Abstract
Interferon (IFN) treatment induces the expression of hundreds of IFN-stimulated genes (ISGs). However, only a selection of their products have been demonstrated to be responsible for the inhibition of rhabdovirus replication in cultured cells; and only a few have been shown to play a role in mediating the antiviral response in vivo using gene knockout mouse models. IFNs inhibit rhabdovirus replication at different stages via the induction of a variety of ISGs. This review will discuss how individual ISG products confer resistance to rhabdoviruses by blocking viral entry, degrading single stranded viral RNA, inhibiting viral translation or preventing release of virions from the cell. Furthermore, this review will highlight how these viruses counteract the host IFN system.
Keywords: interferon, rhabdoviruses, ISG, rabies virus, vesicular stomatitis virus
1. Introduction
The establishment of an antiviral state in cells has resulted in the discovery of interferons (IFNs), and this antiviral activity is still their defining property. IFNs act on target cells to confer resistance to viral infection at various stages of viral replication; including entry, transcription, RNA stability, translation, maturation, assembly and release. The antiviral activities of IFNs are mediated by the induction of IFN-stimulated gene (ISG) products. One interesting property of ISG-mediated antiviral activity is the magnitude by which a single IFN effector can inhibit virus replication. In this review, rhabdovirus replication, IFN production, IFN signaling, ISGs conferring resistance to rhabdoviruses and the mechanisms by which these viruses counteract the IFN pathway will be described.
2. Rhabdoviruses
Rhabdoviruses (order Mononegavirales) are pathogens with a particularly broad host range among a great diversity of organisms including plants, insects, fish, mammals, reptiles and crustaceans. They are associated with significant pathogenicity in humans and livestock [1]. The prototypes of this family are vesicular stomatitis virus (VSV), a member of the Vesiculovirus genus, and rabies virus (RABV), a member of the Lyssavirus genus. VSV is an arthropod-borne virus that primarily affects rodents, cattle, swine and horses. It can induce mild symptoms upon infection of humans and other species and may also cause severe foot- and mouth-like disease in cattle and pigs. The closely related Chandipura virus has recently been associated with outbreaks of fatal acute encephalitis in several parts of India (for a review see [2]). RABV is a prototype neurotropic virus that causes fatal disease in humans and animals. Human rabies is a zoonosis, which still accounts for 50,000 deaths per year worldwide despite the availability of effective vaccines.
Although VSV and RABV have many similarities, they use different strategies to regulate their replication in the host cell, leading to the various pathologies caused by these viruses. VSV replicates rapidly, developing high levels of progenies in a minimum amount of time and strongly interferes with the host’s cell metabolism. VSV inhibits host gene expression and translation [3,4,5], interferes with the host cell’s innate immune response and induces apoptosis of infected cells [6]. In contrast, RABV replicates more slowly, has a reduced interference with the host cell metabolism and is less cytopathic than VSV [7].
Rhabdovirus virions have a bullet-like shape, conical at one end and flat at the other, with a diameter of 75 nm and a length of 100–300 nm [8,9]. The genome consists of a negative sense, single stranded RNA molecule of approximately 11 to 16 kb that encodes the five viral proteins common to all mononegavirales in the order of 3′-N-P-M-G-L-5′. The genome of many rhabdoviruses encodes accessory proteins, whose functions are not yet fully understood. These may occur as alternative or overlapping open reading frames (ORFs) within the major protein genes or as independent ORFs between the genes [10]. The viral RNA is associated with the nucleoprotein (N) to form a helical nucleocapsid (N-RNA). The nucleocapsid contains a significant amount of phosphoprotein (P), some of which is bound to the viral RNA-dependent RNA polymerase (RdRp), the large (L) protein. The N-RNA, together with the viral polymerase complex (P and L proteins) form the ribonucleoprotein (RNP). The RNP is enwrapped in a lipid bilayer, which is acquired from host cell membrane during the budding process. The matrix protein (M) and the glycoprotein (G) are membrane-associated proteins, whereby M is located beneath the viral membrane and maintains the compact structure of the virion by associating with both the nucleocapsid and the lipid bilayer, while the G protein is an integral transmembrane protein involved in viral entry.
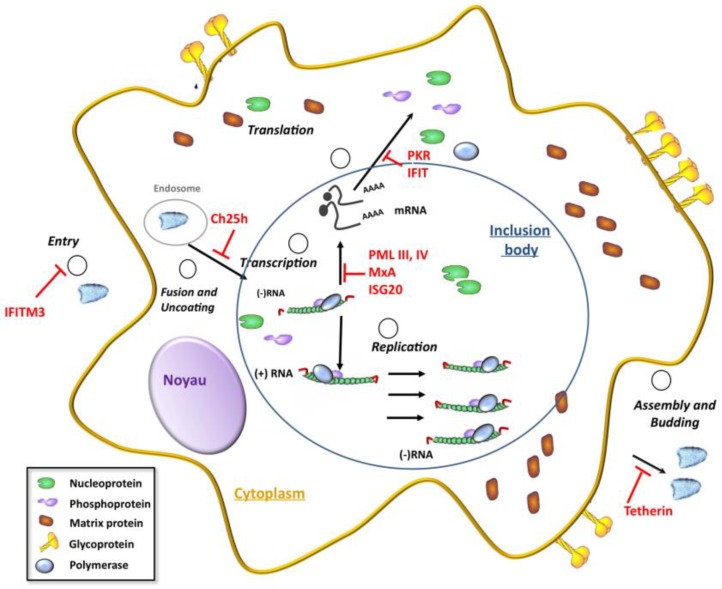
The life cycle of rhabdoviruses is entirely cytoplasmic (Figure 1). After binding to its receptor(s), the virus enters the cell via the endocytic pathway. The acidic environment within early endosomes induces a change in the conformation of the G protein that results in the fusion of the viral envelope with the endosomal membrane. After the fusion event, uncoating of the viral genome results in the release of the viral genomic RNA into the cytoplasm. The RNP constitutes the template for transcription of viral genes and the viral RNA polymerase (the L-P complex) is responsible for replication of the viral genome. RABV transcription and replication take place within Negri bodies, which are characteristic cytoplasmic inclusion bodies formed during viral infection [11]. Similar cytoplasmic inclusions have recently been reported in cells infected with VSV; these inclusions also contain the viral replication machinery [12]. During transcription, a positive-stranded leader RNA, uncapped and non-polyadenylated, and five capped and polyadenylated mRNAs encoding the five viral proteins are synthesized. At a later stage of infection, the polymerase switches to replication of the viral genome, which yields nucleocapsids containing the full-length antigenome (sense) RNA, which in turn serves as template for the synthesis of the (antisense) RNA genome. During their synthesis, both the nascent antigenome and the genome are encapsidated by the N protein. Specific transfer of the N protein to viral RNAs rather than to the cellular mRNAs is mediated by the P protein, which acts as a chaperone by binding the N alone (N°) and maintaining its solubility [13]. The neo-synthesized genomic RNPs then serve either as templates for additional rounds of transcription and/or replication, or are transported to the cell membrane where they are assembled with the M and G proteins into virions, which are then released from the cell through the budding process.
Figure 1.
The different steps of the rhabdovirus cycle inhibited by interferon (IFN)-stimulated gene (ISG) products. IFN-inducible transmembrane (IFITM) proteins block viral entry, CH25h impairs the virus cell-fusion step by inducing cellular membrane changes, MxA inhibits primary transcription, ISG20 and ProMyelocytic Leukemia (PML) inhibit secondary transcription, protein kinase (PKR) and IFIT proteins inhibit viral translation and Tetherin prevents release of virions from the cell.
3. IFN Production upon Viral Infection
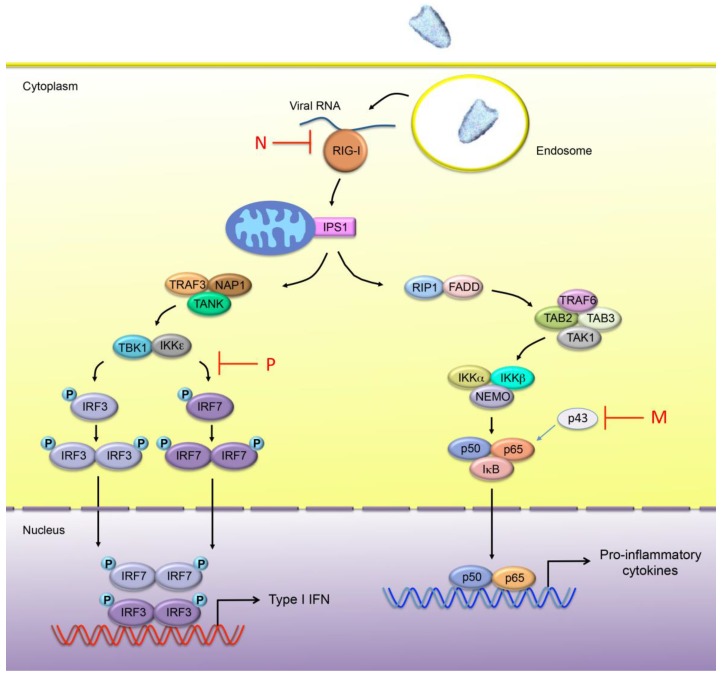
Infection by rhabdoviruses triggers a cellular response through the activation of pattern recognition receptors (PRRs), leading to the production and secretion of IFN and pro-inflammatory cytokines. Two major classes of PRRs are involved in the detection of pathogens: Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs). These sensors can detect exogenous pathogens via the recognition of pathogen-associated molecular patterns (PAMPs). In the case of viral infection, the major PAMPs consist of nucleic acids. Although the diverse PRRs induce distinct signaling pathways, their initiation results in the activation of the nuclear factor κB (NF-κB) and IFN regulatory factor (IRF) 3 and/or IRF7. Once phosphorylated, the IRF3 and IRF7 homodimers as well as NF-κB translocate into the nucleus and induce the activity of the promoter regions of Type I IFNs and pro-inflammatory cytokines, respectively (Figure 2).
Figure 2.
