Abstract
Purpose: One of the advanced cancer therapy strategies is immune-stimulating compound based immunotherapy Staphylococcal enterotoxin B (SEB) is one of the potent superantigens, which can efficiently activate antitumor immune response to eradicate tumor growth and inhibit metastasis. Herein, we evaluated the effect of SEB on the expression of two master microRNAs, mir-335 and mir-10b, involved in metastasis.
Methods: A metastatic breast cancer cell line MDA-MB231was treated with four different concentrations of SEB, including 10, 102, 103 and 104 ng/ml, for 24 and 48 hours. To identify the cytotoxic effect of SEB, treated cells were examined by MTT assay. The stem loop RT-PCR (TaqMan) was used to analyze the mir-335 and mir-10b expression.
Results: Results showed that SEB significantly increased the expression of mir-335 both after 24 and 48 hours (pv < 0.001 and pv < 0.05, respectively). No significant differences were found in the mir-10b expression.
Conclusion: Moreover, our findings demonstrated no cytotoxic effect of SEB on the treated cells. Our results suggest that SEB probably induces its anti-metastatic effect via the expression regulation of the main genes which contributes to metastasis.
Keywords: Breast Cancer, Metastasis, Mir-335, Mir-10b, Staphylococcal Enterotoxin B
Introduction
Breast cancer is the second cause of death in women all around the world. It is estimated that more than 1.38 million women are diagnosed yearly with breast cancer.1 Conventional therapeutic methods such as surgery, chemotherapy, radiotherapy and hormone therapy are considered as non-efficient approaches for metastatic and invasive breast cancer; therefore, new specific methods are required to conquer this disease. Immunotherapy based on immune-stimulating compounds such as immunotoxins, dendritic cells plused with cancerous antigens and bacterial superantigenes have been used in several studies as advanced therapeutic strategies.2 Accordingly, mentioned compounds induce antitumor response of a patient`s immune system. Cancer formation and invasion to other tissues are multi-step genetic and epigenetic alterations.3 Thus, it is essential to explore the influence of novel therapeutic strategies on the expression of key genes.1 On the other hand, in spite of the knowledge limitation on the cellular and molecular factors involved in breast cancer progression, today few gene profiles have been recognized which increases the rate of progression events via regulatory networks.
MicroRNAs, highly conserved 18-25 nucleotide transcripts expressed in the animal and plants, are as robust regulators of processes involved in tumorigenesis and metastasis4 via suppressing mRNA translation and degradation.5 Also, the profile of microRNAs in cancerous conditions differs from normal states. As a result, lack of microRNAs suppressing tumor progression and metastasis could trigger the cancer. Several microRNAs have been recognized in breast cancer progression4 such as mir-10b3 and mir-3356 as a master inducer and suppressor of metastasis in breast cancer, respectively, which affect the cell migration and invasion. Evidently, mir-10b is downregulated in patients with primary breast cancer7 but it is expressed at high levels in approximately 50% of metastatic breast cancer.3 Also, it represses HOXD10 gene expression leading to activate the RHOC gene as a potent pro-metastatic factor. Hence, upregulation of mir-10b results in inducing invasion and metastasis of tumor cells.3 Mir-335, an anti-metastatic microRNA, suppresses cell migration and invasion through inactivating of SOX4 and Tenacin C which are critical agents to promote metastasis.6
Since rising the metastatic phenotype of cancer cause most deaths, designing novel therapeutic methods can be useful to improve human beings’ life. Some advanced agents based on biological compounds are developed, which influence the expression of tumorigenesis and metastasis related genes involved in activation of antitumor immune responses. SEB as a classic superantigen is one of the most potent T-cell mitogens.8,9 In contrast to common antigens, SEB activate a broad spectrum of T-cells unspecifically. Over 25% of both CD4+ and CD8+ T-cells proliferate after SEB exposure.10 Recently, Hosseini et al reported that SEB anchored on Exosomes was able to augment apoptotic genes in the MDA-MB231and Miapaca-2 and induce apoptosis.11,12 Although the antitumor properties of this superantigen were reported in previous studies, but its effect on the regulation of genes which involved in the cancer progression and metastasis has not been completely revealed. In the following study, we aimed to investigate the effect of SEB on the expression of two microRNAs, mir-335 and mir-10b, which regulate metastasis pathway in breast cancer.
