Abstract
Purpose
To examine the efficacy and safety profile of antiepileptic repetitive transcranial magnetic stimulation (rTMS) for refractory status epilepticus (RSE) in the intensive care unit (ICU) setting. In addition, hypothetical concerns about electrical interference of rTMS with ICU equipment have been previously raised.
Methods
We describe two cases of RSE treated with rTMS in the ICU.
Results
In one case, rTMS contributed to decreased seizure frequency; in the second case, rTMS transiently decreased seizure frequency. In both cases, rTMS was safe and did not interfere with the functioning of the ICU equipment.
Conclusion
rTMS is a potential therapy for RSE when conventional therapies have failed. Future studies should investigate the efficacy of various rTMS stimulation parameters, safety issues, and bioengineering considerations in the ICU setting.
Keywords: Transcranial Magnetic Stimulation, Refractory Status Epilepticus, Intensive Care Unit, Epilepsia Partialis Continua
Introduction
Approximately 15-20% of cases of status epilepticus (SE) will become refractory 1, 2. Seizures which have persisted over 120 minutes are considered Refractory status epilepticus (RSE), which often requires intensive care for continuous intravenous antiepileptic drugs (AEDs), general anaesthesia, and/or cardiorespiratory support3. Given its life-threatening nature, when conventional treatments fail, experimental pharmacologic and non-pharmacologic therapies are attempted. Most of the relevant clinical literature on treatment of RSE consists of case reports and series, with few controlled trials and systematic reviews 3.
Repetitive transcranial magnetic stimulation (rTMS) is a potential therapy for RSE. rTMS is a non-invasive technique where pulsed intracranial electrical current is induced by electromagnetic induction. In most subjects, a continuous train of low frequency (≤1 Hz) pulses results in cortical suppression, while intermittent trains of high frequency (≥5 Hz) pulses result in facilitation of cortical excitability 4, 5.
Several case reports describe rTMS application in medication-refractory focal epilepsy or RSE in non-ICU patients, with mixed results6-8. rTMS is well-tolerated by patients with epilepsy, without reports of seizure exacerbation9 and seizure suppression can be achieved by low frequency rTMS 7. The efficacy and safety profile of antiepileptic rTMS in the ICU is poorly understood. Only one prior successful case of rTMS for RSE in the ICU has been reported 10. Hypothetical concerns about interference with ICU electronic equipment may also limit clinical use and warrant further exploration.
We present two patients with focal RSE managed in the ICU. In both cases, a clinical decision to treat the ongoing seizures with rTMS was made. rTMS was delivered by physicians experienced in the clinical application of rTMS. Informed consent was obtained.
Patient 1
A 46 year old patient with intractable epilepsy since childhood presented with increasing seizure frequency. His baseline of 2-3 seizures daily increased to 40-50 seizures daily over the month prior to admission. Typical seizures included brief drop attacks, staring with body stiffening and left arm elevation, and secondarily generalized convulsions. A right temporo-parietal lobectomy in 1991 and vagus nerve stimulator (VNS), placed in 1998 and deactivated in 2001, were ineffective. Multiple AEDs were tried over the years (Table 1).
Table 1.
Therapies attempted during hospitalization to stop focal status epilepticus.
| Patient | Therapy | Dosage | Days of hospitalization |
|---|---|---|---|
| Patient 1 | Phenobarbital | Titrated to high therapeutic level | 1-discharge (47 d) |
| Pregabalin | 150 mg tid | 1–12; 19-discharge | |
| Lamotrigine | 200 mg tid | 1–12; 19-discharge | |
| Phosphenytoin | 150 mg tid | 1-discharge | |
| Lacosamide | 200 mg bid | 12-discharge | |
| Levetiracetam | 2000 mg bid | 13-discharge | |
| Midazolam drip | 0.1 mg/kg/h | 1–2 | |
| Propofol drip | >10 mg/kg/h | 2–4 | |
| Pentobarbital drip | Titrated to burst suppression | 4–22 (attempted wean on day 16) | |
| Ketogenic diet | Titrated to urine ketoacidosis | 6–9 | |
| TMS | 1 Hz, 20 min, 70% MO | 21 | |
| Patient 2 | Lamotrigine | 200 mg/300 mg | 1-discharge (22 d) |
| Levetiracetam | 2000 mg qid | 1-discharge | |
| Felbatol | 1500 mg tid | 1-discharge | |
| Lorazepam | 2 mg every 4 h and prn | 1-discharge | |
| Lacosamide | 250 mg bid | 2-discharge | |
| VNS | Baseline settings and prn magnetic activation | 1-discharge | |
| TMS | 1 Hz, 30 min, 100% MT | 9 |
Prior to hospitalization, patient 1 had a temporo-parietal lobectomy in 1991, a vagus nerve stimulator placed in 1998, and had been tried on multiple antiepileptic medication therapies over the years, Including carbamazepine, valproic acide, tiagabine, levetiracetam, zonisamide, topiramate, felbamate, gabapentin, lacosamide, and rufinamide.
