Abstract
Purpose: Recent developments in the field of cell therapy have led to a renewed interest in treatment of acute kidney injury (AKI). However, the early death of transplanted mesenchymal stem cells (MSCs) in stressful microenvironment of a recipient tissue is a major problem with this kind of treatment. The objective of this study was to determine whether overexpression of a cytoprotective factor, nuclear factor erythroid-2 related factor 2 (Nrf2), in MSCs could protect rats against AKI.
Methods: The Nrf2 was overexpressed in MSCs by recombinant adenoviruses, and the MSCs were implanted to rats suffering from cisplatin-induced AKI.
Results: The obtained results showed that transplantation with the engineered MSCs ameliorates cisplatin-induced AKI. Morphologic features of the investigated kidneys showed that transplantation with the MSCs in which Nrf2 had been overexpressed significantly improved the complications of AKI.
Conclusion: These findings suggested that the engineered MSCs might be a good candidate to be further evaluated in clinical trials. However, detailed studies must be performed to investigate the possible carcinogenic effect of Nrf2 overexpression.
Keywords: NF-E2-Related Factor 2(Nrf2), Mesenchymal Stromal Cells(MSCs), Acute Kidney Injury, Cisplatin
Introduction
Acute kidney injury (AKI) is described as a sudden and prolonged decrease in glomerular filtration rate. AKI is induced by various reasons including low blood volume from any cause, exposure to substances harmful to the kidney, and urinary tract obstruction. Tubular necrosis and apoptosis, changes of the filtration barrier, glomerular misfiltration, vasoconstriction and tubular obstruction, interstitial swelling and activation of proteolytic enzymes characterize the injury.1-3 AKI has been reported in 5 to 7% of hospitalized patients and over 30% of ICU hospitalized patients. The mortality rate of patients with AKI is about 50% and in cases in which dialysis is needed the mortality rate could reach 88%.4 Therefore, AKI has attracted much research interests in recent years because of its significant mortality rate.
A novel strategy for treatment of AKI is MSC transplantation. Promising results of preclinical studies, ease of handling and enormous expansion potential of MSCs, and encouraging preliminary clinical trials of MSC transplantation in other diseases, have made the MSC based approaches very interesting for the treatment of human kidney injury.5,6 According to numerous studies, MSCs exert their repairing effects on kidney via two important mechanisms including repopulation and paracrine actions.6-8
The pathogenesis of AKI is complex, however, it has been shown that ischemia/reperfusion triggers AKI, mainly via aggravating stresses, inflammation and activation of renin–angiotensin system (RAS).9 Hypoxia, serum deprivation and oxidative stress are important factors in the pathogenesis of AKI.10-12 As mentioned before, MSC transplantation could treat AKI; however, the most important problem with the application of MSCs for the treatment is their low survival rate in such a stressful microenvironment. Therefore, protection of MSCs against various stresses is very important for improving their therapeutic potential.
The nuclear factor erythroid-2 related factor 2 (Nrf2) is a critical transcription factor for protection of cells against numerous stresses.13,14 When cells are challenged by various stresses, the Nrf2 induces the transcription of diverse antioxidant and detoxification enzymes which eventually play important roles to ameliorate oxidative stress-induced injuries in the cells.15-18 Previously, in an in vitro study we showed that Nrf2 potentiates MSCs for combating with various stresses.19 Moreover, recent studies have shown that Nrf2 over-expression increases the secretion rate of some growth factors such as VEGF, which might enhance the AKI improvement.20,21 We hypothesized that Nrf2 overexpression in MSCs might exert beneficial therapeutic effects on the cells which could result in their higher survival rate against cisplatin induced stresses, and repairing much tissue than the non-recombinant MSCs by their differentiation to kidney cells and/or secretion of growth factors. Hence, we transiently over-expressed Nrf2 in rat MSCs using an adenoviral transduction system, and then transplanted the cells to cisplatin-induced AKI rats. Then, we assayed that whether improvement rate of the AKI is higher following transplantation of the Nrf2 over-expressing MSCs comparing to non-manipulated control MSCs.
