Abstract
Alpha-fetoprotein (AFP) behavior in patients with hepatocellular carcinoma (HCC) waiting for liver transplant (LT) represents a perfect biological example of a fractal model in which its progressive modification and possible future prediction of its values are very hard to capture. As a consequence, AFP represents a useful but poorly manageable tool to increase the ability to better select HCC patients waiting for LT. Trying to find a “fil-rouge” in the recent literature, no definitive answers can be done to several open questions: (1) the best AFP value to adopt; (2) the best cut-off measurement; and (3) the best way to comfortably capture the effective, time-related, fluctuations of this biological marker. More, structured and prospective, studies using serial determination of AFP values within and without the context of locoregional therapies are needed in order to find the “ideal” (static and dynamic) cut-off values allowing to respond to all the still open questions in this field of transplant oncology.
Keywords: Alpha-fetoprotein, Hepatocellular cancer, Milan criteria, Recurrence, Drop-out
Core tip: Alpha-fetoprotein (AFP) behavior in patients with hepatocellular carcinoma waiting for liver transplant (LT) represents a perfect example of a fractal model. Consequently, AFP represents a useful but poorly manageable selection tool for patients waiting for LT. Looking at the recent literature, we can assume that: (1) last AFP value seem to be the best values to adopt; (2) different cut-offs may be adopted in the two different scenarios of Milan Criteria (MC) IN and MC OUT status; (3) AFP cut-off of 1000 ng/mL represent a good compromise for MC-IN patients; and (4) no definitive conclusion has been reached in relation to MC-OUT patients.
CHAOS THEORY AND BIOLOGICAL SCIENCES
Chaos is the science of surprise, of nonlinearity and of unpredictability, teaching us to expect the unexpected. Sciences are connected with predictable events such as chemical reactions, electricity, gravity, whilst the chaos theory concerns with non-linear processes such as weather, stock market and biological modifications. These last phenomena are typically described by fractal mathematics, a field of study created with the intent to capture the infinite complexity of nature (Figure 1).
Figure 1.
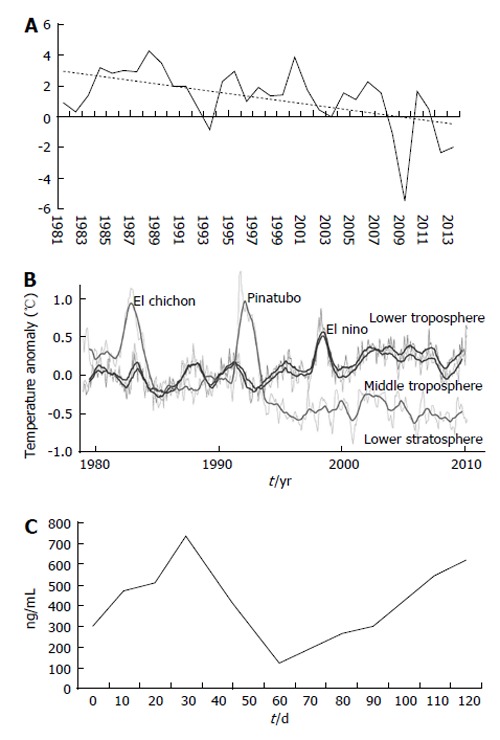
Some examples of systems with chaotic behaviour. A: Annual gross domestic product (GDP) growth of Italy in the last 35 years (%) (from: http://thenextrecession.wordpress.com/2013/08/05/greece-still-bust-spain-depressed-italy-paralysed/); B: Atmospheric temperature from 1979 to 2010, determined by NASA satellites (from: http://earthobservatory.nasa.gov/Features/GlobalWarming/images/msu_1978-2010.png); C: Hypothetical patients’ alpha-fetoprotein fluctuation during his waiting list period before liver transplantation.
The behavior of alpha-fetoprotein (AFP) in patients having hepatocellular carcinoma (HCC) awaiting for liver transplant (LT) represents a perfect biological example of a fractal model in which its progressive modification and possible future prediction of its values are very hard to capture[1].