Materials and Methods
Cell culture
A metastatic human breast cancer cell line MDA-MB231 was obtained from the Pasteur Institute (Tehran, Iran). This cell line was cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin and incubated at 37°C under 5% CO2 atmosphere. All the cell culture reagents were purchased from PAA company, Holland.
Staphylococcal enterotoxin B treatment
To assess the effect of SEB (Sigma–Aldrich, Germany) on the gene expression of breast cancer cells, we cultured 105 MDA-MB231cells in each well of the 24 well plates. After 24 hours, the old medium of wells was replaced with the fresh one. Then, four different concentrations of SEB, including 10,102,103 and 104 ng/ml, were added to separate wells. After 24 and 48 hours, cells of each plate were detached by trypsin/EDTA and applied for extracting microRNAs. All tests were carried out in triplet. PBS (phosphate buffer saline) was used as a negative control.
Proliferation assay
The effect of SEB on the proliferation of MDA MB-231cells was investigated by MTT assay after 24 and 48 hours. Briefly, 104 cells were seeded on each well of a 96 well plate and incubated at the culture condition. After 24 hours, the cells were treated with four different concentrations of the SEB described before. The cells treated with PBS was considered as a negative control. 20 μl MTT reagent (5mg/ml) (Sigma-Aldrich, Germany) was added to each well and incubated for 4 hours at 37°C. The supernatants were replaced with 100 μL dimethyl sulphoxide (Sigma–Aldrich, Germany) and finally, the optical density of each well was measured by micro plate reader (Tecan, Switzerland) at 570 nm. All the tests were carried out in triplicate.
RNA extraction
In order to isolate microRNAs, RNX-Plus kit (Cinnagen, Iran) was utilized to extract total microRNAs according to the manufacturers’ instruction. The microRNAs were precipitated in isopropanol solution and finally resuspended in 50 µl DEPC water. Gel electrophoresis on 1.5% agarose stained by ethidium bromide was used to evaluate the quality of extracted microRNAs. In addition, the concentration of extracted microRNAs was measured by a NanoDrop spectrophotometer (Thermos, USA).
Probe and primer design
To improve the efficiency and specificity of the microRNA quantification, the stem loop RT-PCR (TaqMan) was applied and all the primer and probes were designed according to Chen et al. protocol. The sequences of the mir-10b, mir-335 and the U6 gene, as the internal control, were obtained from the miRBase database (NCBI) and specific primers and probes was designed using AlleleID 7 Software (Palo Alto, CA). Then, the specificity of each probe and primer was evaluated by aligning through BLAST (NCBI). All the primers and probes were synthesized by Metabion Co, Germany, as outlined in Table 1.
Table 1. The sequence of designed primers and probes.
| - | MicroRNA-10b | MicroRNA- 335 | U6 |
| Stem loop RT- primer | 5’-GTCGTATGCAGAGCAGGGTCCGAGGTATTCGCACTGCATACGACCACA-3’ | 5’- GTCGTATGCAGAGCAGGGTCCGAGGTATTCGCACTGCATACGACACAT-3’ | - |
| Forward primer | 5’ GCGTACCCTGTAGAACCGA 3’ | 5’- CGGATCAAGAGCAATAACG -3’ | 5’- GCTTCGGCAGCACATATAC-3’ |
| Reverse primer | 5’- GAGCAGGGTCCGAGGT -3’ | 5’- GAGCAGGGTCCGAGGT-3’ | 5’- AATTTGCGTGTCATCCTTG-3’ |
| Probe | (FAM) 5’-TTTGTGGTCGTATGCAGTGCG-3’ (BHQ-1) | (FAM) 5’-AAAATGTGTCGTATGCAGTGCG-3’ (BHQ-1) | (FAM) 5’- CCATGCTAATCTTTCTCTGTATCGTTCC-3’ (BHQ-1) |
cDNA synthesis and real-time PCR
Following RNA extraction, cDNAs were synthesized using Revert Aid Reverse transcriptase kit (Fermentas, Germany). One μg of total microRNAs was amplified using stem-loop RT primer for mir-10b and mir-335 and reverse primer for U6 as an internal control.
