Abstract
Purpose: Nanogel, a nanoparticle prepared from a cross-linked hydrophilic polymer network, has many biomedical applications. A radiation technique has recently been introduced as one of the appropriate methods for the preparation of polymeric nanogels due to its additive-free initiation and easy control procedure.
Methods: We have investigated the formation of nano-sized polymeric gels, based on the radiation-induced inter- and intra-molecular cross-linking of the inter-polymer complex (IPC) of polyacrylamide (PAAm) and polyacrylic acide (PAAc).
Results: The results indicated that the prepared polymeric complex composed of PAAm and PAAc was converted into nanogel by irradiation under different doses (1, 3, 5 and 7 kGy). This was due to inter- and intra-molecular cross-linking at the range of 446-930 nm as characterized by the photon correlation spectroscopy method. Increasing the irradiation dose reduced the size of nanoparticles to 3 kGy; however, the higher doses increased the size and size distribution. Scanning electron microscopy images indicated the nanogel formation in the reported size by particle size and showed the microcapsule structure of the prepared nanogels. Biocompatibility of nanogels were assessed and proved by MTT assay.
Conclusion: It was concluded that low dose irradiation can be successfully applied for nanometre-ranged hydrogel.
Keywords: Nanogel, Cross-linked, Radiation, Inter-polymer complex
Introduction
Macroscopic hydrogels have been widely studied since the 1960s,1 and to date many applications have been revealed in this field, ranging from filler materials in the coating industry to modern biomaterials.2,3 A polymer gel is a two-compartmental system containing a stable three-dimensional network of linked polymer chains by chemical or physical bonds which is filled with solvent molecules. Nanogels are particles of polymer gels with submicrometre size dimensions.4 Nanogels have shown a rapid progress from being unwanted by-products of polymerization processes to an imperative subject of interdisciplinary research in different areas of polymer chemistry and physics, material, pharmaceutical and medical sciences. Some examples of biomedical applications of nanogels are their usages as potential gene and antisense delivery agents,5 toxin scavengers,6 carriers for encapsulation of enzymes to increase biocatalytic activity and stability,7 usage in cancer chemotherapy8 and controlling cholesterol.9 In these cases of biomedical application, the toxicity issues of materials are considered with more emphasis. The inter-polymer complex (IPC) established between polymer pairs through secondary binding forces, such as hydrogen bonding and electrostatic interaction, has been studied in various research areas, including biological sciences due to the key role of IPCs in biological systems.10,11 The most commonly used polymers for preparing IPCs through electrostatic interaction are polyacrylic acid (PAA) and polyacrylamide (PAAm).12 Ionizing radiation, such as gamma radiation, has been applied for the cross-linking of polymers by the different research groups.13,14 The advantage of this method is the ability for scaling up and the absence of both potentially toxic monomers and cross-linking agents. The only necessary materials are polymer molecules and water. The sterilization of products for biomedical purposes can be achieved by radiation. Radiation sterilization, as a physical cold procedure, has been extensively used in many developed and developing countries for the sterilization of health care products. The simultaneous application of radiation for nanogel preparation and sterilization is the main benefit of this method as well. The aim of this study is to prepare nanogels with IPC (a combination of electron-donor and electron-acceptor polymers) using the gamma radiation technique. The prepared nanogel was characterized with a fourier-transform-infrarot spectrometer (FTIR), scanning electron microscopy (SEM), size and zeta potential. MTT assay was carried out to evaluate the cell toxicity of the nanogels.
Materials and Methods
Materials
PAAm with the molecular weight over 150 kDa and PAAc with the molecular weight around 450 kDa were supplied both from Sigma Alderich (USA).
Nanogel Preparation
PAAm and PAAc were dissolved in different diluted concentrations in distilled water individually and then both were mixed with each other under gentle mixing at room temperature to prepare the IPC. The mixtures were kept refrigerated until irradiation to prevent the complex dissociation at elevated temperatures.11 The pH values of mixtures before and after irradiation were around 3.45 (Metrohm 825, Switzerland). The composition of IPCs and their characterization are mentioned in Table 1 in detail. The IPC of PAAc-PAAm in 15 ml glass tubes were irradiated at room temperature in an atmosphere of air with Co60 source (Gamma celle GC-220, Nordion, Canada) at different doses of 1, 3, 5 and 7 kGy in a dose rate of 4.52 Gy/s.
