Abstract
Cardiovascular disease is associated with proximity to major roadways and highways. We analyzed blood samples from 20 near highway and 20 urban background residents for cytokines and other biomarkers. Near highway participants had significantly lower SES and significantly higher occupational vehicle exhaust exposure and higher LDL levels (p = 0.046). Controlling for exposure to vehicle exhaust on-the-job, IL-6 was borderline significantly higher (p = 0.07) in near highway participants. In logistic regression analyses IL-1β was elevated near highway (OR = 3.27; p = 0.09). It is interesting that we found elevations in IL-1β, a key cytokine linked to inflammation from particulate matter. More research is needed with larger sample sizes to assess the possible role of IL-1β.
Introduction
Living close to heavy traffic appears to increase risk of cardiovascular disease. A leading hypothesis is the gradient of pollution next to highways (Brugge et al. 2007). Particulate matter in this pollution is known to promote inflammation and cardiovascular disease in vitro and in vivo (Steenhof et al. 2011; Miyata et al. 2011) and increases cytokines in human volunteers (Samet 2009). Atherosclerosis is a leading cause of overt cardiovascular illness and many cytokines promote atherosclerosis, while anti-inflammatory cytokines may be atheroprotective (Galkina and Ley 2009).
Elevated CRP has been reported for obese participants living close to major roadways (Rioux et al. 2010). Another study found no association between CRP or fibrinogen and distance of residence to major roads (Hoffmann et al. 2009). A third study looked at postmenopausal overweight women and found no association of CRP or IL-6 levels with residential major roadway proximity. However, this study did find that women who exercised near traffic and also lived close to major roads had lower natural killer cytotoxicity (Williams et al. 2009).
Given the complex nature of inflammatory cascades within the immune system, it is possible that looking only at a couple of well-known and commonly measured cytokines misses associations with immune function.
Methods
The Community Assessment of Freeway Exposure and Health (CAFEH) study is a community-based participatory research study. The blood samples for this analysis came from a subsample of 40 participants (out of 145) from our study area in Somerville, MA. The participants were chosen based on residential proximity to Interstate-93 (150,000 vehicle trips per day). Twenty participants lived in urban background (>1000 meters from the highway and >50 meters from major roadways) and 20 lived near the highway (<100 m). The near highway and urban background residents were matched on age, sex and education to the extent possible.
Venous blood samples were collected, fractionated and frozen at −80°C. Samples of plasma were analyzed for hs-CRP (SPQ High Sensitivity CRP Reagent Set; DiaSorin, Stillwater, MN); fibrinogen (κ-Assay, Kamiya Biomedical, Seattle, WA); TNFα-RII (Quantitative, R & D Systems, Minneapolis, MN); and IL-6 (Quantitative HS, R & D Systems, Minneapolis, MN). Finger-stick blood was analyzed for lipid profile using a CardioChek PA instrument (Polymer Technology Systems, Inc. CardioChek, Indianapolis, IN).
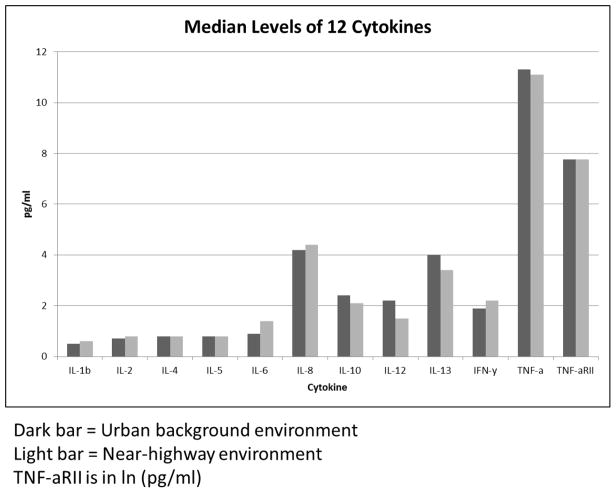
For analysis of 10 additional cytokines (Figure 1), we used a multiplex system manufactured by Meso-Scale Discovery. The reagent used was also by MSD, a human Th1/Th2 cytokine ultra-sensitive 10-plex kit (Cat# K15010C-1). Plasma samples were analyzed with Meso-Scale Discovery human Th1/Th2 cytokine 10-plex reagents.
Figure 1.
Median levels (pg/ml) of 12 cytokines. Note that mean values were substantially higher (>2x) than median values for IL-10, IL-12, IL-13 and IL-5 due to skewed data.