Materials and Methods
MSC isolation and identification
For isolation of MSCs, rats were anesthetized with ketamine (40mg/kg) and xylazine (10mg/kg). Then, femurs and tibias of both legs were dissected out and cleaned under sterile operating conditions. Afterward, the marrow cavity was exposed by cutting the spongious end of each bone. We followed the process as described above. MSCs were characterized by adhesion capacity and differentiation assays. All experiments were performed on cells after the third passage.
Nrf2 isolation and construction of recombinant adenovirus
Gateway pAd/CMV/V5-DEST vector and ViraPower Adenoviral Expression System (Invitrogen) were used to construct recombinant adenoviruses, as described elsewhere.19 Briefly, the human MSCs were UV irradiated for one hour, and then subjected to RNA extraction using TriPure isolation reagent (Roche). Afterwards, full-length Nrf2 cDNA was obtained through RT-PCR using specific primers. Then, the amplified cDNA fragment was cloned into pENTER/TEV/DTOPO vector using pENTER Directional TOPO Cloning kit (Invitrogen). Following confirmation of the cloned sequence, the Nrf2 expression cassette was transferred from pENTR gateway vector to the adenovirus expression plasmid pAd/CMV/V5-DEST (Invitrogen) and designated as pAd/CMV-Nrf2. To obtain virus particles, the recombinant pAd/CMV-Nrf2 plasmid was transfected to 293A cells. As a control, pAd/CMV/V5-GW/lacZ vector (Invitrogen) was transfected into 293A cells to produce lacZ-bearing adenoviruses. After infection of rat MSCs with the recombinant adenovirus, the expression of Nrf2 was evaluated with RT-PCR analyses.
In vitro cytotoxic effect of cisplatin on surveillance of Nrf2-MSCs
The cytotoxic effect of cisplatin on the rat MSCs was determined by MTT assay.19,22 Briefly, 2×104 rat MSCs/well were seeded in a 96-well plate and appropriate MOIs of the recombinant or control adenoviruses were added to the cells after 12 h. Six days post transfection, cisplatin was added to the wells at various concentrations of 0-250µg/ml, (Sigma, Germany) and after 48h, the cells were subjected to cytotoxicity assay. In this regard, 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, Dusseldorf, Germany) was added to the cells at final concentration of 0.5 mg/ml and incubated at 37°C in a 5% CO2 atmosphere for 4 h. Finally, the reaction was stopped by addition of 10% SDS and 0.01 M HCl and the absorbance was read at 570 nm.
Animal model of AKI
Male Wistar rats (2 months old, 200-250 g) were obtained from Pasteur Institute of Iran. Animal care and experiments were according to the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. For induction of acute kidney injury, rats were given an intraperitoneal injection of cisplatin (5 mg/kg body wt). One day after receiving the cisplatin injection (day 1), the rats were divided into three groups of 10 animals, each received an intravenous injection as follows: (1) control group, which received 0.5ml normal saline; (2) Ad-MSCs group, which received 2×106 cells infected with control adenoviruses; (3) Nrf2-MSCs group, which received 2 ×106 cells infected with Nrf2 bearing adenoviruses. Blood samples for assessment of blood urea nitrogen (BUN) and determination of creatinine concentration were collected before the cisplatin dose (baseline) and in surviving rats on days 3, 6, 8 and 11 of the experiment. For determination of renal function, and histology and morphometric analysis, the rats were sacrificed on day eleven after the cisplatin injection.
For morphologic analysis with light microscopy, kidney specimens were fixed in formaldehyde and paraffin sections of 5µm thickness were stained with hematoxylin and eosin. Then, the luminal hyaline casts and tubular necrosis (cell debris in tubular lumen, denudation of tubular basement membrane and nuclear fragmentation) were assessed in non-overlapping fields (up to 30 for each section) using 40x objective (high-power field). Sections were analyzed by a same pathologist, in a single-blind manner.
Statistical Analysis
The statistical significance of the results was evaluated using analysis of variance, ANOVA, and a Student’s t-test. In all tests, p < 0.05 was considered significant.
