Abstract
Background
Transcranial Magnetic Stimulation (TMS) can affect episodic memory, one of the main cognitive hallmarks of aging, but the mechanisms of action remain unclear.
Objectives
To evaluate the behavioral and functional impact of excitatory TMS in a group of healthy elders.
Methods
We applied a paradigm of repetitive TMS -intermittent theta-burst stimulation- over left inferior frontal gyrus in healthy elders (n=24) and evaluated its impact on the performance of an episodic memory task with two levels of processing and the associated brain activity as captured by a pre and post fMRI scans.
Results
In the post-TMS fMRI we found TMS-related activity increases in left prefrontal and cerebellum-occipital areas specifically during deep encoding but not during shallow encoding or at rest. Furthermore, we found a task-dependent change in connectivity during the encoding task between cerebellum-occipital areas and the TMS-targeted left inferior frontal region. This connectivity change correlated with the TMS effects over brain networks.
Conclusions
The results suggest that the aged brain responds to brain stimulation in a state-dependent manner as engaged by different tasks components and that TMS effect is related to inter-individual connectivity changes measures. These findings reveal fundamental insights into brain network dynamics in aging and the capacity to probe them with combined behavioral and stimulation approaches.
Keywords: Transcranial magnetic stimulation, functional magnetic resonance imaging, level of processing, episodic memory, aging
Introduction
Episodic memory is one of the cognitive domains that is most affected by aging[1], and is accompanied by volumetric changes in brain structures, white and grey matter changes and dopamine receptors depletion[2].
Repetitive transcranial magnetic stimulation (rTMS) is able to modulate cortical excitability and produce cognitive[3] and motor[4] changes. Previously, we observed improvements in a face-name memory task after prefrontal rTMS applied to older subjects which was accompanied by increased recruitment of right prefrontal and bilateral posterior areas[5]. Cognitive improvements after transcranial stimulation have also been shown in mild cognitive impairment and Alzheimer’s disease populations[6–8]. However, mechanisms underlying cerebral and behavior responses to rTMS remain unclear.
A mechanism that modulates TMS effects is the state-dependent phenomenon[9–11]. That is, TMS can induce changes revealing the potential to interact with ongoing cognitive processing or physiological states. At a functional level, state-dependency has shown to be related to both, regional activity[11], and connectivity[12–14], therefore representing relevant variables that can help to understand TMS variability, together with other factors such as age[15], genetics[16], technical aspects[17] or anatomical characteristics[18]. Neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), have become a powerful tool to reveal shifts in connectivity and regional activity and its increasingly being used together with TMS[19,20].
Recently, new patterned protocols of stimulation have emerged from animal studies, such as theta-burst stimulation[21] (TBS). Applied in an intermittent fashion (iTBS), it enhances cortical excitability, while continuous TBS (cTBS) produces inhibitory post-effects. When applied to prefrontal areas, TBS has been shown to affect various cognitive functions, such as working memory[12,22], speech repetition[23] and emotional control[24].
The left prefrontal cortex (PFC) is a region that has consistently been implicated in the encoding of verbal material. TMS studies have causally shown the involvement of the PFC during episodic memory formation, both in the left dorsolateral prefrontal cortex[25–27] (DLPFC) and left inferior frontal gyrus[28–30] (IFG). Neuroimaging evidence also supports left PFC involvement in semantic encoding, compared to shallower encodings[31–33]. These findings can be contextualized into a classical psychological theory of level of processing (LoP). That is, different LoP at encoding, such as semantic or perceptual analysis of the incoming information, result in more durable traces, therefore affecting the probability of a successful retrieval[34,35]. Although there is some evidence of age affecting LoP[36–38], this phenomenon has not been thoroughly investigated in aging. Importantly, it seems that if appropriate support is given during encoding phases (i.e. semantic elaboration), aging effects on memory performance can be minimized[36,39].
In the present study, we applied excitatory TMS (iTBS) in combination with fMRI acquisitions at rest and during an encoding memory task with two levels of processing in a sample of elderly volunteers. The main objectives of the study were: 1) to investigate whether iTBS compared to sham stimulation could result in a transient improvement in memory performance, 2) to study the brain networks that support encoding processes and TMS effects on them and 3) to study state-dependent effects of iTBS.
Material and methods
Subjects
Twenty-four healthy older adults, between 61 and 80 years, were recruited (mean age=71.75y.o.; Standard deviation=6.81). Participants had a normal cognitive profile with MMSE scores≥24 and performances not below 1.5SD according to normative scores (adjusted for age, gender and education[40,41]) on a neuropsychological evaluation that covered the major cognitive domains (including: Verbal memory: Rey auditory verbal learning test; visual memory: Rey-Osterrieth complex figure; Language: Benton naming test; semantic and phonetic fluencies; Frontal/Executive functions: direct and inverse digits, symbol digits modalities test, trail making test, Stroop test, London tower test; Visuospatial: line orientation, and visuoperceptive: Popplereuter’s embedded figures test). All participants were right-handed and none of them had any neurological or psychiatric disorder or any contraindications for TMS[26]. All subjects gave informed consent and the protocol was approved by local ethical committee. Subjects were randomly assigned to either the sham or experimental group as described below, although neuroimaging analysis were carried out with 10 subjects in the sham group, due to MRI acquisition problems.
Design and procedure
All subjects previously underwent a neuropsychological assessment and a structural MRI acquisition for subsequent TMS neuronavigation. The main part of the study consisted of two MRI acquisitions, before and after subjects received a real or sham iTBS session (Figure 1a). In each MRI session subjects underwent an episodic memory encoding session in-between two resting-state fMRI acquisitions (Figure 1b). After a wash-out period (≈1 hour), subjects received real or sham iTBS and performed an equivalent fMRI encoding session. After each scanning session, subjects performed a memory retrieval task outside the MRI.
Figure 1.
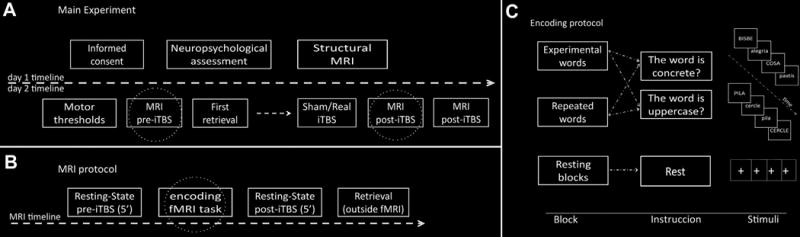
Schematic view of the experiment with a) timeline of the whole experiment, b) the MRI acquisition protocol and c) the encoding protocol realized inside the MRI. Circled boxes are detailed in the following section of the figure.