Innate immune sensing of rhabdovirus infection. In rhabdovirus-infected cells, the viral RNA is mainly detected by RIG-I. Once activated, RIG-I binds to the CARD containing adaptor protein IPS-1 (also known as MAVS, CARDIF or VISA), which phosphorylates IRF3 and/or IRF7 through TRAF3, NAP1 and TBK1/IKKε. Phosphorylated IRF3 and IRF7 homodimerize and translocate into the nucleus where they induce the expression of Type I IFN genes. IPS-1 also interacts with FADD, a death domain-containing adapter involved in death receptor signaling, and RIP1, which induces the activation of the NF-κB pathway. NF-κB is composed of homo- and heterodimeric complexes of members of the Rel family. The most common and best-characterized form of NF-κB is the p65/p50 heterodimer. A new member RelAp43 (p43) of the NF-κB family has been recently identified. Activation of the IκB kinase (IKK) complex, consisting of catalytic kinase subunits (IKKα and/or IKKβ) and the regulatory non-enzymatic scaffold protein NEMO, results in the phosphorylation and subsequence degradation of IκB. This enables free NF-κB to translocate to the nucleus, where it induces target gene expression, including pro-inflammatory cytokine encoding genes. The steps inhibited by the RABV-N and -P proteins, as well as VSV M protein are indicated in the diagram above.
Among the TLR family, a subgroup of endosome localized TLRs (TLR3, 7, 8, 9) detect nucleic acids, whereas the other members, expressed at the cell surface, mainly recognize bacterial cell wall components or viral proteins. TLR3 detects double-stranded (ds) RNA, while TLR7 and TLR8 recognize single-stranded (ss) RNA and TLR9 recognizes unmethylated DNA with CpG motifs. Unlike membrane-bound TLRs, RLRs reside in the cytoplasm and recognize cytoplasmic RNA species. Although TLR7 and TLR9 are essential for the recognition of incoming viruses in plasmacytoid dendritic cells (pDCs), viral replication could stimulate IFN production through RLRs [14,15]. The majority of other cell types also recognize viral RNA in the cytoplasm through RLR-induced sensing. Whereas TLR7 and TLR9 are essential for the recognition of viral nucleic acids in plasmacytoid dendritic cells (pDCs), most other cell types recognize viral RNA through RLRs. The two major RLRs, RIG-I and MDA5, respond to distinct RNA virus species [16]. Indeed, whereas MDA5 is critical for picornavirus detection, RIG-I is able to sense many RNA viruses, including rhabdoviruses (Figure 2). It was demonstrated that uncapped 5′-triphosphate RNA serves as the molecular signature for the detection of viral infections by RIG-I [15,17]. Although RIG-I appears to constitute the main PRR implicated in rhabdovirus sensing, TLR3 and TLR7 may also be involved, although their exact contribution remains unclear. In the case of TLR3, it was first shown that TLR3-deficient mice were as capable as wild-type to clear VSV infection [18]. However, TLR3−/− mice were found to display a reduced susceptibility to a pathogenic RABV strain [19], which could be attributed to the involvement of TLR3 in the formation of viral Negri bodies rather than to its implication in IFN response [19]. TLR7 was found to be involved in VSV sensing in mice [20], whereas it appeared not to be associated with RABV infection; neither in mice [21] nor in human pDCs [15].
Virus-induced IFN production is tightly regulated and the aberrant production of this cytokine is harmful or even fatal to the host. Post-translational modifications of the transcription factors IRF3 and IRF7 are central regulatory mechanisms of Type I IFN mediated antiviral response. It has been reported that VSV infection triggers small ubiquitin modifier (SUMO)-ylation of IRF3 and IRF7 leading to the negative regulation of Type I IFN gene expression [22]. Accordingly, SUMOylation deficient IRF3 and IRF7 mutants lead to higher levels of IFN mRNA induction after viral infection. In contrast, increased MDA5 or RIG-I SUMOylation is correlated with elevated Type I IFN induction [23,24].
4. IFN Signaling and ISGs Conferring Resistance to Rhabdoviruses
4.1. IFN Signaling
The establishment of an antiviral state in cells is the defining function of IFNs and the property that resulted in their discovery in 1957 by Isaacs and Lindenmann [25]. Any stage in viral replication may be a target for inhibition by IFNs; including entry, transcription, RNA stability, translation, maturation, assembly and release. Three classes of IFNs have been identified and are designated Types I to III. In humans, Type I IFNs include IFNα, IFNβ, IFNε, IFNκ and IFNω. A single gene encodes each Type I IFN, with the exception of IFNα, which is comprised of 13 subtypes. IFNγ is the sole Type II IFN, and Type III IFNs are composed of IFNλ1, IFNλ2, IFNλ3 and IFNλ4 [26,27].
The interaction of Type I IFN with IFNAR leads to the activation of the JAK tyrosine kinases (Tyk2 and JAK1) that in turn phosphorylate STAT1 and STAT2. Phosphorylated STAT1 and STAT2 heterodimerize and form, with the DNA binding protein IFN regulatory factor 9 (IRF9), a complex called IFN-stimulated growth factor 3 (ISGF3). There is also growing evidence that Type I IFN activates a STAT2/IRF9 complex that forms an ISGF3-like in the absence of STAT1 [28,29]. ISGF3 translocates to the nucleus and binds to ISG promoters harboring an IFN-stimulated response element (ISRE). The binding of Type II IFN to its receptor, IFNGR, results in the phosphorylation of STAT1 by JAK1 and JAK2. STAT1 homodimers migrate to the nucleus and bind to a DNA element termed GAS (gamma-activated sequence) in the promoter regions of ISGs. Finally, Type III IFNs that are structurally and genetically distinct from Type I IFNs, bind to a different receptor, IFNLR1, but activate the same signal transduction pathway [26]. Expression of IFNLR1 is largely restricted to epithelial cells [30]. Consequently, a large number of cell types respond very poorly or have no response at all to Type III IFNs.
The transcriptional regulation of effector genes downstream of the Jak/STAT pathway contributes to pleiotropic responses induced by IFNs [26]. The products of some ISGs have been shown to mediate the antiviral effects of IFNs. Interestingly, microRNAs (miRNA) induced by IFNs emerged as novel regulators of viral infection [31]. Additionally, miRNAs negatively regulate the RIG-I antiviral pathway. Furthermore, VSV infection increases miR-146a expression within mouse macrophages in a RIG-I-NF-κB-dependent manner. In turn, miR-146a negatively regulates VSV-triggered Type I IFN production, thus promoting VSV replication in macrophages [32]. This aspect will not be discussed in more detail in the review.
4.2. ISG Products Conferring Resistance to Rhabdoviruses
The antiviral restriction factors, which are cellular proteins that confer viral resistance, are themselves encoded by ISGs. Therefore, the ISG products exhibiting antiviral activity are also known as restriction factors and can be implicated in both intrinsic and innate immune activities. They act cooperatively to confer viral resistance by inhibiting various stages of viral replication. Some of these factors are highly constitutively expressed and are able to exert an intrinsic antiviral activity independently of IFN, whereas their innate immune activity requires a pathogen recognition event and/or induction by IFN.
Until 1999, three IFN-responsive antiviral proteins had been considered to be involved in the antiviral processes: the Mx (for myxovirus resistance) dynamins, the double-stranded RNA-dependent protein kinase (PKR) and the 2′-5′ oligoadenylate synthetases that function through RNase L. The demonstration that IFN confers resistance to viral infection in cells derived from mice triply deficient (TD) in PKR, Mx1 and RNase L [33] revealed the implication of other antiviral pathways.
IFN treatment induces the expression of hundreds of ISGs, but only a selection of their products have been demonstrated to be responsible for the inhibition of VSV replication. Indeed, ISG products such as PKR [34], Mx [35], p53 [36], ISG20 [37], Ifit2/ISG54 [38], IFITM3 [39], Tetherin [39], Ch25h [40] and ProMyelocytic Leukemia (PML) [41,42,43] have been reported to confer resistance to VSV infection (Figure 1). Although PKR and the 2′-5′ oligoadenylate synthetases have been shown to be implicated in antiviral defense, their overexpression has not been reported to alter VSV infection.
Recent ectopic expression and gene silencing experiments have identified many novel ISGs with inhibitory activity against viruses from various families [26,44]. In the case of VSV, 34 ISG products have been shown to elicit an antiviral effect [45], which include Mx, Ch25h, IRF1, IFITM3, ISG20 and some members of TRIM protein family (PML/TRIM19, TRIM25, TRIM31, TRIM62).
IFNs inhibit VSV replication at different stages. Individually, some ISG products can interfere with a particular stage of the VSV life cycle. For example IFITM proteins block viral entry [39], CH25h impairs the virus cell-fusion step by inducing cellular membrane changes [40], MxA inhibits primary transcription [35,46], ISG20, a 3′-5′ exonuclease, degrades single stranded viral RNA [37], PML inhibits secondary transcription [41,42], PKR [47] and IFIT [38] proteins inhibit viral translation and Tetherin prevents release of virions from the cell [39] (Figure 1). PML and IFITM3 have been reported to confer resistance to RABV [48,49,50], however the effect of the other human antiviral mediators on RABV is still unknown. The antiviral ISG mediators, also known as host restriction factors, reside in the cytoplasm, the nucleus, the plasma membrane and the viral particle itself. Their localization within the cell often corresponds to the stage of the virus life cycle with which it interferes. In addition to having an intrinsic anti-VSV activity, various ISG products, such as some TRIM proteins have been recognized as important immune signaling mediators [42,51].
One interesting property of ISG-mediated antiviral activity is the magnitude with which a single IFN effector can inhibit virus replication (Table 1). Usually ISGs are known to exert antiviral functions. Intriguingly the IFN-inducible gene IFI35 (also known as IFP35) was identified as factor required for VSV infection [52]. IFI35 negatively regulates RIG-I activation and also mediates its proteasomal degradation through ubiquitination [53]. Whether the requirement of IFI35 is specific to VSV infection is still unknown. This will not be further developed in this review.
Table 1.