Patient 2 had also previously received a sensorimotor corticectomy in 1993, and vagal nerve stimulator in 1993, and had been tried on multiple antiepileptic medications, including tranxene, phenobarbital, carbamazepine, valproic acid, gabapentin, and phenytoin prior to hospitalization. Abbreviations. bid (twice a day), tid (three times a day),qid (four times a day). d (days), mg (milligrams), kg (kilograms), h (hour), prn (as clinically indicated), MT (motor threshold), TMS (transcranial magnetic stimulation).
On admission, he was on phenobarbital, phosphenytoin, pregabalin, and lamotrigine (Table 1). Physical exam showed drowsiness and baseline mild left hemiparesis. Lumbar puncture was unremarkable. An MRI showed right parieto-occipital cortical thickening with mildly restricted diffusion, remote surgical resection and chronic right frontotemporal encephalomalacia.
After intravenous lorazepam, phosphenytoin and phenobarbital were ineffective, he was intubated and started on a midazolam infusion. Because of resulting bradycardia and persistent agitation, he was switched to propofol. While the number of seizures declined, the patient still experienced 30-40 electrographic seizures daily. These were characterized by rhythmic, sharp alpha frequency activity over the right frontotemporal region, with frequent bilateral spread, lasting up to several minutes each, without clinical correlate.
On day 4, due to risk of propofol infusion syndrome, pentobarbital infusion was initiated (Table 1). As pentobarbital was weaned, frequent electrographic seizures recurred. The ketogenic diet was initiated on day 6 but stopped on day 9 due to developing ileus. With attempted pentobarbital wean on day 16, his seizure frequency increased again (Figure 1), requiring a further moderate increase in his standing AEDs.
Fig. 1.
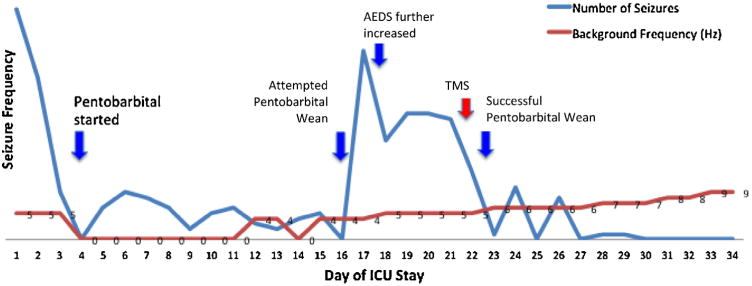
ICU course of Patient Number 1. On admission, the patient experienced between 40–50 seizures daily (blue line, daily seizure frequency). After the initiation of pentobarbital sedation on day 4, the patient's seizure frequency abated but did not completely discontinue. After developing ileus thought likely secondary to pentobarbital, there was an attempt to wean the pentobarbital on day 16 with resulting increased seizure frequency. His intravenous AEDS were further increased with modest improvement. On day 21, TMS was administered in the ICU over the seizure focus. The following day, the pentobarbital was successfully weaned with eventual cessation of seizures. The patient's EEG background (red line) and clinical status gradually improved.
Finally, the clinical team decided to administer rTMS on day 21 over the right centrotemporal region. Neurologically, the patient was intubated and sedated. Prior to starting rTMS, the stimulating coil was discharged repeatedly at 100% machine output (MO) in mid-air to ensure absence of interference with the ICU monitoring equipment. Motor threshold (MT) could not be determined due to the patient's significant peripheral edema. Therefore, the team decided to stimulate at 70% MO 11. Stimulation was applied with a figure-of-8 coil (Cool B56, Tonica, Denmark) centered over electrodes C4/T4 with handle oriented toward vertex. A single train at 1 Hz was applied for 20 minutes with the C4/T4 electrodes removed. The patient tolerated the stimulation uneventfully. ICU electronic equipment, including clinical computer console, vital signs monitor, ventilator, medication pump, feeding pump, bed, pneumatic boot machine, and EEG remained operational during neurostimulation.
