Abstract
Alkyne metathesis is increasingly explored as a reliable method to close macrocyclic rings, but there are no prior examples of an alkyne-metathesis-based homodimerization approach to natural products. In this approach to the cytotoxic C2-symmetric marine-derived bis(lactone) disorazole C1, a highly convergent, modular strategy is employed featuring cyclization through an ambitious one-pot alkyne cross-metathesis/ring-closing metathesis self-assembly process.
Keywords: alkyne metathesis, cytotoxicity, natural products, self-assembly, total synthesis
The use of ring-closing alkyne metathesis (RCAM) in the synthesis of natural products[1] has gained prevalence with the development of reliable, bench-stable catalysts, which have improved substrate scope over their predecessors.[2] These catalysts open up options for the application of RCAM-based strategies to the cyclization of terminal methyl-substituted alkynes,[1] terminal alkynes,[3] and even combinations of the two substitution patterns[4] in the synthesis of natural products. Partial hydrogenation of the resultant macrocyclic alkyne can now be effected under a range of conditions and allows access to either E- or Z-alkene geometry.[1a,5] Yet despite these advances, and in sharp contrast to their alkene counterparts,[6] the development of alkyne cross-metathesis (ACM) reactions remains comparatively underdeveloped.[7] Combining ACM and RCAM reactions to allow self-assembly processes is limited to a handful of examples, including the formation of aryleneethynylene macrocycles and a tetrameric cage structure with 4D2h symmetry.[7a,8] There are no prior examples of such a self-assembly approach to the synthesis of complex natural products.
In order to investigate the application of a combined ACM and RCAM strategy to the synthesis of natural products, our chosen target was the cytotoxic, C2-symmetric bis(lactone) disorazole C1 (1; Figure 1),[9] which was first isolated in 1994 from the fermentation broth of the myxobacterium Sorangium cellulosum.[10] As a family, the disorazoles have been shown to possess cytotoxicity in the nm to pm range and anti-tubulin activity,[11] and current pharmaceutical interest focuses on their potential for the treatment of drug-resistant solid tumors.[12,13] However, much remains to be discovered about the mode of action of these natural products, including how and where they bind to tubulin,[14] as this has been demonstrated to be orthogonal to the binding sites of vincristine and taxol.[14] Until recently, only one total synthesis of this challenging target was reported.[15,16]
Figure 1.
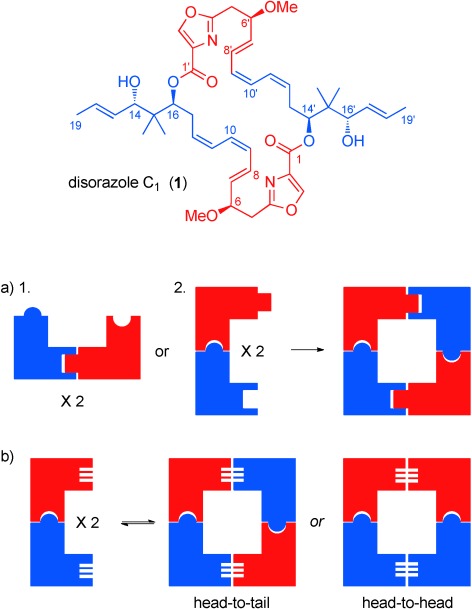
Structure of disorazole C1 and overview of synthetic approaches to disorazole C1. a) Previous approaches to dimerization using 1. lactonization, or 2. cross-coupling reactions. b) This work, self-assembly using alkyne metathesis.