Main human ISG products conferring intrinsic resistance to vesicular stomatitis virus (VSV) and rabies virus.
| ISG Products | Degree of Inhibition of Viral Production | Mechanisms | References |
|---|---|---|---|
| IFITM3 | 1 log | Inhibits a VSV entry step after endocytosis. | [39] |
| Ch25h | 1 log | Inhibits VSV entry by production of 25-Hydroxycholesterol. | [40] |
| MxA | 3 logs | MxA confers resistance to VSV. Inhibits VSV primary transcription. |
[35] [46] |
| PMLIII | 2 logs | Inhibits VSV at transcriptional level. | [41] |
| PMLIV | 3 logs | Inhibits VSV and rabies virus at transcriptional level. | [42] |
| PMLIV | Positively regulates IFNβ synthesis during VSV infection. | [42] | |
| ISG20 | 0.5 log | Reduces VSV mRNA synthesis and requires its exonuclease activity. | [37] |
| Tetherin | 3 logs | Inhibits viral particles release from infected cells. | [39] |
| IFIT3 | 1 log | Reduces VSV production. | [90] |
| GBP1 | 0.5 log | Reduces VSV production. | [97] |
4.2.1. IFITM
The IFN-inducible transmembrane (IFITM) genes are enhanced by cell treatment with IFNs since they contain the ISREs in their promoter. IFITM proteins belong to the CD225 protein superfamily, which can be found in nearly every domain of life, ranging from bacteria to invertebrates to primates. In humans, there are at least four functional members of IFITM proteins: IFITM1, IFITM2 and IFITM3, previously named 9-27, 1-8D and 1-8U, respectively, are expressed in a variety of tissues; IFITM5 is limited to the bone. IFIM4P is a pseudogene (reviewed in [54]). Mouse IFITM1, IFITM2, IFITM3 and IFITM5 genes are orthologues to their human counterparts. In addition, mice have two other IFITM genes: IFITM6 and IFITM7.
IFITM proteins are a group of small ISGs (15 kDa) that have been reported to inhibit infection of Influenza A virus, West Nile virus and Dengue virus [55,56]. These proteins inhibit viral membrane fusion, thus resulting in cellular protection from a diverse range of infections. An early observation of IFITM proteins controlling viral infection was reported in 1996 [57], where overexpression of IFITM1 was shown to inhibit VSV replication, although less potently than the IFN-induced protein MxA [57]. This study is the first description of the antiviral activity of an IFITM protein.
All IFITM proteins have been shown to restrict cell entry of many viruses [54,56,58,59,60]. However, IFITM3 is the most potent IFITM family member in restricting viral replication in cell culture [61]. IFITM3 inhibits a VSV entry step following endocytosis, but at or before the point of primary transcription of incoming viral genomes [39] (Table 1). Both the N-terminal 21 amino acid residues and C-terminal transmembrane region of IFITM3 are required for its antiviral function. In addition, IFITM3 has been shown to inhibit an early stage of the RABV replication cycle by targeting entry mediated by the viral glycoprotein [50].
4.2.2. Ch25h
The cholesterol-25-hydroxylase (Ch25h) has been identified as a broadly antiviral ISG through a systematic functional screen [40]. It has been shown that Ch25h inhibits growth of VSV and a wide-range of enveloped viruses through the production of a soluble antiviral factor that is not IFN. Ch25h is an endoplasmic-reticulum-associated enzyme that catalyzes the oxidation of cholesterol into the soluble component 25 hydroxycholesterol (25HC) [62]. The viral restriction mediated by 25HC occurs at an early stage, resulting in a 1 log inhibition of viral production (Table 1) [40]. The overexpression of factors controlling sterol biosynthesis or the addition of intermediates in the sterol biosynthesis pathway, such as mevalonate, do not rescue 25HC-mediated virus inhibition; suggesting that 25HC blocks membrane fusion. Liu et al. [40] proposed that 25HC impaired viral entry at the virus cell-fusion step by inducing cellular membrane changes due to membrane expansion or aggregation [40]. This inhibition is not specific to a particular structural class of viral fusion proteins (such as Class I, II or III) and is not linked to either pH-dependent or pH-independent fusion processes, but is due to membrane perturbation resulting from the oxidation of the cholesterol. However, the authors do not exclude that the Ch25h protein may have additional antiviral mechanisms to 25HC, perhaps through its association with the endoplasmic reticulum.
4.2.3. MxA
Humans harbor two Mx genes (MX1 and MX2) in the chromosome 21 [63], which are induced only in response to Type I or Type III IFNs via the ISRE present in their gene promoter [64]. Human Mx1 and Mx2 gene products are MxA and MxB, respectively. Mx proteins are classified as large dynamin-like GTPases due to their similarities to dynamins [65]. MxA and dynamins share structural and functional aspects including domain organization, GTPase activity and homo-oligomerization capacity [66]. The Mx proteins inhibit several different viruses by blocking early stages of their replication cycle [65,67].
The human MxA and MxB proteins [35], like those of the rat (Mx2 or Mx3) [68] and the mouse (Mx2) [69], are cytoplasmic, while the Mx1 protein has a speckled nuclear localization in both mouse and rat cells. Mx proteins from different species exhibit distinct antiviral activities with a specificity conferred by their subcellular localization [65,67]. In general, nuclear Mx proteins inhibit viruses that replicate in the nucleus such as influenza virus, whereas cytoplasmic forms inhibit VSV and other RNA viruses that replicate in the cytoplasm.
Rat Mx1, mouse Mx2 and human MxA are active against VSV. More recently, it has been shown that bovine Mx1 inhibits replication of the rabies virus [70]. VSV is among the viruses that are strongly repressed by human MxA. Cells overexpressing MxA acquire a high degree of resistance to VSV in a 3 logs reduction in viral titers compared to wild-type cells [35] (Table 1). MxA inhibits VSV primary transcription, suggesting that MxA alters VSV polymerase function [46]. An interaction between MxA and VSV proteins has not yet been demonstrated.
4.2.4. PML/TRIM19
PML (also named TRIM19 for TRIpartite Motif protein 19) belongs to the TRIM family that comprises over 70 members whose functions span a broad array of physiological processes, including cell proliferation, differentiation and antiviral defense [71]. PML is the organizer of small nuclear-matrix structures named nuclear bodies (NBs) [72]. In response to diverse stimuli, PML NBs recruit a growing number of proteins implicated in different cellular processes including apoptosis, senescence, protein degradation and antiviral defense [73,74,75,76]. PML is covalently conjugated to small ubiquitin modifier (SUMO) [77]. This modification, which is required for PML NB functions, alters PML localization, stability and capacity to interact with other partners.
Several PML isoforms generated by alternative splicing from a single gene are designated PMLI to PMLVIIb [74,78]. They share the same N-terminal region, which encodes the RBCC/TRIM (RING finger, B-box, and Coiled-Coil) motif, but differ in their C-terminal region due to alternative splicing. The variability of the C-terminal region is important for the specific function of each PML isoform [74]. PML confers resistance to RNA and DNA viruses from different families. This has been shown in cells stably expressing individual PML isoforms or in cells depleted for PML by RNA interference (reviewed in [75,76]). The antiviral effect of PML has been observed in vivo, as PML deficiency renders mice more susceptible to VSV infection [43]. A study performed with all PML isoforms revealed that only PMLIII and PMLIV could confer resistance to VSV infection [42]. The anti-VSV activity of PMLIV is higher than that of PMLIII, since the overexpression of PMLIII and PMLIV results in 2 and 3 logs reduction in VSV titers, respectively [41,42] (Table 1). The anti-VSV activity of PMLIII is strictly IFN-independent, whereas resistance to VSV exerted by PMLIV occurs via two independent mechanisms. PMLIV is able to inhibit VSV replication in an IFN-independent manner and is also able to positively regulate IFNβ synthesis via the enhancement of IRF3 phosphorylation. Both activities of PMLIV require its SUMOylation. Among all PML isoforms, only PMLIV is able to recruit the peptidyl-prolyl isomerase (Pin1) [42], known to interact with phosphorylated IRF3 and to induce its proteasomal degradation [79]. The interaction of Pin1 with SUMOylated PMLIV leads to the recruitment of Pin1 within PML NBs and results in an increased stability of phospho-IRF3 and in an enhanced IFNβ production [42]. Further investigations are required to demonstrate how PMLIII and PMLIV exert their intrinsic anti-VSV activity by interacting with a viral or a cellular protein required for VSV replication.
In the case of RABV, among all the PML isoforms tested only PMLIV confers intrinsic resistance to this virus by inhibiting viral transcription, resulting in a 2 logs reduction of viral titer [48]. This anti-RABV effect exerted by PMLIV is independent of IFN production, as PMLIV does not increase IFN production within infected cells ([48] and unpublished data). Further investigations are needed to elucidate the mechanism of action of PMLIV against RABV.
4.2.5. ISG20
IFN-stimulated gene 20 kDa protein is encoded by the human ISG20 gene. ISG20 is a 3′-to-5′ exonuclease specific for single-stranded RNA involved in host defense against RNA viruses. ISG20 is transcriptionally induced by both Types I and II IFNs [80,81]. Its induction by IFN is strictly dependent upon the activation and the binding of IRF1 to a specific ISRE on the ISG20 promoter. ISG20 is also directly induced by synthetic dsRNA via NF-κB and IRF1 activation [82].
ISG20 overexpression in HeLa cells reduces VSV mRNA and protein synthesis resulting in a 0.5 log inhibition of viral production (Table 1). This anti-VSV activity requires the exonuclease activity of ISG20. In addition, the anti-VSV activity of IFN is reduced in cells expressing the inactive exonuclease mutant form of the ISG20 protein, suggesting that the antiviral activity of IFN against VSV is partly mediated by ISG20.