Results
Adenoviral-mediated gene delivery to MSCs
Recombinant adenovirus production results have been presented in our recent article.19 In a few words, Human MSCs were characterized by their adhesion to tissue culture surfaces, immunophenotyping and differentiation capacities. Nrf2 was isolated from human MSCs and cloned into pENTR/D-TOPO. Subsequently, the fidelity of the cloned sequence was confirmed by sequencing (GenBank accession number HM446346). Finally, recombinant adenoviruses were constructed in 293A cells. Our results showed that Nrf2 over-expression by adenovirus system is transient and its expression level increased to the highest at day 6.
Nrf2 protects MSCs against cisplatin induced-toxicities in vitro
To determine whether human Nrf2 can protect bone marrow derived MSCs against cisplatin induced cytotoxicity; the Nrf2 expressing cells were exposed to various concentrations of cisplatin and then subjected to cytotoxicity assay. As it is represented by Figure 1, the rat MSCs infected with Nrf2-adenoviruses were more resistant to 150µg/ml cisplatin comparing to the controls. This confirmed the protective effect of Nrf2 on MSCs against cisplatin induced toxicities.
Figure 1 .
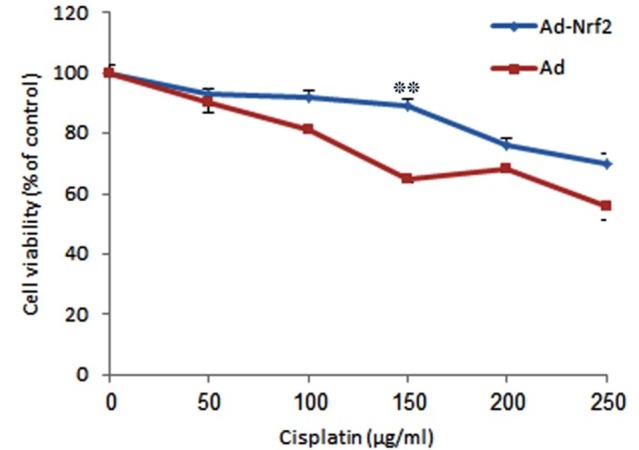
Evaluation of cytotoxicity of various cisplatin concentrations on rat MSCs. (Mean±SE, **: P<0.01)
Nrf2 improves efficacy of MSCs transplantation in animal model of cisplatin induced toxicity
To evaluate whether Nrf2-MSCs exert in vivo protective effects against cisplatin induced toxicity, acute kidney injury was induced in rats by cisplatin administration, an antitumor drug whose clinical use is accompanied by a high incidence of nephrotoxicity, mainly in the form of renal tubular damage. Under light microscope, the kidneys with induced cisplatin toxicity showed AKI-associated tubular lesions including loss of brush border, flattening and loss of the epithelial cells, luminal cell debris and hyaline casts. In addition, renal function tests (BUN and creatinine) showed significant increase (Figure 2). However, in the Ad-MSCs group, intravenous injection of Ad-MSCs strongly protected renal function as reflected by significantly lower amount of BUN and creatinine concentration comparing to the control group which only received normal saline. Furthermore, tubular necrosis and cast formation decreased significantly. Moreover, in the last group, administration of Nrf2-MSCs promoted much more functional and histological improvement. This confirmed the successful function, surveillance and resistance of Nrf2-MSCs in the reactive oxygen species (ROS)-rich microenvironment induced by cisplatin (Figure 3).
Figure 2 .