Pioneering synthetic work was undertaken by Meyers and co-workers before the relative and absolute stereochemistry of disorazole C1 had been fully established.[17] Although the total synthesis of disorazole C1 was not achieved, this work did identify several pivotal issues to be addressed in any future synthesis; most notably that strategies based on the homodimerization of a fully formed seco-acid precursor (strategy 1 in Figure 1 a) were not likely to be successful because of the competing formation of the monomeric 15-membered lactone, and that the correct choice of protecting group(s) would be crucial to the successful completion of the synthesis.[17b These results are mirrored by the recent work from Hoveyda and co-workers on a seco-acid precursor, which has the correct absolute stereochemistry as well as the E,Z,Z-alkene geometry.[16] Hoffmann et al. also explored a direct dimerization approach, but in this instance with the C9=C10 Z-alkene masked as an alkyne.[18] Although an in silico analysis predicted the preferential formation of the desired bis(lactone) over the monomer,[19] neither was formed under a range of conditions. A stepwise coupling to generate the bis(lactone) was achieved by both the Meyers and Hoffmann groups (using C11=C12 and C9=C10 alkyne-masked precursors, respectively) through sequential esterification, unmasking of the second acid and alcohol components, and subsequent lactonization.[17a,18]
Avoiding direct dimerization enabled the first successful total synthesis of disorazole C1 by Wipf and co-workers in 2004.[15] In their approach, the sequential coupling of components to a C9=C10 alkyne-masked seco-acid through esterification, Sonogashira coupling at C8′=C9′, and subsequent lactonization gave the tetradehydro precursor 2, which was converted to the natural product in two further steps through PMB deprotection and Lindlar hydrogenation (Scheme 1).[15] Remarkably, the very recent synthesis of disorazole C1 by Hoveyda et al. shows that by switching the coupling positions to C10=C11/C10′=C11′ and relying on cross-coupling reactions rather than esterification and lactonization reactions, dimerization is possible even on an alkene, rather than an alkyne-masked, precursor (strategy 2 in Figure 1 a).[16]
Scheme 1.
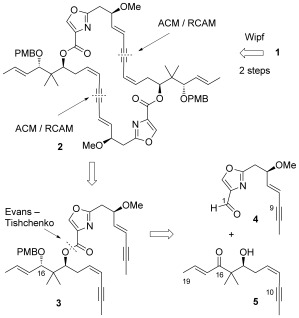
Retrosynthetic analysis of disorazole C1 (1).
Our approach to the construction of the 30-membered bis(lactone) differs markedly from the previously reported approaches in that it relies on self-assembly of the bis(lactone) (strategy in Figure 1 b). To this end, we targeted the interception of the Wipf tetradehydro intermediate 2 through metathesis of the bis(alkyne) precursor 3 (Scheme 1). This direct metathesis approach is unprecedented in the synthesis of complex polyketide-derived natural products, but has some foundation in the formation of cyclophanes.[20] However, it does provide an additional challenge over other approaches in that both head-to-tail and head-to-head coupling products could be formed from the nonsymmetrical bis(alkyne) precursor.
Following on from prior work in our group,[21] we planned the formation of bis(alkyne) 3 through the coupling of aldehyde 4 and β-hydroxy ketone 5 using a heteroaryl Evans–Tishchenko (ET) reaction to set the stereochemistry at C16 (Scheme 1).[22,23] Our initial route to C1–C9 oxazole aldehyde 4 relied upon a lateral lithiation of protected 4-hydroxymethyl-2-methyl-oxazole 6[24] and coupling to enyne aldehyde 7[25] to generate 8 (Scheme 2). Methylation of the resulting free hydroxy group at C6 gave 9, and deprotection of the hydroxy group at C1, followed by oxidation generated racemic aldehyde (±)-4 (Scheme 2). This approach was successful when the protecting group allowed coordination (e.g. PG=MOM), but not for silyl protecting groups, thus suggesting an initial lithiation of the 5-position of the oxazole, with subsequent equilibration to the 2-(lithiomethyl)oxazole species facilitated by diethylamine.[26] Although a few options were explored for converting this route to an asymmetric one,[27] several steps were low-yielding, and we thus pursued an alternative route to a single enantiomer of the desired oxazole aldehyde 4, which had the additional advantage that it would allow a rapid variation of the heterocycle in future studies.
Scheme 2.
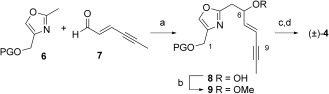
Synthesis of the racemic C1–C9 fragment. Reagents and conditions (PG=MOM): a) 1. nBuLi, THF, −78 °C, 1 h; 2. HNEt2, −78 °C, 45 min; 3. 7, −78 °C, 45 min, 40 %; b) 1. NaH, THF, 0 °C; 2. MeI, 0 °C to RT, 18 h, 29 %; c) HCl, MeOH, RT, 18 h, 74 %; d) DMP, NaHCO3, CH2Cl2, 0 °C, 2 h, 43 %. PG=protecting group.