AFP AND ITS PREDICTION OF HCC RECURRENCE: ROLE OF STATIC VALUES
During the last years, a growing number of studies has been focused on the predictive role of AFP for the diagnosis of tumor recurrence after LT[2]. AFP has been strongly connected with HCC biological behavior, commonly connecting its values with the grade of differentiation as well as the vascular invasiveness of the tumor[3].
As a confirmation of this renewed interest in relation to the role of AFP, the recently published EASL-EORTC guidelines suggest to investigate AFP modification as a clinical selection parameter of patients waiting for LT[4]. However, several questions still remain unsolved in relation to the clinical use of AFP measurements in daily practice, as clearly stated in a recent focused editorial[5]. Among them: (1) the best static value to adopt; (2) the best cut-off measurement; and (3) the best way to comfortably capture the effective, time-related, fluctuations of this biological marker.
Many authors focused on the last pre-transplant value of AFP as the best predictor of recurrence; the threshold level of 400 ng/mL was most frequently advanced.
A large United States experience including 6817 HCC patients listed for LT showed that patients having AFP values superior to 400 ng/mL at the moment of waiting-list inscription and then downstaged (using locoregional therapies) to AFP values ≤ 400 ng/mL immediately before LT showed better intent-to-treat survivals respect to the cases in which their values could not be reduced (3-year survivals: 81% vs 48%; P < 0.001); these downstaged patients had results comparable results to those patients having stable AFP values ≤ 400 ng/mL (74%; P = 0.14). In contrast to AFP at the moment of waiting-list inscription or to modifications of AFP, only last pre-transplant AFP independently predicted survival (P < 0.001)[6]. Another United States study proposed the combination total tumor volume inferior to 115 cm³ and AFP inferior to 400 ng/mL and as a better tool for selecting patients with HCC, showing, 3 years after transplant, survivals inferior to 50% in patients exceeding this cut-off[7]. The Hangzhou group proposed in a study containing 195 patients to combine one of the two following items in order to obtain good tumor free survival rates: total HCC diameter inferior or equal to 8 cm; total HCC diameter superior to 8 cm contemporaneously having pathologic grade I-II and pre-LT AFP ≤ 400 ng/mL[8]. An Italian study showed that the combination of morphological and biological parameters (e.g., total tumor diameter > 8 cm and AFP > 400 ng/mL) conferred scarce survivals: patients having the last AFP value > 400 ng/mL had an eight-times incremented risk of tumor recurrence after transplantation[9].
A monocentric Belgian study similarly identified the last AFP determination > 400 ng/mL as the most important independent predictor for tumor recurrence after LT (HR = 4.86; P = 0.01)[10]. The United Network for Organ Sharing region 6 experience showed that peak AFP value > 400 and AFP at LT > 400 ng/mL were connected with poor outcomes post-LT in patients previously treated with loco-regional treatment (LRT)[11].
Despite many analyses underlined the role of the last AFP measure > 400 ng/mL before LT as a predictive tool, several, greatly differing, cut-off values (100, 200, 210, 300, 1000 ng/mL) have been put forward in the recent literature. The unfollowing paragraph gives an overview of all these different findings published during the period 2009-2014.
A United States study including 101 patients showed that AFP > 100 ng/mL (OR = 5.0, P = 0.006) and tumor size (OR = 4.1, P = 0.013) were correlated with microvascular invasion and post-LT recurrence[12]. Another Polish study including 121 HCC patients confirmed the validity of 100 ng/mL as cut-off value in predicting the risk of post-LT recurrence in patients meeting San Francisco criteria or up-to-seven criteria[13]. An Egyptian study identified AFP value > 200 ng/mL as a predictive tool for HCC recurrence in 170 living donor LT (LDLT)[14]. An Italian study reported that a AFP cut-off measure of 210 ng/mL, significantly influenced 5-year survivals (23.3% vs 76.2%; P < 0.0001)[15]. A Japanese analysis of 167 LDLT patients identified a threshold measure of 300 ng/mL as predictor of HCC recurrence and poor prognosis[16]. Finally some studies identified the value of 1000 ng/mL as significant.
