Abstract
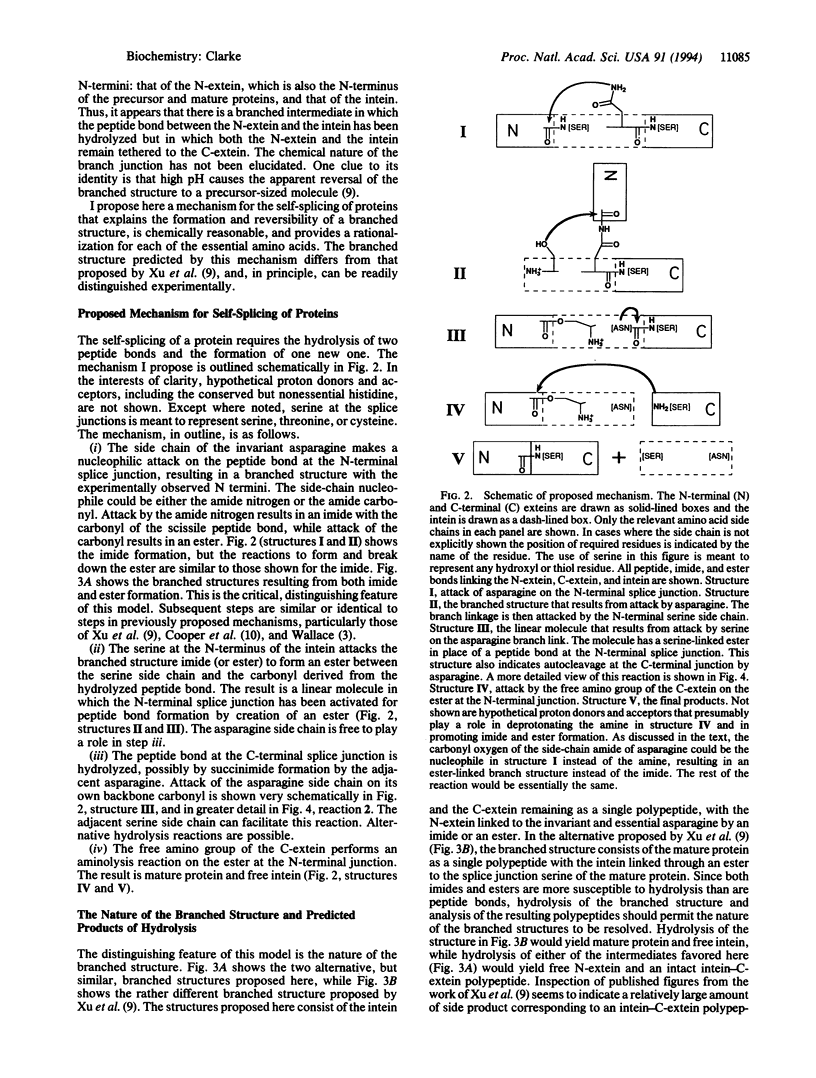
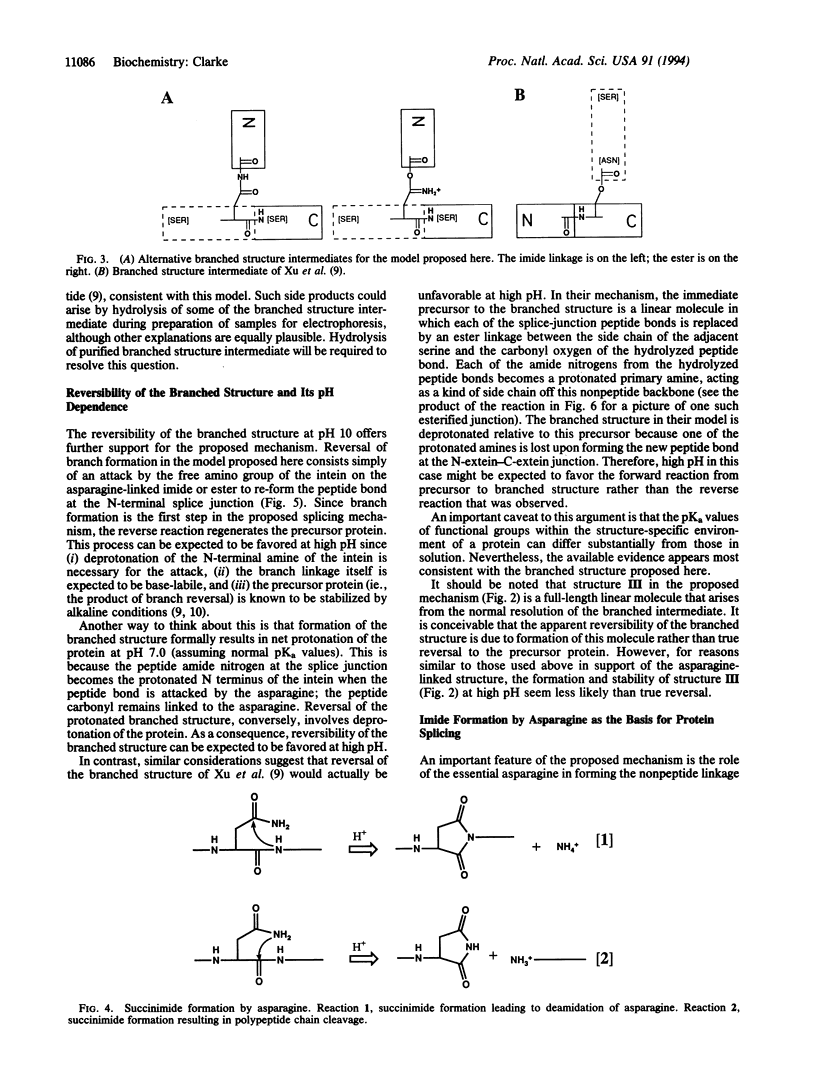
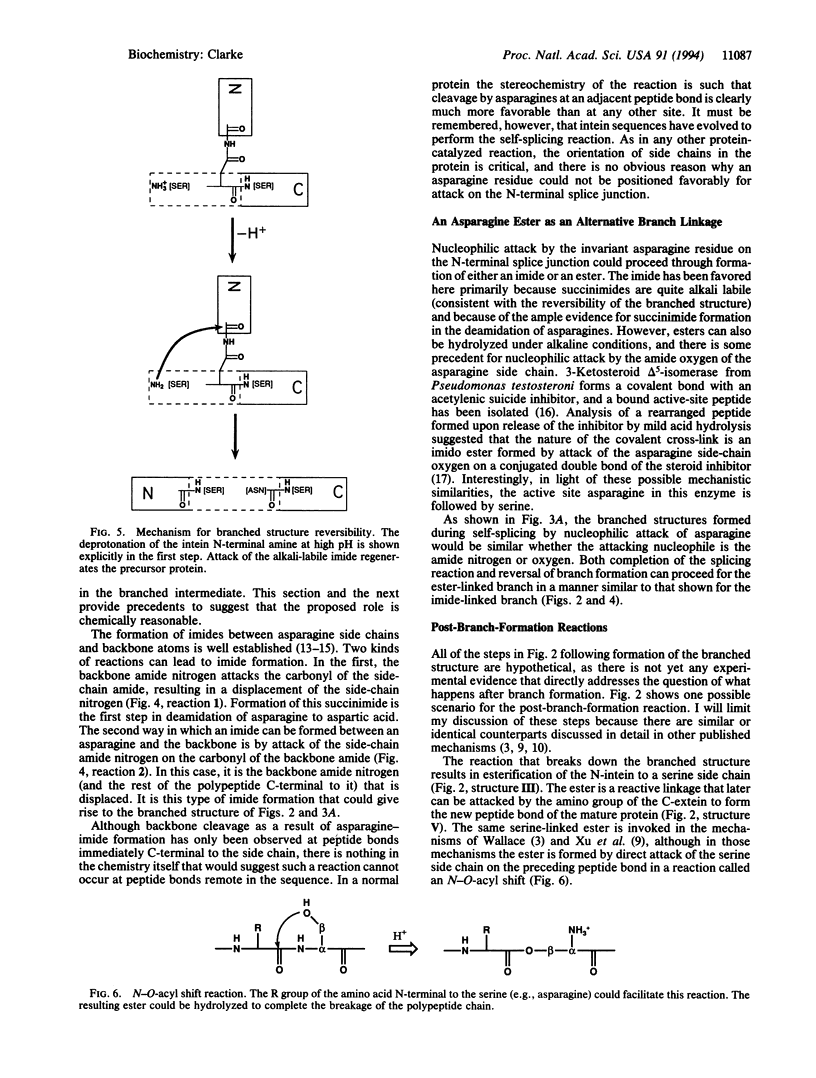
Intervening protein sequences, called inteins, are intronlike elements that are removed posttranslationally, apparently by self-splicing. The conserved and essential residues of precursor proteins consist of an asparagine as the last residue of the intein and a hydroxyl- or thiol-containing residue immediately following both splice junctions. Evidence for a branched intermediate has been reported [Xu, M.-Q., Southworth, M., Mersha, F., Hornstra, L. & Perler, F. (1993) Cell 75, 1371-1377]; however, the chemical nature of the branched structure is unclear. I propose a mechanism that includes the formation of a branched structure, provides an explanation for the reversal of branch formation observed at high pH, and accounts for each of the essential amino acids. The branched structure is formed by nucleophilic attack of the asparagine side chain on the N-terminal splice junction. The nature of this branched structure is a distinguishing feature of the model and can be experimentally tested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper A. A., Chen Y. J., Lindorfer M. A., Stevens T. H. Protein splicing of the yeast TFP1 intervening protein sequence: a model for self-excision. EMBO J. 1993 Jun;12(6):2575–2583. doi: 10.1002/j.1460-2075.1993.tb05913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Jenner P. J., Brooks P. C., Colston M. J., Sedgwick S. G. Protein splicing in the maturation of M. tuberculosis recA protein: a mechanism for tolerating a novel class of intervening sequence. Cell. 1992 Oct 16;71(2):201–210. doi: 10.1016/0092-8674(92)90349-h. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. The comings and goings of homing endonucleases and mobile introns. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5379–5381. doi: 10.1073/pnas.90.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987 Jan 15;262(2):785–794. [PubMed] [Google Scholar]