Commercially available mannitol derivative 10 (Scheme 3) was converted into alkyne 11 by periodate cleavage, Seyferth–Gilbert homologation with the Ohira–Bestmann reagent,[28] and acetal hydrolysis. Vinyl iodide 12 was accessed by a highly (E)-selective palladium-catalyzed hydrostannylation (13, E/Z=30:1) and subsequent iodonolysis.[29] Negishi coupling and selective monotosylation in the presence of catalytic dibutyltin oxide[30] furnished secondary alcohol 14, which then smoothly underwent methylation to give key tosylate 15. Attempts to couple this tosylate directly with the 2-position of a 4-substituted oxazole using either C=H activation or lithiation strategies were unsuccessful.[27] Instead, a step-wise construction of the oxazole was achieved through conversion of 15 into the corresponding acid 16 by cyanide displacement and hydrolysis. Subsequent coupling to serine methyl ester, cyclization, and oxidation provided the fully functionalized oxazole 17, which model studies suggest might be readily converted to the desired oxazole aldehyde 4 through DIBAL-H reduction.
Scheme 3.
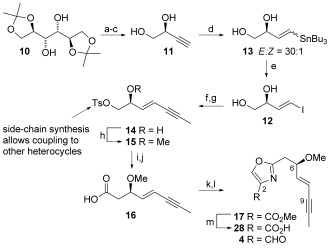
Synthesis of the C1–C9 oxazole fragment. Reagents and conditions: a) NaIO4, NaHCO3 (satd. aq.), MgSO4, CH2Cl2, 0 °C to RT, 2 h 20 min, 88 %; b) dimethyl-1-diazo-2-oxopropylphosphonate, K2CO3 (s), MeOH, 0 °C to RT, 17 h; c) HCl (conc.), MeOH, Et2O, THF, 6 h 15 min, 66 % over 2 steps; d) [PdCl2(PPh3)2], Bu3SnH, Et2O, −30 °C, 30 min, 60 %; e) I2, Et2O, 0 °C, 5 min, 92 %; f) 1. ZnCl2, BrMgC=CCH3, THF, 0 °C, 15 min; 2. 12, [PdCl2(PPh3)2], 0 °C to RT, 25 h; g) Bu2SnO, TsCl, NEt3, CH2Cl2, 0 °C to RT, 11 h, 60 % over 2 steps; h) Me3OBF4, 1,8-bis(dimethylamino)naphthalene; i) KCN, Bu4NI, NaHCO3, DMSO, 60 °C to 70 °C, 4 h, 63 % over 2 steps; j) H2O2, LiOH⋅H2O, EtOH, RT, 34 h, 75 %; k) HBTU, EtN(iPr)2, SerOMe⋅HCl, CH3CN, 0 °C to RT, 10 h, 82 %; l) 1. XtalFluor-E, K2CO3, CH2Cl2, −78 °C to 0 °C, 50 min; 2. BrCCl3, DBU, −20 °C to RT, 4 h 45 min, 76 %; m) LiOH (aq.), THF, RT, 8 h, 96 %.
A number of routes were pursued to the second key fragment, the C10–C19 β-hydroxy ketone 5,[31] but its preparation on a gram scale was eventually achieved as shown in Scheme 4. Thus, known aldehyde 18 was subjected to an enantioselective organoborane-mediated Mukaiyama aldol reaction[32] with the silyl ketene acetal of methyl 2-methylpropionate to afford β-hydroxyketone 19 in 85 % yield and 89 % ee.[19] Silyl protection of the secondary alcohol 20, deprotection (DDQ) of the PMB-ether, Swern oxidation of the primary alcohol and a Stork–Zhao–Wittig reaction[33] gave the key vinyl iodide 21 (Z/E>99:1). A Negishi coupling was used to install the enyne portion of the target fragment 5 in high yield. Subsequent conversion of the ester 22 to the Weinreb amide 24 was best performed on the free alcohol 23, which was reprotected (25) for the following allyl Grignard addition and DBU-mediated isomerization to the enone. Deprotection (with HF) gave the required β-hydroxyketone 5 and completed the fragment in 11 steps and 28 % overall yield from 18.
Scheme 4.