The Seoul National University study including 63 LDLT patients proposed a score based on the following three different variables: (1) tumor size: ≤ 3, 3.1-5, 5.1-6.5, ≥ 6.5 cm; (2) tumor number: 1, 2-3, 4-5, ≥ 6 nodules; and (3) AFP: ≤ 20, 20.1-200, 200.1-1000, > 1000 ng/mL. According to the proposed score, an excellent stratification in relation to recurrence rates and patient survival could be achieved[17]. Another Chinese study in 303 patients similarly found AFP > 1000 ng/mL together with microvascular invasion and tumor size > 6.5 cm as risk factors for fatal recurrence after LT. Interestingly, dead due to tumor recurrence within one year after LT was 85.7% when all three risk factors were present, 37.8% when two factors, 13.6% when one factor and 6.7% when no risk factor were present[18].
A multicentric analysis from France (n = 435 cases) created a mathematical model based on the number of HCC lesions, tumor size and last AFP value. Interestingly, the authors found two different cut-off values in relation to the Milan Criteria (MC) status. When MC status was exceeded, patients experienced high or low 5-year recurrence rates when AFP measures were < 100 or > 1000 ng/mL (47.6% and 14.4%, respectively; P < 0.006). When patients meeting MC had AFP levels > 1000 ng/mL, showed high-risk for recurrence (37.1%; P < 0.001)[19]. An analysis from United States including 211 patients similarly showed that patients meeting MC with last pre-LT AFP > 1000 ng/mL showed a higher number of recurrences 5 years after transplant. An AFP level > 1000 ng/mL strongly predicted vascular invasion (OR = 6.8, P = 0.006), the most important risk factor for recurrence. Five-year recurrence-free survivals were 80.3% and 52.7% for patients meeting or exceeding the AFP threshold measure of 1000 ng/mL (P = 0.026), respectively. Application of the AFP > 1000 ng/mL as a cut-off was connected with the exclusion of 4.7% of cases from the opportunity to be transplanted and with the reduction of 20% of tumor recurrence[20]. All the reported studies are reassumed in Table 1.
Table 1.
Recent articles focused on alpha-fetoprotein static values
| Ref. | Year | n | Country | Cut-off value (ng/mL) |
| McHugh et al[12] | 2010 | 101 | United States | 100 |
| Grąt et al[13] | 2014 | 121 | Poland | 100 |
| Abdel-Wahab et al[14] | 2013 | 170 (LDLT) | Egypt | 200 |
| Lai et al[15] | 2011 | 153 | Italy | 210 |
| Harimoto et al[16] | 2013 | 167 (LDLT) | Japan | 300 |
| Merani et al[6] | 2011 | 6817 | United States | 400 |
| Toso et al[7] | 2009 | 6478 | United States | 400 |
| Zheng et al[8] | 2008 | 195 | China | 400 |
| Lai et al[9] | 2012 | 158 | Italy | 400 |
| Ciccarelli et al[10] | 2012 | 137 | Belgium | 400 |
| Wong et al[11] | 2013 | 211 | United States | 400 |
| Yang et al[17] | 2007 | 63 (LDLT) | South Korea | 1000 |
| Zou et al[18] | 2008 | 303 | China | 1000 |
| Duvoux et al[19] | 2012 | 435 | France | 1000 |
| Hameed et al[20] | 2014 | 211 | United States | 1000 |
LDLT: Living donor liver transplantation.
FROM STATIC TO DYNAMIC
A fascinating way for trying to better define AFP with the intent to completely capture its selective role in HCC patients is to investigate its dynamic behavior more than its static values. During the waiting list period many conditions can indeed occur, some of them being directly connected to the history of the tumor such as progression or need for LRT. Consequently, these conditions may play an important role in conditioning AFP fluctuations. Starting from this statement, different equations able to define AFP modification have been proposed. The San Francisco transplant center underlined the recent implementation in their inclusion policy for LT to include patients with AFP levels > 1000 ng/mL only if LRT enabled to decrease this level beneath 500 ng/mL[21].