In the 72 hours prior to rTMS, a median of 22 electrographic seizures per day (range 5-22), and a median of 75 spike detections (range 57-86) were recorded. This decreased to 9 seizures per day (range 3-11) and 17 spike detections (range 15-355) in the 72 hours following rTMS, which allowed pentobarbital and phenobarbital doses to be weaned and decreased, respectively (Figure 1). EEG background and mental status gradually improved and the patient was discharged to a rehabilitation center on day 47.
Patient 2
A 51 year old man with depression, sleep apnea, and focal epilepsy with dyscognitive features and secondary generalization presented with frequent focal seizures. Seizures originated from the left mid and posterior temporal region and were characterized by right arm and leg clonic movements lasting several minutes. His seizures continued despite a sensorimotor corticectomy in 1993, VNS placement in 1998, and numerous AED trials (Table 1).
By presentation, he experienced 4-5 seizures (lasting 20-70 minutes/day). He was taking lamotrigine, levetiracetam, felbamate, and lorazepam (Table 1) and had received multiple doses of lorazepam and clonazepam without benefit before hospital arrival. Admission exam demonstrated increased right-sided hemiparesis and unsteady gait. Addition of lacosamide, high-dose lorazepam, higher-dose levetiracetam, and frequent VNS magnet activations were ineffective. He was transferred to the ICU for respiratory monitoring but was never intubated. As his seizures were clinically obvious, he was not continuously monitored on EEG.
On hospital day 9 there was a clinical decision to administer rTMS. The patient's VNS was deactivated prior to TMS start. A single 30 minute 1 Hz rTMS train was delivered over the left sensorimotor cortex. Stimulation intensity was 100% hand MT as determined by visual inspection. The stimulation device and orientation was identical to that in case #1. The patient tolerated rTMS without complication. Again, the electronic equipment present, including clinical computer console, vital signs monitor, bed, and pneumatic boot machine were undisturbed during rTMS.
Following rTMS, seizure frequency decreased from a total of 9 prolonged seizures in the 72 hours prior to administration (20-50 minutes each), to a total of one prolonged focal motor seizure (45 minutes) occurring approximately 48 hours after TMS administration. However, 72 hours after rTMS, his seizures increased in frequency.
On day 12, VNS was again turned off in preparation for a second round of rTMS. However, because of seizure recurrence while the VNS was temporarily turned off and resulting alteration in consciousness, he was unable to consent for rTMS. During the last week of his hospital stay, his VNS settings (including output current and duty cycle) were increased. His seizure frequency and duration gradually returned to baseline. He was discharged after 22 days.
Discussion
Our two cases provide preliminary evidence of safety and efficacy of low frequency rTMS for management of focal RSE in the ICU. Our choice of protocol in these two cases of RSE in the ICU setting was based on our experience with epilepsia partialis continua in the outpatient setting (Rotenberg 2009). In these previous cases, low-frequency 1 Hz stimulation has been applied at 90-100% MT over the seizure focus, for 20-30 minutes. In Patient 1, the clinical team spent significant time setting up equipment and testing whether TMS discharge would interfere with ICU electronics. Due to time constraints, the team was only able to apply 1 Hz TMS for 20 minutes. Furthermore, because the patient's significant peripheral edema did not allow for a motor threshold to be assessed, the stimulation intensity was applied at 70% Mo (Fregni 2006). In this patient, rTMS applied over the epileptogenic cortex was associated with decreased seizure frequency, allowing pentobarbital and phenobarbital to be weaned successfully. In the second patient, administration of rTMS temporarily decreased the frequency of seizure activity in the first 72 hours after application. Notably, as in prior reports, rTMS did not exacerbate seizure frequency or induce secondary generalization in either patient (Rotenberg et al 2007; Sun et al 2012). rTMS also did not interfere with ICU equipment and monitoring.
