Abstract
Purpose: The aim of this study was to prepare the optimized oral paste formulation of Triamcinolone acetonide intended to be used in aphtous stomatitis.
Methods: Plastibases were prepared using mineral oil and polyethylene (95:5). Oral paste formulations were prepared with different mixtures of three hydrocolloids solids, including gelatin, pectin and sodium carboxymethylcellulose, with different ratios, as well as Plastibase. Long-term and short-term stability of prepared formulations were studied in the case of color and consistency of pastes. Franz diffusion cell and dialysis membrane were employed for release study. Release data were fitted in the kinetic models to find out the mechanism of drug release.
Results: Formulation containing 60% plastibase, 3.3% pectin, 6.6% gelatin and 30% carboxymethylcellulose showed desired durability of adhesion, spreadability and rheology property in healthy volunteers and was compared with reference formulation (Adcortyl®) in the case of release profile. Although, optimized formulation and Adcortyl followed the Higuchi and first order release kinetics respectively, optimized formulation showed similar release profile to reference formulation.
Conclusion: Optimized oral paste formulation of Triamcinolone Acetonide showed similar characteristics with reference formulation and could be used as an effective drug delivery system for the treatment of recurrent aphthous stomatitis.
Keywords: Aphthous stomatitis, Triamcinolone acetonide, Plastibase, Oral paste
Introduction
The mucosa lining of the oral cavity is susceptible to many inflammatory, atrophic and ulcerative conditions, including aphthous stomatitis, lichen planus, erythema multiforme and Behcet’s syndrome. Recurrent aphthous stomatitis (RAS), usually known as canker sores, is a disease of the oral mucosa involving the repeated development of one or more painful ulcers. The oral symptoms of RAS include pain, weakness and major alterations in oral functions, such as speech, chewing and swallowing. Despite multiple investigations about this disease, its etiology remains unknown.1-3 The main treatment strategy of RAS and other inflammatory conditions of the mouth is the use of corticosteroids. These corticosteroids are normally formulated in ointments, pastes, lozenges and mouthwashes, and applied topically in order to avoid systemic side effects.4-6 Triamcinolone Acetonide, a medium to high potency corticosteroid, is a fluorinated prednisolone derivative and considered an intermediate-acting glucocorticoid. It is effective in the treatment of dermatoses, asthma and allergic rhinitis and is used in the decreasing of the signs and symptoms of many oral inflammatory conditions, including RAS. Triamcinolone Acetonide dental paste was used for adjunctive treatment and temporary relief of symptoms associated with oral inflammation and gingival disorders.7-9 For effective therapy in conditions of the oral mucosa, the concentration of corticosteroid in the buccal mucosa should be preserved with minimal systemic absorption which could be achieved by incorporation of drugs in special dosage forms, resulting in enhanced mucosal concentrations of drugs with reduced systemic absorption. The amount of drug absorption is dependent to the time of exposure of the drug to the buccal membrane.10,11 Drug delivery systems for delivering drugs to the oral mucosa include mouthwashes, tinctures, buccal formulations and ointments. The contact time of mouthwash is very short as compared with the other dosage forms. However, addition of a mucoadhesive polymer in the formulations could provide longer contact time. Mucoadhesive polymers such as hydroxypropylcellulose, carbopol, sodium carboxymethylcellulose, gelatin and pectin have been employed in formulations used for the oral cavity.9,12,13 Tinctures are easily applied to oral lesions but may be swallowed within a rather short time period after application. Buccal formulations retain in the oral cavity for longer periods than tinctures, however, they are usually larger in size and thus uncomfortable to keep in the oral cavity. Conventional ointment formulations may be readily applied, however, in many cases, they removed from the oral cavity during speaking, eating or by salivation, as well as tongue movement and swallowing. In addition, bleeding of active ingredients from ointments during storage or after application to oral lesions results inefficient drug contact to the lesions and/or migration by saliva to other locations in the oral cavity.10,12,14-17 Therefore, a new formulation with suitable adhesion potential with capability for sustained drug release for a period of time is needed. The objective of the present study was to prepare an oromucoadhesive formulation of Triamcinolone Acetonide with desired release of Triamcinolone Acetonide.