MRI acquisition
All subjects were examined on a 3T MRI scanner (Magnetom Trio Tim, Siemens Medical Systems, Germany). A high-resolution 3D structural dataset (T1-weighted magnetization prepared rapid gradient echo [MPRAGE], sagittal plane acquisition, TR=2300ms, TE=2.98ms, 240 slices, slice thickness=1mm, FOV=256mm, matrix size=256×256) was acquired before the main experimental day. fMRI sets of images consisting of 150 and 312 volumes for resting and task runs were also acquired (T2*-weighted GE-EPI sequence, TR=2000, TE=29ms, 36 slices per volume, slice thickness=3mm, interslice gap=25%, FOV=240mm, matrix size=128×128).
Memory task
In each MRI session subjects completed a word encoding memory task followed by a recognition test designed with Presentation software (Presentation v10.1 Neurobehavioral Systems). Four block-designed encoding batteries were generated with LEXESP[42], although subjects completed two encoding batteries (words from the non-presented batteries were used as new words during the retrieval). The presentation of batteries and the button response were counterbalanced. Each encoding battery consisted of 8 fixation, 8 novel words and 8 repeated words 24s-blocks, in which 4 words were constantly repeated. In word blocks, 12 words were presented for 2s, half of them in uppercase and the other in lowercase, while in fixation blocks a white cross was presented. Instructions were presented for 2s asking subjects to rest or to answer according to either deep-semantic (concrete/abstract) or shallow-perceptual judgments (uppercase/lowercase; Figure 1c). Both batteries and within-battery blocks were equivalent according LEXESP normative data. The retrieval memory task started 15min after the encoding and consisted on a recognition task (old/new decision) in which all the encoded words were presented mixed with new words from a non-presented battery (half new).
Transcranial magnetic stimulation
Magnetic pulses were delivered using a MagPro x100 (MagVenture, Denmark) with an eight-figure coil. Resting and active motor threshold (RMT; AMT) were determined for the right first dorsal interosseous (FDI) muscle over the left M1. RMT was acquired according published protocols[43] while AMT was defined as the minimum stimulus intensity that elicited at least 6 of 10 consecutive MEPs of 200-μv during FDI contraction.
iTBS was applied according to the protocol described by Huang et al[21]. Bursts consisted of 3 pulses of 50 Hz at an intensity of 80% AMT, repeated every 200ms during 2′. These trains were repeated once every 10′ for a total of 20 repetitions (600 pulses-190′). Based on a fMRI verbal encoding meta-analysis we selected a region (MNI(x,y,z): −42,14,30) in the left IFG as stimulation point[44]. Neuronavigated stimulation with stereotactic registration was used while sham device was employed for sham iTBS. iTBS was applied in a room next to the scanner and immediately after the stimulation, subjects did the second MRI acquisition.
Data analysis
Behavioral data
To examine any possible effect of iTBS on memory recognition, 2*2*2 repeated measures ANOVA for accuracy was performed, including Group (sham/real iTBS), Time (pre/post stimulation) and Level of Processing (deep/shallow) as independent variables. Accuracy was defined as ([Hits-False Alarms]/Σencoding words) and the range of the index was between −1 and 1, where 1 was an optimal encoding.
Sociodemographic and neuropsychological data
Unpaired t-tests and Chi-square tests were performed when appropriate both for behavioral and sociodemographic data. Tests were performed using the PASW 18.0 (Statistical Package for Social Science, USA). When not specified data is presented as mean (SD), error bars represents standard errors of mean.
MRI analyses
MRI analysis was performed using tools from FSL (http://www.fmrib.ok.ac.uk/fsl/) and AFNI (http://afni.nimh.nih.gov/). Registration of functional images to a 3mm standard template was performed always immediately before group analyses using a two-step linear registration[45].
FMRI data preprocessing included removal of the first 5 volumes, motion correction, skull stripping, spatial smoothing (full width at half maximum [FWHM] = 6 mm), grand mean scaling and filtering. Task-related dataset was filtered with a high pass filter of 150′, while resting data was both high-pass and low-pass filtered (0.1 and 0.01Hz).
Resting-state analyses
From the resting preprocessed dataset, both an independent component (ICA) and an amplitude of low frequency fluctuation (ALFF) analysis were performed to assess any iTBS-related changes produced either in the connectivity of resting-state networks (RSN) or in spontaneous brain activity respectively.
Independent component analysis
A data-driven temporal concatenation ICA approach[46] as implemented in MELODIC followed by a dual-regression algorithm[47] was used to study iTBS effects on RSN connectivity. MELODIC uses probabilistic ICA to decompose 4D data into an estimated number of spatial and temporal components[48,49]. Eight components were selected as reflecting spontaneous brain fluctuation while the remaining ones were considered artifactuals after comparison with existing RSN reported in the literature (Biswal 2010, Smith 2009): the anterior default mode network (DMN), the posterior DMN, the cerebellar network, the frontal executive network, the left and right frontoparietal networks, the sensorimotor network and a visual network[50,51].
Selected components were introduced into a dual-regression analysis involving a spatial and a temporal regression for each session. A subtraction between post-iTBS and pre-iTBS was performed to obtain one map reflecting differences between sessions. Maps were registered to a template and entered into a group analysis. Comparisons were made using a voxel-wise nonparametric permutation testing[52] (5,000 permutations). Threshold-free cluster enhancement correction[53] was used and maps were thresholded at p<0.05 family-wise error (FWE).
ALFF
An analysis based on the ALFF, thought to be a measure of spontaneous intrinsic brain activity[54], was performed with resting data. Preprocessed datasets[55] were transformed to frequency domain by a fast Fourier transform in order to calculate the power spectrum. ALFF is then computed as the square root of the band-pass filtered power spectrum, and the index arises from the mean amplitude within the frequencies of interest for each voxel. Fractional ALFF[56] (fALFF), was computed by dividing the ALFF index for the sum of amplitude of the entire frequency range (0–0.25Hz). Both ALFF and fALFF values were Z-standardized and introduced in the same two-step pipeline as that described in the ICA section.
TASK-RELATED fMRI ANALYSIS
Functional task-related images were analyzed with FEAT. After the abovementioned preprocessing, a three-level general linear model (GLM)-based statistical analysis was performed for all the fMRI-task sessions.
