Abstract
Purpose
Surgical resection of gastric cancer has produced suboptimal survival despite multiple randomized trials that used postoperative chemotherapy or more aggressive surgical procedures. We performed a randomized phase III trial of postoperative radiochemotherapy in those at moderate risk of locoregional failure (LRF) following surgery. We originally reported results with 4-year median follow-up. This update, with a more than 10-year median follow-up, presents data on failure patterns and second malignancies and explores selected subset analyses.
Patients and Methods
In all, 559 patients with primaries ≥ T3 and/or node-positive gastric cancer were randomly assigned to observation versus radiochemotherapy after R0 resection. Fluorouracil and leucovorin were administered before, during, and after radiotherapy. Radiotherapy was given to all LRF sites to a dose of 45 Gy.
Results
Overall survival (OS) and relapse-free survival (RFS) data demonstrate continued strong benefit from postoperative radiochemotherapy. The hazard ratio (HR) for OS is 1.32 (95% CI, 1.10 to 1.60; P = .0046). The HR for RFS is 1.51 (95% CI, 1.25 to 1.83; P < .001). Adjuvant radiochemotherapy produced substantial reduction in both overall relapse and locoregional relapse. Second malignancies were observed in 21 patients with radiotherapy versus eight with observation (P = .21). Subset analyses show robust treatment benefit in most subsets, with the exception of patients with diffuse histology who exhibited minimal nonsignificant treatment effect.
Conclusion
Intergroup 0116 (INT-0116) demonstrates strong persistent benefit from adjuvant radiochemotherapy. Toxicities, including second malignancies, appear acceptable, given the magnitude of RFS and OS improvement. LRF reduction may account for the majority of overall relapse reduction. Adjuvant radiochemotherapy remains a rational standard therapy for curatively resected gastric cancer with primaries T3 or greater and/or positive nodes.
INTRODUCTION
In the 1990s, Intergroup 0116 (INT-0116), a randomized phase III trial, was conducted to compare observation versus adjuvant radiochemotherapy following curative gastric cancer resection. Before the initiation of INT-0116, survival following curative surgery had changed little in the prior three decades. The American College of Surgeons1 and National Cancer Data Base2,3 reported large cohorts resected in the 1980s and 1990s. Five-year survival ranged from 29% to 37% in stage II and 13% to 20% in stage III, depressingly similar to data from the late 1950s and 1960s.4–6 Postoperative adjuvant chemotherapy was extensively tested with disappointing results in Western patients. Some meta-analyses reported no significant benefit with postoperative chemotherapy;7–9 others found benefit either confined to10 or driven by Asian trials.11,12
Strategies to decrease locoregional failure (LRF) have also been explored. Surgical series document LRF in 40% to 60% of patients in stage II or III disease.5,13–15 LRF consistently occurs in the tumor bed, anastomosis, and undissected regional nodes in these clinical, reoperative, and autopsy series. Surgical trials have attempted to reduce the nodal component of LRF by more comprehensive nodal resection. All five phase III trials that examine various degrees of Japanese D-level guided nodal surgery report higher operative morbidity and/or mortality with more extensive procedures but no convincing increase in survival.16–20 Several reports of aggressive nodal surgery continued to observe substantial LRF.5,15,16 The Dutch randomized trial reported LRF in 58% with D1 and 45% with D2 dissection.16 This suggested that a more comprehensive strategy to address all LRF sites was needed. INT-0116 investigated whether postoperative adjuvant radiochemotherapy in patients with completely resected tumors but at substantial risk of LRF would improve relapse-free survival (RFS) and overall survival (OS). Previous methodologically flawed evaluations of adjuvant radiotherapy observed reduced LRF,21–23 which may improve OS.21,22 Quality assurance problems with radiotherapy had been described,23 requiring a robust radiotherapy quality assurance program. This study's primary analysis in 2001 demonstrated significant improvement in RFS and OS with adjuvant radiochemotherapy.24 This update, with more than 10 years of follow-up, confirms the benefits of radiochemotherapy and explores associations of this type of therapy in selected subsets of patients with resected gastric cancer.
PATIENTS AND METHODS
Details of INT-0116 have been previously reported.24 Eligibility criteria included R0 resection of adenocarcinoma of the stomach or gastroesophageal junction (GEJ), presence of complete penetration of the tumor through the muscularis propria and/or involved regional nodes (including 1988 American Joint Committee on Cancer [AJCC] stages IB to IV with M0), performance status 0 to 2, more than 1,500 kcal/d intake, and adequate general medical condition and laboratory parameters. Institutional review board–approved informed consent was obtained, registration occurred 20 to 41 days postoperatively, and treatment began within 7 working days of registration.