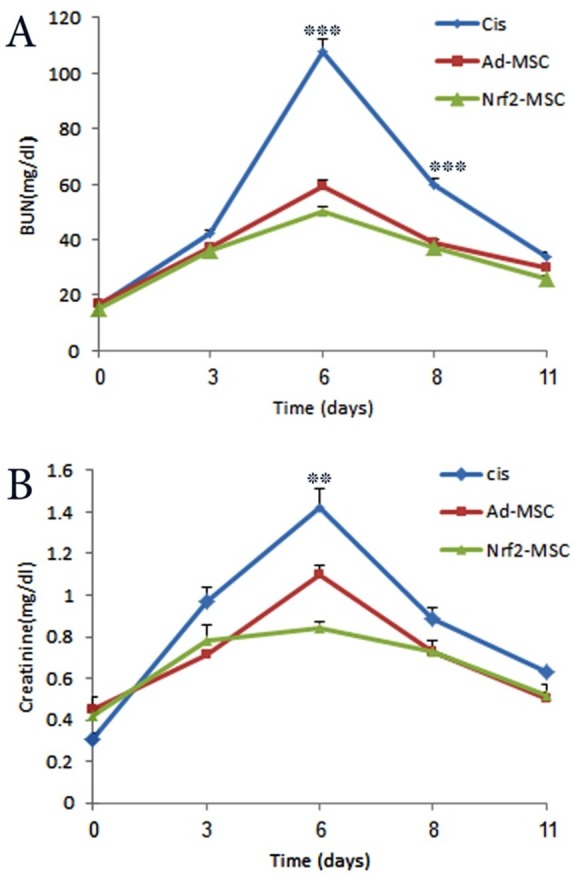
Protective effects of Nrf2 on MSCs following their transplantation in rats suffering from cisplatin-induced acute kidney injury. A; Assessment of renal function by measuring BUN values. B; Evaluation of serum creatinine concentrations as an indicator of renal function (Mean±SE, **: P<0.01 and ***: P<0.001).
Figure 3 .
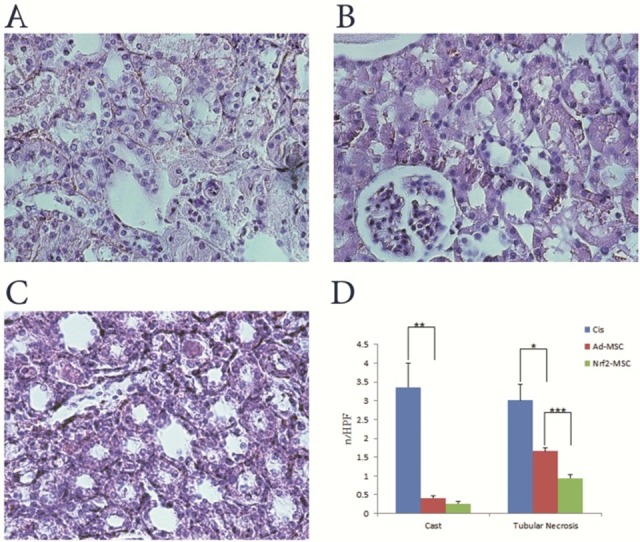
Renal histology following transplantation of Nrf2-MSCs and Ad-MSCs. A, B and C; Light microscopy images of renal tissue from rats (H&E stained kidney sections, 400×). A; Cisplatin group: tubules showed extensive and marked changes consisting of flattening, loss of epithelial cells and brush border. B; Ad-MSC group: less severe tubular damage and very mild cell swelling were observed. C; Nrf2-MSC group: higher recovery and similarity to normal morphology of kidney. D; Renal histology (casts and tubular necrosis, quantified as number per high power field (HPF)) of cisplatin treated rats. Tubular necrosis and lesions included loss of brush border and epithelial cells, flattening, , and luminal cell debris. (Mean±SE, *: P<0.05, **: P<0.01 and ***: P<0.001)
Discussion
The prevalence of acute kidney injury ranges from 5% of all hospitalized patients to 30 to 50% in critical care units.23 Application of some growth factors including insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF) and epidermal growth factor (EGF) for enhancement of tubular regeneration in experimental AKI has been one of the successful strategies.24,25 These growth factors rescue surviving tubular epithelial cells which consequently their regeneration repairs the injured kidneys. However, this approach requires plenty of surviving cells.24,25 Another critical strategy that involves a lot of studies in the world is the administration of MSCs. Various studies have shown that infusion of MSCs accelerates the recovery rate and prolongs survival of AKI animals.6,26-28 The mechanism of this treatment is based on the current theory about MSCs, stating that tissue stem cells replace dead tissue cells under physiologic conditions. Following tissue injury, mobilized stem cells replenish stem cell repertoire lost. When cell lost prevail stem cell repair, tissue injury occurs. Cytokines and chemokines secreted from injured tissue attract mobilized stem cells to repair site. Therefore, mesenchymal stem cells could not contribute in tissue repair and not enter organ in absence of injury.29
Different studies have shown that MSCs exert their therapeutic effects via two distinct mechanisms including differentiation to target tissue cells and secretion of growth factors. Morigi et al. and Herrera et al. reported that MSC mediated protection is a result of the capability of MSCs to engraft into a damaged kidney.24,30 In addition, differentiation of MSCs into renal cell types has been observed after AKI. Moreover, Poulsom et al. showed that when Y chromosome containing MSCs are injected into female mouse with AKI, the transplanted MSCs are recruited into peritubular sites.31 In contrast, other studies have shown that the valuable effects of MSCs are mediated via paracrine activities. Some growth factors and chemokines, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, monocyte-chemoattractant protein-1, HGF, and insulin-like growth factor-1 (IGF-1), are secreted by MSCs which enhance epithelial proliferation, modulate inflammation, or promote angiogenesis, and therefore are good candidates for therapy of AKI.6