For assessing task-related iTBS changes, we modeled the encoding deep (EncD), repeated deep (RepD), encoding shallow (EncS) and repeated shallow (RepS) task blocks as first-level regressors, which were convolved. The regressors, their temporal derivatives and realignment motion parameters were also entered into the GLM for each session[57]. In the second-level fixed-effect analysis, pre-iTBS contrasts of interest resulting from the first-level analysis (Memory Effect [Mem>Rep], LoP [Deep>Shallow] and Interaction [(EncD>RepD)>(EncsS>RepS)]) were subtracted from post-iTBS contrasts for every subject. Finally, a third-level mixed-effect groupal analysis was performed[58]. This analysis allowed the study of an iTBS state-dependent effect over Memory, Level of processing, and the interaction of Memory*LoP, focused on assessing the rTMS effect over Deep Encoding. As we found iTBS effect over Interaction contrast, the model was re-run to explore the iTBS effect on the EncD>EncS and EncD>RepD first-level contrasts, that is, separating the Interaction contrast into two simpler first-level contrasts to gain further insight in the results.
Additionally baseline, pre-stimulation, patterns of task-related activation were analyzed to investigate the specific BOLD patterns of activation of every task. First-level pre-stimulation contrasts were entered in a matrix and mixed-effects analysis was performed. In both analyses, corrections for multiple comparisons were made at cluster level using Gaussian random field theory (min Z>2.3; cluster significance p<0.05).
PPI
A post-hoc connectivity analysis was performed to test whether there was a task-dependent change in connectivity between the stimulation coordinates and the regions in which iTBS effect was found. We hypothesized that state-dependent iTBS-induced effects on deep encoding would be related to a task-dependent shift in connectivity in the pre-stimulation encoding. Psychophysiological interaction[59] (PPI) allow the study of regions that differ in connectivity by context or condition. In this regard, applying this technique, we expected increased connectivity between posterior and IFG regions during (pre-stimulation) semantic encoding as well as a correlation of the connectivity shift (PPI measure) with distal rTMS effects. For the PPI, we established five psychological regressors, consisting of the convolved task conditions plus a resting condition spanning all the experimental time[60]. In addition, a physiological BOLD signal regressor from a ROI was created in the stimulated area (5mm-radius sphere). Finally five PPI variables were created by multiplying a vector of each psychological condition with the physiological regressor. We generated PPI maps by testing the same contrasts as those mentioned in the task-related GLM analysis using instead the PPI regressors. We created a 5mm-ROI around the peak activation of the task-related Interaction contrast (MNI(x,y,z): −6, −82, −16) and examined ROI-to-ROI task-dependent change in connectivity. Finally, a correlation between pre-stimulation PPI measures and iTBS posterior changes in BOLD activity was performed to establish a relationship between both measures.
RESULTS
The sham and real iTBS groups did not differ statistically in age, gender, education, motor thresholds or performance on neuropsychological memory tests (p>0.05; Table 1).
Table 1.
Mean sociodemographic variables (standard deviations), Motor Threshold intensities and Neuropsychological memory test scores (Rey Auditory Verbal Learning Test (RAVLT) and Rey-Osterrieth Complex Figure Test (ROCF) both assessed at 30 minutes for real and sham iTBS group.
| Real iTBS (n=12) | Sham iTBS (n=12) | t/x (P) | |
|---|---|---|---|
| Age | 73.00 (6.28) | 70.08 (7.17) | 1.06 (0.300) |
| Education | 0:5:5:2 | 0:4:2:6 | 3.40 (0.183) |
| Sex | 6:6 | 6:6 | 0.00 (1.000) |
| RMT | 57.75 (7.45) | 53.08 (6.95) | 1.59 (0.127) |
| AMT | 52.83 (7.23) | 48.80 (6.06) | 1.47 (0.156) |
| RAVLT | 7.67 (3.80) | 8.08 (3.55) | −0.28 (0.784) |
| ROCF-30’ | 17.68 (5.27) | 18.18 (3.55) | 0.29 (0.776) |
Education and Sex had been tested by Chi-square tests while for the other measures unpaired t-tests were used. Education levels are classified as basic:primary:secondary:superior whereas Sex levels are men:woman.
Behavioral analysis
Mean memory performance was 0.21(0.11). The three-way repeated-measures ANOVA to assess effects on retrieval accuracy only revealed main effects of LoP (F=143.78, p<0.001) being the semantic encoded material more easily recognized than the perceptual one. No main effects of Time nor Group were found (F=1.37, p=0.258; F=1.22, p=0.281). Concerning iTBS effects, neither Time*Group (F=0.83, p=0.776) nor Time*Group*LoP interaction (F=3.22, p=0.087) effects were found, indicating that iTBS had no impact on the main behavioral memory measures (Table 2; Figure 2).
Table 2.
Behavioral descriptive results from the recognition test assessed after each MRI encoding session.
| Real iTBS (n=12) | Sham iTBS (n=12) | |
|---|---|---|
| Pre-stimulation retrieval | ||
| Deep accuracy | 0.32 (0.19) | 0.38 (0.16) |
| Shallow accuracy | 0.10 (0.09) | 0.12 (0.11) |
| Post-Stimulation retrieval | ||
| Deep accuracy | 0.31 (0.14) | 0.32 (0.14) |
| Shallow accuracy | 0.04 (0.09) | 0.14 (0.15) |
Statistical comparisons are given in the main text.
Figure 2.
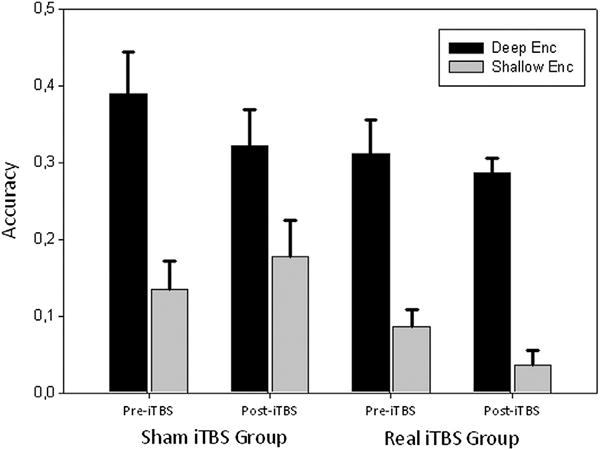
Accuracy in the recognition memory task. Only significant main effect of Level of Processing was found significant (Deep>Shallow accuracy; F=143.780, p<0.001). Error bars represent Standard Error of Mean.