Treatment Plan
Patients were randomly assigned to surgery alone versus postoperative radiochemotherapy. Radiochemotherapy consisted of bolus fluorouracil (FU) and leucovorin (LV) before, during, and after radiotherapy. FU 425 mg/m2/d and LV 20 mg/m2/d on days 1 through 5 began on day 1. Radiation to a total of 45 Gy (1.8 Gy/d 5 d/wk for 5 weeks) began on day 28. FU 400 mg/m2/d and LV 20 mg/m2/d was given the first four and the last three days of radiotherapy. beginning 1 month after radiotherapy, two additional cycles of FU + LV were given once every 28 days. Radiotherapy targeted common LRF sites such as the tumor bed, regional nodes, and anastomoses. Nodal volumes were previously described.24 The radiotherapy plan required approval by the radiation-oncology coordinator (S.R.S.) before radiotherapy could be initiated. Because of excessive toxicity risk, 9.5% of initial plans were rejected but all were corrected before radiotherapy. At initial review, 35% of treatment plans contained major or minor protocol deviations and most were corrected before the start of radiotherapy. Final quality assurance review (conducted after the delivery of radiation) revealed major deviations in 6.5% of the treatment plans.
Statistical Analysis
INT-0116 was designed to accrue 550 eligible patients, which ensured 90% power to detect a 40% difference in OS (hazard ratio [HR] of 1.4) and a 40% difference in RFS. The two stratification factors, T stage (T1-2 v T3 v T4) and N stage (N0 v one to three positive nodes v > three positive nodes), were included as covariates in the Cox regression analysis. All eligible patients were included in the OS and RFS analyses according to the intention-to-treat principle.
Patterns of failure (POF; based on Notice of Recurrence form required at first failure indicating all relapse sites and means of detection) were classified (J.S.M.) as follows: local if tumor relapsed in the surgical anastomosis, residual stomach, or gastric bed; regional if tumor recurred in the peritoneal cavity (including the liver, intra-abdominal lymph nodes, and peritoneum); and distant for relapse outside the peritoneal cavity. Relapse patterns were compared by using the χ2 test. Histologic evaluation was centrally reviewed (G.N.S.). We also explored the effect of therapy by selected patient characteristics: sex, race, T stage, N stage, D level of nodal surgery, site of the primary tumor in the stomach (proximal or other), histology (diffuse or intestinal),24a and Maruyama index (MI).25 This was initially done by including a treatment-by-characteristic interaction in the Cox regression model. Lack of a significant interaction suggested insufficient evidence for selective treatment effects by level of the variable. To further explore potential relationships between treatment and variables, we estimated the treatment effects separately within levels of the factors (presented as forest plots). Where interaction terms were nonsignificant, it should be stressed that these individual analyses need to be interpreted with caution. P values are two-sided.
RESULTS
In all, 603 patients were registered from 1991 to 1998. Forty-four (8%) were ineligible because of positive surgical margins or nonadenocarcinoma histology, or they registered after the specified time limit. Of the 559 eligible patients, 277 were randomly assigned to observation and 282 to radiochemotherapy. Among those last known alive (n = 121), median follow-up was 10.3 years. GEJ primaries occurred in approximately 20% of patients. Patients had a high risk of LRF (more than two thirds of patients had stage T3,4 primaries, and 85% had nodal metastases).
Treatment
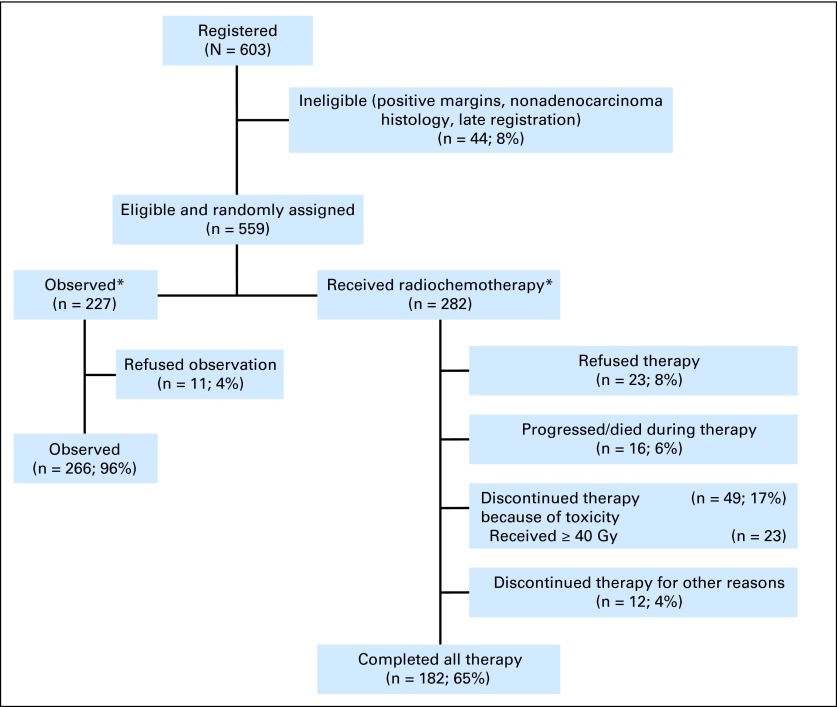
In the group that received radiochemotherapy, 182 (65%) completed planned treatment, 49 (17%) stopped treatment because of toxicity (23 of these 49 received ≥ 40 Gy), 5% progressed during treatment, 1% died during therapy, and 4% discontinued treatment for other reasons. Twelve percent (8% assigned to treatment; 4% assigned to observation) declined to continue the assigned therapy, but they are included in the assigned study group according to intention to treat (CONSORT diagram; Fig 1).