Many stresses are involved in AKI pathogenesis. Over 50% of hospitalized cases of AKI are caused by renal ischemia.32 Ischemia is thought to create an inhospitable environment by inducing local expression of inflammatory cytokines that promote cell apoptosis.33 Ischemia has two important components including hypoxia and serum deprivation.34 Also, it has been demonstrated that ischemia can cause oxidative stress.35 In addition to tubular cells, these stresses cause apoptosis in transplanted MSCs.19,34 MSCs are more susceptible to death than other tissue cells.36,37 Therefore, not only MSCs alleviate the stresses, but they are died by stresses. Various factors such as strength and length of stresses, number of infused MSCs and the level of growth factors determine the outcome of this battle.
Previously, we showed that overexpression of Nrf2 in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity.19 Also, the protective role of Nrf2 has been previously shown by numerous studies.14,16,38 Higher surveillance rate of MSCs in stressful microenvironments could result in successful regeneration of the damaged tissue by the MSCs. Nrf2 is a transcription factor which activates expression of multiple antioxidants and detoxifying enzymes.39,40 Therefore, Nrf2 overexpression in MSCs might decrease cytotoxic effect of stresses. Furthermore, previous studies, demonstrated that Nrf2 overexpression can increases the amount of some growth factors and detoxifying enzymes such as VEGF, HO-1 and SOD-1 &2.19,41-44 As mentioned previously, administration of growth factors is one of the current strategies in AKI treatment. Consequently, Nrf2 overexpression in MSCs has a dual and synergistic protective effect on surveillance of MSCs which can result in successful treatment of AKI.
Because of homology between human and rat Nrf2 proteins,45 here we used the recombinant adenovirus vector harboring human Nrf2 coding sequence constructed in our previous study.19 Previously, we showed that the adenovirus mediated expression of Nrf2 protein is transient. In addition, Nrf2 overexpression has no deleterious effect on differentiation capacity of MSCs into adipocytic, osteoblastic and chonderocytic lineages. In this study we showed that human Nrf2 could protect rat MSCs against cisplatin in vitro. This capability makes Nrf2 an excellent protein for improving transplantation process, especially for treatment of acute kidney injury.
In this study, we hypothesized that Nrf2, as a potent cytoprotective transcription factor, could protect MSCs against stresses during transplantation. To verify this hypothesis, Nrf2-MSCs were transplanted into cisplatin-induced AKI rats and their therapeutic efficacy was assessed by measuring blood urea nitrogen and creatinine concentration and morphologic analysis.
AKI was diagnosed according to RIFLE criteria. These criteria include three categories of injury (Risk, Injury, and Failure with increasing severity) and two classes of kidney outcome (loss of function, and end-stage renal disease). Serum creatinine increases to 1.5-fold from baseline in the risk class. In the injury and failure classes, serum creatinine increases to 2.0 and 3.0-fold, respectively.46-49 In this study, it has been shown that while cisplatin treated rats developed failure class of AKI, but rats transplanted with Ad-MSCs and Nrf2-MSCs developed injury and risk classes of AKI, respectively. It showed that MSC transplantation can reduce kidney damage and overexpression of Nrf2 in MSCs potentiates them against AKI.
