Abstract
The high prevalence of sexual reproduction is considered a paradox mainly for two reasons. First, asexuals should enjoy various growth benefits because they seemingly rid themselves of the many inefficiencies of sexual reproduction—the so-called costs of sex. Second, there seems to be no lack of asexual origins because losses of sexual reproduction have been described in almost every larger eukaryotic taxon. Current attempts to resolve this paradox concentrate on a few hypotheses that provide universal benefits that would compensate for these costs and give sexual reproduction a net advantage. However, are new asexual lineages really those powerful invaders that could quickly displace their sexual ancestors? Research on the costs of sex indicates that sex is often stabilized by highly lineage-specific mechanisms. Two main categories can be distinguished. First are beneficial traits that evolved within a particular species and became tightly associated with sex (e.g., a mating system that involves sexual selection, or a sexual diapausing stage that allows survival through harsh periods). If such traits are absent in asexuals, simple growth efficiency considerations will not capture the fitness benefits gained by skipping sexual reproduction. Second, lineage-specific factors might prevent asexuals from reaching their full potential (e.g., dependence on fertilization in sperm-dependent parthenogens). Such observations suggest that the costs of sex are highly variable and often lower than theoretical considerations suggest. This has implications for the magnitude of universal benefits required to resolve the paradox of sex.
Keywords: evolution of sex, genetic variation, mating systems, clonality, parthenogenesis
The dominance of sex as a reproductive mode in multicellular eukaryotes constitutes a paradox that has stimulated an enormous amount of research (1–3). Most of this research directly or indirectly addresses the question why most organisms shuffle their genetic information instead of just producing exact copies of themselves. This question has spurred many hypotheses to identify the mechanisms that might stabilize the sexual reproductive mode against recurrent asexual invasions. The costs of sex play a central role in the paradox because they define the gap between expected and observed prevalence of sex. In addition, the identification of individual-level costs has prompted researchers to find mechanisms for the maintenance of sex that provide a significant advantage and operate in the short term (2, 3).
Most of these sexual costs rely on simple, model-based or qualitative considerations or on presumed differences in fitness between sexuals and asexuals (for a recent summary, see ref. 4). For example, sexuals and asexuals may differ in the amount of resources they invest into male production, or the time, energy, and risk exposure they take to engage in sexual activities. This has led to the situation that costs are usually assumed to exist and to be significant. In fact, the existence of substantial individual-level costs of sexual reproduction is established to the degree of a dogma. In this study, I will examine whether this is justified. I will examine the various costs of sex, their underlying assumptions, and possible violations due to biological or ecological details of the species involved. I will mainly view “costs of sex” as the benefits an asexual invader gains by skipping sexual processes and activities.
There is some inconsistency in the literature regarding the use of the term “asexuality.” In the most general sense, asexuality can be understood as the negation of sexuality. However, the exact meaning depends on whether one adopts a loose definition, such as “some sexual elements missing” vs. a strict definition, such as “all sexual elements missing.” For instance, according to the loose definition an automictic parthenogen would be considered an asexual organism, because it strongly deviates from a pattern of “normal (outbreeding) sex with two parents.” If one adheres to the tight definition of asexuality, automictic parthenogens would not be considered asexual because they still have an element of sex (meiotic recombination). In this study I will adopt a loose definition of asexuality. That is, I will not only consider organisms that are strictly clonal, but also include those cases of uniparental reproduction where a small amount recombination may be still present.
Universal vs. Lineage-Specific Factors
A major problem in evolutionary biology is identifying the benefits of sex that are responsible for its maintenance in most eukaryotic species, or in other words, the factors that cause the failure of most asexual invasions at an early stage. It is useful to distinguish between universal and lineage-specific factors in this context. Universal factors apply to all sexual species. For example, recombination due to the mechanisms of random segregation or crossing over is a universal feature shared by all sexual eukaryotes. In population genetic terms, recombination can reduce linkage disequilibrium because it breaks up associations between alleles of different loci. Depending on the exact pattern of the underlying gene combinations, recombination can be either beneficial or detrimental.