After determining distributional properties, we assessed the relationship between near-highway or urban background and demographic and biomarker levels using t-tests for continuous variables and tabular analysis with Fisher’s exact test for variables that were categorical. For cytokines or other biomarkers with p-values below 0.20 we further assessed the relationship with living area. For cytokines with all or most of their values above the limit of detection (LOD), near highway versus urban background was investigated using linear regression, without adjusting values below the LOD. For cytokines with significant numbers of readings below the LOD, analysis was done using logistic regression with the variable dichotomized as over or under the LOD. For all cytokine linear regression analyses, levels were first transformed using the natural log to better approximate normality.
Results
Demographics, SES, and biomarker levels are summarized in Table 1. There were no significant differences in age, height, or weight comparing people in near highway and urban background areas. Urban background participants were more likely to earn >$75,000, to have a graduate degree, and to be less exposed to occupational vehicle exhaust (p < 0.01). Employment status approached significance (p = 0.08).
Table 1.
Demographic characteristics (means), income, employment, on the job exposure to vehicle exhaust (number), and biomarker levels (means) of study populations.
| Urban Background | Near Highway | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 53.7 | 54.7 | 0.79 |
| Height (males, cm) | 178.9 | 173.4 | 0.59 |
| Weight (males, kg) | 87.3 | 82.9 | 0.77 |
| Height (females, cm) | 166.9 | 157.7 | 0.38 |
| Weight (females, cm) | 69.6 | 72.8 | 0.38 |
| Annual income | < 0.001 | ||
| 1 (<25,000) | 1 | 4 | |
| 2 (25,000–49,999) | 0 | 7 | |
| 3 (50,000–74,999) | 2 | 7 | |
| 4 (≥75,000) | 13 | 1 | |
| Highest Degree | < 0.001 | ||
| 1 (did not complete high school) | 1 | 1 | |
| 2 (completed high school) | 1 | 9 | |
| 3 (completed junior college) | 1 | 1 | |
| 4 (completed college) | 7 | 7 | |
| 5 (completed graduate school) | 10 | 2 | |
| Work status | 0.08 | ||
| 1 (working full-time) | 13 | 7 | |
| 2 (working part-time) | 2 | 7 | |
| 3 (Unemployed) | 0 | 2 | |
| 4 (retired) | 4 | 3 | |
| 5 (disabled) | 1 | 0 | |
| Exposure to vehicle exhaust at work (excludes 10 non-working subjects) | <0.01 | ||
| 1 (multiple hours per day) | 1 | 4 | |
| 2 (daily for less than one hour) | 0 | 3 | |
| 3 (once per week) | 0 | 2 | |
| 4 (once per month) | 0 | 0 | |
| 5 (never) | 16 | 7 | |
| Total cholesterol (mg/dl) | 144 (154) | 175 (176) | 0.11 |
| Triglyceride (mg/dl) | 50 (78.8) | 53 (79.1) | 0.91 |
| LDL (mg/dl) | 82 (92) | 110 (113.3) | 0.046 |
| HDL (mg/dl) | 43 (45.4) | 41 (43.9) | 0.87 |
| Fibrinogen (mg/dl) | 421.3 (422.7) | 396.2 (418.3) | 0.70 |
| CRP (mg/L) | 0.9 (1.1) | 1.2 (3.4) | 0.14 |
Females had somewhat higher levels of all biomarkers but p > 0.10 (not shown) so males and females were combined. The near highway population had significantly higher LDL (p < 0.05). The difference in CRP values was also below the p < 0.20 cutoff and was further assessed. There were no other significant differences. Median values for all cytokines are provided in Figure 1. Differences in both IL-1β and IL-6 were below p < 0.20, but above p=0.10 and were further assessed.
Linear regression was used to assess IL-6, total cholesterol, CRP, and LDL while logistic regression was used to assess IL-1β. Controlling for standard demographic or employment variables did not substantively alter the p-values for CRP or total cholesterol (not shown). Controlling for occupational vehicle exhaust, IL-6 was borderline significant (p = 0.07) and LDL was significantly higher in near highway participants (p = 0.033). In the logistic regression analyses, IL-1β had an odds ratio of 3.27 in a univariate model. Multivariate models controlling for age, sex, income, employment variables, and health history variables did not materially alter this result.
Discussion
In a separate analysis from the CAFEH study we included 260 people in whom we could test for associations with highway proximity with IL-6, CRP, fibrinogen and TNFα-RII. In that analysis we found associations for CRP and IL-6, but not for fibrinogen or TNFα-RII, while controlling for many factors that could not be considered here (Brugge et al. in process). It is encouraging that the smaller sample reported here was consistent with analysis of the larger data set.