- Gimble F. S., Thorner J. Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature. 1992 May 28;357(6376):301–306. doi: 10.1038/357301a0. [DOI] [PubMed] [Google Scholar]
- Hirata R., Anraku Y. Mutations at the putative junction sites of the yeast VMA1 protein, the catalytic subunit of the vacuolar membrane H(+)-ATPase, inhibit its processing by protein splicing. Biochem Biophys Res Commun. 1992 Oct 15;188(1):40–47. doi: 10.1016/0006-291x(92)92347-z. [DOI] [PubMed] [Google Scholar]
- Hodges R. A., Perler F. B., Noren C. J., Jack W. E. Protein splicing removes intervening sequences in an archaea DNA polymerase. Nucleic Acids Res. 1992 Dec 11;20(23):6153–6157. doi: 10.1093/nar/20.23.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H(+)-adenosine triphosphatase. Science. 1990 Nov 2;250(4981):651–657. doi: 10.1126/science.2146742. [DOI] [PubMed] [Google Scholar]
- Penning T. M., Heller D. N., Balasubramanian T. M., Fenselau C. C., Talalay P. Mass spectrometric studies of a modified active-site tetrapeptide from delta 5-3-ketosteroid isomerase of Pseudomonas testosteroni. J Biol Chem. 1982 Nov 10;257(21):12589–12593. [PubMed] [Google Scholar]
- Penning T. M., Talalay P. Linkage of an acetylenic secosteroid suicide substrate to the active site of delta 5-3-ketosteroid isomerase. Isolation and characterization of a tetrapeptide. J Biol Chem. 1981 Jul 10;256(13):6851–6858. [PubMed] [Google Scholar]