Baseline fMRI task-related analysis
The Memory network (Mem>Rep) included activation of cerebellum, occipital cortex (OC), orbitofrontal cortex and left IFG and deactivation of right angular gyrus. LoP (Deep>Shallow) activation was mostly localized in the Left PFC including the IFG, the middle and the superior frontal gyrus (SFG). The paracingulate cortex (PAC) and right cerebellum were also found to be implicated in the network. The Interaction contrast ([EncD>RepD]>[EncS>RepS]; also referred as deep encoding contrast) resulted in activation patterns in the left IFG and the dorsomedial PFC, encompassing the supplementary motor area (SMA) and the SFG (Table 3, Figure 3).
Table 3.
Baseline task-related activations for the main contrasts investigated and the whole sample of subjects (N=22; see also Figure 3).
| Task | Region | Location (x,y,z) | mm3 | Z-max |
|---|---|---|---|---|
| Mem > Rep | Cerebellum/OC | 14, −98, 0 | 115,748 | 5.05 (25.80) |
| Cerebellum/OC | −44, 28, −14 | 21,856 | 4.97 (7.53) | |
| Rep > Mem | Right AG | 36, −68, 38 | 4596 | 4.02 (1.06) |
| Deep > Shallow | Left PFC/PAC | 34, −78, −40 | 42,984 | 5.39 (13.70) |
| Right cerebellum | −60, −52, −2 | 10,084 | 4.41 (4.30) | |
| Left TOC | −60, −52, −2 | 3940 | 4.19 (1.62) | |
| (EncD > RepD)> | Left IFG | 10, 10, 60 | 5068 | 3.58 (1.73) |
| (EncS > RepS) | SMA/SFG | −52, 16, 18 | 4264 | 3. (1.40) |
OC= Occipital Cortex; AG = Angular Gyrus; PFC = Prefrontal Cortex; PAC= Paracingulate Cortex TOC= Temporoccipital Cortex; IFG= Inferior Frontal Gyrus; SMA= Supplementary Motor Area; SFG= Superior Frontal Gyrus. Z-max(−log10(p)).
Coordinates are referred in MNI coordinates system.
Figure 3.
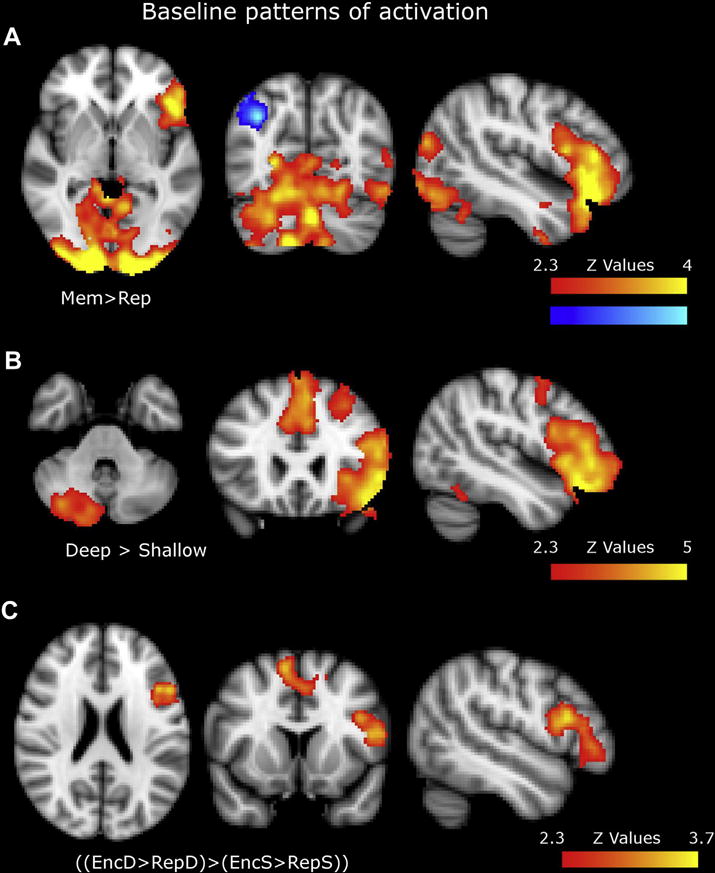
Baseline patterns of activation for a) Memory b) LoP and c) Interaction contrast task contrasts (images shown in radiological convention). For precise anatomical localizations see table 3.
Pre vs. post iTBS analyses
The following analyses were carried to test iTBS impact over neuronal networks both in task and rest context.
Resting fMRI ICA and ALFF analysis
In the eight RSN networks extracted from ICA, the FWE-corrected analysis did not reveal any significant clusters that indicated iTBS effects on the functional architecture of the brain. Even at an uncorrected level (p<0.001) no relevant clusters emerged from the analysis. Results of the ALFF analyses did not demonstrate any effect of iTBS on FWE-corrected maps neither at an uncorrected level (p < 0.001).
Task-related fMRI analysis
Task-related analysis was initially focused on studying the effect of iTBS on Memory, LoP and Interaction BOLD contrasts. Results from these analyses would indicate a state-dependent effect of iTBS for the specific contrast (i.e. results on memory contrast would reflect an specific effect of iTBS during memory compared to repeated condition). Only in the Interaction contrast a cluster emerged encompassing primary visual areas, lateral OC, ventral occipitotemporal areas and the cerebellum (Table, Figure 4). In other words, ITBS compared to sham, induced increased activation specifically for deep encoding (LoP*Memory Interaction).
Figure 4.