Table 1. Formulation composition and its size and zeta potential values before and after radiation; data are reported as mean ± standard deviation (n = 3).
| Formulation code | Irradiation (kGy) | e PdI | Z-average (nm) | Peak-1 | Peak-2 | Zeta potential (mV) | ||
| Size (nm) | Intensity (%) | Size (nm) | Intensity (%) | |||||
| a F1 | 0 | 0.83 ± 0.06 | 886 ± 28 | 411 ± 21 | 85 ± 0.3 | 66 ± 11 | 16 ± 0.9 | -36 ± 2.1 |
| 1 | 0.63 ± 0.05 | 584 ± 41 | 424 ± 26 | 63 ± 0.5 | 4234 ± 21 | 35 ± 2.7 | -31 ± 1.8 | |
| 3 | 0.54 ± 0.04 | 529 ± 39 | 207 ± 28 | 59 ± 0.4 | 4300 ± 28 | 39 ± 3.5 | -22 ± 1.2 | |
| 5 | 0.64 ± 0.07 | 886 ± 26 | 318 ± 31 | 61 ± 2.6 | 4602 ± 32 | 37 ± 2.2 | -32 ± 1.9 | |
| 7 | 0.68 ± 0.05 | 930 ± 23 | 532 ± 39 | 55 ± 0.3 | 4850 ± 24 | 36 ± 2.1 | -30 ± 2.3 | |
| b F2 | 0 | 1.12 ± 0.09 | 870 ± 33 | 429 ± 37 | 63 ± 0.8 | 45 ± 12 | 24 ± 0.9 | -35 ± 2.7 |
| 1 | 0.66 ± 0.08 | 653 ± 41 | 469 ± 41 | 75 ± 0.2 | 5316 ± 24 | 23 ± 1.8 | -33 ± 2.6 | |
| 3 | 0.59 ± 0.06 | 618 ± 24 | 270 ± 36 | 59 ± 0.9 | 3905 ± 31 | 44 ± 3.4 | -34 ± 2.9 | |
| 5 | 0.81 ± 0.08 | 621 ± 12 | 378 ± 28 | 54 ± 0.9 | 4852 ± 41 | 39 ± 2.6 | -34 ± 3.4 | |
| 7 | 0.89 ± 0.09 | 661 ± 27 | 558 ± 31 | 57 ± 0.7 | 5461 ± 34 | 41 ± 3.2 | -33 ± 1.4 | |
| c F3 | 0 | 0.67 ± 0.07 | 768 ± 19 | 480 ± 42 | 85 ± 1.1 | 62 ± 14 | 14 ± 0.9 | -37 ± 3.8 |
| 1 | 0.55 ± 0.06 | 553 ± 32 | 263 ± 39 | 74 ± 0.4 | 5101 ± 27 | 22 ± 1.2 | -32 ± 3.1 | |
| 3 | 0.41 ± 0.04 | 492 ± 26 | 268 ± 29 | 66 ± 1.2 | 4041 ± 36 | 31 ± 2.8 | -30 ± 1.9 | |
| 5 | 0.47 ± 0.05 | 536 ± 31 | 340 ± 42 | 51 ± 1.6 | 3757 ± 38 | 39 ± 2.9 | -35 ± 2.6 | |
| 7 | 0.55 ± 0.06 | 561 ± 42 | 385 ± 39 | 56 ± 0.9 | 4324 ± 29 | 42 ± 3.4 | -29 ± 2.1 | |
| d F4 | 0 | 0.71 ± 0.07 | 781 ± 34 | 519 ± 28 | 85 ±1.3 | 69 ± 10 | 11 ± 0.6 | -36 ± 2.8 |
| 1 | 0.61 ± 0.06 | 567 ± 26 | 243 ± 19 | 75 ± 1.7 | 5242 ± 22 | 23 ± 1.1 | -34 ± 3.2 | |
| 3 | 0.55 ± 0.06 | 446 ± 35 | 178 ± 21 | 74 ± 0.5 | 5201 ± 27 | 21 ± 1.6 | -29 ± 2.7 | |
| 5 | 0.58 ± 0.06 | 487 ± 22 | 310 ± 33 | 72 ± 1.6 | 5001 ± 19 | 28 ± 1.3 | -29 ± 3.1 | |
| 7 | 0.57 ± 0.06 | 510 ± 31 | 379 ± 31 | 67 ± 0.8 | 4631 ± 23 | 31 ± 2.6 | -30 ± 3.1 | |
a F1: Polyacrylamide (PAAm):Polyacrylic acid (PAAc) - 1:1 (w:w), b F2: PAAm: PAAc –1:0.8 (w:w), c F3: PAAm: PAAc – 0.8:1 (w:w), d F4: PAAm: PAAc – 0.6:1 (w:w), e Polydispersity index
Size and Zeta Potential Measurement
Sizes based on z-average and zeta potentials of the IPCs and prepared nanogels by irradiation were measured by photon correlation spectroscopy (PCS, Zetasizer-ZS, Malvern Instrument, Malvern, UK). Samples were diluted in distilled water without using a sonicator to avoid any effect of sonication on the results. Measurements were repeated 5-10 min apart to ensure that no dissolution or agglomeration of particles occurred. Each sample was measured in triplicate.
