029. [Google Scholar]
- 189.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–189. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 189a.Ellingson BM, Malkin MG, Rand SD, et al. Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and antiangiogenic treatments in malignant gliomas. J Neurooncol. 2011;102:95–103. doi: 10.1007/s11060-010-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189b.Ellingson BM, Cloughesy TF, Lai A, et al. Nonlinear registration of diffusion-weighted images improves clinical sensitivity of functional diffusion maps in recurrent glioblastoma treated with bevacizumab. Magn Reson Med. 2012;67:237–245. doi: 10.1002/mrm.23003. [DOI] [PubMed] [Google Scholar]
- 189c.Hwang EJ, Cha Y, Lee AL, et al. Early response evaluation for recurrent high grade gliomas treated with bevacizumab: A volumetric analysis using diffusion-weighted imaging. J Neurooncol. 2013;112:427–435. doi: 10.1007/s11060-013-1072-z. [DOI] [PubMed] [Google Scholar]
- 190.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: A pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 191.Schiepers C, Dahlbom M, Chen W, et al. Kinetics of 3′-deoxy-3′-18F-fluorothymidine during treatment monitoring of recurrent high-grade glioma. J Nucl Med. 2010;51:720–727. doi: 10.2967/jnumed.109.068361. [DOI] [PubMed] [Google Scholar]
- 192.Wardak M, Schiepers C, Dahlbom M, et al. Discriminant analysis of 18F-fluorothymidine kinetic parameters to predict survival in patients with recurrent high-grade glioma. Clin Cancer Res. 2011;17:6553–6562. doi: 10.1158/1078-0432.CCR-10-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hutterer M, Nowosielski M, Putzer D, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52:856–864. doi: 10.2967/jnumed.110.086645. [DOI] [PubMed] [Google Scholar]
- 194.Colavolpe C, Chinot O, Metellus P, et al. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012;14:649–657. doi: 10.1093/neuonc/nos012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Galldiks N, Rapp M, Stoffels G, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40:22–33. doi: 10.1007/s00259-012-2251-4. [DOI] [PubMed] [Google Scholar]
- 196.Vidiri A, Pace A, Fabi A, et al. Early perfusion changes in patients with recurrent high-grade brain tumor treated with bevacizumab: Preliminary results by a quantitative evaluation. J Exp Clin Cancer Res. 2012;31:33. doi: 10.1186/1756-9966-31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196a.Ellingson BM, Cloughesy TF, Lai A, et al. Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2012;106:111–119. doi: 10.1007/s11060-011-0638-x. [DOI] [PubMed] [Google Scholar]
- 197.Gupta A, Young RJ, Karimi S, et al. Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. AJNR Am J Neuroradiol. 2011;32:1301–1306. doi: 10.3174/ajnr.A2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mong S, Ellingson BM, Nghiemphu PL, et al. Persistent diffusion-restricted lesions in bevacizumab-treated malignant gliomas are associated with improved survival compared with matched controls. AJNR Am J Neuroradiol. 2012;33:1763–1770. doi: 10.3174/ajnr.A3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.LaViolette PS, Cohen AD, Prah MA, et al. Vascular change measured with independent component analysis of dynamic susceptibility contrast MRI predicts bevacizumab response in high-grade glioma. Neuro Oncol. 2013;15:442–450. doi: 10.1093/neuonc/nos323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Grommes C, Karimi S, Beal K, et al. FLAIR, T1 contrast enhancement, MR perfusion, and FDG PET following hypofractionated stereotactic radiotherapy (HFSRT), bevacizumab (BEV), and temozolomide (TMZ) for glioblastoma (GBM) J Clin Oncol. 2011;29(suppl):152s. abstr 2048. [Google Scholar]
- 200a.Pope WB, Lai A, Mehta R, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol. 2011;32:882–889. doi: 10.3174/ajnr.A2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200b.Emblem KE, Bjornerud A, Mouridsen K, et al. T(1)- and T(2)(*)-dominant extravasation correction in DSC-MRI: Part II. Predicting patient outcome after a single dose of cediranib in recurrent glioblastoma patients. Cereb Blood Flow Metab. 2011;31:2054–2064. doi: 10.1038/jcbfm.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200c.Emblem KE, Batchelor TT, Gerstner ER, et al. Glioblastoma patients with arterio-venous normalization during anti-angiogenic therapy have prolonged survival. Eur J Cancer. 2013;49:S29. (abstr MC13-0062) [Google Scholar]
- 201.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 202.O'Neill Blakeley J, Fisher JD, Lieberman FS, et al. Imaging biomarkers of ramucirumab and olaratumab in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(suppl 15s):125s. abstr 2044. [Google Scholar]
- 202a.Sorensen AG, Jenning D, Wang M, et al. Use of neurovascular imaging in GBM patients (pts) to quantify early physiologic changes after treatment with XL184, an inhibitor of multiple receptor tyrosine kinases: Results from a phase II study. J Clin Oncol. 2009;27(suppl 15S):99s. abstr 2048. [Google Scholar]
- 203.Lupo JM, Essock-Burns E, Molinaro AM, et al. Using susceptibility-weighted imaging to determine response to combined anti-angiogenic, cytotoxic, and radiation therapy in patients with glioblastoma multiforme. Neuro Oncol. 2013;15:480–489. doi: 10.1093/neuonc/nos325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Essock-Burns E, Lupo JM, Cha S, et al. Assessment of perfusion MRI-derived parameters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:119–131. doi: 10.1093/neuonc/noq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cha S, Knopp EA, Johnson G, et al. Dynamic contrast-enhanced T2-weighted MR imaging of recurrent malignant gliomas treated with thalidomide and carboplatin. AJNR Am J Neuroradiol. 2000;21:881–890. [PMC free article] [PubMed] [Google Scholar]
- 206.Akella NS, Twieg DB, Mikkelsen T, et al. Assessment of brain tumor angiogenesis inhibitors using perfusion magnetic resonance imaging: Quality and analysis results of a phase I trial. J Magn Reson Imaging. 2004;20:913–922. doi: 10.1002/jmri.20202. [DOI] [PubMed] [Google Scholar]
- 207.Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neyns B, Sadones J, Chaskis C, et al. A phase II trial of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103:491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 209.Neyns B, Chaskis C, Dujardin M, et al. Phase II trial of sunitinib malate in patients with temozolomide refractory recurrent high-grade glioma. J Clin Oncol. 2009;27(suppl 15S):96s. abstr 2038. [Google Scholar]
- 210.Wen PY Prados M, Schiff D, et al. Phase II study of XL184 (BMS 907351), an inhibitor of MET, VEGFR2, and RET, in patients (pts) with progressive glioblastoma (GB) J Clin Oncol. 2010;28(suppl):181s. abstr 2006. [Google Scholar]