Real time PCR was accomplished by SLAN® Real-Time PCR Detection System (Shanghai, China). Three distinct PCR reactions were carried out for each test using specific oligonucleotide sets. Each PCR reaction containing10 μl of premix Ex Taq (Takara, Japan), 0.2 μM of desired reverse and forward primer, 0.2 μM of a specific TaqMan probe, 0.5 μl of ROX reference Dye (50X) and 2 μl of synthesized cDNA in a final volume of 25 μl using the following thermal conditions: 95°C for 10 min, followed by 50 cycles of 95°C for 5 s and 60°C for 31 s. Analysis of gene expression was done using REST -2009 software which is designed by Qiagen Co. of Germany.
Statistical analysis
Data obtained from all tests were assessed by the nonparametric Mann–Whitney test using SPSS.15 software (SPSS, Chicago, IL). p value less than 0.05 was considered statistically significant.
Results
Cell proliferation analysis (MTT assay)
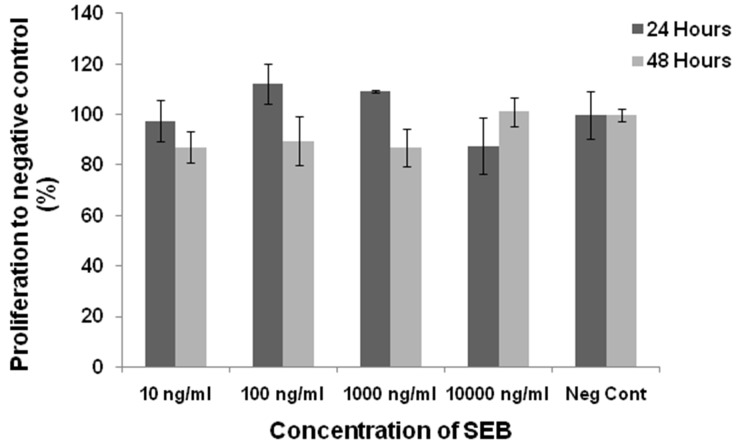
MDA MB-231 cells were treated with different concentrations of the SEB for 24 and 48 hours and cell proliferation was examined by MTT assay. After 24 hours, none concentrations of SEB had the inhibitory effect on the treated cells compared to negative controls. Although, 48-hour exposure to SEB showed a negative effect on the cell proliferation, it is not statistically significant to the control group (Figure 1).
Figure 1.
The cell proliferation rate of MDA MB-231 treated with the SEB after 24 and 48 hours. The cells were treated with 10, 100, 103 and 104 ng/ml of EXO/SEB for 24 and 48 hours. Also PBS was examined as a control. No significant different proliferation rate was determined in all concentrations of SEB after 24 and 48 hours.
Gene expression
In order to assess the influence of SEB on the expression of mir-10b and mir-335, MDA- MB 231 breast cell line was treated with four discrepant concentrations of SEB for 24 and 48 hours. microRNAs expression was evaluated via stem loop RT-PCR method.
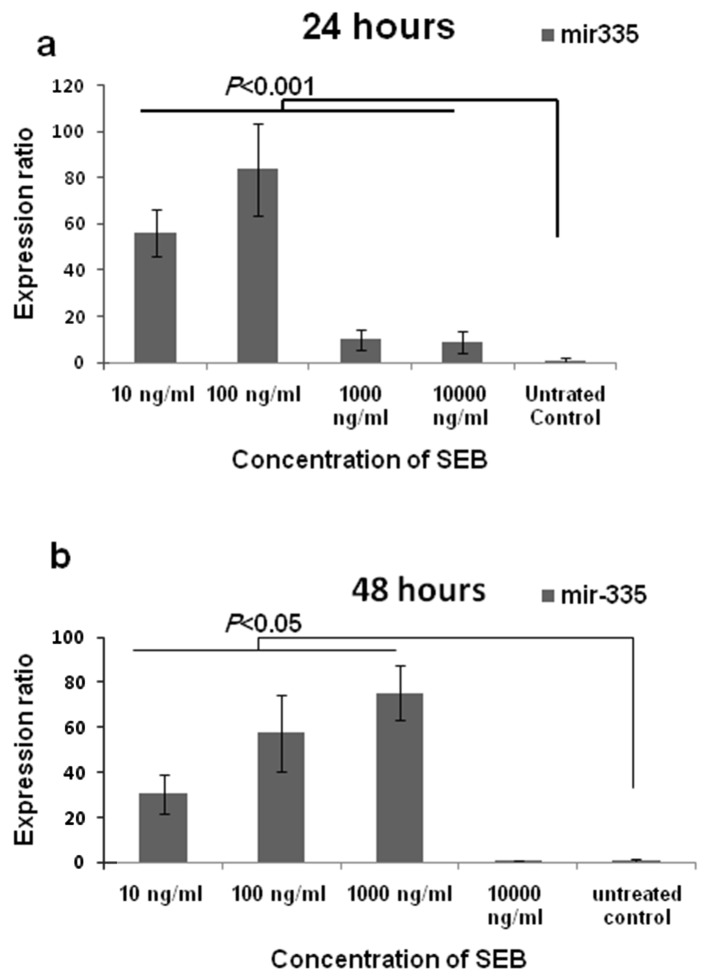