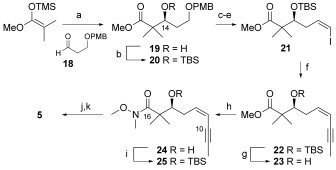
Synthesis of the C10–C19 β-hydroxy ketone fragment 3. Reagents and conditions: a) 1. N-Ts-D-Valine, BH3⋅THF, CH2Cl2, −78 °C, 5 h, 2. HCl, THF:H2O (1:1), 85 %, 89 % ee; b) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C, 2.5 h, 94 %; c) DDQ, CH2Cl2:H2O (18:1), RT, 1.5 h, quant.; d) Swern, 95 %; e) ICH2PPh3+I−, NaHMDS, HMPA, THF, −78 °C, 2 h, 75 %; f) 1. BrMgC≡CCH3, ZnCl2, THF, 0 °C, 30 min, 2. 20, [PdCl2(PPh3)2], 0 °C to RT, 16 h, 92 %; g) HF (40 % aq.), MeCN, 0 °C to RT, 1 h, 99 %; h) 1. HNMe(OMe)⋅HCl, nBuLi, −78 °C to RT, 30 min, 2. 22, THF:hexane (1:1), −78 °C to RT, 1.5 h, 82 %; i) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C to RT, 2 h, 96 %; j) 1. allyl-MgBr, Et2O −20 to −78 °C, 2 h, 2. DBU, Et3N, 50 °C, 18 h, 81 %; k) HF (40 % aq.), MeCN, 0 °C to RT, 45 min, 85 %.
The preparation of β-hydroxyketone 5 on a gram scale allowed the examination of the scope of the ET reaction with model aryl and heteroaryl aldehydes. Electron-deficient aldehydes (in particular pyridines and nitrobenzaldehydes) were shown to undergo a SmIII-catalyzed ET reaction, giving 1,3-anti diol monoester products in good to excellent yields (>95:5 d.r.). Unfortunately, indoles, pyrroles, and pyrazoles,[23] even those containing electron-withdrawing substituents at the heteroatom, gave poor yields or failed to react. Thus, the critical anti stereorelationship at C14–C16 was set using an ET coupling with 3-nitrobenzaldehyde to give 26 (Scheme 5); a PMB protecting group was installed, and the hydroxy group at C14 was unmasked through subsequent ester hydrolysis to give 27. Yamaguchi coupling, through the portionwise addition of the activated acid derived from the C1–C9 fragment 28 (Scheme 3) to fragment 27 gave the PMB-protected bis(alkyne) 3 spanning the C1–C9/C10′–C19′ portion of disorazole C1. This bis(alkyne) could be readily deprotected under the controlled conditions used in the total synthesis reported by Wipf[15] to give the bis(alkyne) alcohol 29.
Scheme 5.
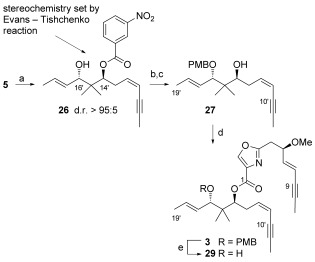
Coupling of the C1–C9 and C10′–C19′ fragments to give bis(alkyne) intermediate 3. Reagents and conditions: a) 3-NO2PhCHO, SmI2, THF, −20 °C, 4 h, 94 %; b) PMB-TCA, Sc(OTf)3, PhCH3, 0 °C to RT, 1 h, 79 %; c) LiOH, MeOH:H2O (10:1), reflux, 18 h, 91 %; d) 1. 2,4,6-trichlorobenzoyl chloride, Et3N, 28, PhCH3, RT, 30 min, added portionwise to 27 (0.05 m in toluene), DMAP, 40 °C, 30 min each addition, 2. 40 °C, 18 h 71 %; e) DDQ, CH2Cl2:phosphate buffer (1:1), RT, 30 min, 77 %.
With bis(alkynes) 3 and 29 in hand, we could now explore the ambitious ACM/RCAM self-assembly process (Scheme 6). We were encouraged by the computational studies of Hoffmann et al., which suggested that dimer formation was favored for a C9–C10 based bis(alkyne),[19] and by our own preliminary modelling studies, which indicated a thermodynamic preference for the desired head-to-tail coupling mode for the parent disorazole C1 structure. For the metathesis reaction, we chose the recently published molybdenum alkylidyne catalyst 30 from the Fürstner group.[2a The reaction was initiated in a glovebox and we found that the overall yields and conversion were considerably improved if the substrate was stirred with a mixture of 4 Å and 5 Å activated molecular sieves prior to the addition of the catalyst.[4] The outcome seemed to depend on the initial amount of catalyst added and could not be improved by the later addition of further portions of catalyst. The reaction was best followed by reverse-phase LC-MS (λ=254 nm), which allowed distinct peaks corresponding to each of the linear (31, 32, 33) and cyclic (2, 34) dimers to be identified. Under optimum conditions (Scheme 6), cyclic dimers 2 and 34 could be isolated in an overall yield of 62 %; the ratio of the desired head-to-tail-coupled product 2 to its head-to-head-coupled regioisomer 34 was approximately 5:1. The formation of the unwanted cyclic monomer was not discernible by LC-MS, thus supporting computational predictions.[34] Data for tetradehydrido disorazole C1 (2) was found to be identical in all regards to that reported by the Wipf group in 2004, and this intermediate could be converted in two steps via the known alcohol 35 to the target natural product, disorazole C1 (1), using the previously reported procedures.[15,35] When we attempted the self-assembly reaction with the deprotected bis(alkyne) 29, a complex mixture of products was produced. While LC-MS indicated a possible correlation with the predicted linear (36, 37, 38) and cyclic (35, 39) dimers, isolation of the major peak with the desired m/z[36] gave material which had distinctly different NMR data to that which we had already determined for compound 35, thus indicating a more complex process (perhaps with accompanying double bond isomerism) for the deprotected material.