4.2.6. IFIT
IFIT (IFN-induced proteins with tetratricopeptide repeats) genes encode a family of cytoplasmic proteins that are induced after Type I IFN or IRF3-dependent signaling. They contain the ISREs in their promoter that are recognized by members of the IRF family of transcription factors [83]. IFN and many inducers that activate IRFs induce their expression. The strongest IFIT inducers are Types I and III IFNs, whereas induction by Type II IFN is much weaker. IFIT genes are also induced in cells infected with various RNA viruses, including VSV. All IFIT proteins contain several full and partial tetratricopeptide repeat (TPR) motifs [84], which are critical for viral regulation. By convention, the human IFIT genes are spelt using capital letters whereas the mouse ifit genes are spelt in lowercase. In humans, four members have been characterized, IFIT1 (also named ISG56), IFIT2 (also named ISG54), IFIT3 (also named ISG60) and IFIT5 (also named ISG58), whereas three members are expressed in mice, ifit1 (also named ISG56), ifit2 (also named ISG54) and ifit3 (also named ISG49). The cognate members of two species have distinct sequences; for example, human IFIT2 and mouse Ifit2 are only 62% homologous. Thus, they share names but not equating properties.
IFIT proteins contribute to an antiviral state against some viruses through multiple mechanisms by binding components of the eIF3 translation initiation complex and inhibiting protein translation, binding viral RNA and sequestering viral RNA or proteins in the cytoplasm [85,86]. Both IFIT1 and Ifit1 can specifically bind mRNA 5′ ends whose caps lack the 2′-O-methylation of the first ribose, a specificity of some viral but not cellular mRNAs thus preventing viral mRNA translation [87]. Remarkably, IFIT1 inhibits viral translation by two different mechanisms (eIF3 and cap-RNA binding), it uses its N-terminal and central region to bind to RNA, whereas its C-terminal binds eIF3 [88,89].
Depletion of IFIT1, IFIT2 or IFIT3 in HeLa cells with siRNA results in an increase in the rate of infection by VSV. It has been reported that overexpression of IFIT3 in Vero cells results in a 1 log decrease of virus titer (Table 1) and IFIT3 depletion in human cells reduces the anti-VSV activity of IFNα [90].
4.2.7. Tetherin
Tetherin is an IFN-inducible, 28- to 36-kDa transmembrane protein also known as BST2 (Bone marrow stromal Ag 2, also named CD317 or HM1.24). Tetherin is constitutively expressed in several cell types and its expression is strongly induced by all three types of IFNs [91]. Tetherin is a Type II transmembrane protein that contains an N-terminal cytoplasmic domain, a single membrane-spanning α-helix, an extracellular domain, and a C-terminal glycosylphosphatidylinositol (GPI) anchor attaching to the cell membrane lipid bilayer [92]. Tetherin associates with cholesterol-enriched lipid rafts, which are involved in both virus budding and cell-to-cell spread [93].
Expression of Tetherin in HEK293 cells confers resistance to VSV resulting in a 3 logs reduction of viral production (Table 1). Tetherin impairs a late step in the VSV replication cycle, most likely virion particle release from infected cells [39]. Tetherin depletion reduces the capacity of IFNα to inhibit VSV at lower concentrations (1 to 10 IU/mL), but the attenuation effect decreases in cells treated with high IFN concentration, probably due to the participation of other ISG products.
4.2.8. GBP1
There are at least two forms of IFN-induced Guanylate Binding Proteins (GBPs) in human and murine cells [94]. They have an affinity for guanylate and function as GTPases. They belong to the dynamin superfamily of large GTPases characterized by an oligomerization-dependent GTPase activity [95]. GBP1 is highly induced by IFNγ [96]. Overexpression of human GBP-1 inhibits 0.5 log VSV production, protecting cells from the subsequent cytopathic effect (Table 1). In addition, decreasing GBP-1 levels using antisense RNA to GBP1 reduces the anti-VSV activity of IFN. Further investigations are required to determine the exact viral stage that is inhibited by GBP-1 [97].
4.3. Results Obtained from VSV-Infected Knockout Mice and Their Derived Cell Lines
Studies using mice defective in ISGs revealed their implication in antiviral defense (Table 2). Compared to the parental mice, PKR−/− mice are more susceptible to VSV infection and die from acute infection of the respiratory tract [47]. In addition, IFNα/β are unable to rescue PKR−/− mice from VSV infection [34]. However, the overexpression of human PKR in culture cells does not alter VSV replication [98]. The generation of triply deficient (TD) PKR, Mx1 and RNase L mice showed that IFN still inhibited VSV replication in their derived cell lines revealing the existence of other pathways [33].
Table 2.
Results obtained from VSV-infected knockout mice and their derived cell lines.
| ISG | Mechanisms | References |
|---|---|---|
| PKR−/− mice | Are more susceptible to VSV infection and die from acute infection of the respiratory tract. | [47] |
| IFNα/β is unable to rescue PKR−/− mice from VSV infection. | [34] | |
| PKR−/−, RNaseL−/−, Mx1−/− MEFs | IFN still inhibits VSV replication revealing the existence of other pathways. | [33] |
| PML−/− mice | Have an increased susceptibility to VSV compared to parental mice | [43] |
| p53−/− mice | Are more sensitive to VSV infection compared to parental. mice. | [36] |
| Ifit2 (ISG54)−/− mice | Are very vulnerable to neuropathogenesis caused by VSV infection. | [38] |
| Tetherin−/− mice | Intranasal infection of VSV results in lower viral titers in lungs. | [99] |
Ablation of the PML gene by homologous recombination has shown that mice are viable and are able to develop normally. However, the loss of PML alters viral replication. Analysis of PML−/− mice when compared to parental mice revealed that they were more susceptible to VSV infections [43]. Additionally, fibroblasts derived from PML knockout mice, PML−/− MEFs, exhibited enhanced VSV and RABV multiplication [42,48].
P53 was discovered in 1980, and its connection to the IFN pathway was revealed over 20 years later by Taniguchi and colleagues who showed that Type I IFN directly induced p53 expression via an active ISRE motif in its promoter [36]. They also reported in the same study that p53−/− mice were more susceptible to VSV compared to parental mice and that the VSV yield was more than 30-fold higher in p53−/− MEFs than in wild-type.
Using Ifit2 (ISG54) knockout mice, Fensterl et al. showed that they were more vulnerable to neuropathogenesis caused by intranasal VSV infection compared to parental or Ifit1−/− mice [38]. Interestingly, resistance to VSV conferred by Ifit2 was restricted to neurons.
Tetherin−/− mice were generated and challenged with VSV [99]. Both Tetherin−/− and parental mice have similar tissue viral burdens after systemic infection with VSV. Intriguingly, only at short times post-infection did local intranasal infection of VSV result in lower viral titers in the lungs of Tetherin−/− mice compared to parental mice. Determination of IFN titers in the lung tissue of VSV-infected mice revealed that Tetherin−/− mice also had lower levels of IFNα compared with their parental counterparts [99]. This suggests that Tetherin may play a role in IFNα production.
5. Subversion of Antiviral Response or Viral Evasion of Host Immunity
Viruses use the cellular machinery to replicate, and they have developed various strategies to inhibit the actions of IFNs by altering IFN production, IFN signaling or antiviral mediators (reviewed in [100,101]).
Although VSV and RABV share a similar organization of their genomes, they differ in their ability to interplay with the cellular host defense system. VSV is a fast and highly cytopathic virus, which can shut down host cell gene expression to evade the host cellular antiviral response and the Matrix (M) protein plays an essential role in this process (see Section 5.1). In contrast, RABV replicates slowly and requires the host cell to remain intact; it must therefore counteract the innate antiviral response mediated by IFNs. The RABV phosphoprotein, cofactor of the RNA polymerase, is known to antagonize several stages of the IFN pathway (see Section 5.2, Section 5.3 and Section 5.4) and to disrupt PML NBs (see Section 5.5).
5.1. Shutdown of Host Gene Expression by VSV M
The matrix protein (M) (27 kDa) is a major structural component of the VSV particle and plays a major role in viral assembly and budding. The VSV M protein is also responsible for the cytopathic effects associated with VSV infection. The ability of VSV to counteract the host IFN system has been related to the highly cytopathogenic nature of the virus. Indeed, the differential capacity of VSV to inhibit IFN induction in distinct cell types following infection is correlated to the difference in the efficiency with which the virus is able to affect global shutoff of the host cell [102]. The VSV M protein is responsible for the cytopathic effects associated with VSV infection. Indeed, expression of M alone results in the rounding of cells [103,104], and it also inhibits cellular transcription [105]. This is done by inactivating the basal transcription factor TFIID, subsequently inhibiting Polymerase II-mediated transcription of host genes [106], however the precise molecular mechanisms underlying this inhibition are not known. Importantly, mutation of critical residues M51, E213, V221 and S226 in VSV M have been shown to abolish the ability of M to inhibit host RNA synthesis and thereby IFN production [3]. Importantly, subcellular trafficking of M appears to play roles in the disregulation of cellular functions, as the portion of M protein that is located in the nucleus blocks nuclear export of all cellular mRNAs by interacting with the nucleoporin Nup98 and the export factor Rae [107,108,109,110]. Significantly, both Nup98 and Rae are ISGs, implying that their role in nuclear export may contribute to the IFN response. Thus it has been proposed that inhibition of nuclear export by M may affect aspects of the cellular IFN response [110].
5.2. Inhibition of IFN Production by RABV P and N
The RABV phosphoprotein P (40 kDa) is a multifunctional protein that plays a central role in the network of viral and host protein interactions (Figure 4). First, P protein is an essential noncatalytic cofactor of the viral dependent RNA polymerase L and a regulatory protein that plays a role in viral transcription and replication. The second most important role of the RABV phosphoprotein is its ability to inhibit IFNβ induction, as demonstrated by experiments in which recombinant RABV defective for P expression lost the ability to prevent IFNβ production [111]. Specifically, P is able to block the critical phosphorylation of IRF3 by the cellular kinase TBK1 (Figure 2). Although the mechanism of this inhibition remains unclear, this inhibition results in a blockage of IFNβ production in RABV-infected cells.
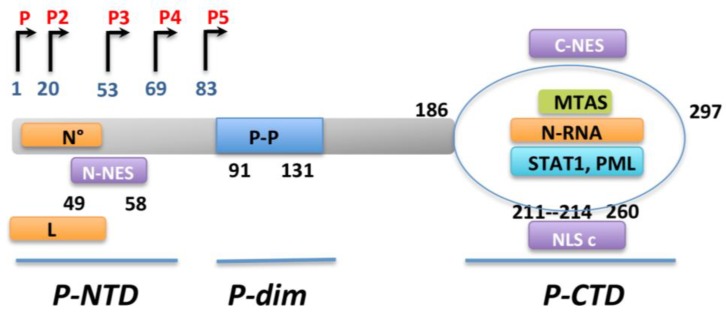
Figure 4.