In the morphologic aspect, necrosis and apoptosis of tubular cells lead to tubular obstruction by cast formation. Casts are composed of shed epithelial cells and necrotic debris. Cytoskeletal disruption, ATP depletion, loss of cell polarity, and cell-cell and cell-matrix attachment are the consequences of ischemia.50-55 These morphologic changes have been also seen in cisplatin treated rats. Because of the beneficial therapeutic effect of MSCs, morphological abnormalities decreased in Ad-MSCs rats. However, the Nrf2-MSCs were more effective in decreasing the morphological abnormalities comparing to the Ad-MSCs. Therefore, it could be deduced that Nrf2 overexpression protects MSCs from stress-related conditions in vivo.
Nevertheless, a possible disadvantage of Nrf2 overexpression is the risk of tumor progression.56 Recent studies have shown that an Nrf2 suppressor, Keap1, is often mutated in cancer cells resulting in a constitutive activation of Nrf2.57,58 In addition, it has been demonestrated that a hypoxic tumor microenvironment leads to increased transactivation of Nrf2.59 Considering the role of Nrf2 in regulating a battery of genes that act to detoxify cancer drugs and/or attenuate drug-induced oxidative stress, it is possible that Nrf2 overexpression may play role in increasing resistance to treatment in case of tumor progression.60-63 However, in the present study, no sign of tumorogenic transformational changes have been observed.
Conclusion
The obtained results showed that Nrf2 could inhibit apoptosis induction in MSCs transplanted into the cisplatin-induced AKI rats. In fact, despite rare reports on the existence of MSCs within the renal interstitium,64 transplantation of MSCs satisfactorily contributed to restoring renal tubule structure and improving renal function, as reported by other studies.23,24,64-67 Therefore, MSCs probably exert their protective effect on cisplatin-induced AKI in a paracrine manner, i.e. via production of growth factors and anti-inflammatory cytokines. Localization of MSCs in peritubular interstitial areas indicates that the MSCs acted by exerting paracrine activity.23 The Nrf2-MSCs produced higher amount of growth factors and therefore, can be more effective than the non-Nrf2 overexpressing Ad-MSCs in improvement of kidney functions.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1. Racusen LC. The morphologic basis of acute renal failure. In: Molitoris BA, Finn WF, editors. Acute renal failure: a companion to Brenner and Rector’s the kidney, 1st ed. Philadelphia: WB Saunders; 2001:1-12.
- 2.Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36(4 Suppl):S187–92. doi: 10.1097/ccm.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- 3.Bussolati B, Hauser PV, Carvalhosa R, Camussi G. Contribution of stem cells to kidney repair. Curr Stem Cell Res Ther. 2009;4(1):2–8. doi: 10.2174/157488809787169129. [DOI] [PubMed] [Google Scholar]
- 4.De Fatima Fernandes Vattimo M, Da Silva NO. Uncaria tomentosa and acute ischemic kidney injury in rats. Rev Esc Enferm USP. 2011;45(1):194–8. doi: 10.1590/s0080-62342011000100027. [DOI] [PubMed] [Google Scholar]
- 5.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–94. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59(1):311–25. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 7.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Li Q, Zhang L, Lin H, Hu J, Li D. et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012;7(8):e43768. doi: 10.1371/journal.pone.0043768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efrati S, Berman S, Hamad RA, Siman-Tov Y, Ilgiyaev E, Maslyakov I. et al. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant. 2012;27(1):136–45. doi: 10.1093/ndt/gfr256. [DOI] [PubMed] [Google Scholar]
- 10.Yo Y, Morishita R, Nakamura S, Tomita N, Yamamoto K, Moriguchi A. et al. Potential role of hepatocyte growth factor in the maintenance of renal structure: anti-apoptotic action of HGF on epithelial cells. Kidney Int. 1998;54(4):1128–38. doi: 10.1046/j.1523-1755.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoudi M, Willgoss D, Cuttle L, Yang T, Pat B, Winterford C. et al. In vivo and in vitro models demonstrate a role for caveolin-1 in the pathogenesis of ischaemic acute renal failure. J Pathol. 2003;200(3):396–405. doi: 10.1002/path.1368. [DOI] [PubMed] [Google Scholar]
- 12.Yamada M, Pat B, Gobe G, Wojcikowski K. Angelica sinensis has inherent endothelial cell toxicity at high concentrations but can also protect the vascular endothelium from oxidative stress-induced injury at moderate concentrations. Altern Med Stud. 2011;1(1):e8. doi: 10.4081/ams.2011.e8. [DOI] [Google Scholar]