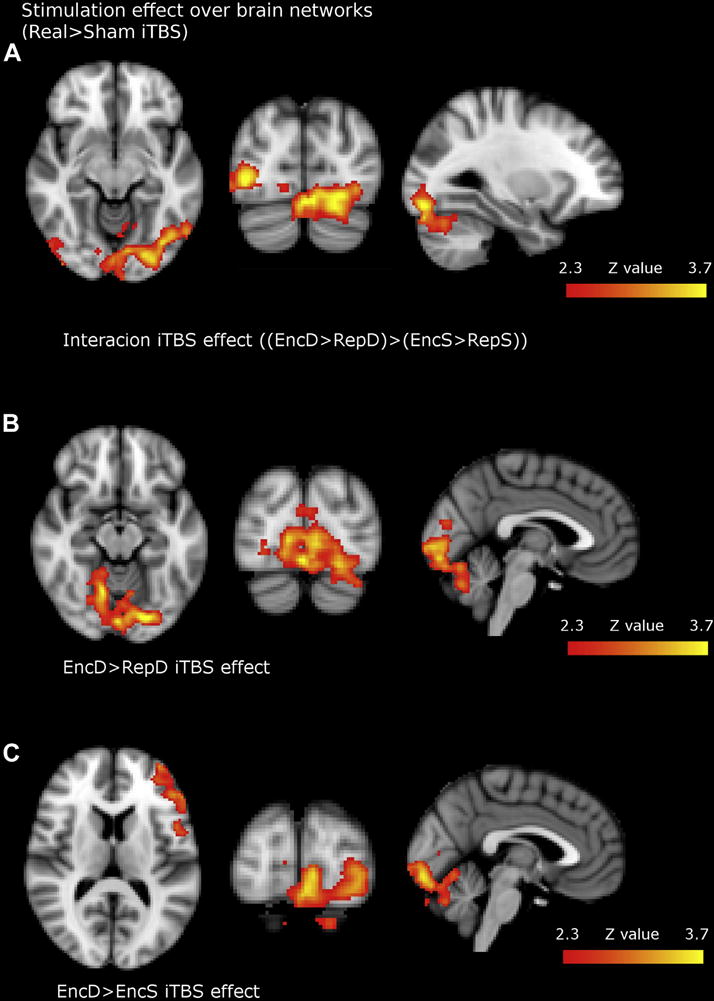
Brain regions where iTBS effects (Time*Group interaction) were found after unpaired two sample t-tests. All contrasts showed increased BOLD activity of real compared to sham group. Figure 4a) showed increased activity for Interaction (Memory*LoP) first-level contrast, namely increased activity specifically for deep encoding. Figure 4b) and 4c) represents increased activity after real iTBS for EncD>RepD and EncD>EncS task contrasts respectively (images shown in radiological convention). For precise anatomical localizations see table 4.
We additionally explored iTBS effect over EncD>RepD and EncD>EncS contrasts to confirm a more specific involvement of EncD condition in the interaction contrast (LoP*Memory) as opposite of an effect related to RepS condition. Congruently, similar clusters emerged in posterior areas for both contrasts. Additionally, the EncD>EncS contrast showed an additional cluster in the left IFG, the area underlying stimulation (Table, Figure 4). These results indicate that iTBS had an effect on brain networks specifically when subjects were performing a deep encoding task, both in local and distal areas that belong to memory encoding network. As we wanted to gain some insight regarding the mechanisms underlying rTMS modulation over posterior areas, we performed a post-hoc connectivity analysis.
PPI – relationship between baseline task-dependent connectivity and iTBS effects
PPI ROI-to-ROI analysis revealed significant changes in connectivity during the pre-iTBS encoding, in a task-dependent fashion ((EncD>RepD)>(EncS>RepS)), between the stimulated region and the region in which maximum effects of iTBS during deep encoding were observed (t=2.53, p=0.029). Hence, coupling between both areas was greater when subjects performed a deep encoding than in the other ‘psychological’ states. To note, during pre-stimulation encoding, posterior ROI was more coupled with LIFG ROI in semantic condition even when no BOLD changes were observed in posterior areas for this specific contrast (see Figure 3). Finally, PPI changes were associated with an iTBS effect on the BOLD signal in this posterior ROI (r=0.63, p=0.028) in the active group (Figure 5), but not in the sham group (r=−0.22, p=0.551). In other terms, greater task-dependent shifts in coupling between both areas in the pre-stimulation encoding correlated with greater state-dependent iTBS BOLD modulation in distal areas after stimulation.
Figure 5.
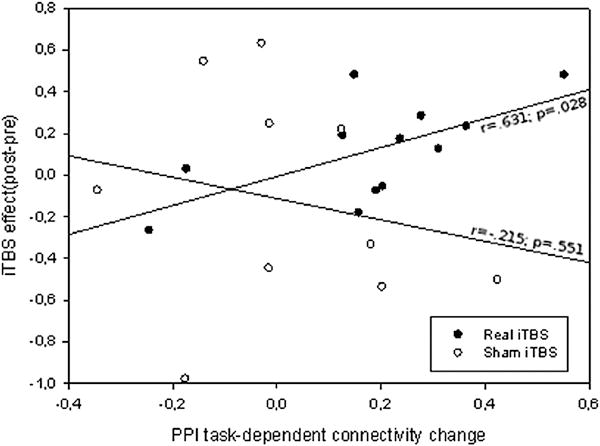
Correlation between PPI task-dependent connectivity changes at baseline and the iTBS induced effects. PPI values reflect a deep encoding specific increased connectivity (positive values) between stimulated area and a ROI localized in the area where maximum iTBS effects were found. iTBS effect reflects relative change of signal in this area induced by stimulation (arbitrary units). See main text for ROI and contrast definitions.
Discussion
We applied real or sham iTBS over the left IFG in healthy elders in order to study behavioral and brain functional changes during an episodic memory task. Main conclusions could be summarized as: 1) iTBS did not produce changes in performance in the memory task. 2) Functional brain TMS-induced changes were found specifically when subjects were performing a deep encoding task in local (left IFG) and distal areas (OC and cerebellum). 3) Areas where TMS effects were found belong to memory encoding networks. 4) TMS effects correlated with task-related connectivity shifting measures at pre-ITBS encoding task. Finally, 5) when subjects were at “resting-state” there were no stimulation changes in connectivity or in spontaneous brain activity measures.
iTBS does not produce behavioral changes in memory retrieval
Although behavioral changes were hypothesized in accordance with existing literature, lack of cognitive effect of the stimulation in our study can be explained by a number of factors. First, most of the TMS protocols reporting effects on memory employed on-line protocols[30], while off-line protocols usually involve stimulation over a number of days[3]. Second, elderly people may be more resistant to show behavioral effects. Bihemispheric[5] or contralateral stimulation may be needed in the elderly to induce behavioral effects according with literature on cognitive aging compensation processes[2,61,62]. Third, functional increases in OC and cerebellum may be unrelated to behavioral improvements since magnitude of activation it is not fundamental in remembered items, compared to forgotten ones[44,62]. Four, mechanisms modulated by rTMS in the LIFG may be more related to encoding processes that do not strongly influence subsequent retrieval in terms of recognition accuracy[29,30]. Although unexpected, absence of behavioral changes allow us to interpret brain function avoiding complications of interpreting neural changes associated to behavioral modifications[10,63,64].