FTIR (Fourier-Transform-Infrared spectrometer)
FTIR spectrophotometry was used to study the possible interaction between PAAc and PAAm in IPC and nanogel. PAAc, PAAm, IPC and nanogel were mixed with KBr and samples were pressed to disk. Infrared (IR) spectra of samples were scanned in the range from 400 to 4000 cm-1 and recorded on a FTIR spectrometer (Shimadzu 8400, Kyoto Japan). FTIR spectra were obtained at a resolution of 4 cm-1 with a minimum of 256 scan per spectrum. All measurements were taken at room temperature. An empty KBr disk was used as reference and its spectrum was subtracted from the sample spectrum to suppress the spectral artifacts caused by KBr impurities and water.
Scanning Electron Microscopy
The shape and surface morphology of the particles were studied by a scanning electron microscope (MV2300, Czech Republic). Prior to scanning, the samples were coated with a thin layer of gold, using a direct current sputter technique (EMITECH K450X, England). The surface topographies of the carriers and the attachment of microparticles to the carrier surface were assessed qualitatively from the taken photomicrographs.
Cell Viability Assessment (In Vitro Cytotoxicity Assay)
The in vitro cytotoxicity was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay.15 The in vitro cytotoxicity was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A549 human lung cancer cells were cultured in RPMI 1640 (Roswell Park Memorial Institute’s Medium) supplemented with 10% FBS (Fetal Bovine Serum) at 37 °C and 5% CO2. At the 80% confluency, the cells were seeded at the density of 2×104 cell/cm2 on 96-well plates and were incubated. The cells were exposed with different dilution ratios of nanogel formulation (60-300 µl/ml) and were incubated for 24, 48 and 72 hours at 37°C. Then, the cells were washed once with phosphate buffered saline (PBS, pH=7.2, 10 mM) and culture medium was replaced with 150 µl fresh media and 50 µl MTT reagent (2 mg/ml in PBS). After 4 hours incubation at 37°C, medium was removed and the cells were exposed to 200 µl DMSO and 25 µl of Sorenson buffer (0.1 M glycine, 0.1 M NaCl, pH 10.5). The cultures were incubated for 30 minutes at 37°C to ensure dissolving of formazan crystals and then absorbance was measured at 570 nm (absorbance value) using a spectrophotometric plate reader, ELx 800 (Biotek, CA, USA). The following formula was used to calculate the cell viability of each group:
| Eq1 |
Statistical Analysis
All results are expressed as mean ± standard deviation (SD). An independent Student’s t-test was used to compare the mean differences between two independent groups and a one-way analysis of variance (one-way ANOVA) for multiple comparisons. When the differences between the means were significant, post hoc pair wise comparisons were carried out using Tukey multiple comparison tests (SPSS, version 13.0, Chicago, IL, USA). For all the statistical tests performed, the level of significance (P) was set at > 0.05.
Results
Radiation-Induced Formation of PAAc–PAAm Nanogels
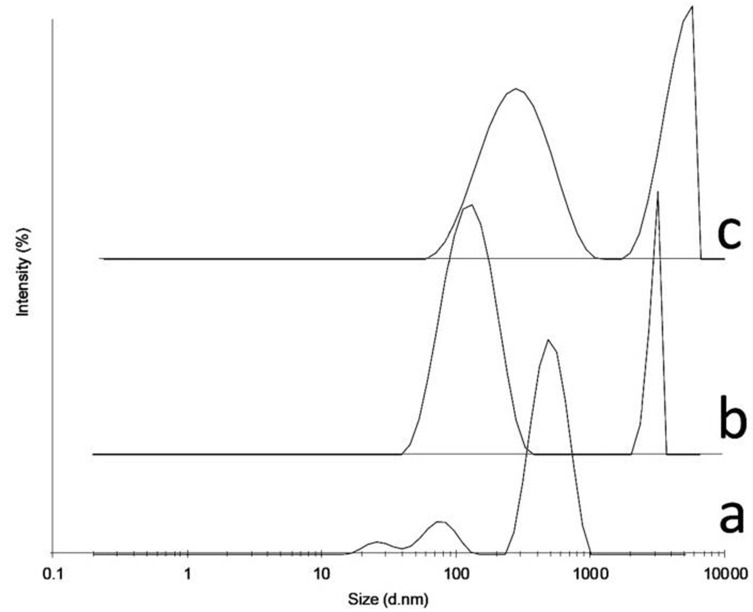
Zeta potential and size results for IPCs and nanogels at different irradiation doses are presented in Table 1. Zeta potential values for PAAc-PAAm complexes are in a negative range between -22 mV and -37 mV. IPCs in all formulations (F1-F4) showed higher amounts (more negative) than irradiated formulations (nanogels). Zeta potential did not show a direct relationship with irradiation dose, but in general it can be concluded that zeta potential of particles increased due to the positive values by irradiation. IPC formation is confirmed by size data. Table 1 shows that IPCs have a size range around 768-886 nm. The lower size of particles after irradiation confirmed the nanogel preparation. Among the different applied doses, the dose of 3 kGy resulted in the lowest particle size. The highest ratio of PAAc to PAAm (1:0.6) resulted in lower particle size after irradiation with the 3 kGy dose. Therefore, F4 formulation was selected for further investigations as the optimized formulation. The comparison of size patterns of F4 formulation in different conditions (before irradiation (IPC), after irradiation with the 3 kGy and 7 kGy doses) in Figure 1 indicated that the size was reduced after irradiation at lower doses, while an increase in irradiated dose resulted in elevated size compared to low irradiation dose.