Universal features of sexual species, such as recombination, are attractive starting points for hypotheses trying to resolve the paradox of sexual reproduction with a single, universal explanation. For example, according to the drift-based explanation of the maintenance of sex, most finite populations should exhibit an excess of genotypes in which beneficial alleles are tied to deleterious genetic backgrounds [owing to the Hill–Robertson effect (5)]. Under such conditions, recombination has usually beneficial effects (6, 7). The drift-based explanation tolerates a wide range in parameter space, such as different types of epistasis, large differences in population size, or different forms of selection (8). Thus, the beneficial effects of recombination in such a setting may serve as a paradigm for a universal factor that could explain why high rates of sex are maintained in most eukaryotic species. However, not all features of a sexual species are universal.
Most features are the result of long-term evolution of sexuality within a lineage. For example, coevolutionary processes between males and females, such as sexual selection or sexual conflict, can give rise to morphological or behavioral traits that are highly lineage-specific. Nevertheless, even though the evolutionary path might be unique for each lineage, there is ultimately a limited set of such features as they may exhibit patterns of convergent evolution (e.g., sexual selection may have evolved independently in different lineages). Such lineage-specific features can act as powerful factors in the maintenance of sex because they may affect the cost of sex both in positive and negative direction. In extreme cases, a lineage-specific benefit of sex may neutralize any preexisting costs, which would mean that a universal explanation for the maintenance of sex would not be required for this lineage.
Asexual Invasions
Theoretical considerations of sexual costs usually involve an invasion scenario, that is, the competition between an initially rare asexual mutant and its sexual relatives (2, 3). Because asexuality is a derived state in all eukaryotes (9), each newly formed asexual lineage can be traced back to one sexual ancestor (or two, in case of hybrid origin). This invasion scenario deviates from the framework of many theoretical models on the maintenance of sex, which are the so-called modifier models and assume gradual changes in recombination rates. In these models, an allele that suppresses recombination completely is only considered as a special case (8). However, the invasion scenario is well in agreement with empirical observations of the origins of asexuality, which commonly involve all-or-nothing events (10).
The invasion scenario provides two complementary ways to look at costs of sex, the perspective of a sexual resident and that of an asexual invader. Defining the costs of sex from the resident focuses on compensatory benefits of sex. For example, a twofold cost of sex implies that the benefits of sex should allow a sexual resident to produce at least twice as many offspring to resist an asexual invader. Defining the costs of sex from the perspective of an asexual invader focuses on the fitness advantage gained by skipping sexual reproduction. This requires that the fitness advantage be measured in the absence of any universal benefits of sex (e.g., in a benign, simple, and constant laboratory environment). Here I will adopt the invader’s perspective and focus on the following questions: (i) Will an asexual invader outcompete the sexual residents? (ii) Which factors will facilitate, restrict, or prevent its spread? (iii) When (at which stage) can asexual invasions fail?