IL-1β had an OR high enough that we suspect that a larger sample size would be unlikely to render it consistent with 1.0. To our knowledge this is the first report to find an association of IL-1β with proximity to highways or heavy traffic. The IL-1 family of cytokines is involved in mediating autoinflammatory diseases and IL-1β plays a critical role in local and systemic inflammation (Dinarello 2009). Particularly relevant to our findings, sterile inflammation from particles appears to be driven by production of IL-1β in response to phagocytosis of the particles. However, these studies have not tested ambient pollution, but rather used occupational exposures such as asbestos and silica (Rock et al. 2010).
Intratracheal installation of ambient PM in mice leads to increases of IL-1, IL-6 and TNFα in bronchial alveolar lavage fluids (Park et al. 2011). A substantial literature (Miyata and Eeden 2011) has shown that ambient particulate matter activates NFkB leading to production of IL-1β, IL-6 and TNFα in alveolar macrophages.
The primary limitations of our analysis are the small sample size and the large number of biomarkers tested. Thus, we present this as preliminary work that is hypothesis generating. Of particular interest to us is the possibility that IL-1β could be a valuable addition to the blood markers more commonly assayed in near highway and more generally in air pollution research.
Acknowledgments
We would like to thank the members of the CAFEH Steering Committee, Ellin Reisner, John Durant, Baolian Kuang, Lydia Lowe, Edna Carrasco, M. Barton Laws, Yuping Zeng, Emmanuel Owusu, Christina Hemphill Fuller, Mae Fripp, Tina Wang, Michelle Liang, and Mario Davia for their contributions to the project. Aaron Marden selected participants from the larger data set for use in this analysis. Funding for CAFEH was provided by the National Institute of Environmental Health Sciences (ES015462). Support for Kevin Lane was provided by an EPA STAR Fellowship (FP-917349-01-0). The project described was also supported by the National Center for Research Resources Grant Number UL1 RR025752 and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number UL1 TR000073.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Doug Brugge, Email: dbrugge@aol.com, Department of Public Health and Community Medicine, Tufts University School of Medicine, 136 Harrison Ave., Boston MA 02111, V: 617 636 0326.
Kevin J. Lane, Email: k.lanejr@gmail.com, Department of Environmental Health, Boston University School of Public Health, 715 Albany Street, Boston MA, 02118.
Andrea Stewart, Email: andrea.leigh.stewart@gmail.com, College of Arts and Sciences, Tufts University, Medford, MA 02155.
Albert K Tai, Email: albert.tai@tufts.edu, Department of Pathology, Tufts University School of Medicine, 150 Harrison Ave., Boston MA 02111.
Mark Woodin, Email: mark.woodin@tufts.edu, Department of Civil and Environmental Engineering, Tufts School of Engineering, Anderson Hall, Medford, MA 02155.
References
- Brugge D, Durant JL, Rioux C. Near-highway pollutants in motor vehicle exhaust: a review of epidemiologic evidence of cardiac and pulmonary health risks. Environ Health. 2007;6:23. doi: 10.1186/1476-069X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the Interleukin-1 family. Annual Review of Immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Immune and inflammatory mechanisms of astherosclerosis. Annual Review of Immunology. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata R, van Eeden SF. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicology and Applied Pharmacology. 2011;257:209–226. doi: 10.1016/j.taap.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Park E-J, Roh J, Kim Y, Park K, Kim D-S, Yu S-D. PM 2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Environmental Research. 2011;111:348–355. doi: 10.1016/j.envres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Rioux CL, Tucker KL, Mwamburi M, Gute DM, Cohen SA, Brugge D. Residential Traffic Exposure, Pulse Pressure, and C-reactive Protein: Consistency and Contrast among Exposure Characterization Methods. Environ Health Perspect. 2010;118(6):803–811. doi: 10.1289/ehp.0901182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Review of Immunology. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Rappold A, Graff D, Cascio WE, Berntsen JH, Huang YC, Herbst M, Bassett M, Montilla T, Hazucha MJ, Bromberg PA, Devlin RB. Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am J Respir Crit Care Med. 2009;179:1034–1042. doi: 10.1164/rccm.200807-1043OC. [DOI] [PubMed] [Google Scholar]
- Williams LA, Ulrich CM, Larson T, Wener MH, Wood B, Campbell P, Potter J, McTiernan A, De Roos A. Proximity to Traffic, Inflammation, and Immune Function among Women in the Seattle, Washington, Area. Environ Health Perspect. 2009;117(3):374–378. doi: 10.1289/ehp.11580. [DOI] [PMC free article] [PubMed] [Google Scholar]