A Canadian study including 48 patients showed by multivariate analysis that preoperative slope of AFP was the unique independent tool able to predict tumor recurrence. Receiver operating characteristic analysis showed that the best discriminant cut-off value was 50 ng/mL per month (sensitivity: 36%; specificity: 97%). Cases having a pre-LT AFP slope > 50 ng/mL per month experienced a much worse one-year recurrence-free survival rate (40% vs 90%, P < 0.001)[22].
The Paris Paul Brousse experience including 153 patients transplanted during the period 1985-2005 revealed that patients exceeding the cut-off value of 15 ng/mL per month had lower five-year overall (54% vs 77%) and recurrence-free survival rates (47% vs 74%). At multivariate analysis, progression of AFP > 15 ng/mL per month and presence of more than three nodules at LT were poor prognostic factors[23].
Another study from Canada based on 92 patients transplanted during the period 1992-2010 showed that patients with an AFP slope exceeding 0.1 ng/mL per day had an increased risk of recurrence. Such slope was able to strongly predict post-LT recurrence, and microvascular invasion[24].
Finally, the European multicenter experience (EURHECALT study) performed on 306 patients meeting and 116 exceeding MC showed that mRECIST progression during waiting time and AFP slope > 15 ng/mL per month were the sole predictors of tumor recurrence and post-LT death[25]. All the reported studies are reassumed in Table 2.
Table 2.
Recent articles focused on alpha-fetoprotein dynamic values
| Ref. | Year | n | Country | Cut-off value (ng/mL per month) |
| Han et al[22] | 2007 | 48 | Canada | 50 |
| Vibert et al[23] | 2010 | 153 | France | 15 |
| Dumitra et al[24] | 2013 | 92 | Canada | 0.11 |
| Lai et al[25] | 2013 | 422 | Europe2 | 15 |
ng/mL per day;
Austria, Belgium, Germany, Italy.
It should be underlined that in all these mentioned studies, AFP slope was calculated using only two data points. Vibert et al[23] adopted the value obtained from the difference between the lowest and highest measured divided by the lapse of time passed between the two measurements; our group (Lai et al[25]) adopted the measures at the moment of waiting-list inscription and at moment of LT. Both methods insufficiently show the real behavior of AFP changes overtime because they are not able to completely capture the AFP oscillations during the time.
Until now, neither “dynamic” vs “static” values nor the proposed cut-off value of AFP slope (15 or 50 ng/mL per month, 0.1 ng/mL per day) have been validated.
CONSIDERATION FOR AN INTEGRATED MODEL
Several questions are thus still open in relation to the possible adoption of AFP as a refinement selector of patients with HCC awaiting for transplant. The, growing, recent literature focused on the prognostic role of AFP in relation to tumoral features, recurrence and overall patient survival, did not yet identify the best way to integrating this marker into the morphologic tumor behavior. It is however clear that besides the fundamental starting point, namely tumor morphology (based on MC), biologic tumor behaviour must obtain a valid place within the construction of every LT selection model. In a fascinating editorial, Marsh stressed that biological features, typically considered the “king” among all prognostic variables in oncology, have not enough space in the “Metroticket” paradigm (the longer the distance the higher the price; the more the tumor is advanced, the higher is the risk of recurrence) proposed by Marsh et al[3] and Mazzaferro et al[26]. Lai et al[27] reported that biology is like a dwarf on the shoulder of a giant (the MC), but thanks to this “privileged position”, the dwarf is able to see further, this means to identify risk factors and so to refine selection criteria for LT[27]. Despite these “visionary” statements, AFP appears not to be a manageable variable. Firstly, AFP may increase due to tumor-unrelated events such as viral- and toxic- (due to LRT or medication) related events; secondly, this marker frequently is not secreted by the tumor, explaining its poor sensitivity and specificity in the diagnostic process of HCC. As a consequence, all high AFP values are not equal to aggressive tumors and not all the low-value are equal to good-prognosis HCC. Moreover, the chaotic fluctuations of AFP make it difficult to find the best variable/equation able to capture them and finally, no definitive answer has been found to identify the best cut-off value to adopt.