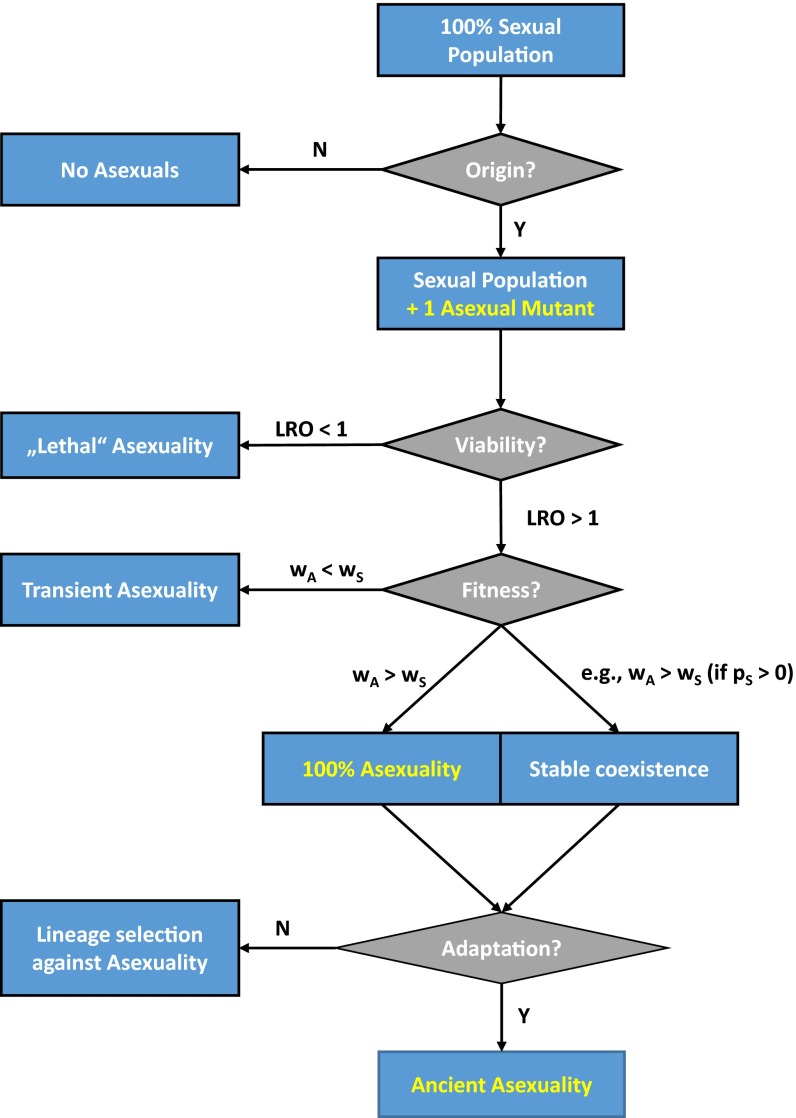
An asexual invasion can be considered successful if the asexual mutant permanently replaced its direct sexual ancestor or if both reproductive types reach a point of stable long-term coexistence. Fig. 1 briefly summarizes the steps of a successful asexual invasion—or, conversely, the points at which an invasion might fail or halt. The first limiting step in an invasion is an origin of asexuality. In fact, in several taxa intrinsic constraints seem to prevent a natural origin of asexual reproduction (11). The second limiting step is that asexuals are at least viable enough to reproduce themselves across all possible environments. If this were not the case, it would be irrelevant to consider competition between asexuals and their sexual ancestors. For example, if asexuality involves automixis or gamete duplication and if such asexuals derive from a normally outbreeding sexual population, most offspring will have extremely low fitness because deleterious recessive alleles become homozygous.
Fig. 1.
Stages of asexual invasions. LRO, lifetime reproductive output; N, no; p, frequency; w, fitness; Y, yes.
There are several scenarios in which asexuals compete against sexuals. First, asexuality may be transient, if asexuals are globally less fit than sexuals (geometric mean fitness wA < wS). In this case the invasion will ultimately fail, but locally or temporarily there may be situations in which asexuals have a competitive edge. For example, if asexuals are competitively superior under benign conditions, but have a low probability of surviving through harsh periods, they might be temporarily favored, but in the long term they will die out. Second, asexual invasions may reach a point of stable coexistence between sexuals and asexuals. This may be the case if the fitness of asexuals depends on a certain frequency of sexuals, such as in sperm-dependent parthenogens (which depend on a sexual host for reproduction and must live in sympatry with their sperm donor). Third, if asexuals are globally fitter than sexuals (geometric mean fitness wA < wS) they should ultimately displace their sexual ancestors. Nevertheless, an asexual invasion might ultimately fail, if asexuals are not able to deal with challenges that recur on longer time scales. In this case, lineage selection can result in an equilibrium of origin and long-term extinction of asexuals (12, 13). For instance, asexuals will be doomed if they are unable to withstand accumulation of mutations, or if they are unable to generate sufficient genetic variation to adapt to changes in their environment. “Ancient asexuals” are hypothesized to possess the traits to deal with such long-term challenges. Bdelloid rotifers, one of the best examples of ancient asexuality, show some characteristics that might be interpreted as alternative forms of genetic exchange (14, 15), exceptional DNA repair mechanisms (16), and nongenetic means of evading fungal parasites (17).