Fig 1.
CONSORT diagram. OS, overall survival; RFS, relapse-free survival. (*) All 277 patients randomly assigned to observation and 282 patients randomly assigned to radiochemotherapy are included in the assigned study group according to the intention to treat in RFS/OS analyses
Surgical Procedures
A form defining the extent of lymphadenectomy was required before surgery. In all, 552 patients had a review of nodal dissection type; 54 (9.6%) received D2 dissection; 199 (36%) received D1 dissection (removal of Japanese N1 nodal stations), and 54% received a less than D1 dissection (resection of less than Japanese N1 nodal groups).
Toxicity
Acute toxicity.
Acute toxicity effects were previously reported, the predominate types being hematologic and gastrointestinal. Four patients (1%) died secondary to therapy: two before radiation therapy (one from cardiotoxicity, one from neutropenic sepsis), one from radiation pulmonary fibrosis, and one from central line fungemia.
Long-term toxicity.
We had no reports of excess treatment-related toxicities during long-term follow-up. Table 1 lists second malignancies. In the radiochemotherapy arm, there were 21 patients (representing 25 separate cancers) with second malignancies versus eight in the observation group (P = .21). These results must be viewed with caution, because there is potential bias by treatment arm for completeness of reporting for second primaries.
Table 1.
Second Tumor Sites
| Site | No. of Tumor Sites |
|---|---|
| Radiochemotherapy | 25* |
| Skin | 6 |
| Melanoma | 2 |
| Colorectal | 4 |
| Breast | 1 |
| Prostate | 4 |
| Hematologic | 3 |
| Lymphoma | 2 |
| Myelodysplastic syndrome | 1 |
| Bladder | 3 |
| Lung | 1 |
| Renal pelvis | 1 |
| Larynx | 1 |
| Unknown primary | 1 |
| Control | 8 |
| Skin | 2 |
| Pancreas | 2 |
| Breast | 1 |
| Lung | 1 |
| Hematologic | 1 |
| Renal | 1 |
No. of patients = 21.
Survival and Relapse Sites
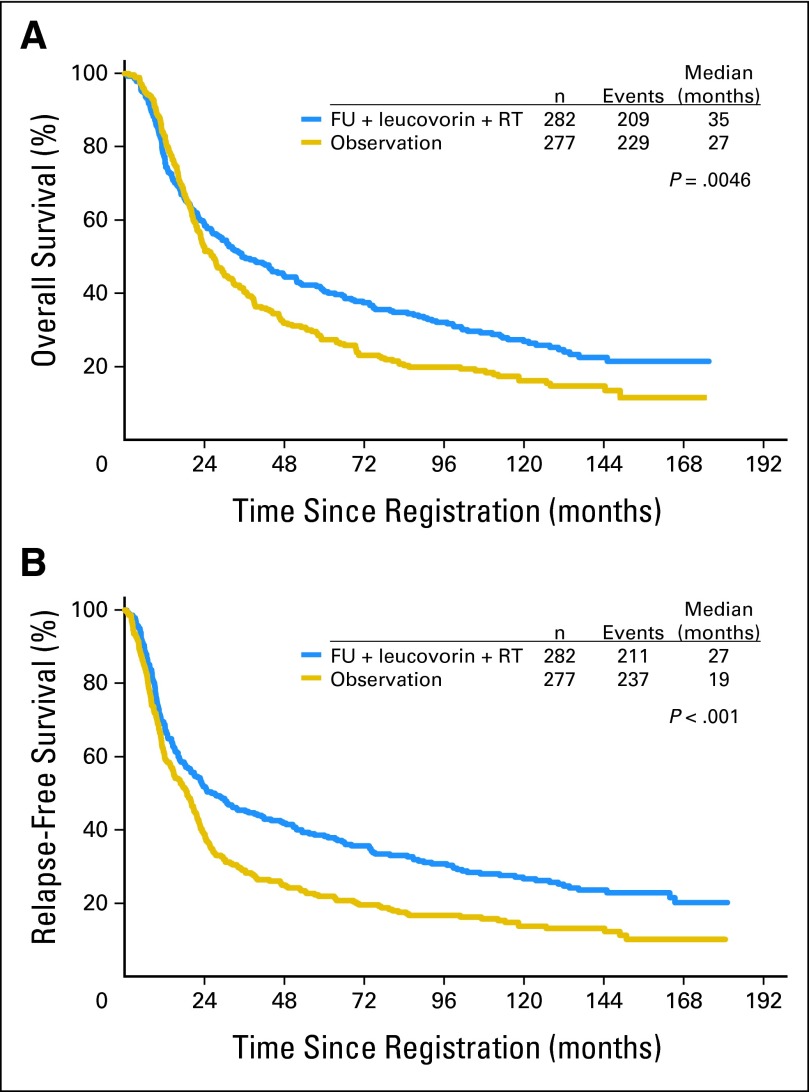
With more than 10 years of follow-up, the OS and RFS data demonstrate continued strong benefit from postoperative radiochemotherapy. HRs were virtually unchanged since the original report. HR for OS (Fig 2A) was 1.32 (95% CI, 1.10 to 1.60; P = .0046), and the HR for RFS (Fig 2B) was 1.51 (95% CI, 1.25 to 1.83; P < .001). This shows highly significant benefit for radiochemotherapy. POF comparison was a prespecified major objective of INT-0116 and the details are provided in Table 2. χ2 comparison for relapse versus no relapse had P < .001. For sites of relapse (among patients for whom sites were reported), χ2 test for trend had P = .012.