iTBS did not induce resting-state brain network changes in connectivity or in spontaneous brain activity measures
Our results show that the functional architecture of the brain as measured by resting fMRI was not modified by iTBS in contrast with published literature[65–68]. However, only two previous studies examined TMS effects over large-scale resting-state networks[66,68] both modulating DMN connectivity. As the stimulated area in the present study was not clearly part of DMN or any well-established large-scale RSN, it is possible that effect of stimulation could be quite inconsistent due to subjects variability in its connectivity patterns and, thus, difficult to assess with groupal analysis. Other methodological approximations, though, such as graph-theory methods[69], seed-based analyses[70] could be more sensitive to reveal connectivity-induced changes in such conditions. ALFF analyses also failed to detect TMS effects on spontaneous activity during the resting state. Similarly, other studies that used MRI techniques failed to detect changes of activity at rest after TBS[71], suggesting that TBS may have a greater impact during a task than during resting-state conditions.
iTBS induced specific changes in BOLD signal during deep encoding task in local and distal related areas
Results strongly indicate that iTBS modulated brain network expression in a task-dependent manner. Specifically, we found that stimulation only produced differences in BOLD activity when subjects were performing a semantic encoding task while brain networks remained unaltered when subjects were at rest or doing a perceptual encoding task. Hence, LoP modulated LIFG stimulation effects in a state-dependent fashion similarly to other paradigms in TMS literature[10,72]. This task-dependent effect might be related to the regional state[31–33] of the targeted area and its functional implication during semantic encoding tasks [10,71–73] and it can be linked with recent TMS findings on the LoP theory[27]. Innocenti et al. found that deep encoding processes were interfered by the application of on-line interfering TMS over left PFC while shallow encoding processes remained unaffected during a memory task. This suggested that left PFC is a node engaged during long-term memory and crucial for semantic processing, and that TMS methods can selectively interact with semantic encoding networks. However the targeted area differed in both studies, being left DLPFC in the previous study and left IFG in ours. It has been proposed that DLPFC may be implicated in executive-monitoring processes[74] while left IFG seems to be specifically involved in phonological and semantic control[75] and semantic elaboration processes[30].
Our results are in good agreement with a role of semantic control of LIFG, suggesting that this area may selectively exert top-down modulation over posterior areas. rTMS modulations were observed in the ventral visual stream, from primary visual areas to the ventral occipitotemporal cortex (see Figure 4a), while areas included in the semantic network, such as the anterior temporal lobe[75,76], remained unaffected. Moreover, modulation was observed in relatively unspecific areas, hierarchically located before visual areas that are selectively involved in word and semantic recognition[77,78] such as the visual word form area. However, early visual activity has been shown to be modulated by semantic properties such as non-predictive semantic cues[79], semantic priming paradigms[80], bilingual literacy[81], age of acquisition[82], or shifting in attention to semantic category[83]. Since these areas do not specifically respond to semantic properties of the stimuli, results were generally interpreted as evidences of top-down modulations. Further, EEG studies reported early sensitivity of posterior areas to semantics[84,85] before the widely described N400 evoked-related potential linked to semantic processing emerges[86]. However, off-line TMS and fMRI does not allow extracting conclusions of such temporal resolution, so we cannot ascertain whether IFG modulation over posterior regions reflects an early attentional shift towards facilitating deep encoding, such as the ones proposed by cuing paradigms or instead it reveals a continuous feedforward network that leads to enhanced recognition during semantic encoding[82]. Involvement of LIFG in encoding during a wide temporal window[29,30,87] in chronometric TMS approaches suggest a continuous involvement of prefrontal areas during semantic processing.
Interindividual changes in network connectivity with increasing task demands at baseline predict iTBS modulation of deep memory encoding networks
Our results showed a correlation of IFG-posterior coupling shifts towards deep encoding condition and task-dependent rTMS modulation. This result strengthens the idea of a semantic-selective top-down modulation of IFG to posterior areas probably through Inferior Fronto-Occipital Fascicle[88,89], a bundle that connects both areas. Relevance of connectivity parameters as a factor of variability in rTMS has been observed in several studies[14]. Both, connectivity from targeted region to rTMS distal modulations and brain intrinsic connectivity dynamics have been shown to modulate stimulation responses [10,12,13,90]. In our study, the PPI analyses revealed that the amount of posterior modulation is mediated by individual’s subject capacity to shift between psychological contexts. The influence of IFG to posterior areas is not static and fixed but dynamically changes with different task conditions (in our study, depending on semantic and encoding demands) as suggested in other elegant studies that modulated activity in visual processing areas after IFG stimulation[64,91,92] in a content and context specific manner. PPI analysis does not measure coupling but instead examines connectivity shifts in interaction with different psychological contexts. Although “absolute” connectivity patterns have been usually used in conjunction with rTMS, PPI measures may represent an appropriate approximation when considering task-dependent modulations of TMS. However, more research is needed in order to know whether absolute or relative patterns better predict TMS effect[36,39][93].
Regarding aging, the recruitment of networks were highly constrained by the specific task-demands on each condition which probably eliminated a differential engagement (compared to young subjects) of effective learning strategies which are known to be affected during intentional encoding in aging[36,39]. Experimentally establishing encoding strategies, has shown to minimize memory decrements in aging. Also, age-related functional changes seem to consist on task-related decreased deactivations and overrecruitment of additional areas while role of primary cognitive networks (those engaged in young subjects) usually remains partially preserved. Still, it is not possible to rule-out the possibility that age-related changes over networks are conditioning our results, similarly to other paradigms[26,94]. In the future, a young group and an intentional encoding condition should be considered, as well as stimulation over overrecruited areas, to fully explore the role of left IFG over memory encoding and its subsequence age-related changes. Our results, in conjunction with Innocenti et al.[27], highlight the relevance of combining stimulation within a specific strategy of encoding in order to obtain functional modifications of memory networks. These results have to be seriously taken into account in future studies involving both cognitive and transcranial stimulation. In conclusion, these results highlight the role of the left IFG in semantic encoding, probably by exerting top-down modulation effects, enforce the idea of task-dependency for higher cognitive functions, and emphasize the role of connectivity in order to understand TMS-induced changes.
Table 4.