Functional and structural characterization of RABV-P protein. The RABV-P gene encodes a full length (P1) and four N-terminally truncated isoforms (P2-P5) from the initial and subsequent internal, in frame Met codons at indicated residues. RABV-P protein contains three functional domains separated by two intrinsically disordered regions: the N-terminal domain (P-NTD, residues 1 to 52), interacting with the soluble N protein (called N°) and L protein; the dimerization domain (Pdim, residues 91 to 131); and the C-terminal domain (P-CTD, residues 186 to 297), involved in binding to the N-RNA, as well as cellular proteins STAT1 and PML. The P protein also contains several targeting sequences including two NESs, a conformational NLSc, formed by the globular fold of the CTD.
It has also been shown that RABV P can inhibit phosphorylation of IRF7 by the kinase IKKi, thereby blocking the activation of ”later” IFNα subtypes which are important for propagation of the IFN response [111]. A small region of the P protein, within residues 176–186, has been shown to be important for the IRF blocking activity of RABV P. This region is also involved in viral pathogenicity in vivo, although the precise molecular interactions involved remain to be resolved [112].
The RABV N protein (50 kDa) encapsidates the genomic RNA [113] and thus is essential for the replication of the genome. It plays also an important role in blocking the early stages of IFN induction in infected cells. This has been shown using a recombinant chimeric rabies virus strain, CE(NiN), in which the N gene of the virulent Nishigahara strain has been inserted into the genetic background of a non-lethal attenuated derivative strain of Nishigahara (Ni-CE strain), in the place of the endogenous Ni-CE N gene. Masatani et al. have shown that CE(NiN) induced the expression of the IFNβ gene less efficiently than in the parental Ni-CE strain in human neuroblastoma cells; indicating that the N protein is able to inhibit the innate immune response [114]. This suggests that such a deficiency in the N protein contributes to the defective pathogenicity of the Ni-CE strain [114]. Importantly, the N protein does not act as an independent antagonist of IFN signaling and is only active in the context of viral infection. In addition, the activation of RIG-I was severely inhibited in cells infected with the Nishigahara and CE(NiN) strains, but not with the Ni-CE strain [114]. Thus, it was hypothesized that rather than acting as an inhibitor of the IFN signaling pathway, N protein acts by encapsidating the viral genomic RNA and inhibiting its recognition by RIG-I (Figure 2). Similar observations were made using other viral IFN antagonist proteins including Ebola virus VP35, which acts by masking viral PAMPs from cellular receptors [115].
5.3. Inhibition of the NF-κB Pathway by RABV M
NF-κB is activated by viral infections to induce the expression of antiviral cytokines. RelA, one of the members of the NF-κB family, has been proposed to be crucial for early IFNβ expression [116]. A new member RelAp43 has been recently identified [117]. Luco et al. [117] have shown that although RelAp43 lacks the transactivation domain, it is able to potentiate RelA-mediated transactivation and to stabilize dimers comprising p50, leading to the induction of the expression of IFNβ (Figure 2). Interestingly, RelAp43 is specifically targeted by the matrix protein of lyssaviruses resulting in the inhibition of the NF-κB pathway (Figure 2). It should be mentioned that the M protein of laboratory adapted or vaccine strains (SADB19) has lost the ability to interact with RelAp43 most likely during the virus adaptation to cell culture or during selection for vaccine preparation [117]. This suggests that the inhibition of the NF-κB pathway contributes to the pathogenesis of the virus and its escape from innate immune response.
5.4. Inhibition of IFN Signaling by RABV-P
The RABV-P is not the only protein derived from the P gene acting as an IFN antagonist. The P gene encodes the full length P protein, as well as four N-terminally truncated protein isoforms (P2–P5) (Figure 4), which are produced from the P mRNA transcript by a leaky ribosomal scanning mechanism [118]. Whereas P translation is initiated from the initial Methionine (Met), the shorter products are translated from the four subsequent internal in-frame Met codons [118]. In addition, the P-protein isoforms have distinct trafficking properties depending on multiple targeting signals (two Nuclear Export signal (NES), two nuclear localization signal (NLS). P1 and P2 localize mainly in the cytoplasm, while P3 accumulates in the nucleus (Figure 4) [119,120,121].
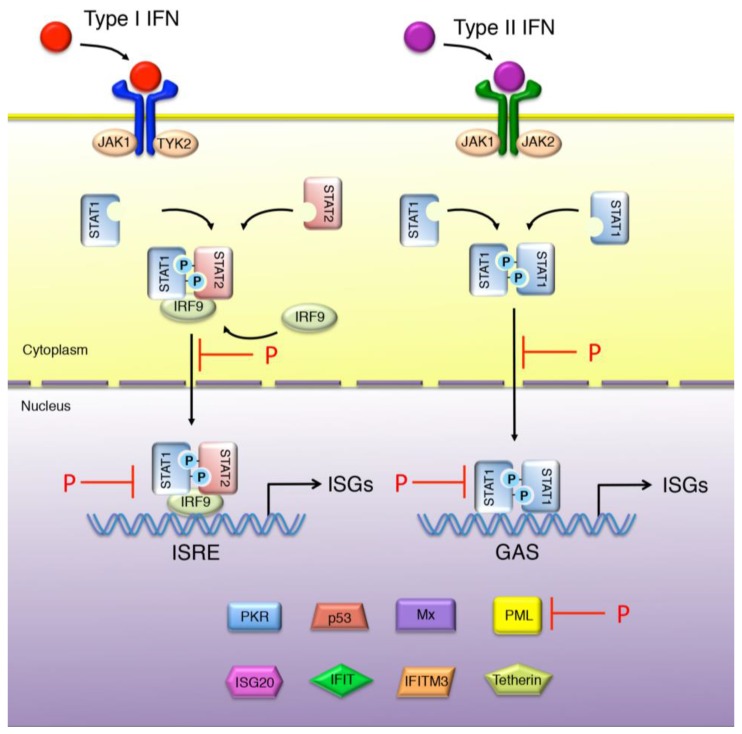
In addition to antagonizing IFN production (Figure 2), RABV also has a mechanism to inhibit IFN signaling (Figure 3). Therefore, virus infected cells are resistant to IFN action. RABV P interacts with STAT1 and STAT2, and thereby blocks IFN signaling through several mechanisms, including sequestration of STAT in the cytoplasm and inhibition of pSTAT1 and ISGF3 binding to DNA promoters [122,123,124]. However, RABV P neither induces STAT1 degradation nor interferes with STAT1 phosphorylation. It has been shown that the last 30 residues of P are required to bind STAT. As these residues are maintained in all five RABV P isoforms, P1-5 retain the capacity to interact with STAT1 [123]. The retention of STAT proteins in the cytoplasm is mediated by P1 and P2 proteins, which are excluded from the nucleus due to the NES signal. Indeed P1 alone can cause the cytoplasmic retention of STAT1, and treatment of cells with leptomycin B reverses or inhibits cytoplasmic sequestration of STAT1 [122]. However`, RABV P neither induces STAT1 degradation nor interferes with STAT1 phosphorylation. It has been shown that the last 30 residues of P are required to bind STAT. As these residues are maintained in all five RABV P isoforms’, P1-5 retain the capacity to interact with STAT1 [123]. The retention of STAT proteins in the cytoplasm is mediated by P1 and P2 proteins, which are excluded from the nucleus due to the NES signal. Indeed P1 alone can cause the cytoplasmic retention of STAT1, and treatment of cells with leptomycin B reverses or inhibits cytoplasmic sequestration of STAT1 [122]. The P3 isoform is also able to retain STAT1 in the cytoplasm using a distinct mechanism. P3 has been shown to interact with microtubules (MT) via a specific sequence MTAS, and this interaction mediates the sequestration of STAT proteins on the cytoplasmic MTs [125]. The capacity of P to inhibit nuclear translocation of STAT1 is conserved between the most distantly related members of the lyssavirus genus except for the attenuated strain derived from Nishigahara strain, NiCE for which P is mutated in the NES signal [126,127]. Consequently, P from NiCE is unable to be actively exported into the cytoplasm and subsequently inhibit STAT nuclear translocation and IFN signaling [127]. This illustrates the importance of active nuclear export of the RABV P protein in its ability to block IFN signaling and thereby in viral pathogenicity.
Figure 3.
Inhibition of IFN signaling by RABV-P. The interaction of Type I IFN with IFNAR leads to the activation of the JAK tyrosine kinases (Tyk2 and JAK1) resulting in the phosphorylation of STAT1 and STAT2, which form, with IRF9, the complex ISGF3. ISGF3 translocates to the nucleus and induces the expression of ISGs that harbor an ISRE. The binding of Type II IFN to its receptor, IFNGR, results in the phosphorylation of STAT1 by JAK1 and JAK2. pSTAT1 homodimers migrate to the nucleus and bind to the GAS in the promoter region of specific ISGs. The steps counteracted by the RABV-P are indicated: P interacts with STAT1 and STAT2, and thereby blocks IFN signaling by STAT1 sequestration in the cytoplasm and the inhibition of pSTAT1 and ISGF3 binding to DNA promoters.
The P protein has also been reported to be able to inhibit the interaction of STAT1 with the target DNA, thereby inhibiting STAT1 signaling at an intranuclear stage [122]. This nuclear activity is presumably mediated by RABV P isoforms P3–P5 which are reported to localize into the nucleus [119]. Binding and sequestration of pSTAT1 in either subcellular compartment inhibits the interaction of pSTAT1 with ISRE promoter elements in the DNA, and subsequently impairs the pSTAT1-mediated expression of ISGs [122] (Figure 3). The ability of the P protein to target pSTAT1 in both of these compartments thus appears to be yet another demonstration of the functional efficiency of this protein. Accordingly, RABV infection before IFN treatment abolishes the capacity of this cytokine to induce the expression of ISGs [123]. Similar mechanisms of inhibition of STAT1 activity have also been described for the P proteins of the Hendra and Nipah Viruses (HeV and NiV, respectively) [128,129].