Fig 2.
(A) Overall survival by arm; (B) relapse-free survival by arm. FU, fluorouracil; RT, radiotherapy.
Table 2.
Patterns of Failure by Arm
| Relapse Status | Radiochemotherapy |
Control(surgery alone) |
Total |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No relapse* | 135 | 48 | 67 | 24 | 202 | 36 |
| Relapse* | 147 | 52 | 210 | 76 | 357 | 64 |
| Sites of relapse (% of those randomly assigned)* | ||||||
| Local | 7 | 2 | 21 | 8 | 28 | 5 |
| Regional | 62 | 22 | 109 | 39 | 171 | 31 |
| Distant | 46 | 16 | 49 | 18 | 95 | 17 |
| Unknown site | 32 | 11 | 31 | 11 | 63 | 11 |
| Total | 282 | 277 | 559 | |||
Indicates statistically significant comparisons. P < .001 for relapse v no relapse (χ2); P = .012 for sites of relapse (among those with sites reported, χ2 test for trend).
Tests of Interaction and Exploration of Patient Subsets
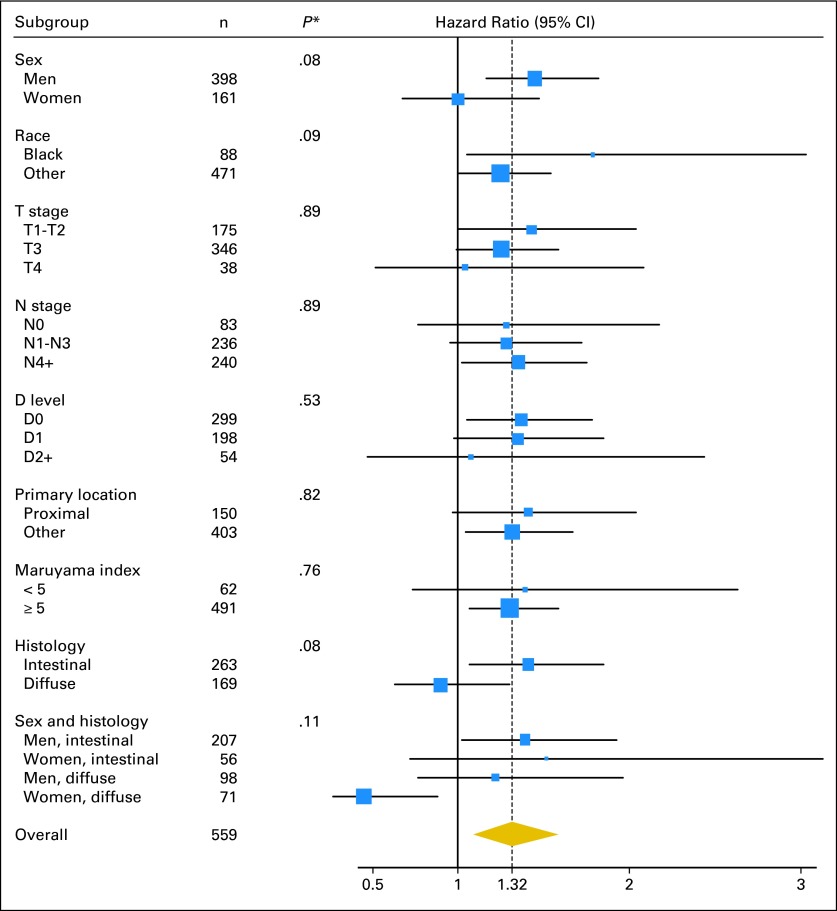
Exploratory subgroup analyses were carried out for the following factors: sex, race, T stage, N stage, D level of resection, primary tumor location, histology, and MI. Forest plots (Fig 3) show overall survival HRs and CIs for treatment within selected variable subsets as well as tests of the treatment by variable interactions. The lack of significant interactions suggest no strong evidence for a differential treatment effect between variable levels. One exception is a potential trend for an interaction of sex and histology. Individual plots for sex and histology suggest that treatment did not improve outcome for women or for those with diffuse tumors. Examination of histology pattern by sex revealed some confounding. Nearly twice as many females (56%) had diffuse disease compared with males (30%). Multivariate analysis assessed whether both of these variables were related to outcome. Only histology was significant, suggesting that any effect related to sex may be related to histology rather than to sex itself. The Data Supplement includes selected exploratory subset evaluations by arm. Extreme caution in interpretation is mandatory in view of the low numbers and unplanned subset nature of the data. Evaluation of the D2 group must be tempered by poor statistical power from having small numbers and possible unknown biases influencing selection. The test of treatment interaction for D level was not significant (P = .53) and thus does not provide evidence of a lack of treatment benefit for the patients with D2 level of resection. Treatment interaction testing with diffuse histology had P = .077. Although not significant at P < .05, this raises the question of whether radiochemotherapy improves outcomes in patients with tumors having diffuse histology. In addition to the variables presented in Figure 3, we also explored whether the magnitude of the treatment effect differed depending on the time between surgery and random assignment. We were unable to detect any such effects, either with respect to OS (P = .47) or disease-free survival (P = .71).
Fig 3.
Forest plot for overall survival hazard ratios and CIs for treatment within subsets of the selected variables. Hazard ratios greater than one favor the chemoradiation arm. The dotted line represents the overall hazard ratio for treatment benefit.
DISCUSSION