Scheme 6.
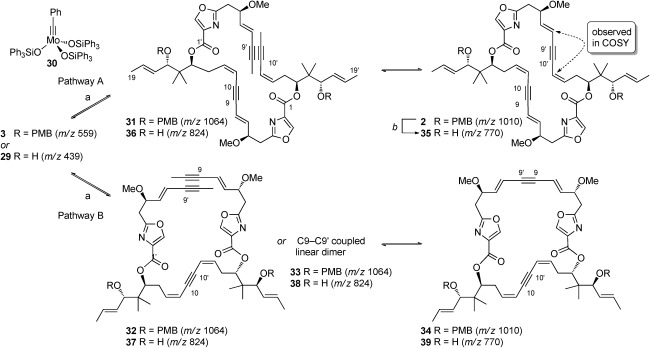
ACM/RCAM self-assembly reaction products. Pathway A: desired head-to-tail bis(alkyne) coupling proceeding via a C9=C10 [or C9′=C10′] coupled linear dimer intermediate. Pathway B: undesired head-to-head coupling proceeding via a C10=C10′ (shown) or C9=C9′ coupled linear dimer intermediate. Reagents and conditions: a) 1. 4 Å/5 Å MS (1:1), PhCH3, RT, 20 min; 2. 30 (20 mol %), RT, 16 h; b) DDQ, CH2Cl2:phosphate buffer (1:1), RT, 30 min, 61 %.
In conclusion, we have demonstrated the first example of an alkyne metathesis self-assembly process, using cross-metathesis and ring-closing metathesis reactions, to give the C2-symmetric bis(lactone) disorazole C1. The synthetic results give an intriguing glimpse into the possibility of using such an approach in the synthesis of complex natural product architectures and open up new routes to the synthesis of analogues of this fascinating natural product.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201501922.
References
- [1a].Fürstner A. Angew. Chem. Int. Ed. 2014;53:8587–8598. doi: 10.1002/anie.201402719. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014;126 [Google Scholar]
- [1b].Fürstner A. Angew. Chem. Int. Ed. 2013;52:2794–2819. doi: 10.1002/anie.201204513. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013;125 [Google Scholar]
- [2a].Heppekausen J, Stade R, Kondoh A, Seidel G, Goddard R, Fürstner A. Chem. Eur. J. 2012;18:10281–10299. doi: 10.1002/chem.201200621. [DOI] [PubMed] [Google Scholar]
- [2b].Wu X, Tamm M. Beilstein J. Org. Chem. 2011;7:82–93. doi: 10.3762/bjoc.7.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2c].Jyothish K, Zhang W. Angew. Chem. Int. Ed. 2011;50:8478–8480. doi: 10.1002/anie.201102678. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011;123 [Google Scholar]
- [3].Haberlag B, Freytag M, Daniliuc CG, Jones PG, Tamm M. Angew. Chem. Int. Ed. 2012;51:13019–13022. doi: 10.1002/anie.201207772. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012;124 [Google Scholar]
- [4].Willwacher J, Fürstner A. Angew. Chem. Int. Ed. 2014;53:4217–4221. doi: 10.1002/anie.201400605. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014;126 [Google Scholar]
- [5].Fürstner A. Science. 2013;341:1357–1363. doi: 10.1126/science.1229713. [DOI] [PubMed] [Google Scholar]
- [6].Chatterjee AK, Choi T-L, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- [7].For recent examples, see:
- [7a].Wang Q, Zhang C, Noll BC, Long H, Jin Y, Zhang W. Angew. Chem. Int. Ed. 2014;53:10663–10667. doi: 10.1002/anie.201404880. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014;126 [Google Scholar]
- [7b].Chen Z, Paley DW, Wei L, Weisman AL, Friesner RA, Nuckolls C, Min W. J. Am. Chem. Soc. 2014;136:8027–8033. doi: 10.1021/ja502706q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7c].Lhermet R, Fürstner A. Chem. Eur. J. 2014;20:13188–13193. doi: 10.1002/chem.201404166. [DOI] [PubMed] [Google Scholar]
- [7d].Li ST, Schnabel T, Lysenko S, Brandhorst K, Tamm M. Chem. Commun. 2013;49:7189–7191. doi: 10.1039/c3cc43108h. [DOI] [PubMed] [Google Scholar]
- [7e].Persich P, Llaveria J, Lhermet R, de Haro T, Stade R, Kondoh A, Fürstner A. Chem. Eur. J. 2013;19:13047–13058. doi: 10.1002/chem.201302320. [DOI] [PubMed] [Google Scholar]
- [8].Jin Y, Wang Q, Taynton P, Zhang W. Acc. Chem. Res. 2014;47:1575–1586. doi: 10.1021/ar500037v. [DOI] [PubMed] [Google Scholar]
- [9].Hopkins CD, Wipf P. Nat. Prod. Rep. 2009;26:585–601. doi: 10.1039/b813799b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jansen R, Irschik H, Reichenbach H, Wray V, Höfle G. Liebigs Ann. Chem. 1994:759–773. [Google Scholar]
- [11a].Hopkins CD, Schmitz JC, Chu E, Wipf P. Org. Lett. 2011;13:4088–4091. doi: 10.1021/ol2015994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11b].Schäckel R, Hinkelmann B, Sasse F, Kalesse M. Angew. Chem. Int. Ed. 2010;49:1619–1622. doi: 10.1002/anie.200906450. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010;122 [Google Scholar]
- [11c].Wipf P, Graham TH, Vogt A, Sikorski RP, Ducruet AP, Lazo JS. Chem. Biol. Drug Des. 2006;67:66–73. doi: 10.1111/j.1747-0285.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- [12].Lazo JS, Reese CE, Vogt A, Vollmer LL, Kitchens CA, Günther E, Graham TH, Hopkins CD, Wipf P. J. Pharmacol. Exp. Ther. 2010;332:906–911. doi: 10.1124/jpet.109.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13a].Irschik H, Jansen R, Sasse F, Baasner S, Schmidt P, Günther E. 2004. Chem. Abstr2004140 Patent PCT Int. Appl., WO 2004024149,, [ , 264487]
- [13b].Günther E, Schaefer O, Teifel M, Paulini K. 2008. Chem. Abstr2008148 Patent PCT Int. Appl., WO 2008028934,, [ , 355564]
- [13c].Günther E, Schaefer O, Teifel M, Paulini K. 2010. Patent PCT Int. Appl., US 20100323963.