Brain regions where iTBS effects (Time*Group interaction) were found (see also Figure 4).
| Task | Region | Location (x,y,z) | mm3 | Z-max |
|---|---|---|---|---|
| ((EncD > RepD) > (EncS > RepS)) | Cerebellum/OC | −6, −82, −16 | 15,532 | 3.87 (5.44) |
| EncD > RepD | Cerebellum/OC | −20, −82, −16 | 15,720 | 3.98 (6.53) |
| EncD > EncS | Cerebellum/OC | 6, −88, −30 | 22,692 | 3.84 (8.13) |
| Left IFG | −46, 34, 10 | 6668 | 3.42 (2.80) |
OC= Occipital Cortex; IFG= Inferior Frontal Gyrus. Z-max(−log10(p)).
Coordinates are referred in MNI coordinates system.
Acknowledgments
We are indebted to the IDIBAPS Medical Imaging Core Facility for technical help.
Financial disclosures: This work was supported by a research grant from the Spanish Ministerio de Economia y Competitividad (PSI2012-38257) to Dr. David Bartrés-Faz. Dr. Alvaro Pascual-Leone was supported by a grant from the National Institutes of Health – Harvard Clinical and Translational Science Center/Harvard Catalyst (UL1 RR025758).
Footnotes
Conflicts of Interest
Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI).
References
- 1.Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65:405–15. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm Vienna Austria 1996. 2010;117:105–22. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka S, Sandrini M, Cohen LG. Modulation of motor learning and memory formation by non-invasive cortical stimulation of the primary motor cortex. Neuropsychol Rehabil. 2011;21:650–75. doi: 10.1080/09602011.2011.605589. [DOI] [PubMed] [Google Scholar]
- 5.Solé-Padullés C, Bartrés-Faz D, Junqué C, Clemente IC, Molinuevo JL, Bargalló N, et al. Repetitive transcranial magnetic stimulation effects on brain function and cognition among elders with memory dysfunction. A randomized sham-controlled study. Cereb Cortex N Y N 1991. 2006;16:1487–93. doi: 10.1093/cercor/bhj083. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71:493–8. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 7.Cotelli M, Manenti R, Rosini S, Calabria M, Brambilla M, Bisiacchi PS, et al. Action and Object Naming in Physiological Aging: An rTMS Study. Front Aging Neurosci. 2010;2:151. doi: 10.3389/fnagi.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2011;82:794–7. doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MFS. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–90. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, et al. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex N Y N 1991. 2008;18:1281–91. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–54. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Lee TG, D’Esposito M. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J Neurosci Off J Soc Neurosci. 2012;32:15458–66. doi: 10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cárdenas-Morales L, Volz LJ, Michely J, Rehme AK, Pool E-M, Nettekoven C, et al. Network Connectivity and Individual Responses to Brain Stimulation in the Human Motor System. Cereb Cortex N Y N. 1991;2013 doi: 10.1093/cercor/bht023. [DOI] [PubMed] [Google Scholar]
- 14.Buch ER, Johnen VM, Nelissen N, O’Shea J, Rushworth MFS. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J Neurosci Off J Soc Neurosci. 2011;31:17669–79. doi: 10.1523/JNEUROSCI.1513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheeran BJ, Ritter C, Rothwell JC, Siebner HR. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009;164:156–63. doi: 10.1016/j.neuroscience.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 17.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 18.Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–84. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 19.Stagg CJ, O’Shea J, Johansen-Berg H. Imaging the effects of rTMS-induced cortical plasticity. Restor Neurol Neurosci. 2010;28:425–36. doi: 10.3233/RNN-2010-0553. [DOI] [PubMed] [Google Scholar]
- 20.Reithler J, Peters JC, Sack AT. Multimodal transcranial magnetic stimulation: using concurrent neuroimaging to reveal the neural network dynamics of noninvasive brain stimulation. Prog Neurobiol. 2011;94:149–65. doi: 10.1016/j.pneurobio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Morgan HM, Jackson MC, van Koningsbruggen MG, Shapiro KL, Linden DEJ. Frontal and parietal theta burst TMS impairs working memory for visual-spatial conjunctions. Brain Stimulat. 2013;6:122–9. doi: 10.1016/j.brs.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Restle J, Murakami T, Ziemann U. Facilitation of speech repetition accuracy by theta burst stimulation of the left posterior inferior frontal gyrus. Neuropsychologia. 2012;50:2026–31. doi: 10.1016/j.neuropsychologia.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Volman I, Roelofs K, Koch S, Verhagen L, Toni I. Anterior prefrontal cortex inhibition impairs control over social emotional actions. Curr Biol CB. 2011;21:1766–70. doi: 10.1016/j.cub.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 25.Sandrini M, Cappa SF, Rossi S, Rossini PM, Miniussi C. The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J Cogn Neurosci. 2003;15:855–61. doi: 10.1162/089892903322370771. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci Off J Soc Neurosci. 2004;24:7939–44. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innocenti I, Giovannelli F, Cincotta M, Feurra M, Polizzotto NR, Bianco G, et al. Event-related rTMS at encoding affects differently deep and shallow memory traces. NeuroImage. 2010;53:325–30. doi: 10.1016/j.neuroimage.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Köhler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: a two-stage fMRI-rTMS study. J Cogn Neurosci. 2004;16:178–88. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- 29.Kahn I, Pascual-Leone A, Theoret H, Fregni F, Clark D, Wagner AD. Transient disruption of ventrolateral prefrontal cortex during verbal encoding affects subsequent memory performance. J Neurophysiol. 2005;94:688–98. doi: 10.1152/jn.01335.2004. [DOI] [PubMed] [Google Scholar]
- 30.Machizawa MG, Kalla R, Walsh V, Otten LJ. The time course of ventrolateral prefrontal cortex involvement in memory formation. J Neurophysiol. 2010;103:1569–79. doi: 10.1152/jn.90937.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci U S A. 1994;91:2008–11. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 33.Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain J Neurol. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- 34.Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. J Verbal Learn Verbal Behav. 1972;11:671–84. [Google Scholar]
- 35.Craik F, Tulving E. Depth of processing and the retention of words in episodic memory. J Exp Psychol Gen. 1975;104:268–94. [Google Scholar]
- 36.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–40. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 37.Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, et al. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- 38.Kalpouzos G, Chételat G, Landeau B, Clochon P, Viader F, Eustache F, et al. Structural and metabolic correlates of episodic memory in relation to the depth of encoding in normal aging. J Cogn Neurosci. 2009;21:372–89. doi: 10.1162/jocn.2008.21027. [DOI] [PubMed] [Google Scholar]
- 39.Kirchhoff BA, Anderson BA, Barch DM, Jacoby LL. Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb Cortex N Y N 1991. 2012;22:788–99. doi: 10.1093/cercor/bhr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peña-Casanova J, Blesa R, Aguilar M, Gramunt-Fombuena N, Gómez-Ansón B, Oliva R, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): methods and sample characteristics. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2009;24:307–19. doi: 10.1093/arclin/acp027. [DOI] [PubMed] [Google Scholar]