This final update of INT-0116 results extends and contextualizes our earlier report.24 Median follow-up for living patients is more than 10 years, and OS and RFS continue to demonstrate dramatic benefit for patients who received adjuvant radiochemotherapy. HRs remain virtually unchanged from our initial report. POF analysis allows several observations. Twenty-four percent more patients in the treatment arm remained free of disease than in the surgery alone arm (nearly twice as many without disease recurrence with radiochemotherapy). This degree of benefit is almost precisely that predicted by the seminal failure pattern analyses of Gunderson and Sosin.5 That original work, supported by the work of others,13–16 provided the rationale for INT-0116 and observed that 23% of patients experienced treatment failure only in the LRF sites without other sites of relapse. We acknowledge that failure site coding is subject to clinical error, although failure site designation was required at the time of first failure in all patients with relapse. Our POF analysis, however, suggests that our therapy effectively sterilized subclinical LRF sites which would otherwise have resulted in relapse and death. LRF, as defined, was observed much less frequently with radiochemotherapy. However, distant relapse was similar in the treatment and observation groups, suggesting that the improvement in LRF associated with radiochemotherapy may have driven the demonstrated improvement in disease-free survival and OS. Corollaries of this statement are that adjuvant radiochemotherapy should be offered only to those at moderate risk of LRF; further improvements in OS will likely come from improvements in systemic disease control.
Toxicity evaluation demonstrates significant acute morbidity. INT-0116 was activated before serotonin 5-HT3 receptor antagonists were available. These drugs markedly decrease the nausea and nutritional toxicity of upper abdominal radiochemotherapy.26 The 1% treatment-related mortality seems justified by the substantial OS and RFS benefit. The long follow-up provides some reassurance regarding long-term toxicity. We underscore that the data do not allow evaluation of nonlethal toxicity (eg, hypertension, peptic ulcer disease, or coronary atherosclerosis). Our analyses of treatment effects by patient subsets are exploratory in nature, and must be viewed with caution. In general, the data demonstrate robust benefit of radiochemotherapy in most subsets. Figure 3 shows no statistically significant interactions between any of the variables and treatment effect. Therefore, there is no compelling evidence that the effect of radiochemotherapy is different among any of these explored treatment variables. Forest plots further provide evidence of the wide-ranging benefit of therapy among the various subsets. Therapy seems beneficial regardless of race, T stage, N stage, or location of the primary within the stomach or GEJ. The exception for histology is interesting and unexplained. Diffuse histology, in most other series, carries a much poorer prognosis occurring in younger, more frequently female patients (all of these associations were described in the original report by Lauren24a). There is evidence that diffuse histology is associated with differences in mucin class, sonic hedgehog profile, gene signature array, and ongoing epidemiologic shift in North America. Whether our observation of reduced treatment effect in patients with diffuse histology is reflective of these biologic variations or is a random observation of an unplanned subset analysis is unknown (histologic type was known in only 77% of patients).