Results showed the positive effect of SEB exposure on giving rise the expression of mir-335 compared to untreated control. Figure 2 illustrates the alterations of the mir-335 amount corresponding to concentration and time. In addition, the results have shown that SEB exerted its effect dose dependently. The rate of mir-335 expression at the concentration of 10, 102, 103 and 104 ng/ml was 56.1, 83.6, 10.1 and 8.8 folds more than untreated control after 24 hours, respectively (pv < 0.001). Moreover, after 48 hours, the expression of mir-335 was 30.4, 57.4, 75.4 and 0.7 folds higher than control under the effect of 10, 102, 103 and 104 ng/ml of SEB, respectively. The expression levels under the effect of mentioned concentrations were significantly more than the expression in untreated control cells (pv < 0.05) except to the amount of microRNAs under the effect of 104 ng/ml SEB, after 48 hours. Also, cells exposed to102 ng/ml and 103 ng/ml of SEB showed the most increase in mir-335 expression at 24 and 48 hours, respectively.
Figure 2.
Mir-335 expression in MDA-MB231 cell line after treating with staphylococcal enterotoxin B. As shown in the graph (a), after 24 hours, all concentrations of SEB were resulted in the increase of mir-335 significantly (pv< 0.001). After 48 hours (b), except to the concentration of 104 ng/ml, the rest concentrations significantly cause to give rise the expression (pv< 0.05).
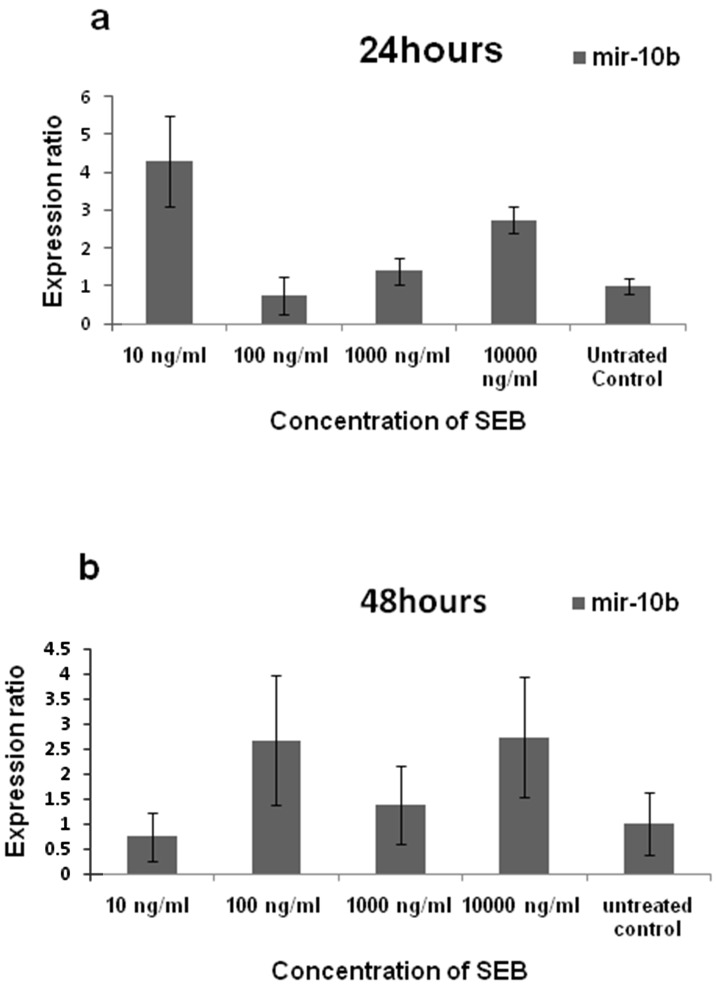
Figure 3 showed the increase of mir-10b expression after treating the cells with SEB both after 24 and 48 hours. The rate of mir-10b expression at the concentration of 10, 102, 103 and 104 ng/ml was 4.3, 0.758, 1.398 and 2.738 folds more than untreated control after 24 hours, respectively. Furthermore, after 48 hours, the expression of mir-10b was 0.744, 2.67, 1.39 and 2.74 folds higher than control under the effect of 10, 102, 103 and 104 ng/ml of SEB, respectively. However, these differences were not significant (pv> 0.05).