- [14].Brisson Tierno M, Kitchens CA, Petrik B, Graham TH, Wipf P, Xu FL, Saunders WS, Raccor BS, Balachandran R, Day BW, Stout JR, Walczak CE, Ducruet AP, Reese CE, Lazo JS. J. Pharmacol. Exp. Ther. 2009;328:716–722. doi: 10.1124/jpet.108.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wipf P, Graham TH. J. Am. Chem. Soc. 2004;126:15346–15347. doi: 10.1021/ja0443068. [DOI] [PubMed] [Google Scholar]
- [16].Speed AWH, Mann TJ, O’Brien RV, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2014;136:16136–16139. doi: 10.1021/ja509973r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17a].Hillier MC, Price AT, Meyers AI. J. Org. Chem. 2001;66:6037–6045. doi: 10.1021/jo010249e. [DOI] [PubMed] [Google Scholar]
- [17b].Hillier MC, Meyers AI. Tetrahedron Lett. 2001;42:5145–5147. [Google Scholar]
- [18].Niess B, Hartung IV, Haustedt LO, Hoffmann HMR. Eur. J. Org. Chem. 2006:1132–1143. [Google Scholar]
- [19].Hartung IV, Niess B, Haustedt LO, Hoffmann HMR. Org. Lett. 2002;4:3239–3242. doi: 10.1021/ol026468j. [DOI] [PubMed] [Google Scholar]
- [20].Beer S, Brandhorst K, Grunenberg J, Hrib CG, Jones PG, Tamm M. Org. Lett. 2008;10:981–984. doi: 10.1021/ol800154y. [DOI] [PubMed] [Google Scholar]
- [21].Aird JI, Hulme AN, White JW. Org. Lett. 2007;9:631–634. doi: 10.1021/ol062932z. [DOI] [PubMed] [Google Scholar]
- [22a].Evans DA, Hoveyda AH. J. Am. Chem. Soc. 1990;112:6447–6449. [Google Scholar]
- [22b].Ralston KJ, Hulme AN. Synthesis. 2012:2310–2324. [Google Scholar]
- [23].Dorgan PD, Durrani J, Cases-Thomas MJ, Hulme AN. J. Org. Chem. 2010;75:7475–7478. doi: 10.1021/jo1015689. [DOI] [PubMed] [Google Scholar]
- [24].Prepared from 4-hydroxymethyl-2-methyl-oxazole: Benoit G-E, Carey JS, Chapman AM, Chima R, Hussain N, Popkin ME, Roux G, Tavassoli B, Vaxelaire C, Webb MR, Whatrup D. Org. Process Res. Dev. 2008;10:2813–2816. [Google Scholar]
- [25a].Boland W, Schroer N, Sieler C, Feigel M. Helv. Chim. Acta. 1987;70:1025–1040. [Google Scholar]
- [25b].Koop U, Handke G, Krause N. Liebigs Ann. Chem. 1996:1487–1499. [Google Scholar]
- [26].Evans DA, Cee VJ, Smith TE, Santiago KJ. Org. Lett. 1999;1:87–90. doi: 10.1021/ol990027r. [DOI] [PubMed] [Google Scholar]
- [27].H. S. Niblock, PhD Thesis, The University of Edinburgh, 2012
- [28a].Seyferth D, Marmor RS, Hilbert P. J. Org. Chem. 1971;36:1379–1386. [Google Scholar]
- [28b].Gilbert JC, Weerasooriya U. J. Org. Chem. 1982;47:1837–1845. [Google Scholar]
- [28c].Müller S, Liepold B, Roth GJ, Bestmann HJ. Synlett. 1996:521–522. [Google Scholar]
- [28d].Roth SJ, Liepold B, Müller SG, Bestmann HJ. Synthesis. 2004:59–62. [Google Scholar]
- [29a].Zhang HX, Guibé F, Balavoine G. J. Org. Chem. 1990;55:1857–1867. [Google Scholar]
- [29b].Liron F, Gervais M, Peyrat J-F, Alami M, Brion J-D. Tetrahedron Lett. 2003;44:2789–2794. [Google Scholar]
- [30].Martinelli MJ, Nayyar NK, Moher ED, Dhokte UP, Pawlak JM, Vaidyanathan R. Org. Lett. 1999;1:447–450. [Google Scholar]
- [31].P. D. Dorgan, PhD Thesis, The University of Edinburgh, 2010
- [32a].Mukaiyama T, Banno K, Narasaka K. J. Am. Chem. Soc. 1974;96:7503–7509. [Google Scholar]
- [32b].Kiyooka S, Kaneko Y, Komura M, Matsuo H, Nakano M. J. Org. Chem. 1991;56:2276–2278. [Google Scholar]
- [33a].Stork G, Zhao K. Tetrahedron Lett. 1989;30:2173–2174. [Google Scholar]
- [33b].Conway JC, Quayle P, Regan AC, Urch CJ. Tetrahedron. 2005;61:11910–11923. [Google Scholar]
- [34].Whether this self-assembly process is under kinetic or thermodynamic control has not yet been established
- [35].The yield of the Lindlar reduction was considerably lower than reported in reference [15], most probably because of the Lindlar catalyst, a problem encountered previously by others. For examples, see
- [35a].Nicolaou KC, Petasis NA, Zipkin RE. J. Am. Chem. Soc. 1982;104:5560–5562. [Google Scholar]
- [35b].Parker KA, Lim HN. Strategies and Tactics in Organic Synthesis, Vol. 10. Oxford: Academic Press; 2014. pp. 51–78. (Ed.:, pp. . [Google Scholar]
- [36].Multiple peaks of different intensities were observed in the LC corresponding to this desired mass
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