Materials and Methods
Materials
Triamcinolone Acetonide was kindly donated from Jaber Ebne Hayyan Pharmaceutical Company (Iran). Gelatin was supplied from Fluka biochemika Company (Germany). Pectin (from citrus peel, Galacturonic acid ≥74.0 % (dried basis)) and high viscosity sodium carboxymethylcellulose (NaCMC) (average Mw ~700,000) were bought from Sigma-Aldrich (USA) and mineral oil was purchased from Merck Company (Germany). Low density and high density polyethylene were prepared from Tabriz Petrochemical Company (Iran). Adcortyl in Orabase (Triamcinolone Dental Paste) was provided from the market manufactured by Bristol-Myers Squibb Pharmaceuticals Ltd.
Preparation of Plastibase
Plastibases were prepared using mineral oil and polyethylene (95:5). Two types of polyethylene (PE), high density polyethylene (HDPE) and low density polyethylene (LDPE) were dissolved in mineral oil at 120-130 °C. After complete dissolving of polyethylene in mineral oil, the mixtures were cooled immediately by pouring the oil on a metal surface cold by ice. The rapid cooling process is crucial in an appropriate gel formation.
Preparation of oral paste
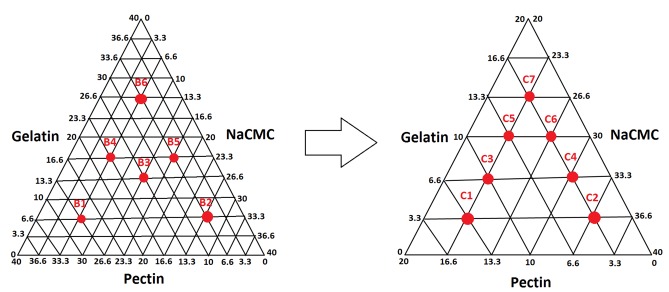
Oral drug delivery systems were provided by compositions which included three hydrocolloids solids, including gelatin, pectin and NaCMC, with different ratios, and base comprising of mineral oil containing polyethylene (Plastibase®). For prevention of formulations from hardening upon prolonged storage due to the presence of humidity, solid colloids were dried in an oven (60 °C) for at least 16 hrs. The first step (A) formulations (Table 1) were designed to find the most appropriate Plastibase® ratio. To obtain the best formulation with desired characteristics, different formulations with various ratios of gelatin, pectin and NaCMC were prepared at two pre-formulation stages, formulations B and C based on triangle phase diagram (Figure 1 and Table 2).
Table 1. Plastibase and colloid ratios of different formulation of pastes containing equivalent amount of gelatin, pectin and sodium carboxymethylcellulose.
| Formulation code | Plastibase: Colloids (w/w) |
| A1 | 30:70 |
| A2 | 40:60 |
| A3 | 50:50 |
| A4 | 60:40 |
| A5 | 70:30 |
| A6 | 80:20 |
Figure 1.
Formulation development of oromucoadhesive paste based on triangle phase diagram.
Table 2. Formulations with different composition of gelatin, pectin and sodium carboxymethylcellulose (NaCMC).
| Formulation code | Pectin (%w/w) | Gelatin (%w/w) | NaCMC (%w/w) |
| B1 | 26.6 | 6.6 | 6.6 |
| B2 | 6.6 | 6.6 | 26.6 |
| B3 | 13.3 | 13.3 | 13.3 |
| B4 | 16.6 | 16.6 | 6.6 |
| B5 | 6.6 | 16.6 | 16.6 |
| B6 | 6.6 | 26.6 | 6.6 |
| C1 | 13.3 | 3.3 | 23.3 |
| C2 | 3.3 | 3.3 | 33.3 |
| C3 | 10 | 6.6 | 23.3 |
| C4 | 3.3 | 6.6 | 30 |
| C5 | 6.6 | 10 | 23.3 |
| C6 | 3.3 | 10 | 26.6 |
| C7 | 3.3 | 13.3 | 23.3 |
In vitro release study
Dialysis membrane was mounted between the donor and receptor chamber of Franz diffusion cells filled with 50% ethanol solution in receptor phase ensuring that there were no air bubbles between the formulation and donor surface to keep the sink condition for Tramcinolone Acetonide. The surface area available for diffusion was 2.5 cm2 and receptor volume was 25 cm3. Accurately weighed of different formulations (0.5 g of Triamcinolone Acetonide paste, 0.1 % w:w) was applied to the surface of the dialysis membranes and spread by means of a spatula. The receptor fluid was maintained at 37 ± 0.5 °C and continuously stirred at 700 rpm using a transdermal tester (Erweka HDT6, Germany). Samples from receptor medium (2 mL) were withdrawn at specific time intervals over a 6 hrs period and immediately replaced with fresh prewarmed hydroethanolic solution. The samples were quantified spectrophotometrically at 240 nm using a calibration curve which was linear in the range of 1.25 - 20 µg/mL (r2=0.9997). Each formulation was investigated in 3 cells. Based on release study, dissolution parameters indicating drug release behavior were calculated and reported as the following. The dissolution efficiency (DE) of a pharmaceutical dosage form is defined as the area under the dissolution curve up to the time t, expressed as the percentage of the area of the rectangle:
Where y is the percentage of Triamcinolone Acetonide dissolved at time t.