Trying to find a “fil-rouge” in the recent literature, we assume that: (1) last AFP value or AFP slope seem to be the best values to adopt; (2) different cut-offs may be adopted in the two different scenarios of MC-IN and MC-OUT, adopting lower values in this latter context; (3) the possible use of 1000 ng/mL as cut-off for MC-IN patients seems to represent a good compromise between the necessity to exclude high-risk patients from LT and the desire to give the transplant opportunity to the highest number of patients; (4) the latter considerations can be potentially extended also to University California San Francisco criteria, eventually adopting a more stringent AFP parameter (necessity of post-LRT AFP reduction from 1000 to 500 ng/mL? eventually a lower value?); (5) no definitive conclusion has been reached in relation to the best cut-off value to adopt in case of MC-OUT patients (400 ng/mL or less?) and finally (6), no definitive cut-off has been investigated in relation to AFP slope in the two different published scenarios, so more studies are required (Table 3).
Table 3.
Proposal for the integration of alpha-fetoprotein values and morphological tumor criteria into the selection process for liver transplantation in hepatocellular carcinoma cirrhotic patients
| Criteria | No. of lesions | Maximum diameter (cm) | Last AFP value (ng/mL) | AFP slope (ng/mL per month) |
| MC | 1 | 5 | 1000 | 15, 50, higher? |
| 2-3 | 3 | 1000 | 15, 50, higher? | |
| UCSFC | 1 | 5.1-6.5 | 1000? | 50 or higher? |
| 1000 → 500? lower? | ||||
| 2-3 | 3.1-4.5 (total sum 8) | 1000? | 50 or higher? | |
| 1000 → 500? lower? | ||||
| Out of conventional criteria | 400? lower? | 50 or higher? | ||
AFP: Alpha-fetoprotein; MC: Milan Criteria; UCSFC: University of California San Francisco Criteria.
CONCLUSION
AFP represents a useful but poorly manageable tool to increase the ability to better select HCC patients waiting for LT. More, structured and prospective, studies using serial determination of AFP values within and without the context of locoregional therapies are needed in order to find the “ideal” (static and dynamic) cut-off values allowing to respond to all the still open questions in this field of transplant oncology.
Footnotes
P- Reviewer: Shen T, Zhang J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: None of the authors has any conflict of interest to declare in relation to this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 2, 2015
First decision: January 20, 2015
Article in press: June 16, 2015
References
- 1.Lai Q, Lerut J. α-Fetoprotein and hepatocellular cancer recurrence: a paradigm of the chaos theory. Liver Transpl. 2014;20:1283. doi: 10.1002/lt.23962. [DOI] [PubMed] [Google Scholar]
- 2.Lai Q, Melandro F, Pinheiro RS, Donfrancesco A, Fadel BA, Levi Sandri GB, Rossi M, Berloco PB, Frattaroli FM. Alpha-fetoprotein and novel tumor biomarkers as predictors of hepatocellular carcinoma recurrence after surgery: a brilliant star raises again. Int J Hepatol. 2012;2012:893103. doi: 10.1155/2012/893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh JW, Schmidt C. The Milan criteria: no room on the metro for the king? Liver Transpl. 2010;16:252–255. doi: 10.1002/lt.22037. [DOI] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Mehta N, Yao FY. Moving past “One size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl. 2013;19:1055–1058. doi: 10.1002/lt.23730. [DOI] [PubMed] [Google Scholar]
- 6.Merani S, Majno P, Kneteman NM, Berney T, Morel P, Mentha G, Toso C. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol. 2011;55:814–819. doi: 10.1016/j.jhep.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 7.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 8.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 9.Lai Q, Avolio AW, Manzia TM, Sorge R, Agnes S, Tisone G, Berloco PB, Rossi M. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant. 2012;26:E125–E131. doi: 10.1111/j.1399-0012.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 10.Ciccarelli O, Lai Q, Goffette P, Finet P, De Reyck C, Roggen F, Sempoux C, Doffagne E, Reding R, Lerut J. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int. 2012;25:867–875. doi: 10.1111/j.1432-2277.2012.01512.x. [DOI] [PubMed] [Google Scholar]