Figure 3.
Mir-10b expression in MDA-MB231 cell line after treating with staphylococcal enterotoxin B. As shown in the figure, after 24 (a) and 48 (b) hours, no significant differences were observed after SEB treating compared to control.
Discussion
Metastasis is a frequent event after classic treatment of cancer. It is usually because of inefficient anti-tumor activity of the immune system due to the avoidance of cancerous cells to present their antigens to T lymphocyte.13 Therefore, designing an immunotherapy approach which activate the tumor-specific T cells and tumor eradication is always seem considerably essential.
Superantigens (SAgs) such as viral and bacterial proteins are potent activators of a vast population of T lymphocytes regardless of their antigen specificity.14 In addition to inducing the antitumor activity and releasing respective cytokins, these kind of compounds can inhibit the tumor growth and metastasis. Generation of significant antitumor response is one of the critical steps in appropriate immunotherapy. In our previous reports, SEB, one of the most strongest superantigen, showed an antitumoric properties in a dose-dependent manner.15 It was demonstrated that 100 ng/ml of SEB causes rising the proliferation of CD4+ and CD8+ T-lymphocytes in the animal model. Moreover, IV-injection of SEB significantly increased levels of IFN-γ and IL-4.15,16 Beside antitumor immune responses, this superantigen has a potential effect on inducing of necrosis and decreasing tumor size that could lead to alleviate the rate of metastasis.17
Furthermore, Pulaski et al applied SEB as an adjuvant combined with MHC class II and CD80 for treating metastatic breast cancer in an animal model. Their result showed that SEB can extend the survival time and limit the metastasis.8
Since the antimetastatic effect of superantigenes has been reported by previous studies,8,17 further investigation is necessary to recognize which mechanism is involved in . In the current study, we assessed the role of SEB on the expression of two master regulatory microRNAs, mir-10b and mir-335, involving in the metastasis of breast cancers. The expression of mir-335 is highly down-regulated in primary breast cancer cells which promotes metastasis.6
In the current study, it was observed that SEB can alter the expression pattern of microRNAs in a dose and time dependent manner. Data from MTT assay showed no cytotoxic effect of SEB on the cells. Lack of cytotoxic effect along with alterations in gene pattern indicates that this toxin is probably able to enter to the cell and induce biological changes in the gene expression. In addition, lack of cellular death by SEB recognized the same cell population in both control and treatment cell. Consistent with our previous study,17 100 ng/ml of SEB after 24 hours incubation was the most efficient condition to increase the anti metastatic mir-335 level. Despite the highly positive effect of above mentioned concentration after 48 hours incubation, 1000 ng/ml of SEB was the most efficient concentration to rise mir-335 expression. Besides, 104 ng/ml of SEB has a quite inefficient effect on microRNA expression. Higher efficient concentrations at 48 hours are probably related to maintenance of intracellular concentration of SEB. During the period of 48 hours, the content of SEB in the concentration of 100 ng/ml may reduce whereas the intracellular levels of 1000ng/ml become constant. Moreover, loss of inducing effect of SEB after 48 hours incubation in the concentration of 100 ng/ml may lead to promote decay of mir-335.