Another approach to achieve a parameter that explains the dissolution rate is the mean dissolution time (MDT). This parameter is the most likely time taken for a molecule to be dissolved from a solid dosage form. In other words, MDT is the mean time for the drug to dissolve under in vitro dissolution conditions. This is calculated using the following equation:
Where j is the sample number, tj is the midpoint of the jth time period (easily calculated with ((t+t−1)/2) and Mj is the additional amount of drug dissolved between tj and t−1. The mean dissolution rate (MDR) can be calculated according to the following equation:
Where n is the number of dissolution sample times, t is the time at the midpoint between t and t−1 (easily calculated with [t+(t−1)/2]).
Stability study
Long-term and short-term stability of prepared formulations were studied in the case of color and consistency of pastes. Long-term stability of prepared formulations was conducted for 6 months at three temperatures, 4 °C, 25 °C and 40 °C. Short-term stability test was performed as four 24 hr temperature cycles (4 °C and 40 °C).
Drug release kinetic study
The dissolution results have served as a means to evaluate different formulations (Table 3). The kinetic models have been used in interpretation of drug release data and dissolution data of formulations were fitted to the kinetic models. The equations of the kinetic models and coefficient of correlation of each formulation are presented in Table 4.
Table 3. Drug dissolution parameters.
| Formulation code | DE (%)a | MDR (%.h-1)b | MDT (h)c |
| A1 | 28.9 | 9.3 | 2.23 |
| A2 | 48.4 | 15.7 | 2.22 |
| A3 | 44.2 | 14.7 | 2.08 |
| A4 | 41.7 | 13.3 | 1.76 |
| A5 | 36.4 | 11.9 | 2.03 |
| A6 | 13.9 | 4.6 | 1.87 |
| B1 | 40.9 | 13.4 | 2.06 |
| B2 | 34.5 | 11.3 | 2.17 |
| B3 | 34.1 | 11.2 | 2.23 |
| B4 | 23.2 | 7.5 | 2.08 |
| B5 | 34.3 | 11.6 | 1.55 |
| B6 | 34.3 | 11.6 | 1.55 |
| C1 | 43.5 | 14.2 | 2.18 |
| C4 | 34.6 | 11.5 | 2.24 |
| Adcortyl® | 30.0 | 11.15 | 2.24 |
aDissolution efficiency
bMean dissolution rate
cMean dissolution time
Table 4. Kinetic models used for analysis of selected formulations release data.
| Formulation code | Zero order (F=kt) | First order (ln (1-F) = - kft) | Higuchi (F= k t0.5) | |||
| MRSQ | K | MRSQ | K | MRSQ | K | |
| A1 | 0.955 | 0.144 | 0.986 | 0.001 | 0.996 | 2.865 |
| A2 | 0.960 | 0.192 | 0.998 | 0.004 | 0.996 | 4.811 |
| A3 | 0.973 | 0.143 | 0.997 | 0.002 | 0.999 | 3.558 |
| A4 | 0.915 | 0.001 | 0.958 | 0.002 | 0.979 | 0.031 |
| A5 | 0.978 | 0.001 | 0.995 | 0.001 | 0.993 | 0.283 |
| A6 | 0.979 | 0.003 | 0.982 | 0.004 | 0.983 | 0.008 |
| B1 | 0.978 | 0.003 | 0.999 | 0.002 | 0.998 | 0.329 |
| B2 | 0.990 | 0.001 | 0.999 | 0.002 | 0.990 | 0.282 |
| B3 | 0.960 | 0.001 | 0.990 | 0.002 | 0.998 | 0.033 |
| B4 | 0.957 | 0.001 | 0.976 | 0.001 | 0.998 | 0.205 |
| B5 | 0.972 | 0.004 | 0.997 | 0.003 | 0.998 | 0.034 |
| B6 | 0.954 | 0.001 | 0.974 | 0.001 | 0.994 | 0.184 |
| C1 | 0.974 | 0.001 | 0.999 | 0.002 | 0.999 | 0.032 |
| C4 | 0.977 | 0.001 | 0.977 | 0.002 | 0.999 | 0.032 |
| Adcortyl® | 0.985 | 0.001 | 0.998 | 0.002 | 0.996 | 0.349 |
F denotes fraction of drug released up to time t.