With regard to D resection level and MI, our results support the benefit of radiation. Certainly, those with MI > 5 and D1 or greater nodal surgery (the overwhelming majority of our patients) had markedly improved outcome with radiochemotherapy. Only a small proportion of patients in our data set had MI < 5 surgery (11%) or D2 resection (9.6%). There is no statistically significant interaction of either MI or D strata with therapy, and the HRs favor therapy in both the MI < 5 and D2 cohorts, although with wide confidence intervals. Although MI reflects multiple demographic, T stage, histologic, and other variables in addition to extent of nodal surgery, it does serve as a quantitative estimate of the adequacy of nodal surgery in an individual patient. Every patient included in this study could have had an MI of zero; this variable was under the surgeon's control. Low MI is more likely with more favorable T stage and other variables regardless of nodal resection extent. It is, therefore, not surprising that patients with low MI had improved outcome compared with those with higher MIs. However, by previous multivariate analysis that included T stage and N stage, MI was an independent predictor of survival.25 The RFS and OS curves in the MI < 5 curves show a nonsignificant trend in favor of therapy (for example RFS two-sided P = .07). The lack of significant interactions of MI strata and the trend of the MI < 5 subset to benefit from therapy provide assurance that adjuvant radiochemotherapy is reasonable in this group. Likewise, D level shows no significant interaction with treatment. Several studies report that D2 dissection is more frequently performed in younger, healthier patients,27–29 resulting in a potential selection bias likely to produce improved outcome regardless of therapy. In our study, the RFS and OS curves show no trend favoring therapy in the D2 group. Whether this is due to small sample sizes, random fluctuations in subset analyses, or a true clinicopathologic principle is conjectural.
We would comment, regarding INT-0116 surgery, that INT-0116 reflects the real world of gastric surgery performed in North America. Multiple large data sets demonstrate that retrieval of 15 or more nodes in gastric cancer—a suggested surrogate for greater than D1 nodal surgery30,31—is infrequently performed.28,31,32 Surgeons' knowledge of quality indicators for gastric surgery is less than optimal,31 and evaluations of nodal count suggest minimal increases in recent years.33,34 This is understandable in view of the five randomized phase III trials16–20 of nodal resection in gastric cancer (usually D1 v D2 or D2+ comparisons) and a meta-analysis of these randomized trials,35 which uniformly show no OS benefit with more extensive surgery but do show greater mortality and/or morbidity. Furthermore, several reports of D2 surgery continue to observe substantial LRF. The Dutch randomized study16,36 reported significant LRF in the D2 arm overall (45%), and in both MI < 5 (27%) and MI 5+ (57%) subsets (autopsy series). Memorial Sloan-Kettering Cancer Center15 reported LRF in 54% of patients with relapse (76% had D2 or greater resections) and noted no LRF reduction with D2 surgery. Others5,37 report continued substantial LRF rates with D2 or greater resection. In contrast, Surveillance, Epidemiology, and End Results (SEER) data show improved survival with adjuvant radiochemotherapy independent of nodal clearance.32 Kim et al38 evaluated 990 patients with D2 dissection and found consistent OS and LRF benefit with adjuvant radiochemotherapy. Therefore, regardless of nodal surgery, some subsets of patients with gastric cancer remain at significant risk of LRF and benefit from radiochemotherapy.
The role of adjuvant radiochemotherapy, used either postoperatively or preoperatively in the setting of effective induction chemotherapy is important to consider. Although several phase III randomized trials39,40 and a meta-analysis41 of all published trials have shown no beneficial effect of induction chemotherapy, the MAGIC trial established perioperative ECF (epirubicin-cisplatin-fluorouracil) chemotherapy as an acceptable standard therapy for resectable lower esophageal and stomach cancer.42 HR, RFS, and OS improvements were similar to INT-0116. The composition of the MAGIC cohort is dissimilar to that of the INT-0116 cohort (twice the node-negative incidence, for example), and we emphasize that RFS and OS absolute outcomes cannot be compared across studies. Nevertheless, perioperative chemotherapy is rational, and consideration of adjuvant radiochemotherapy in this context is important. The MAGIC trial noted similar frequency of noncurative (R1-R2) resections in both groups (31% to 34%). R1 resection was especially common in the GEJ (D. Cunningham, personal communication, January 2009). Within a group of patients accrued preoperatively, there will be some subsets at low risk of LRF, rendering radiochemotherapy unreasonable. However, other groups (including uT3,4 GEJ lesions) will have substantial risk of R1-R2 resections and LRF. Preoperative radiochemotherapy may more effectively reduce LRF in these cohorts by improving R0 resection rates. The trial described by Stahl et al44 is illustrative of this issue. Patients with lower esophageal/gastric cardia uT3-T4 lesions were randomly assigned to induction chemotherapy alone versus induction chemotherapy followed by low-dose radiochemotherapy. The addition of radiochemotherapy increased pathologic complete response rate, decreased node positivity, and improved 3-year OS from 28% to 47%. With regard to the high frequency of R1 resection in GEJ tumors noted in the MAGIC trial, Zhang et al45 reported improved survival with preoperative radiation alone in a phase III study of 370 patients with GEJ primaries. Similarly, the Gaast et al46 trial demonstrated improved OS with preoperative radiochemotherapy versus surgery alone in esophageal and gastroesophageal tumors. The MD Anderson Cancer Center has reported serial phase II studies44–49 of induction chemotherapy followed by radiochemotherapy before resection and observed high R0 resection rate and local control in patient cohorts with unfavorable prognoses. Preoperative radiochemotherapy can, therefore, be judiciously implemented with induction chemotherapy in settings in which induction chemotherapy alone will result in a high R1-R2 rate or in significant LRF after surgery. The current ongoing randomized trials of radiochemotherapy after induction chemotherapy will need to carefully evaluate cohorts with high risk of LRF. INT-0116 accrued only patients at high risk of LRF and established that radiochemotherapy improves locoregional control in such cohorts, which effectively improves RFS and OS. Many issues regarding optimization of radiochemotherapy remain, including optimal timing of radiation with respect to surgery.