Figure 1.
The particle size distributions of IPC (inter polymer complexe) (a), nanogel (F4) irradiated with 3 kGy (b) and 7 kGy (c)
Morphology
Scanning electron microscopy (SEM) was used to characterize the morphology of PAAc-PAAm complexes and nanogels (F4). Figure 2 shows SEM micrographs of IPC (a), nanogel irradiated with 1 kGy (b) and 3 kGy (c). The formation of IPC was confirmed by Figure 2a. As shown in Figure 2, the particles were spherical and had a smooth surface without pores or cavities. The same appearance was observed for all formulations prepared under various irradiation doses. The SEM micrographs also revealed that nanogels have a capsule structure rather than a spherical structure.
Figure 2.
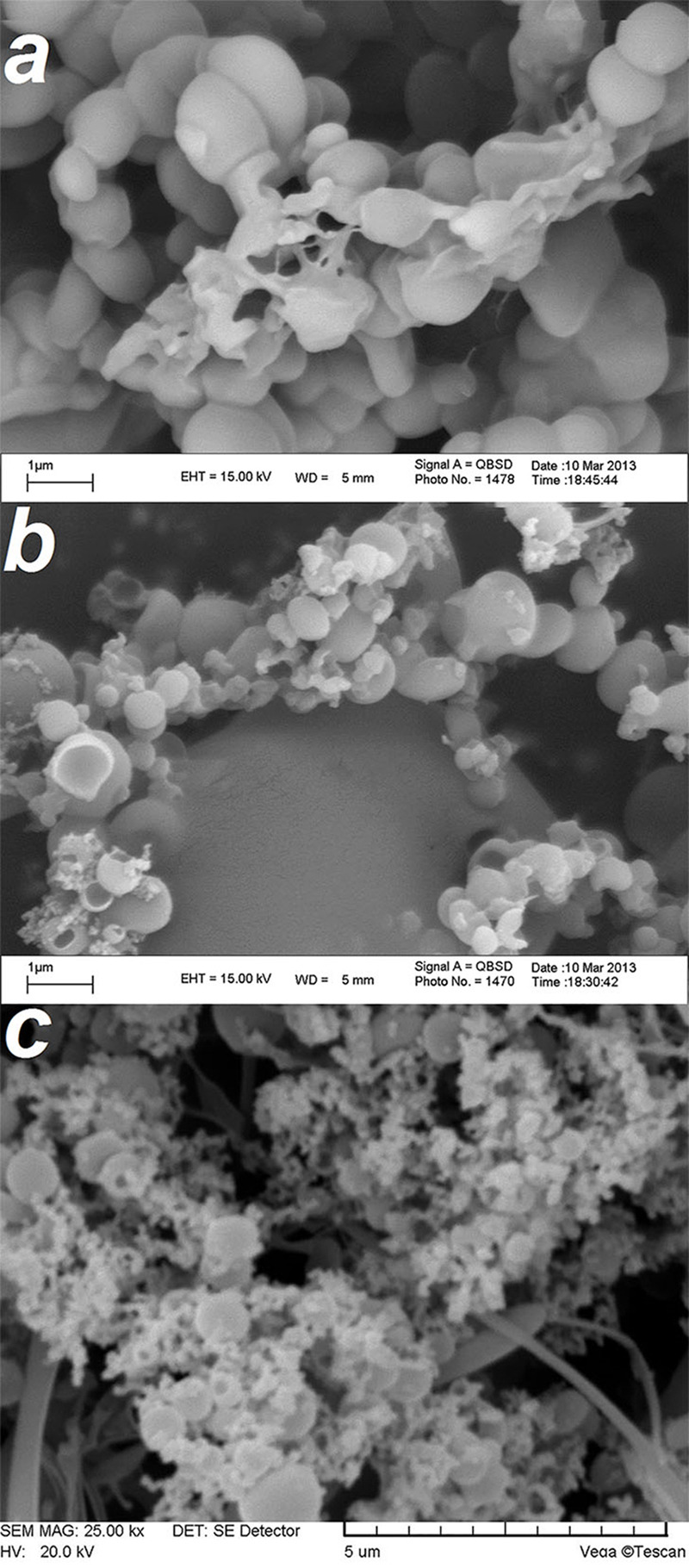
SEM micrographs of IPC (inter polymer complexe) (a), nanogel (F4) irradiated with 1 kGy (b) and 7 kGy (c).