5.5. Counteraction of PML Nuclear Bodies by RABV
During early viral infection, different viral proteins transiently colocalize with PML on NBs before disrupting them (reviewed in [75]). The functional consequences of the altered localization of the PML NBs could be a specific viral strategy to block cellular systems that may hamper viral replication.
PML NBs are not altered during VSV infection (unpublished data), whereas RABV infection reorganizes PML NBs, causing them to become larger and appear as dense aggregates [49]. Expression of RABV P (P1) can cause relocalization of one particular PML isoform, PMLIII from the NBs into cytoplasmic dots where both proteins colocalize. Interestingly, the expression of the P3 isoform, which lacks the dominant N-NES and can localize into the nucleus, results in reorganization of PML NBs analogous to that observed in RABV infected cells, indicating an intranuclear function of this P isoform and highlighting once again the importance of nuclear trafficking of the P products.
Both P1 and P3 bind to the RING finger motif of PMLIII in transfected or in infected cells, via a PML binding site in the CTD of P [49]. Thus, disrupting of PMLIII’s localization within the NBs could counteract its antiviral action. Accordingly, the anti-VSV and anti-RABV properties of PMLIV require its localization within the NBs [42,48].
6. Conclusions
In this manuscript, we have reviewed the mechanisms by which VSV and RABV trigger IFN production, the different ISG products that inhibit their lifecycle and the mechanisms by which these viruses counteract the IFN pathway. Elucidating the various strategies used by these viruses to usurp the cellular machinery for their own benefit will provide a better understanding of the relationship between the viruses and their hosts.
The functions of the majority of antiviral ISGs still remain unknown. In recent years, new antiviral ISGs have been identified and characterized for their contributions to intrinsic and innate immune antiviral activities. These ISGs encode distinct proteins with a diverse range of biological functions that directly block various stages of the viral life cycle. In addition, particular ISGs have also been implicated in innate immunity, by positively regulating IFN production, thus reinforcing viral resistance. Determining the mechanisms of antiviral ISGs and their implication in IFN response are main goals of future research. In cells treated with IFN, ISGs are the primary genes controlling the replication of viruses. Among the hundreds of known ISGs, only few have been validated in vivo for their implication in antiviral defense. While it is most likely that inhibition of the infection of any given virus by IFNs is through the induction of multiple ISGs that work cooperatively to disrupt a number of stages of viral replication, identification of individual antiviral ISGs and the subsequent elucidation of their modes of action are essential to uncover the antiviral mechanism of IFNs and viral pathogenesis. Understanding the intrinsic antiviral activity of the ISG products may introduce new ways for developing targeted antiviral therapy that would directly activate these genes and bypass the requirement for IFN treatment.
Acknowledgments
This work was partly supported by the Agence Nationale de la Recherche (ANR 11BSV3002803). We are grateful to Zara Hannoun for the critical reading of the manuscript.
Author Contributions
M.K. C.-A. and D. B. wrote the review. D.B., G.M., S.N. and M.K.C-A were involved in gathering information, reading articles and critically revising the manuscript. All of the authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Kuzmin I.V., Novella I.S., Dietzgen R.G., Padhi A., Rupprecht C.E. The rhabdoviruses: Biodiversity, phylogenetics, and evolution. Infect. Genet. Evol. 2009;9:541–553. doi: 10.1016/j.meegid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Basak S., Mondal A., Polley S., Mukhopadhyay S., Chattopadhyay D. Reviewing Chandipura: A vesiculovirus in human epidemics. Biosci. Rep. 2007;27:275–298. doi: 10.1007/s10540-007-9054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed M., McKenzie M.O., Puckett S., Hojnacki M., Poliquin L., Lyles D.S. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor J.H., Lyles D.S. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 2002;76:10177–10187. doi: 10.1128/JVI.76.20.10177-10187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor J.H., Lyles D.S. Inhibition of host and viral translation during vesicular stomatitis virus infection. eIF2 is responsible for the inhibition of viral but not host translation. J. Biol. Chem. 2005;280:13512–13519. doi: 10.1074/jbc.M501156200. [DOI] [PubMed] [Google Scholar]
- 6.Kopecky S.A., Willingham M.C., Lyles D.S. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnell M.J., McGettigan J.P., Wirblich C., Papaneri A. The cell biology of rabies virus: Using stealth to reach the brain. Nat. Rev. Microbiol. 2010;8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S. Electron microscopy of nerve cells infected with street rabies virus. Virology. 1962;17:198–202. doi: 10.1016/0042-6822(62)90099-5. [DOI] [PubMed] [Google Scholar]
- 9.Tordo N., Poch O., Ermine A., Keith G., Rougeon F. Completion of the rabies virus genome sequence determination: Highly conserved domains among the L (polymerase) proteins of unsegmented negative-strand RNA viruses. Virology. 1988;165:565–576. doi: 10.1016/0042-6822(88)90600-9. [DOI] [PubMed] [Google Scholar]
- 10.Walker P.J., Firth C., Widen S.G., Blasdell K.R., Guzman H., Wood T.G., Paradkar P.N., Holmes E.C., Tesh R.B., Vasilakis N., et al. Evolution of genome size and complexity in the rhabdoviridae. PLoS Pathog. 2015;11:e1004664. doi: 10.1371/journal.ppat.1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahaye X., Vidy A., Pomier C., Obiang L., Harper F., Gaudin Y., Blondel D. Functional characterization of Negri bodies (NBs) in rabies virus-infected cells: Evidence that NBs are sites of viral transcription and replication. J. Virol. 2009;83:7948–7958. doi: 10.1128/JVI.00554-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrich B.S., Cureton D.K., Rahmeh A.A., Whelan S.P. Protein expression redirects vesicular stomatitis virus RNA synthesis to cytoplasmic inclusions. PLoS Pathog. 2010;6:e1000958. doi: 10.1371/journal.ppat.1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavrakis M., Mehouas S., Real E., Iseni F., Blondel D., Tordo N., Ruigrok R.W. Rabies virus chaperone: Identification of the phosphoprotein peptide that keeps nucleoprotein soluble and free from non-specific RNA. Virology. 2006;349:422–429. doi: 10.1016/j.virol.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Bruni D., Chazal M., Sinigaglia L., Chauveau L., Schwartz O., Despres P., Jouvenet N. Viral entry route determines how human plasmacytoid dendritic cells produce type I interferons. Sci. Signal. 2015;8:ra25. doi: 10.1126/scisignal.aaa1552. [DOI] [PubMed] [Google Scholar]
- 15.Hornung V., Ellegast J., Kim S., Brzózka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 16.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 17.Pichlmair A., Schulz O., Tan C.P., Naslund T.I., Liljestrom P., Weber F., Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 18.Edelmann K.H., Richardson-Burns S., Alexopoulou L., Tyler K.L., Flavell R.A., Oldstone M.B. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Menager P., Roux P., Megret F., Bourgeois J.P., le Sourd A.M., Danckaert A., Lafage M., Prehaud C., Lafon M. Toll-like receptor 3 (TLR3) plays a major role in the formation of rabies virus Negri Bodies. PLoS Pathog. 2009;5:e1000315. doi: 10.1371/journal.ppat.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., Iwasaki A., Flavell R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faul E.J., Wanjalla C.N., Suthar M.S., Gale M., Wirblich C., Schnell M.J. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog. 2010;6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota T., Matsuoka M., Chang T.H., Tailor P., Sasaki T., Tashiro M., Kato A., Ozato K. Virus infection triggers SUMOylation of IRF3 and IRF7, leading to the negative regulation of type I interferon gene expression. J. Biol. Chem. 2008;283:25660–25670. doi: 10.1074/jbc.M804479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu J., Xiong Y., Xu Y., Cheng G., Tang H. MDA5 is SUMOylated by PIAS2beta in the upregulation of type I interferon signaling. Mol. Immunol. 2011;48:415–422. doi: 10.1016/j.molimm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi Z., Fu J., Xiong Y., Tang H. SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell. 2010;1:275–283. doi: 10.1007/s13238-010-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1957;147:258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 26.Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prokunina-Olsson L., Muchmore B., Tang W., Pfeiffer R.M., Park H., Dickensheets H., Hergott D., Porter-Gill P., Mumy A., Kohaar I., et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013;45:164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaszczyk K., Olejnik A., Nowicka H., Ozgyin L., Chen Y.L., Chmielewski S., Kostyrko K., Wesoly J., Balint B.L., Lee C.K., et al. STAT2/IRF9 directs a prolonged ISGF3-like transcriptional response and antiviral activity in the absence of STAT1. Biochem. J. 2015;466:511–524. doi: 10.1042/BJ20140644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fink K., Grandvaux N. STAT2 and IRF9: Beyond ISGF3. JAK-STAT. 2013;2:e27521. doi: 10.4161/jkst.27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommereyns C., Paul S., Staeheli P., Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen I.M., Cheng G., Wieland S., Volinia S., Croce C.M., Chisari F.V., David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J., Wang P., Lin L., Liu X., Ma F., An H., Wang Z., Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 33.Zhou A., Paranjape J.M., Der S.D., Williams B.R., Silverman R.H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- 34.Balachandran S., Roberts P.C., Brown L.E., Truong H., Pattnaik A.K., Archer D.R., Barber G.N. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/S1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 35.Pavlovic J., Zurcher T., Haller O., Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J. Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaoka A., Hayakawa S., Yanai H., Stoiber D., Negishi H., Kikuchi H., Sasaki S., Imai K., Shibue T., Honda K., et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 37.Espert L., Degols G., Gongora C., Blondel D., Williams B.R., Silverman R.H., Mechti N. ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA genomic viruses. J. Biol. Chem. 2003;278:16151–16158. doi: 10.1074/jbc.M209628200. [DOI] [PubMed] [Google Scholar]