- Perler F. B., Comb D. G., Jack W. E., Moran L. S., Qiang B., Kucera R. B., Benner J., Slatko B. E., Nwankwo D. O., Hempstead S. K. Intervening sequences in an Archaea DNA polymerase gene. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5577–5581. doi: 10.1073/pnas.89.12.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F. B., Davis E. O., Dean G. E., Gimble F. S., Jack W. E., Neff N., Noren C. J., Thorner J., Belfort M. Protein splicing elements: inteins and exteins--a definition of terms and recommended nomenclature. Nucleic Acids Res. 1994 Apr 11;22(7):1125–1127. doi: 10.1093/nar/22.7.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shub D. A., Goodrich-Blair H. Protein introns: a new home for endonucleases. Cell. 1992 Oct 16;71(2):183–186. doi: 10.1016/0092-8674(92)90345-d. [DOI] [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J Biol Chem. 1989 Apr 15;264(11):6164–6170. [PubMed] [Google Scholar]
- Tyler-Cross R., Schirch V. Effects of amino acid sequence, buffers, and ionic strength on the rate and mechanism of deamidation of asparagine residues in small peptides. J Biol Chem. 1991 Nov 25;266(33):22549–22556. [PubMed] [Google Scholar]
- Wallace C. J. The curious case of protein splicing: mechanistic insights suggested by protein semisynthesis. Protein Sci. 1993 May;2(5):697–705. doi: 10.1002/pro.5560020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H. T. Nonenzymatic deamidation of asparaginyl and glutaminyl residues in proteins. Crit Rev Biochem Mol Biol. 1991;26(1):1–52. doi: 10.3109/10409239109081719. [DOI] [PubMed] [Google Scholar]
- Xu M. Q., Southworth M. W., Mersha F. B., Hornstra L. J., Perler F. B. In vitro protein splicing of purified precursor and the identification of a branched intermediate. Cell. 1993 Dec 31;75(7):1371–1377. doi: 10.1016/0092-8674(93)90623-x. [DOI] [PubMed] [Google Scholar]