FTIR Analysis
The absorption infrared spectra of PAAc, PAAm, IPC and nanogels irradiated with the 3 kGy dose are shown in Figure 3. The absorption infrared spectrum of PAAc shows a peak at 1710 cm−1 for the C=O of the carboxylic group and a broad region at 3201 cm−1, which can be allocated to the –OH of the carboxylic group; peaks at 1172 cm-1 and 1254 cm-1 are attributed to the C-O of the carboxylic group or may be related to the coupling between inplane OH bending and COO stretching vibrations of neighbouring carboxyl groups. Weaker bands associated with scissor and bending vibrations of -CH2- and CH-CO groups are located at 1452 and 1417 cm-1, respectively. The absorption infrared spectrum of PAAm bands located at 3349, 3190, 1675, and 1613 cm-1 are attributed to the asymmetric and symmetric NH2 stretching vibrations, amide I (-CO and -CN), and amide II (-NH and -CN), respectively. The bands located at 3349 and 3190 cm-1 are attributed to tensional stretching of –NH amide I and at 1675 to tensional stretching of C=O amide I. The band at 1613 is for flexural stretching of –NH amide I and II. The bands at 1441, 1351 and 1282 are ascribed to the C-N groups of amides. The band descriptions of PAAc and PAAm FTIR spectrums are matched with previously reported articles.14,16 The band related to tensional stretching of –NH amide I appeared at 3430 cm-1 in IR spectra of the IPC, which shows a shift from 3349 cm-1 of its location at IR spectra of PAAm. The band related to –OH groups with Hydrogen bonds appeared at 3430 and 3222 cm-1 in IR spectra of the IPC. The band appeared in 3201 cm-1 in PAAc IR spectra related to -OH stretching of the carboxylic group was shifted to 2937 cm-1 in IR spectra of IPC. The comparison of IR spectra of nanogel with IPC showed that the band appears in 1251 cm-1, attributed to the coupling between inplane OH bending and COO stretching vibrations of neighbouring carboxyl groups in IPC spectra, was moved to 1213 cm-1 in nanogel IR spectra.
Figure 3.
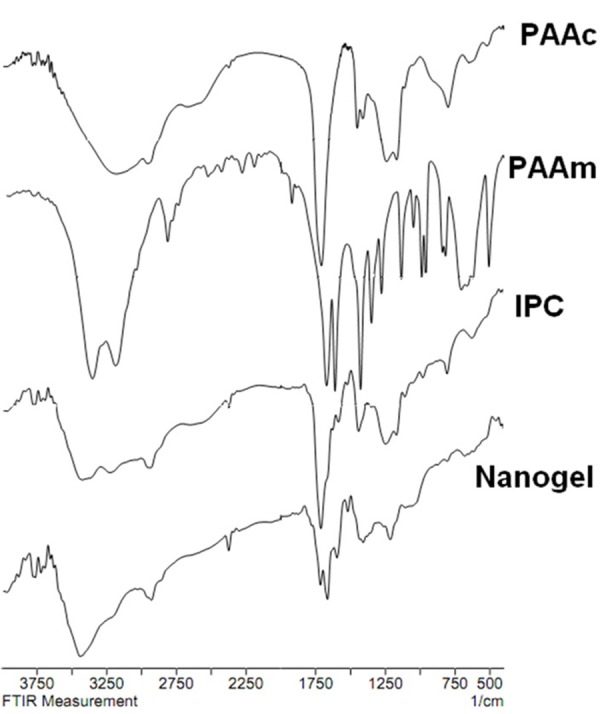
FTIR spectrum of Polyacrylicacid (PAAc), Polyacrylamide (PAAm), Inter polymer complex of PAA-PAAm (IPC) and Nanogel (F4).
MTT Assay
The cell viability is assayed to estimate the toxicity of nanogel (PAAc-PAAm) in different concentrations (60, 120, 180, 240 and 300 µl of final product/ml) quantitatively by MTT assay. The samples did not display any cytotoxicity to A549 cells (no significant differences in the proliferation of the cells) up to 72 h as statistically compared with the control (P>0.05) (Figure 4).
Figure 4.