- 38.Fensterl V., Wetzel J.L., Ramachandran S., Ogino T., Stohlman S.A., Bergmann C.C., Diamond M.S., Virgin H.W., Sen G.C. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 2012;8:e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weidner J.M., Jiang D., Pan X.B., Chang J., Block T.M., Guo J.T. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S.Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., Pernet O., Guo H., Nusbaum R., Zack J.A., et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chelbi-Alix M.K., Quignon F., Pelicano L., Koken M.H., de The H. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J. Virol. 1998;72:1043–1051. doi: 10.1128/jvi.72.2.1043-1051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Asmi F., Maroui M.A., Dutrieux J., Blondel D., Nisole S., Chelbi-Alix M.K. Implication of PMLIV in both intrinsic and innate immunity. PLoS Pathog. 2014;10:e1003975. doi: 10.1371/journal.ppat.1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonilla W.V., Pinschewer D.D., Klenerman P., Rousson V., Gaboli M., Pandolfi P.P., Zinkernagel R.M., Salvato M.S., Hengartner H. Effects of promyelocytic leukemia protein on virus-host balance. J. Virol. 2002;76:3810–3818. doi: 10.1128/JVI.76.8.3810-3818.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoggins J.W. Interferon-stimulated genes: Roles in viral pathogenesis. Curr. Opin. Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S.Y., Sanchez D.J., Aliyari R., Lu S., Cheng G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. USA. 2012;109:4239–4244. doi: 10.1073/pnas.1114981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staeheli P., Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J. Virol. 1991;65:4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stojdl D.F., Abraham N., Knowles S., Marius R., Brasey A., Lichty B.D., Brown E.G., Sonenberg N., Bell J.C. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 2000;74:9580–9585. doi: 10.1128/JVI.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blondel D., Kheddache S., Lahaye X., Dianoux L., Chelbi-Alix M.K. Resistance to rabies virus infection conferred by the PMLIV isoform. J. Virol. 2010;84:10719–10726. doi: 10.1128/JVI.01286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blondel D., Regad T., Poisson N., Pavie B., Harper F., Pandolfi P.P., de The H., Chelbi-Alix M.K. Rabies virus P and small P products interact directly with PML and reorganize PML nuclear bodies. Oncogene. 2002;21:7957–7970. doi: 10.1038/sj.onc.1205931. [DOI] [PubMed] [Google Scholar]
- 50.Smith S.E., Gibson M.S., Wash R.S., Ferrara F., Wright E., Temperton N., Kellam P., Fife M. Chicken interferon-inducible transmembrane protein 3 restricts influenza viruses and lyssaviruses in vitro. J. Virol. 2013;87:12957–12966. doi: 10.1128/JVI.01443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Versteeg G.A., Benke S., Garcia-Sastre A., Rajsbaum R. InTRIMsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014;25:563–576. doi: 10.1016/j.cytogfr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panda D., Das A., Dinh P.X., Subramaniam S., Nayak D., Barrows N.J., Pearson J.L., Thompson J., Kelly D.L., Ladunga I., et al. RNAi screening reveals requirement for host cell secretory pathway in infection by diverse families of negative-strand RNA viruses. Proc. Natl. Acad. Sci. USA. 2011;108:19036–19041. doi: 10.1073/pnas.1113643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das A., Dinh P.X., Panda D., Pattnaik A.K. Interferon-inducible protein IFI35 negatively regulates RIG-I antiviral signaling and supports vesicular stomatitis virus replication. J. Virol. 2014;88:3103–3113. doi: 10.1128/JVI.03202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey C.C., Zhong G., Huang I.C., Farzan M. IFITM-Family Proteins: The Cell’s First Line of Antiviral Defense. Annu. Rev. Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegrist F., Ebeling M., Certa U. The small interferon-induced transmembrane genes and proteins. J. Interferon Cytokine Res. 2011;31:183–197. doi: 10.1089/jir.2010.0112. [DOI] [PubMed] [Google Scholar]
- 56.Brass A.L., Huang I.C., Benita Y., John S.P., Krishnan M.N., Feeley E.M., Ryan B.J., Weyer J.L., van der Weyden L., Fikrig E., et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alber D., Staeheli P. Partial inhibition of vesicular stomatitis virus by the interferon-induced human 9-27 protein. J. Interferon Cytokine Res. 1996;16:375–380. doi: 10.1089/jir.1996.16.375. [DOI] [PubMed] [Google Scholar]
- 58.Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., Brass A.L., Ahmed A.A., Chi X., Dong L., et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7:e1001258. doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang D., Weidner J.M., Qing M., Pan X.B., Guo H., Xu C., Zhang X., Birk A., Chang J., Shi P.Y., et al. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J. Virol. 2010;84:8332–8341. doi: 10.1128/JVI.02199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li K., Markosyan R.M., Zheng Y.M., Golfetto O., Bungart B., Li M., Ding S., He Y., Liang C., Lee J.C., et al. IFITM proteins restrict viral membrane hemifusion. PLoS Pathog. 2013;9:e1003124. doi: 10.1371/journal.ppat.1003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feeley E.M., Sims J.S., John S.P., Chin C.R., Pertel T., Chen L.M., Gaiha G.D., Ryan B.J., Donis R.O., Elledge S.J., et al. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7:e1002337. doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holmes R.S., Vandeberg J.L., Cox L.A. Genomics and proteomics of vertebrate cholesterol ester lipase (LIPA) and cholesterol 25-hydroxylase (CH25H) 3 Biotech. 2011;1:99–109. doi: 10.1007/s13205-011-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aebi M., Fah J., Hurt N., Samuel C.E., Thomis D., Bazzigher L., Pavlovic J., Haller O., Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Boil. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang K.C., Hansen E., Foroni L., Lida J., Goldspink G. Molecular and functional analysis of the virus- and interferon-inducible human MxA promoter. Arch. Virol. 1991;117:1–15. doi: 10.1007/BF01310488. [DOI] [PubMed] [Google Scholar]
- 65.Verhelst J., Hulpiau P., Saelens X. Mx proteins: Antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Boil. Rev. 2013;77:551–566. doi: 10.1128/MMBR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haller O., Kochs G. Interferon-induced mx proteins: Dynamin-like GTPases with antiviral activity. Traffic. 2002;3:710–717. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- 67.Haller O., Staeheli P., Schwemmle M., Kochs G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbial. 2015;23:154–163. doi: 10.1016/j.tim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Meier E., Fah J., Grob M.S., End R., Staeheli P., Haller O. A family of interferon-induced Mx-related mRNAs encodes cytoplasmic and nuclear proteins in rat cells. J. Virol. 1988;62:2386–2393. doi: 10.1128/jvi.62.7.2386-2393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zurcher T., Pavlovic J., Staeheli P. Mouse Mx2 protein inhibits vesicular stomatitis virus but not influenza virus. Virology. 1992;187:796–800. doi: 10.1016/0042-6822(92)90481-4. [DOI] [PubMed] [Google Scholar]
- 70.Leroy M., Pire G., Baise E., Desmecht D. Expression of the interferon-alpha/beta-inducible bovine Mx1 dynamin interferes with replication of rabies virus. Neurobiol. Dis. 2006;21:515–521. doi: 10.1016/j.nbd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Nisole S., Stoye J.P., Saib A. TRIM family proteins: Retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- 72.Ishov A.M., Sotnikov A.G., Negorev D., Vladimirova O.V., Neff N., Kamitani T., Yeh E.T., Strauss J.F., 3rd, Maul G.G. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Boil. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernardi R., Papa A., Pandolfi P.P. Regulation of apoptosis by PML and the PML-NBs. Oncogene. 2008;27:6299–6312. doi: 10.1038/onc.2008.305. [DOI] [PubMed] [Google Scholar]
- 74.Nisole S., Maroui M.A., Mascle X.H., Aubry M., Chelbi-Alix M.K. Differential Roles of PML Isoforms. Front. Oncol. 2013;3 doi: 10.3389/fonc.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geoffroy M.C., Chelbi-Alix M.K. Role of promyelocytic leukemia protein in host antiviral defense. J. Interferon Cytokine Res. 2011;31:145–158. doi: 10.1089/jir.2010.0111. [DOI] [PubMed] [Google Scholar]
- 76.Everett R.D., Chelbi-Alix M.K. PML and PML nuclear bodies: Implications in antiviral defence. Biochimie. 2007;89:819–830. doi: 10.1016/j.biochi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Kamitani T., Nguyen H.P., Kito K., Fukuda-Kamitani T., Yeh E.T. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J. Biol. Chem. 1998;273:3117–3120. doi: 10.1074/jbc.273.6.3117. [DOI] [PubMed] [Google Scholar]
- 78.Jensen K., Shiels C., Freemont P.S. PML protein isoforms and the RBCC/TRIM motif. Oncogene. 2001;20:7223–7233. doi: 10.1038/sj.onc.1204765. [DOI] [PubMed] [Google Scholar]
- 79.Saitoh T., Tun-Kyi A., Ryo A., Yamamoto M., Finn G., Fujita T., Akira S., Yamamoto N., Lu K.P., Yamaoka S., et al. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat. Immunol. 2006;7:598–605. doi: 10.1038/ni1347. [DOI] [PubMed] [Google Scholar]
- 80.Gongora C., Degols G., Espert L., Hua T.D., Mechti N. A unique ISRE, in the TATA-less human Isg20 promoter, confers IRF-1-mediated responsiveness to both interferon type I and type II. Nucleic Acids Res. 2000;28:2333–2341. doi: 10.1093/nar/28.12.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen L.H., Espert L., Mechti N., Wilson D.M., 3rd The human interferon- and estrogen-regulated ISG20/HEM45 gene product degrades single-stranded RNA and DNA in vitro. Biochemistry. 2001;40:7174–7179. doi: 10.1021/bi010141t. [DOI] [PubMed] [Google Scholar]
- 82.Espert L., Rey C., Gonzalez L., Degols G., Chelbi-Alix M.K., Mechti N., Gongora C. The exonuclease ISG20 is directly induced by synthetic dsRNA via NF-kappaB and IRF1 activation. Oncogene. 2004;23:4636–4640. doi: 10.1038/sj.onc.1207586. [DOI] [PubMed] [Google Scholar]