- 11.Wong LL, Naugler WE, Schwartz J, Scott DL, Bhattacharya R, Reyes J, Orloff SL. Impact of locoregional therapy and alpha-fetoprotein on outcomes in transplantation for liver cancer: a UNOS Region 6 pooled analysis. Clin Transplant. 2013;27:E72–E79. doi: 10.1111/ctr.12056. [DOI] [PubMed] [Google Scholar]
- 12.McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12:56–61. doi: 10.1111/j.1477-2574.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grąt M, Kornasiewicz O, Lewandowski Z, Hołówko W, Grąt K, Kobryń K, Patkowski W, Zieniewicz K, Krawczyk M. Combination of morphologic criteria and α-fetoprotein in selection of patients with hepatocellular carcinoma for liver transplantation minimizes the problem of posttransplant tumor recurrence. World J Surg. 2014;38:2698–2707. doi: 10.1007/s00268-014-2647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Wahab M, Sultan AM, Fathy OM, Salah T, Elshobary MM, Elghawalby NA, Yassen AM, Elsarraf WM, Elsaadany MF, Zalatah K. Factors affecting recurrence and survival after living donor liver transplantation for hepatocellular carcinoma. Hepatogastroenterology. 2013;60:1847–1853. [PubMed] [Google Scholar]
- 15.Lai Q, Avolio AW, Manzia TM, Agnes S, Tisone G. Role of alpha-fetoprotein in selection of patients with hepatocellular carcinoma waiting for liver transplantation: must we reconsider it? Int J Biol Markers. 2011;26:153–159. doi: 10.5301/JBM.2011.8557. [DOI] [PubMed] [Google Scholar]
- 16.Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013;96:1008–1012. doi: 10.1097/TP.0b013e3182a53f2b. [DOI] [PubMed] [Google Scholar]
- 17.Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, Kim IH, Yi NJ, Lee KU. A revised scoring system utilizing serum alphafetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery. 2007;141:598–609. doi: 10.1016/j.surg.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Zou WL, Zang YJ, Chen XG, Shen ZY. Risk factors for fatal recurrence of hepatocellular carcinoma and their role in selecting candidates for liver transplantation. Hepatobiliary Pancreat Dis Int. 2008;7:145–151. [PubMed] [Google Scholar]
- 19.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994.e3; quiz e14-15. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao FY, Hameed B, Mehta N, Roberts JP. Response to letter to the editors. Liver Transpl. 2014;20:1285. doi: 10.1002/lt.23982. [DOI] [PubMed] [Google Scholar]
- 22.Han K, Tzimas GN, Barkun JS, Metrakos P, Tchervenkov JL, Hilzenrat N, Wong P, Deschênes M. Preoperative alpha-fetoprotein slope is predictive of hepatocellular carcinoma recurrence after liver transplantation. Can J Gastroenterol. 2007;21:39–45. doi: 10.1155/2007/206383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129–137. doi: 10.1111/j.1600-6143.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 24.Dumitra TC, Dumitra S, Metrakos PP, Barkun JS, Chaudhury P, Deschênes M, Paraskevas S, Hassanain M, Tchervenkov JI. Pretransplantation α-fetoprotein slope and milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation. 2013;95:228–233. doi: 10.1097/TP.0b013e31827743d7. [DOI] [PubMed] [Google Scholar]
- 25.Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, Goffette P, Vogel W, Pitton MB, Lerut J. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108–1118. doi: 10.1002/lt.23706. [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 27.Lai Q, Avolio AW, Graziadei I, Lerut J. Response to locoregional treatment and alpha-fetoprotein trend in liver transplant candidates for HCC: dwarfs standing on the shoulders of giants. J Hepatol. 2014;60:1331–1332. doi: 10.1016/j.jhep.2014.01.030. [DOI] [PubMed] [Google Scholar]