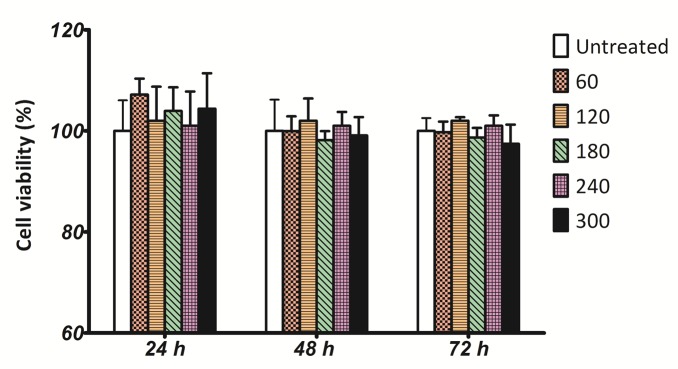
Cell viability values (%) estimated by MTT proliferation tests versus incubation time (h) of PAAc-PAAm nanogels (F4) with various dilution ratios (60, 120, 180, 240, 300 μl/mL). All values are reported as the mean ± standard deviation (n=6).
Discussion
The application of irradiation for hydrogel preparation is generally based on cross-linking reactions caused by inter- and intra-molecular recombination of polymer radicals. Several cross-linking techniques, such as ionic cross-linking, self-assembly, crystallization, polymerization cross-linking, radiation cross-linking and functional group cross-linking can be applied to fabricate nanogels.17 Polymer cross-linking with the aid of radiation has been reported as a proper method for preparing hydrogels and nanogels of different types of polymers, e.g., neutral hydrophilic polymers such as poly(vinyl alcohol),18 poly(vinyl methyl ether),13 as well as synthetic polyelectrolytes like PAAc,10,12,19 PAAm20 and Polyvinylpyrrolidone (PVP).12 The combination of PAAc with a polymer with an opposite charge such as PVP has also been used for preparation of nanogels with the irradiation technique.10,21 Facile process control, sterilization in one technological step accompanying with nanogel formation, as well as a cost-benefit procedure without wastage or the need to add chemical initiators or cross-linking agents, which are mostly toxic materials, offer the irradiation method as an advantageous choice in the synthesis of nanogels.10,22 In this study, IPC (formed with PAAc and PAAm) in different concentrations were irradiated in different doses to prepare different nanogels (Table 1). The size reduction after the radiation can be attributed to the creation of inter- and intra-molecular cross-linking in IPC. When IPC of PAAc and PAAm is exposed to gamma radiation in a dilute aqueous solution, the radiation energy is mostly absorbed by water. Accordingly, free radicals of hydroxyl radicals are formed, which are capable of getting hydrogen atoms out of macromolecules, producing polymer radicals and finally creating a nanogel.12,22 Nanoparticles with a zeta potential above (±) 30 mV have been shown to be stable in colloidal systems.23 The zeta potential results of formulations are mostly less than –30 mV, thus the electrostatic effect contributes significantly to their stability against aggregation. The combination of PAAc and PAAm resulted in the preparation of IPCs of these polymers with sizes around 800 nm in z-average and 450 nm in the most dominant peak. It was shown that a stable complex of PAAc-PAAm was formed at pH below 4 (pH range of our experiments).24,25 Table 1 and Figure 1 indicate the initial decrease in the size of particles after low doses (1 and 3 kGy) followed by a size increase after higher irradiation doses (5 and 7 kGy). These findings can be interpreted by the fact that intra-molecular recombination in dilute polymer solutions was established by low irradiation doses, while high doses were responsible for intermolecular cross-linking as well.19 As F4 (fabricated with the highest weight ratio of PAAc to PAAm) showed the lowest size after irradiation with the 3 kGy dose among all formulations, it was selected for further studies (FTIR, SEM and MTT assay). SEM images of nanogels showed a good agreement with the obtained results from particle size analysis. The microcapsule structure of nanogels offers a suitable potential to carry hydrophilic active ingredients. The results of FTIR experiments indicated that ionic interaction and hydrogen bonding both played the primary roles in IPC and nanogel formation. Hydrogen bonding was introduced as the primary mechanism of interaction among these polymers.26 Our findings in comparison of IR spectra of IPCs and nanogels confirm the primary role of hydrogen bonding in nanogel formation after irradiation. This may be interpreted by the fact that inter- and intra-molecular interaction formation following the irradiation creates a near "wall to wall" contact between PAAc and PAAm, which in turn enhances the formation of new hydrogen bonding between these polymers. The energy of a single hydrogen bond is relatively low (2–167 kJ/mol) and its length is in the 1.2–3.0 Å range.27 However, in the case of simultaneous formation of a large number of intermolecular hydrogen bonds between two