Statistical analysis
The data was demonstrated as mean ± SD. Analysis of variance (ANOVA) was performed for multiple comparisons (SPSS 15). A level of significance of P< 0.05 was set to determine any significant difference between the formulations.
Results and Discussion
The preparation of Plstibase with both LDPE and HDPE showed that LDPE provides better Plastibase based on the least paraffin oil leak during the storage for one month in different storage conditions. Preliminary studies revealed that formulations containing about 60% of Plastibase® and 40 % of solid colloids with equivalent amount of gelatin, pectin and NaCMC (A4), suitable characteristics (Table 1). In the next stage, B2 formulation was selected the most proper formulation in the point of mucoadhesion and consequently the next triangle phase diagram was designed based on B2 formulation. Finally, after further investigations on the formulations with different ratios of solid colloids, formulation C4 and C1 were chosen as a formulation with desired organoleptic properties durability of adhesion, spreadability and rheology property in healthy volunteers.
In vitro release study
Figure 2 illustrates the cumulative release of different formulations of Triamcinolone Acetonide over 6 hrs through the formulations with different base to colloids ratios. In formulations A2-A6, by increase in Plastibase® ratio drug release was decreased probably as a result of drug entrapment into PE network. The lower drug release rate observed in formulation A1 may be attributed to the high viscosity of paste caused by the presence of high amounts of hygroscopic colloids in the formulation composition. The lower DE and MDR values for formulations A1 and A6 than other formulations implies the sustained release pattern of these formulations (Table 3). Accordingly, in formulations B1-B6, by increasing concentration of gelatin and Sodium CMC concentrations a decrease in drug release were observed (Figure 3). The role of Sodium CMC in the control of drug release through the paste was much clear in the comparison of C1 and C4 in Figure 4. Both optimized formulations especially formulation C4 showed similar release profile to that of the reference product. The dissolution parameters (DE, MDT and MDR) corresponding to C4 were also similar to the reference commercial brand (Adcortyl®) (Table 3).
Figure 2.
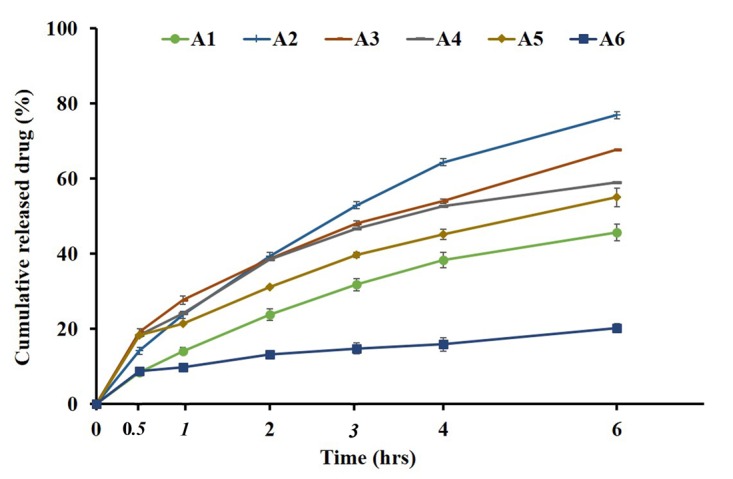
In vitro drug release patterns of different formulations vary in the base/mucoadhesive ingredients ratio.
Figure 3.

In vitro drug release patterns of different formulations vary in the ratios of mucoadhesive ingredients.
Figure 4.

In vitro drug release patterns of the optimized formulations and Reference formulation (Adcortyl®).
Stability study
Short-term stability results of optimized formulation (C4) showed that the consistency and odor of formulations did not change significantly. Accordingly, long-term storage of formulations at 4 °C and 25 °C did not change consistency and odor of formulations. However, storage of formulation at 40 °C caused to the change in consistency without any effect on odor.