Supplementary Material
Acknowledgment
Supported in part by Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA22433, CA04919, CA46441, CA20319, CA58348, CA46113, CA27057, CA32734, CA45450, CA58882, CA46368, CA63844, CA37981, CA58686, CA12644, CA58416, CA28862, CA42777, CA46136, CA45461, CA76447, CA45807, CA45377, CA35176, CA58658, CA58415, CA58723, CA16385, CA52654, CA35281, CA35192, CA76448, CA35261, CA67663, CA46282, CA12213, CA58861, CA35431, CA52420, CA35178, CA35090, CA52772, CA63845, CA67575, CA45560, CA76429, CA14028, CA35262, CA35117, CA33601, CA31946, CA21661, CA37422, CA 25224, CA15488, and CA21115 from the National Cancer Institute, Department of Health and Human Services.
Footnotes
See accompanying editorial on page 2297
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Scott A. Hundahl, Stewart Fund, Pathfinder Fund Expert Testimony: None Other Remuneration: Scott A. Hundahl, National Cancer Institute
AUTHOR CONTRIBUTIONS
Conception and design: Stephen R. Smalley, Jacqueline K. Benedetti, Daniel G. Haller, Norman C. Estes, Leonard L. Gunderson, James A. Martenson, Grant N. Stemmermann, John S. Macdonald
Administrative support: Jaffer A. Ajani, Grant N. Stemmermann
Provision of study materials or patients: Stephen R. Smalley, Scott A. Hundahl, James A. Martenson, Grant N. Stemmermann
Collection and assembly of data: Stephen R. Smalley, Jacqueline K. Benedetti, Scott A. Hundahl, Bryan Goldman, Grant N. Stemmermann, John S. Macdonald
Data analysis and interpretation: Stephen R. Smalley, Jacqueline K. Benedetti, Scott A. Hundahl, Jaffer A. Ajani, Bryan Goldman, James A. Martenson, J. Milburn Jessup, Grant N. Stemmermann, Charles D. Blanke, John S. Macdonald
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583–592. doi: 10.1097/00000658-199321850-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hundahl SA, Menck HR, Mansour EG, et al. The National Cancer Data Base report on gastric carcinoma. Cancer. 1997;80:2333–2341. doi: 10.1002/(sici)1097-0142(19971215)80:12<2333::aid-cncr15>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 4.Dockerty M. Pathologic aspects of primary malignant neoplasms of the stomach. In: ReMine W, Priestley J, Berkson J, editors. Cancer of the Stomach. Philadelphia, PA: WB Saunders; 1964. p. 173. [Google Scholar]
- 5.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: Areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy BJ. TNM classification for stomach cancer. Cancer. 1970;26:971–983. doi: 10.1002/1097-0142(197011)26:5<971::aid-cncr2820260503>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Mari E, Floriani I, Tinazzi A, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: A meta-analysis of published randomised trials. A study of GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente) Ann Oncol. 2000;11:837–843. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 8.Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: Revisiting a meta-analysis of randomized trials. Eur J Cancer. 1999;35:1059–1064. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 9.Hermans J, Bonenkamp JJ., Boon MC, et al. Adjuvant therapy after curative resection for gastric cancer: Meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–1447. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 10.Janunger K, Hafström L, Nygren P, et al. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309–326. doi: 10.1080/02841860151116385. [DOI] [PubMed] [Google Scholar]
- 11.GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 12.Shah MA, Ajani JA. Gastric cancer: An enigmatic and heterogeneous disease. JAMA. 2010;303:1753–1754. doi: 10.1001/jama.2010.553. [DOI] [PubMed] [Google Scholar]
- 13.Smalley S, Gunderson L. Stomach. In: Perez C, Brady L, editors. Principles and Practice of Radiation Oncology. ed 3. Philadelphia, PA: Lippincott-Raven; 1996. [Google Scholar]
- 14.Landry J, Tepper JE, Wood WC, et al. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357–1362. doi: 10.1016/0360-3016(90)90344-j. [DOI] [PubMed] [Google Scholar]
- 15.D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch Gastric Cancer Group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonemura Y, Wu CC, Fukushima N, et al. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132–137. doi: 10.1007/s10147-007-0727-1. [DOI] [PubMed] [Google Scholar]
- 19.Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–112. doi: 10.1002/bjs.1800750206. [DOI] [PubMed] [Google Scholar]
- 20.Robertson CS, Chung SC, Woods SD, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176–182. doi: 10.1097/00000658-199408000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi M, Abe M. Intra-operative radiotherapy for carcinoma of the stomach. Eur J Surg Oncol. 1986;12:247–250. [PubMed] [Google Scholar]