macromolecules, the strength of the interaction is considerable, which leads to the formation of an adequately stable ladder-type structure of IPC. These ladder-type structures begin compacting after irradiation to lower sizes with the formation of intramolecular cross-linking. The question posed in the present work is whether synthesized nanogel is nontoxic or toxic. The toxicity of nanogels is very important, especially when they are intended for a medical application. Therefore, the nontoxic characteristics concluded from MTT assay for our prepared nanogels suggested their potential for biomedical application. Using the MTT assay, the polymer-treated cells were able to metabolize the mitochondrial substrate MTT by conversion into formazan crystals, and no damage was observed at the intracellular level. This metabolic activity of cells is a suitable method for evaluating the number of viable cells, since damaged or dead cells do not show any mitochondrial dehydrogenase activity.28,29 In a study, the cytotoxicity of nanometre-sized PAAc hydrogels synthesized by emulsion polymerization of methyl acrylate against T-lymphoblastic Jurkat cells was conducted using the MTT assay method. It was shown that all tested concentrations were non-toxic, even after 72 h of treatment of the cells with PAAc nanogels.30 It has been proposed that nanogels prepared from synthetic polymers offer well-defined morphologies that can be customized to gel networks with biocompatible and degradable characteristics.17
Conclusion
It was shown that PAAc formed complexes in dilute aqueous solution with PAAm. The complexes could be internally cross-linked by gamma irradiation in an aqueous solution. It is suggested that the radiation method can be a practical and feasible way to prepare composite nanogels based on complexes of macromolecules. A smaller particle size and biodegradability and/or biocompatibility have been suggested as the main common characteristics of nanogels, which are well matched with our PAAc-PAAm nanogel prepared by the irradiation technique.
Acknowledgments
This project is financially supported by the Drug Applied Research Center of Tabriz University of Medical Sciences.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Wichterle O, Lim D. Hydrophilic Gels for Biological Use. Nature. 1960;185(4706):117–8. doi: 10.1038/185117a0. [DOI] [Google Scholar]
- 2.Rosiak JM. Hydrogel dressings. In: Clough RL, Shalaby SW, editors. Radiation Effects on Polymers. Washington D.C: ACS; 1991. [Google Scholar]
- 3.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17(16):1553–61. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 4.Peppas NA, Lustig SR. Solute diffusion in hydrophilic network structures. In: Peppas NA, editor. Hydrogels in Medicine and Pharmacy. Boca Raton, Florida: CRC Press; 1986. [Google Scholar]
- 5.Mcallister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ. et al. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J Am Chem Soc. 2002;124(51):15198–207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- 6.Somasundaran P, Liu F, Chakraborty S, Deo N, Somasundaram T, Gryte C. Polymeric Matrices and Drug Particle Engineering. In: Svenson S, editor. Polymeric Drug Delivey II. New York: American Chemical Society; 2006. [Google Scholar]
- 7.Yan M, Ge J, Liu Z, Ouyang P. Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J Am Chem Soc. 2006;128(34):11008–9. doi: 10.1021/ja064126t. [DOI] [PubMed] [Google Scholar]
- 8.Kohli E, Han HY, Zeman AD, Vinogradov SV. Formulations of biodegradable Nanogel carriers with 5'-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Control Release. 2007;121(1-2):19–27. doi: 10.1016/j.jconrel.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nizam El-Din HMM, El-Naggar AWM. Radiation synthesis of acrylic acid/polyethyleneimine interpenetrating polymer networks (IPNs) hydrogels and its application as a carrier of atorvastatin drug for controlling cholesterol. Eur Polym J. 2012;48(9):1632–40. doi: 10.1016/j.eurpolymj.2012.06.014. [DOI] [Google Scholar]
- 10.Ulanski P, Kadlubowski S, Rosiak JM. Synthesis of poly (acrylic acid) nanogels by preparative pulse radiolysis. Radiat Phys Chem. 2002;63(3-6):533–7. doi: 10.1016/s0969-806x(01)00549-7. [DOI] [Google Scholar]