In the study by Barmack et al, the decay of mir-335 transcripts has been reported 3 hours after stopping exposure of cerebellar Purkinje cell by horizontal optokinetic stimulation.18 Png et al. showed that two mechanisms involve in the silencing and downregulation of these microRNAs: promoter hypermethylation and genetic deletion. They also observed that 5-aza-deoxycytidine can inhibit the promoter methylation of mir-335 in MDA-MB231 and subsequently increases its expression.19 Accordingly, we suggest that SEB may reduce promoter methylation. However, further studies are required to confirm it.
Mir-10b is an inducer of metastasis, which is up-regulated only in metastatic cancer cell lines. The increase of this microRNA contributes to triggering cell migration and invasion via suppressing the translation of HOXD10.3
Though we observed increasing of mir-10b expression after both 24 and 48 hours, it was not a significant elevation. This result is benefited because high levels of mir-10b promote the metastasis. However, further studies are required to verify this finding.
In order to approve SEB as a therapeutic adjuvant for cancer therapy, it is needed to study its effect on expression of expanded sets of genes involved in cancer progression and metastasis. In addition, the mechanisms of SEB effect on gene expression must be completely determined.
Conclusion
Our results suggest that SEB probably induces its anti-metastatic effect via the expression regulation of the main genes which contributes to metastasis.
Acknowledgments
This work was supported by the grant from the Iranian National Sciences Foundation (INSF), molecular biology research center and applied microbiology research center of Baqiyatallah University of Medical Sciences, Tehran, Iran.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Zhong Y. Breast cancer immunotherapy. Cell Mol Immunol. 2004;1(4):247–55. [PubMed] [Google Scholar]
- 3.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–8. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 4.O'day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010;12(2):201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J. et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–9. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 6.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD. et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–52. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–70. doi: 10.1158/0008-5472.can-05-1783. [DOI] [PubMed] [Google Scholar]
- 8.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60(10):2710–5. [PubMed] [Google Scholar]
- 9.Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A. et al. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244(4906):811–3. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 10.Si SY, Hu PZ, Huang YY, Ye J, Huang Y, Li ZS. et al. Tumor cells with B7.1 and transmembrane anchored staphylococcal enterotoxin A generate effective antitumor immunity. Biochem Biophys Res Commun. 2006;347(1):208–14. doi: 10.1016/j.bbrc.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J, Nourani MR, Davaran S, Mahdavi M. Staphylococcal entorotoxin B anchored exosome induces apoptosis in negative esterogen receptor breast cancer cells. Tumour Biol. 2014;35(4):3699–707. doi: 10.1007/s13277-013-1489-1. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J, Nourani MR, Mahdavi M. Exosome/staphylococcal enterotoxin B, an anti tumor compound against pancreatic cancer. J BUON. 2014;19(2):440–8. [PubMed] [Google Scholar]
- 13.Ostrand-Rosenberg S. Tumor immunotherapy: the tumor cell as an antigen-presenting cell. Curr Opin Immunol. 1994;6(5):722–7. doi: 10.1016/0952-7915(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 14.Fischer H, Dohlsten M, Andersson U, Hedlund G, Ericsson P, Hansson J. et al. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J Immunol. 1990;144(12):4663–9. [PubMed] [Google Scholar]
- 15.Fooladi AA, Sattari M, Nourani MR. Study of T-cell stimulation and cytokine release induced by Staphylococcal enterotoxin type B and monophosphoryl lipid A. Arch Med Sci. 2009;3:335–41. [Google Scholar]
- 16.Imani Fooladi AA, Sattari M, Nourani MR. Synergistic effects between Staphylococcal enterotoxin type B and Monophosphoryl lipid A against mouse fibrosarcoma. J BUON. 2010;15(2):340–7. [PubMed] [Google Scholar]
- 17.Fooladi AA, Sattari M, Hassan ZM, Mahdavi M, Azizi T, Horii A. In vivo induction of necrosis in mice fibrosarcoma via intravenous injection of type B staphylococcal enterotoxin. Biotechnol Lett. 2008;30(12):2053–9. doi: 10.1007/s10529-008-9805-3. [DOI] [PubMed] [Google Scholar]
- 18.Barmack NH, Qian Z, Yakhnitsa V. Climbing fibers induce microRNA transcription in cerebellar Purkinje cells. Neuroscience. 2010;171(3):655–65. doi: 10.1016/j.neuroscience.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Png KJ, Yoshida M, Zhang XH, Shu W, Lee H, Rimner A. et al. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25(3):226–31. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]