- 83.Lewin A.R., Reid L.E., McMahon M., Stark G.R., Kerr I.M. Molecular analysis of a human interferon-inducible gene family. Eur. J. Biochem. 1991;199:417–423. doi: 10.1111/j.1432-1033.1991.tb16139.x. [DOI] [PubMed] [Google Scholar]
- 84.Fensterl V., Sen G.C. The ISG56/IFIT1 gene family. J. Interferon Cytokine Res. 2011;31:71–78. doi: 10.1089/jir.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diamond M.S., Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat. Rev. Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vladimer G.I., Gorna M.W., Superti-Furga G. IFITs: Emerging Roles as Key Anti-Viral Proteins. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., Lin T.Y., Schneller S., Zust R., Dong H., et al. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbas Y.M., Pichlmair A., Gorna M.W., Superti-Furga G., Nagar B. Structural basis for viral 5′-PPP-RNA recognition by human IFIT proteins. Nature. 2013;494:60–64. doi: 10.1038/nature11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo J., Sen G.C. Characterization of the interaction between the interferon-induced protein P56 and the Int6 protein encoded by a locus of insertion of the mouse mammary tumor virus. J. Virol. 2000;74:1892–1899. doi: 10.1128/JVI.74.4.1892-1899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmeisser H., Mejido J., Balinsky C.A., Morrow A.N., Clark C.R., Zhao T., Zoon K.C. Identification of alpha interferon-induced genes associated with antiviral activity in Daudi cells and characterization of IFIT3 as a novel antiviral gene. J. Virol. 2010;84:10671–10680. doi: 10.1128/JVI.00818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blasius A.L., Giurisato E., Cella M., Schreiber R.D., Shaw A.S., Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 92.Kupzig S., Korolchuk V., Rollason R., Sugden A., Wilde A., Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 93.Rollason R., Korolchuk V., Hamilton C., Jepson M., Banting G. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Boil. 2009;184:721–736. doi: 10.1083/jcb.200804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wynn T.A., Nicolet C.M., Paulnock D.M. Identification and characterization of a new gene family induced during macrophage activation. J. Immunol. 1991;147:4384–4392. [PubMed] [Google Scholar]
- 95.Vopel T., Syguda A., Britzen-Laurent N., Kunzelmann S., Ludemann M.B., Dovengerds C., Sturzl M., Herrmann C. Mechanism of GTPase-activity-induced self-assembly of human guanylate binding protein 1. J. Mol. Boil. 2010;400:63–70. doi: 10.1016/j.jmb.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 96.Cheng Y.S., Colonno R.J., Yin F.H. Interferon induction of fibroblast proteins with guanylate binding activity. J. Biol. Chem. 1983;258:7746–7750. [PubMed] [Google Scholar]
- 97.Anderson S.L., Carton J.M., Lou J., Xing L., Rubin B.Y. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology. 1999;256:8–14. doi: 10.1006/viro.1999.9614. [DOI] [PubMed] [Google Scholar]
- 98.Meurs E.F., Watanabe Y., Kadereit S., Barber G.N., Katze M.G., Chong K., Williams B.R., Hovanessian A.G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 1992;66:5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swiecki M., Wang Y., Gilfillan S., Lenschow D.J., Colonna M. Cutting edge: Paradoxical roles of BST2/tetherin in promoting type I IFN response and viral infection. J. Immunol. 2012;188:2488–2492. doi: 10.4049/jimmunol.1103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goodbourn S., Didcock L., Randall R.E. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 101.Regad T., Chelbi-Alix M.K. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene. 2001;20:7274–7286. doi: 10.1038/sj.onc.1204854. [DOI] [PubMed] [Google Scholar]
- 102.Wertz G.W., Youngner J.S. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J. Virol. 1970;6:476–484. doi: 10.1128/jvi.6.4.476-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blondel D., Harmison G.G., Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J. Virol. 1990;64:1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melki R., Gaudin Y., Blondel D. Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: Possible implications in the viral cytopathic effect. Virology. 1994;202:339–347. doi: 10.1006/viro.1994.1350. [DOI] [PubMed] [Google Scholar]
- 105.Black B.L., Rhodes R.B., McKenzie M., Lyles D.S. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan H., Yoza B.K., Lyles D.S. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology. 1998;251:383–392. doi: 10.1006/viro.1998.9413. [DOI] [PubMed] [Google Scholar]
- 107.Petersen J.M., Her L.S., Varvel V., Lund E., Dahlberg J.E. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Boil. 2000;20:8590–8601. doi: 10.1128/MCB.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Enninga J., Levy D.E., Blobel G., Fontoura B.M. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- 109.Von Kobbe C., van Deursen J.M., Rodrigues J.P., Sitterlin D., Bachi A., Wu X., Wilm M., Carmo-Fonseca M., Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell. 2000;6:1243–1252. doi: 10.1016/S1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 110.Faria P., Chakraborty P., Lavay A., Barber G., Ezelle H., Enninga J., Fontoura B. VSV disrupts the Rae/mrnp40 mRNA nuclear export pathway. Mol. Cell. 2005;17:93–102. doi: 10.1016/j.molcel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 111.Brzozka K., Finke S., Conzelmann K.K. Identification of the rabies virus alpha/beta interferon antagonist: Phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J. Virol. 2005;79:7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rieder M., Brzozka K., Pfaller C.K., Cox J.H., Stitz L., Conzelmann K.K. Genetic dissection of interferon-antagonistic functions of rabies virus phosphoprotein: Inhibition of interferon regulatory factor 3 activation is important for pathogenicity. J. Virol. 2011;85:842–852. doi: 10.1128/JVI.01427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patton J.T., Davis N.L., Wertz G.W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J. Virol. 1984;49:303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masatani T., Ito N., Shimizu K., Ito Y., Nakagawa K., Sawaki Y., Koyama H., Sugiyama M. Rabies virus nucleoprotein functions to evade activation of the RIG-I-mediated antiviral response. J. Virol. 2010;84:4002–4012. doi: 10.1128/JVI.02220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cardenas W.B., Loo Y.M., Gale M., Jr., Hartman A.L., Kimberlin C.R., Martinez-Sobrido L., Saphire E.O., Basler C.F. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J., Basagoudanavar S.H., Wang X., Hopewell E., Albrecht R., Garcia-Sastre A., Balachandran S., Beg A.A. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J. Immunol. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luco S., Delmas O., Vidalain P.O., Tangy F., Weil R., Bourhy H. RelAp43, a member of the NF-kappaB family involved in innate immune response against Lyssavirus infection. PLoS Pathog. 2012;8:e1003060. doi: 10.1371/journal.ppat.1003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chenik M., Chebli K., Blondel D. Translation initiation at alternate in-frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J. Virol. 1995;69:707–712. doi: 10.1128/jvi.69.2.707-712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pasdeloup D., Poisson N., Raux H., Gaudin Y., Ruigrok R.W., Blondel D. Nucleocytoplasmic shuttling of the rabies virus P protein requires a nuclear localization signal and a CRM1-dependent nuclear export signal. Virology. 2005;334:284–293. doi: 10.1016/j.virol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 120.Oksayan S., Wiltzer L., Rowe C.L., Blondel D., Jans D.A., Moseley G.W. A novel nuclear trafficking module regulates the nucleocytoplasmic localization of the rabies virus interferon antagonist, P protein. J. Biol. Chem. 2012;287:28112–28121. doi: 10.1074/jbc.M112.374694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moseley G.W., Filmer R.P., DeJesus M.A., Jans D.A. Nucleocytoplasmic distribution of rabies virus P-protein is regulated by phosphorylation adjacent to C-terminal nuclear import and export signals. Biochemistry. 2007;46:12053–12061. doi: 10.1021/bi700521m. [DOI] [PubMed] [Google Scholar]
- 122.Vidy A., el Bougrini J., Chelbi-Alix M.K., Blondel D. The nucleocytoplasmic rabies virus P protein counteracts interferon signaling by inhibiting both nuclear accumulation and DNA binding of STAT1. J. Virol. 2007;81:4255–4263. doi: 10.1128/JVI.01930-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vidy A., Chelbi-Alix M., Blondel D. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J. Virol. 2005;79:14411–14420. doi: 10.1128/JVI.79.22.14411-14420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brzozka K., Finke S., Conzelmann K.K. Inhibition of interferon signaling by rabies virus phosphoprotein P: Activation-dependent binding of STAT1 and STAT2. J. Virol. 2006;80:2675–2683. doi: 10.1128/JVI.80.6.2675-2683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moseley G.W., Lahaye X., Roth D.M., Oksayan S., Filmer R.P., Rowe C.L., Blondel D., Jans D.A. Dual modes of rabies P-protein association with microtubules: A novel strategy to suppress the antiviral response. J. Cell Sci. 2009;122:3652–3662. doi: 10.1242/jcs.045542. [DOI] [PubMed] [Google Scholar]
- 126.Wiltzer L., Larrous F., Oksayan S., Ito N., Marsh G.A., Wang L.F., Blondel D., Bourhy H., Jans D.A., Moseley G.W., et al. Conservation of a unique mechanism of immune evasion across the Lyssavirus genus. J. Virol. 2012;86:10194–10199. doi: 10.1128/JVI.01249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ito N., Moseley G.W., Blondel D., Shimizu K., Rowe C.L., Ito Y., Masatani T., Nakagawa K., Jans D.A., Sugiyama M., et al. Role of interferon antagonist activity of rabies virus phosphoprotein in viral pathogenicity. J. Virol. 2010;84:6699–6710. doi: 10.1128/JVI.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaw M.L., Garcia-Sastre A., Palese P., Basler C.F. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 2004;78:5633–5641. doi: 10.1128/JVI.78.11.5633-5641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shaw M.L., Cardenas W.B., Zamarin D., Palese P., Basler C.F. Nuclear localization of the Nipah virus W protein allows for inhibition of both virus- and toll-like receptor 3-triggered signaling pathways. J. Virol. 2005;79:6078–6088. doi: 10.1128/JVI.79.10.6078-6088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]