Previous commentaries have suggested that AEDs and anesthetics, by altering synaptic plasticity, could decrease the efficacy of neurostimulation (Rotenberg et al, 2007). Typically, electroconvulsive therapy (ECT) protocols used for RSE include decreased or withdrawn AEDs and anesthetics to allow a generalized convulsion12. This hypothesis has not been tested in the clinical setting with rTMS. Here, a reduction in seizure frequency following 1 Hz rTMS in a patient (case #1) who is intubated and sedated by a moderate-dose pentobarbital infusion as well as multiple AEDs, suggests that cortical plasticity can be effectively modulated by rTMS even under barbiturate anesthesia
In a recent review and suggested treatment algorithm for super-refractory SE, rTMS was considered to be of low potential utility 3. In contrast, our report and another recent case 10 suggest rTMS in RSE merits further exploration. New studies should investigate various rTMS stimulation parameters, safety issues, and bioengineering considerations in the ICU setting.
Acknowledgments
Anli Liu MD MA has received funding from the Epilepsy Foundation Clinical Research Training Fellowship (2011), ABF/AES/EF Susan Spencer Clinical Research Training Fellowship (2012-14), and the NIH-NINDS T32 Institutional Research Training Grant in Cognitive Neurology (2011-12). Trudy Pang MD has received research support from Harvard Catalyst and the Center for Integration of Medicine and Technology (CIMIT). Dr. Alvaro Pascual-Leone has received research support from the Harvard-Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center and the Harvard Clinical and Translational Science Center (grants M01-RR-01066 and UL1 RR025758 from the National Center for Research Resources, National Institutes of Health). Dr. Alexander Rotenberg has received research support from the from the National Institutes of Health, Department of Defense, the Epilepsy Therapy Project, and by the Translational Research Program at Boston Children's Hospital. The authors would like to acknowledge Rachel Jurd PhD, who provided editorial assistance in the preparation of this manuscript.
Susan T. Herman MD has received support from Lundbeck, Inc; UCB Pharmaceuticals, and Fidelity Biosciences Research Institute. Dr. Alvaro Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, starlab Neuroscience, Neosync, and Novavision.. Drs. Alvaro Pascual-Leone and Alexander Rotenberg are inventors on patents and patent applications related to noninvasive brain stimulation and real-time integration of TMS with EEG and fMRI. Dr. Alexander Rotenberg has served as a paid consultant for Sage Therapeutics Inc.
Footnotes
Disclosures: The remaining authors have no disclosures to report.
References
- 1.Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43:483–488. doi: 10.1212/wnl.43.3_part_1.483. [DOI] [PubMed] [Google Scholar]
- 2.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–210. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 4.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 5.Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 6.Misawa S, Kuwabara S, Shibuya K, Mamada K, Hattori T. Low-frequency transcranial magnetic stimulation for epilepsia partialis continua due to cortical dysplasia. J Neurol Sci. 2005;234:37–39. doi: 10.1016/j.jns.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Rotenberg A, Bae EH, Takeoka M, Tormos JM, Schachter SC, Pascual-Leone A. Repetitive transcranial magnetic stimulation in the treatment of epilepsia partialis continua. Epilepsy Behav United States. 2009:253–257. doi: 10.1016/j.yebeh.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff-Guerrero A, Gonzales-Olvera J, Ruiz-Garcia M, Avila-Ordonez U, Vaugier V, Garcia-Reyna JC. rTMS reduces focal brain hyperperfusion in two patients with EPC. Acta Neurol Scand. 2004;109:290–296. doi: 10.1046/j.1600-0404.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 9.Bae EH, Schrader LM, Machii K, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav United States. 2007:521–528. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Thordstein M, Constantinescu R. Possibly lifesaving, noninvasive, EEG-guided neuromodulation in anesthesia-refractory partial status epilepticus. Epilepsy Behav. 2012;25:468–472. doi: 10.1016/j.yebeh.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Fregni F, Otachi PT, Do Valle A, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–455. doi: 10.1002/ana.20950. [DOI] [PubMed] [Google Scholar]
- 12.Lisanby SH, Bazil CW, Resor SR, Nobler MS, Finck DA, Sackeim HA. ECT in the treatment of status epilepticus. The journal of ECT. 2001;17:210–215. doi: 10.1097/00124509-200109000-00013. [DOI] [PubMed] [Google Scholar]