- 13.Zhu H, Zhang L, Itoh K, Yamamoto M, Ross D, Trush MA. et al. Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radic Biol Med. 2006;41(1):132–43. doi: 10.1016/j.freeradbiomed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74(13):1526–39. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37(2):139–43. doi: 10.5483/BMBRep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 16.Osburn WO, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush MA. et al. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454(1):7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkanen HK, Kansanen E. et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol. 2007;27(4):741–7. doi: 10.1161/01.atv.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 18.Jin W, Wang H, Ji Y, Zhu L, Yan W, Qiao L. et al. Genetic ablation of Nrf2 enhances susceptibility to acute lung injury after traumatic brain injury in mice. Exp Biol Med (Maywood) 2009;234(2):181–9. doi: 10.3181/0807-rm-232. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadzadeh M, Halabian R, Gharehbaghian A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM. et al. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones. 2012;17(5):553–65. doi: 10.1007/s12192-012-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afonyushkin T, Oskolkova OV, Binder BR, Bochkov VN. Involvement of CK2 in activation of electrophilic genes in endothelial cells by oxidized phospholipids. J Lipid Res. 2011;52(1):98–103. doi: 10.1194/jlr.m009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D, Lee YM. et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 2011;71(6):2260–75. doi: 10.1158/0008-5472.can-10-3007. [DOI] [PubMed] [Google Scholar]
- 22.Roudkenar MH, Ghasemipour Z, Halabian R, Roushandeh AM, Yaghmai P, Gharehbaghian A. et al. Lipocalin 2 acts as a cytoprotective factor against cisplatin toxicity, an in vitro study. DARU. 2008;16(2):106–11. [Google Scholar]
- 23.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L. et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18(11):2921–8. doi: 10.1681/asn.2006121318. [DOI] [PubMed] [Google Scholar]
- 24.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M. et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 25.Morigi M, Benigni A, Remuzzi G, Imberti B. The regenerative potential of stem cells in acute renal failure. Cell Transplant. 2006;15 Suppl 1:S111–7. doi: 10.3727/000000006783982449. [DOI] [PubMed] [Google Scholar]
- 26. Proceedings from the Cleveland Clinic Workshop on Innovation in Treatment of Uremia. Seminars in dialysis 2009;22(6):597-708. [PubMed]
- 27.Togel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nature reviews Nephrology. 2010;6(3):179–83. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 28.Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A. et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol. 2011;300(1):F254–62. doi: 10.1152/ajprenal.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 30.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14(6):1035–41. doi: 10.3892/ijmm.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 31.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S. et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195(2):229–35. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 32. Grenz A, Hong JH, Badulak A, Ridyard D, Luebbert T, Kim JH, et al. Use of a hanging-weight system for isolated renal artery occlusion. J Vis Exp 2011;19(53). doi: 10.3791/2549 [DOI] [PMC free article] [PubMed]
- 33.Forrester JS, Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am J Cardiol. 2007;99(5):732–8. doi: 10.1016/j.amjcard.2006.09.125. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24(2):416–25. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 35.Park MK, Kim WS, Lee YS, Kang YJ, Chong WS, Kim HJ. et al. Glucose oxidase/glucose induces apoptosis in C6 glial cells via mitochondria-dependent pathway. J Appl Pharmacol. 2005;13(4):207. [Google Scholar]