Common Costs of Sex
Costs of sex can be conceptualized based on simple, model-based, or qualitative considerations or about presumed differences in fitness between sexuals and asexuals. I will focus on the assumptions of these theoretical models and on how well these assumptions may be met (or violated) in real organisms (Table 1). The combined cost of sex will consist of several, but not all, of the costs listed below.
Table 1.
Common costs of sex and their sources of variation
| Cost | Assumption | Violation | Example of violation | Refs. | Effect |
| Cost of males | Males contribute only genetic information | Males provide extra benefits | Paternal care, males provide direct benefits to females | 18 | ↓ |
| Males interfere with female fitness | Sexual conflict | 19, 20 | ↑ | ||
| Genetic information provided by males is of average quality | Males carry sexually antagonostic genes | Intersexual ontogenetic conflict | 22 | ↑ | |
| Males provide genes with above average fitness | “Good genes” sexual selection | 21 | ↓ | ||
| 1:1 sex ratio | Female-biased sex ratio | Local mate competition | 23 | ↓ | |
| “All else is equal” | Asexuals have lower lifetime reproduction | Automixis in previously outbreeding population | 11 | ↓ | |
| Asexuals have higher lifetime reproduction | Heterosis in asexuals of hybrid origin | 31 | ↑ | ||
| Cost of genome dilution | Gene pool of asexuals still “connected” to sexuals | Asexuals have 'female-only' populations | Most species with separate sexes | 32, 33 | ↓ |
| Viable males or male function | Fertility of asexual males is compromised | Distorted meiosis produces inviable pollen in asexual plants | 36 | ↓ | |
| Allocation to male function 50% | Allocation to male function <50% | Hermaphrodites with reduction in male function | 23 | ↓ | |
| Costs of recombination | Population highly adapted to environment | Adaptation prevented by rapidly changing environment | Environmental changes, migration, coevolutionary arms races | ↓ | |
| Adaptation is mainly due to nonadditive gene interactions | Adaptation is mainly due to additive gene interactions | Sexual ancestor had sex every generation (vs. occasional sex) | 40 | ↓ | |
| Asexuals lack recombination | Asexuality involves some sort of recombination | Automictic pathenogenesis in a previously outbreeding sexual population | 11 | ↓ | |
| Costs of mating | Sexuals invest 100% into mating activities, asexuals 0% | Asexuals still engage in mating activities or have costly physical structures for mating | Showy flowers in asexual plants | 47 | ↓ |
| Dependence on fertilization (gynogenesis, hybridogenesis) | 48 | ↓ | |||
| Sexual males avoid fertilizing asexuals (which depend on it) | 48 | ↑ | |||
| Cellular mechanical cost of meiosis | Minimum duration of meiotic processes limits generation time | Long generation times (relative to meiosis) | Most multicellular organisms have long development times, or egg-to-egg times, relative to the time needed for meiosis | 50 | ↓ |
The Cost of Males.
The cost of males is essentially a problem of resource allocation. Consider a female that uses its resources to produce exactly two offspring. Under equal sex allocation, half of these offspring are males, whose only contribution is the genetic information provided at the time of fertilization. An asexual female would thus have a significant reproductive advantage, and if both reproductive forms compete for the same resources, clonal females should rapidly displace all sexual females. Given a set of assumptions, the “cost of males” model predicts a twofold advantage for an asexual mutant.