- 41.Strauss EM, Carone DA, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Appl Neuropsychol. 2007;14:62–3. [Google Scholar]
- 42.Gallés NS, Vega FC, Valiña MFC, Antonin MAM. Lexesp-Lexico Informatizado Del Español. Publicacions i Edicions de la Universitat de Barcelona; 2000. [Google Scholar]
- 43.Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- 44.Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–61. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 45.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 46.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minka TP. Automatic choice of dimensionality for PCA. 2000 [Google Scholar]
- 49.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 50.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 54.Li Z, Zhu Y, Childress AR, Detre JA, Wang Z. Relations between BOLD fMRI-derived resting brain activity and cerebral blood flow. PloS One. 2012;7:e44556. doi: 10.1371/journal.pone.0044556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–41. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 58.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 59.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 60.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 62.Spaniol J, Grady C. Aging and the neural correlates of source memory: over-recruitment and functional reorganization. Neurobiol Aging. 2012;33:425 e3–18. doi: 10.1016/j.neurobiolaging.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Josephs O, Deichmann R, et al. Studying the role of human parietal cortex in visuospatial attention with concurrent TMS-fMRI. Cereb Cortex N Y N 1991. 2010;20:2702–11. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feredoes E, Heinen K, Weiskopf N, Ruff C, Driver J. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc Natl Acad Sci U S A. 2011;108:17510–5. doi: 10.1073/pnas.1106439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci Off J Soc Neurosci. 1997;17:3178–84. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van der Werf YD, Sanz-Arigita EJ, Menning S, van den Heuvel OA. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci. 2010;11:145. doi: 10.1186/1471-2202-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44:725–31. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A. 2011;108:21229–34. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polanía R, Paulus W, Antal A, Nitsche MA. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. NeuroImage. 2011;54:2287–96. doi: 10.1016/j.neuroimage.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 70.Esslinger C, Schüler N, Sauer C, Gass D, Mier D, Braun U, et al. Induction and quantification of prefrontal cortical network plasticity using 5 Hz rTMS and fMRI. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cárdenas-Morales L, Grön G, Kammer T. Exploring the after-effects of theta burst magnetic stimulation on the human motor cortex: a functional imaging study. Hum Brain Mapp. 2011;32:1948–60. doi: 10.1002/hbm.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sack AT, Kohler A, Bestmann S, Linden DEJ, Dechent P, Goebel R, et al. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex N Y N 1991. 2007;17:2841–52. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- 73.Bestmann S, Baudewig J, Frahm J. On the synchronization of transcranial magnetic stimulation and functional echo-planar imaging. J Magn Reson Imaging JMRI. 2003;17:309–16. doi: 10.1002/jmri.10260. [DOI] [PubMed] [Google Scholar]
- 74.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–48. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 75.Jefferies E. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex J Devoted Study Nerv Syst Behav. 2013;49:611–25. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex N Y N 1991. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song Y, Tian M, Liu J. Top-down processing of symbolic meanings modulates the visual word form area. J Neurosci Off J Soc Neurosci. 2012;32:12277–83. doi: 10.1523/JNEUROSCI.1874-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain J Neurol. 2002;125:1054–69. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- 79.Goffaux V, Martin R, Dormal G, Goebel R, Schiltz C. Attentional shifts induced by uninformative number symbols modulate neural activity in human occipital cortex. Neuropsychologia. 2012;50:3419–28. doi: 10.1016/j.neuropsychologia.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 80.Kherif F, Josse G, Price CJ. Automatic top-down processing explains common left occipito-temporal responses to visual words and objects. Cereb Cortex N Y N 1991. 2011;21:103–14. doi: 10.1093/cercor/bhq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szwed M, Qiao E, Jobert A, Dehaene S, Cohen L. Effects of Literacy in Early Visual and Occipitotemporal Areas of Chinese and French Readers. J Cogn Neurosci. 2013 doi: 10.1162/jocn_a_00499. [DOI] [PubMed] [Google Scholar]
- 82.Urooj U, Cornelissen PL, Simpson MIG, Wheat KL, Woods W, Barca L, et al. Interactions between visual and semantic processing during object recognition revealed by modulatory effects of age of acquisition. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 83.Çukur T, Nishimoto S, Huth AG, Gallant JL. Attention during natural vision warps semantic representation across the human brain. Nat Neurosci. 2013;16:763–70. doi: 10.1038/nn.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pulvermüller F, Assadollahi R, Elbert T. Neuromagnetic evidence for early semantic access in word recognition. Eur J Neurosci. 2001;13:201–5. doi: 10.1046/j.0953-816x.2000.01380.x. [DOI] [PubMed] [Google Scholar]
- 85.Moseley RL, Pulvermüller F, Shtyrov Y. Sensorimotor semantics on the spot: brain activity dissociates between conceptual categories within 150 ms. Sci Rep. 2013;3:1928. doi: 10.1038/srep01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu Rev Psychol. 2011;62:621–47. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grafman J, Pascual-Leone A, Alway D, Nichelli P, Gomez-Tortosa E, Hallett M. Induction of a recall deficit by rapid-rate transcranial magnetic stimulation. Neuroreport. 1994;5:1157–60. doi: 10.1097/00001756-199405000-00034. [DOI] [PubMed] [Google Scholar]
- 88.Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex J Devoted Study Nerv Syst Behav. 2010;46:691–9. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 89.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Elsevier; 2005. [Google Scholar]
- 90.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Higo T, Mars RB, Boorman ED, Buch ER, Rushworth MFS. Distributed and causal influence of frontal operculum in task control. Proc Natl Acad Sci U S A. 2011;108:4230–5. doi: 10.1073/pnas.1013361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–61. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bilek E, Schäfer A, Ochs E, Esslinger C, Zangl M, Plichta MM, et al. Application of high-frequency repetitive transcranial magnetic stimulation to the DLPFC alters human prefrontal-hippocampal functional interaction. J Neurosci Off J Soc Neurosci. 2013;33:7050–6. doi: 10.1523/JNEUROSCI.3081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke J Cereb Circ. 2012;43:2185–91. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]