- 36.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1(4):32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues M, Blair H, Stockdale L, Griffith L, Wells A. Surface tethered epidermal growth factor protects proliferating and differentiating multipotential stromal cells from FasL-induced apoptosis. Stem Cells. 2013;31(1):104–16. doi: 10.1002/stem.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisman SA, Yeager RL, Yamamoto M, Klaassen CD. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci. 2009;108(1):35–47. doi: 10.1093/toxsci/kfn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ. et al. Nrf2, a multi-organ protector? FASEB J 2005;19(9):1061-6. Nrf2, a multi-organ protector? FASEB J. 2005;19(9):1061–6. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 40.Tufekci KU, Civi Bayin E, Genc S, Genc K. The Nrf2/ARE Pathway: A Promising Target to Counteract Mitochondrial Dysfunction in Parkinson's Disease. Parkinsons Dis. 2011;2011:314082. doi: 10.4061/2011/314082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY. et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26(2):175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 42.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH. et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290(5):H1862–70. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M. et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29(11):1843–50. doi: 10.1161/atvbaha.109.189480. [DOI] [PubMed] [Google Scholar]
- 44.Kweider N, Fragoulis A, Rosen C, Pecks U, Rath W, Pufe T. et al. Interplay between Vascular Endothelial Growth Factor (VEGF) and Nuclear Factor Erythroid 2-related Factor-2 (Nrf2): Implications for preeclampsia. J Biol Chem. 2011;286(50):42863–72. doi: 10.1074/jbc.m111.286880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher J, Yamamoto M. The rise of antioxidant signaling--the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244(1):4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Biesen W, Vanholder R, Lameire N. Defining acute renal failure: RIFLE and beyond. Clin J Am Soc Nephrol. 2006;1(6):1314–9. doi: 10.2215/cjn.02070606. [DOI] [PubMed] [Google Scholar]
- 48.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–9. doi: 10.1681/asn.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7(4):201–8. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 50.Wang YH, Li F, Schwartz JH, Flint PJ, Borkan SC. c-Src and HSP72 interact in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol. 2001;281(5):C1667–75. doi: 10.1152/ajpcell.2001.281.5.C1667. [DOI] [PubMed] [Google Scholar]
- 51.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–20. doi: 10.1681/asn.2006010017. [DOI] [PubMed] [Google Scholar]
- 52.Havasi A, Wang Z, Gall JM, Spaderna M, Suri V, Canlas E. et al. Hsp27 inhibits sublethal, Src-mediated renal epithelial cell injury. Am J Physiol Renal Physiol. 2009;297(3):F760–8. doi: 10.1152/ajprenal.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kathleen D, Liu GMC. Acute renal failure. In: Jamison RL, Wilkinson R, editors. Nephrology. London: New York: Chapman & Hall; 2010.
- 54.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 55.Bajwa SJ, Sharma V. Peri-operative renal protection: The strategies revisited. Indian J Urol. 2012;28(3):248–55. doi: 10.4103/0970-1591.102691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244(1):66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M. et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21(5):689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO. et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67(2):546–54. doi: 10.1158/0008-5472.can-06-2401. [DOI] [PubMed] [Google Scholar]
- 60.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y. et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 61.Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3(5):481–90. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004;37(4):433–41. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 63.Kim YJ, Baek SH, Bogner PN, Ip C, Rustum YM, Fakih MG. et al. Targeting the Nrf2-Prx1 pathway with selenium to enhance the efficacy and selectivity of cancer therapy. J Cancer Mol. 2007;3(2):37–43. [Google Scholar]
- 64.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18(9):2486–96. doi: 10.1681/asn.2007020140. [DOI] [PubMed] [Google Scholar]
- 65.Kucic T, Copland IB, Cuerquis J, Coutu DL, Chalifour LE, Gagnon RF. et al. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol. 2008;295(2):F488–96. doi: 10.1152/ajprenal.00044.2008. [DOI] [PubMed] [Google Scholar]
- 66.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C. et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–82. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 67.Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y, Liu CY. et al. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;300(1):F207–18. doi: 10.1152/ajprenal.00073.2010. [DOI] [PubMed] [Google Scholar]