Drug release kinetic study
Kinetic study of drug release is often useful for comparative purposes and relating to the release parameters with bioavailability. To clarify the mechanism of release, the in vitro release data were fitted to different kinetic models. The accuracy and prediction ability of the models were compared by calculation of mean squared correlation coefficients (MRSQ) for all data sets (Table 4). Our finding revealed that, almost in all of the formulations the drug release data were fitted best to Higuchi models, even though some formulations also follow relatively from first order model. Therefore, diffusion could be considered as a major mechanism of drug release from the different formulations made from Plastibase®. Release of drug from Adcortyl® and formulation C4 followed from first order and Higuchi release kinetics, respectively.
Conclusion
Oral paste formulations of Triamcinolone Acetonide were prepared using plastibase as well as different ratios of pectin, gelatin and carboxymethylcellulose. Formulations were optimized after preliminary studies and formulation with desired durability of adhesion, spreadability and rheology property was compared with reference formulation in the case of release profile and kinetic. Release study and fitting release data in to kinetics models revealed that optimized formulation followed Higuchi release kinetics and had similar release pattern to the reference formulation.
Acknowledgments
The authors would like to thank Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. This article is based on a thesis submitted for Pharm D degree (No. 3019) in Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Albrektson M, Hedstrom L, Bergh H. Recurrent aphthous stomatitis and pain management with low-level laser therapy: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):590–4. doi: 10.1016/j.oooo.2014.01.228. [DOI] [PubMed] [Google Scholar]
- 2.Porter SR, Hegarty A, Kaliakatsou F, Hodgson TA, Scully C. Recurrent aphthous stomatitis. Clin Dermatol. 2000;18(5):569–78. doi: 10.1016/s0738-081x(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 3.Ship JA. Recurrent aphthous stomatitis. An update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(2):141–7. doi: 10.1016/s1079-2104(96)80403-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Chien YW. Oral mucosa controlled delivery of LHRH by bilayer mucoadhesive polymer systems. J Control Release. 1995;37(3):251–61. doi: 10.1016/0168-3659(95)00082-8. [DOI] [Google Scholar]
- 5.Miles DA, Bricker SL, Razmus TF, Potter RH. Triamcinolone acetonide versus chlorhexidine for treatment of recurrent stomatitis. Oral Surg Oral Med Oral Pathol. 1993;75(3):397–402. doi: 10.1016/0030-4220(93)90158-z. [DOI] [PubMed] [Google Scholar]
- 6.Scully C, Porter S. Oral mucosal disease: recurrent aphthous stomatitis. Br J Oral Maxillofac Surg. 2008;46(3):198–206. doi: 10.1016/j.bjoms.2007.07.201. [DOI] [PubMed] [Google Scholar]
- 7.Browne RM, Fox EC, Anderson RJ. Topical triamcinolone acetonide in recurrent aphthous stomatitis. A clinical trial. Lancet. 1968;1(7542):565–7. doi: 10.1016/s0140-6736(68)92833-x. [DOI] [PubMed] [Google Scholar]
- 8.Quijano D, Rodriguez M. Topical corticosteroids in recurrent aphthous stomatitis. Systematic review. Acta Otorrinolaringol Esp. 2008;59(6):298–307. doi: 10.1016/s2173-5735(08)70242-4. [DOI] [PubMed] [Google Scholar]
- 9.Sveinsson SJ, Peter Holbrook W. Oral mucosal adhesive ointment containing liposomal corticosteroid. Intl J Pharm. 1993;95(1-3):105–9. doi: 10.1016/0378-5173(93)90396-w. [DOI] [Google Scholar]
- 10.Bernkop Schnurch A. Mucoadhesive systems in oral drug delivery. Drug Discov Today Technol. 2005;2(1):83–7. doi: 10.1016/j.ddtec.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Rossi S, Sandri G, Caramella CM. Buccal drug delivery: A challenge already won? Drug Discov Today Technol. 2005;2(1):59–65. doi: 10.1016/j.ddtec.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Smart JD. Drug delivery using buccal-adhesive systems. Adv Drug Deliv Rev. 1993;11(3):253–70. doi: 10.1016/0169-409x(93)90012-s. [DOI] [Google Scholar]
- 14.Giunchedi P, Juliano C, Gavini E, Cossu M, Sorrenti M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur J Pharm Biopharm. 2002;53(2):233–9. doi: 10.1016/s0939-6411(01)00237-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Chien YW. Development and evaluation of a mucoadhesive drug delivery system for dual-controlled delivery of nonoxynol-9. J Control Release. 1996;39(1):93–103. doi: 10.1016/0168-3659(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 16.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm. 1999;178(1):11–22. doi: 10.1016/s0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]