- 22.Moertel CG, Childs DS, Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–867. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 23.Hallissey MT, Dunn JA, Ward LC, et al. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: Five-year follow-up. Lancet. 1994;343:1309–1312. doi: 10.1016/s0140-6736(94)92464-3. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med, 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 24a.Lauren P. The two histologic main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Hundahl SA, Macdonald JS, Benedetti J, et al. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: The effect of undertreatment. Ann Surg Oncol. 2002;9:278–286. doi: 10.1007/BF02573066. [DOI] [PubMed] [Google Scholar]
- 26.Lévy E, Paillarse JM, Votan B. Efficacy of ondansetron in radiation-induced nausea and vomiting: Review of the literature. Bull Cancer Radiother. 1994;81:179–185. [PubMed] [Google Scholar]
- 27.Dhar DK, Kubota H, Tachibana M, et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59:18–23. doi: 10.1159/000012131. [DOI] [PubMed] [Google Scholar]
- 28.Coburn NG, Swallow CJ, Kiss A, et al. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143–2151. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 29.Orsenigo E, Tomajer V, Palo SD, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer. 2007;10:39–44. doi: 10.1007/s10120-006-0409-0. [DOI] [PubMed] [Google Scholar]
- 30.van de Velde C. Resection for gastric cancer in the community. Semin Oncol. 2005;32:S90–S93. doi: 10.1053/j.seminoncol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Helyer LK, O'Brien C, Coburn NG, et al. Surgeons' knowledge of quality indicators for gastric cancer surgery. Gastric Cancer. 2007;10:205–214. doi: 10.1007/s10120-007-0435-6. [DOI] [PubMed] [Google Scholar]
- 32.Coburn NG, Govindarajan A, Law CH, et al. Stage-specific effect of adjuvant therapy following gastric cancer resection: A population based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500–507. doi: 10.1245/s10434-007-9640-0. [DOI] [PubMed] [Google Scholar]
- 33.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J Clin Oncol. 2005;28:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 34.Le A, Berger D, Lau M, et al. Secular trends in the use, quality, and outcomes of gastrectomy for noncardia gastric cancer in the United States. Ann Surg Oncol. 2007;14:2519–2527. doi: 10.1245/s10434-007-9386-8. [DOI] [PubMed] [Google Scholar]
- 35.McCulloch P, Nita ME, Kazi H, et al. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2003;4:CD001964. doi: 10.1002/14651858.CD001964. [DOI] [PubMed] [Google Scholar]
- 36.Hundahl SA, Peeters KC, Kranenbarg EK, et al. Improved regional control and survival with “low Maruyama Index” surgery in gastric cancer: Autopsy findings form the Dutch D1–D2 Trial. Gastric Cancer. 2007;10:84–86. doi: 10.1007/s10120-007-0426-7. [DOI] [PubMed] [Google Scholar]
- 37.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279–1285. doi: 10.1016/j.ijrobp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Hartgrink HH, van de Velde CJ, Putter H, et al. Neo-adjuvant chemotherapy for operable gastric cancer: Long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643–649. doi: 10.1016/j.ejso.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organization for Research and Treatment of Cancer Randomized Trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu AW, Xu GW, Wang HY, et al. Neoadjuvant chemotherapy versus none for resectable gastric cancer. Cochrane Database Syst Rev. 2007;18:CD005047. doi: 10.1002/14651858.CD005047.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 43. Reference deleted.
- 44.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advance adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 45.Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of the gastric cardia (AGC): Report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929–934. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 46.Gaast AV, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patient with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study. J Clin Oncol. 2010;28(suppl):302s. abstr 4004. [Google Scholar]
- 47.Allal AS, Zwahlen D, Bründler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: Long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2005;63:1286–1289. doi: 10.1016/j.ijrobp.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 48.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–2780. doi: 10.1200/JCO.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Ajani J.A., Winter K., Okawara G.S., et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): Quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.