- 11.Khutoryanskiy VV. Hydrogen-bonded interpolymer complexes as materials for pharmaceutical applications. Int J Pharm. 2007;334(1-2):15–26. doi: 10.1016/j.ijpharm.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Ulanski P, Rosiak JM. The use of radiation technique in the synthesis of polymeric nanogels. Nucl Instrum Methods Phys Res B. 1999;151(1-4):356–60. doi: 10.1016/s0168-583x(99)00085-3. [DOI] [Google Scholar]
- 13.Schmidt T, Janik I, Kadlubowski S, Ulanski P, Rosiak JM, Reichelt R. et al. Pulsed electron beam irradiation of dilute aqueous poly (vinyl methyl ether) solutions. Polymer. 2005;46(23):9908–18. doi: 10.1016/j.polymer.2005.07.077. [DOI] [Google Scholar]
- 14.Abd El-Rehim HA, Hegazy ESA, Hamed AA, Swilem AE. Controlling the size and swellability of stimuli-responsive polyvinylpyrrolidone–poly (acrylic acid) nanogels synthesized by gamma radiation-induced template polymerization. Eur Polym J. 2013;49(3):601–12. doi: 10.1016/j.eurpolymj.2012.12.002. [DOI] [Google Scholar]
- 15.Eskandani M, Hamishehkar H, Ezzati Nazhad Dolatabadi J. Cyto/Genotoxicity study of polyoxyethylene (20) sorbitan monolaurate (tween 20) DNA Cell Biol. 2013;32(9):498–503. doi: 10.1089/dna.2013.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao XC, Chu LY, Chen WM, Zhu JH. Monodispersed thermoresponsive hydrogel microspheres with a volume phase transition driven by hydrogen bonding. Polymer. 2005;46(9):3199–209. doi: 10.1016/j.polymer.2005.01.075. [DOI] [Google Scholar]
- 17.Yallapu MM, Jaggi M, Chauhan SC. Design and engineering of nanogels for cancer treatment. Drug Discov Today. 2011;16(9-10):457–63. doi: 10.1016/j.drudis.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao RS, You QD, Liu PJ, Xu YF. Synthesis and pH-induced phase transition behavior of PAA/PVA nanogels in aqueous media. J Appl Polym Sci. 2009;111(1):358–62. doi: 10.1002/app.29078. [DOI] [Google Scholar]
- 19.Kadlubowski S, Grobelny J, Olejniczak W, Cichomski M, Ulanski P. Pulses of fast electrons as a tool to synthesize poly (acrylic acid) nanogels. intramolecular cross-linking of linear polymer chains in additive-free aqueous solution. Macromolecules. 2003;36(7):2484–92. doi: 10.1021/ma021628s. [DOI] [Google Scholar]
- 20.Abd El-Rehim HA. Swelling of radiation crosslinked acrylamide-based microgels and their potential applications. Radiat Phys Chem. 2005;74(2):111–7. doi: 10.1016/j.radphyschem.2005.01.002. [DOI] [Google Scholar]
- 21.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog Polym Sci. 2008;33(4):448–77. doi: 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- 22.Darwis D. Role of radiation processing in production of hydrogels for medical applications. Atom Indones. 2009;35(2):85–104. [Google Scholar]
- 23.Mohanraj VJ, Chen Y. Nanoparticles - A review. Trop J Pharm Res. 2006;5(1):561–73. [Google Scholar]
- 24.Sivadasan K, Somasundaran P, Turro NJ. Fluorescence and viscometry study of complexation of poly (acrylic acid) with poly (acrylamide) and hydrolysed poly (acrylamide) Colloid Polym Sci. 1991;269(2):131–7. doi: 10.1007/bf00660302. [DOI] [Google Scholar]
- 25.Sudre G, Tran Y, Creton C, Hourdet D. pH/Temperature control of interpolymer complexation between poly(acrylic acid) and weak polybases in aqueous solutions. Polymer. 2012;53(2):379–85. doi: 10.1016/j.polymer.2011.11.055. [DOI] [Google Scholar]
- 26.Moharram MA, Rabie SM, El-Gendy HM. Infrared spectra of gamma-irradiated poly (acrylic acid)–polyacrylamide complex. J Appl Polym Sci. 2002;85(8):1619–23. doi: 10.1002/app.10702. [DOI] [Google Scholar]
- 27.Desiraju GR. Hydrogen bonding. In: Atwood JL, Steed JW, editors. Encyclopedia of Supramolecular Chemistry. New York: Marcel Dekker Inc; 2004. [Google Scholar]
- 28.Liu P, Davis P, Liu H, Krishnan TR. Evaluation of cytotoxicity and absorption enhancing effects of melittin - a novel absorption enhancer. Eur J Pharm Biopharm. 1999;48(1):85–7. doi: 10.1016/s0939-6411(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 29.Eskandani M, Nazemiyeh H. Self-reporter shikonin-Act-loaded solid lipid nanoparticle: formulation, physicochemical characterization and geno/cytotoxicity evaluation. Eur J Pharm Sci. 2014;59:49–57. doi: 10.1016/j.ejps.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Argentiere S, Blasi L, Ciccarella G, Barbarella G, Cingolani R, Gigli G. Nanogels of poly(acrylic acid): Uptake and release behavior with fluorescent oligothiophene-labeled bovine serum albumin. J Appl Polym Sci. 2010;116(5):2808–15. doi: 10.1002/app.31691. [DOI] [Google Scholar]