The first assumption is that males contribute nothing to the offspring except their genetic material. This assumption is often violated, for example, in species that have paternal care, or if males provide other benefits (18). It is also violated in the case of sexual conflict. In this case, males exert a negative impact on females by preventing them from reaching their full lifetime-reproductive output (19). Sexual conflict can theoretically elevate the cost of males above the level of twofold (20). There is one caveat, though: Sexual conflict is only relevant as a factor in the cost of sex, if asexual females do not suffer from such costs. This requires that males be able to discriminate against asexual females. Although it should be adaptive for males to ignore asexual females, it is questionable that this condition would be given at an early stage of an asexual invasion. The quality of the genetic material provided by the males is also important. For example, sexual selection may enable females to choose a male with an above-average genetic constitution. Thus, breeding systems in which males provide genetic benefits may effectively compensate for the cost of male production (21). The converse may apply in the case of intersexual ontogenetic conflict (IOC) (22), which arises when alleles are expressed in both sexes but are selected diametrically between the sexes (i.e., sexually antagonistic alleles). Similar to other forms of sexual conflict, IOC can lead to fitness impairments in females that carry many or particularly large-effect “male-benefit” alleles. IOC may elevate the cost of sex, for example, if an asexual lineage originates by chance in a female with a lower number of male-benefit alleles than the mean of the population. The second assumption is that exactly 50% of the offspring are males. This may also be violated; for example, local mate competition in some species can lead to highly female-biased sex ratios, which reduces the cost of males (e.g., ref. 23). The third assumption means that essentially all fitness-related traits should be equivalent in sexual vs. asexual females (i.e., both reproductive types should have the same number of offspring, the same quality of offspring, etc.). Several studies have provided tests of this assumption by comparing the life histories of sexuals and asexuals in species where both forms occur. In some studies, asexuals and sexuals had similar fecundity and survival schedules (24–26), implying that this assumption about the cost of males is fulfilled. However, in many other studies, asexual lines actually had lower fitness than sexuals, either due to higher mortality (27, 28) or due to reduced fecundity (29, 30). Thus, in the latter cases the cost of sex will be strongly reduced. The assumption of equal life-history patterns might also be violated as a consequence of the mechanism of asexuality. For example, if asexuality is the result of a hybridization event, heterosis might influence fitness-related traits and might thus result in a higher lifetime reproduction of asexuals (31).
The Cost of Genome Dilution.
Genome dilution, according to its original definition (3), refers to the fact that asexual mothers transmit all their genes to their offspring whereas sexual mothers transmit only half. There has been some controversy about how this contributes to the cost of sex (32, 33). Meanwhile, it has been recognized that genome dilution can be highly relevant in the context of asexual hermaphrodites, but that it does not apply in most species with separate sexes (4). The arguments are as follows. For genome dilution to be a cost of sex it is only relevant that a gene for sex is replaced by a gene that codes for asex. Dilution at other parts of the genome is irrelevant, because the crucial question is whether the reproductive system allows “asexuality genes” to reenter the sexual gene pool. This can be mediated, for instance, by functional males or functional male organs in the asexuals. Thus, the cost of genome dilution does not apply in situations in which asexuals form all-female populations, because these are instantly separated from the sexual gene pool. Lehtonen et al. (4) illustrated these points with several examples (e.g., an asexual origin involving a dominant meiosis-suppressing allele, or a recessive loss-of-sex allele). Theoretical models have shown that there is a one-and-a-half–fold cost of genome dilution in a hermaphroditic species competing against its asexual counterpart (34). In the case of a dominant allele for asexuality A2 this value arises as follows: An asexual hermaphrodite (genotype A1A2) with the same male fertility as a sexual hermaphrodite (A1A1) will transfer an average of half a A2 copy via its male gametes, whereas each offspring produced via its ameiotic eggs will contain one copy of A2 (34).
One assumption of such models is that allocation to male function in the hermaphrodite is exactly 50%. This is equivalent to an absence of any cost of male production. If this assumption is violated (e.g., male allocation is reduced), the cost of males reappears and a mixture of both costs applies (35). The second assumption is that there is indeed a transfer of asexuality genes into sexual populations. This requires functional male organs that produce viable sperm or pollen. For example, most seed plants are hermaphrodites. Asexual lineages in plants may originate via the two basic mechanisms, apomixis (i.e., asexual seed production) and vegetative propagation. Transitions to apomixis usually involve a ploidy elevation, such that asexuals are triploid or of higher ploidy. It has been argued that this will inevitably lead to a highly distorted gametogenesis and thus to a very low proportion of functional pollen (e.g., ref. 36). In the case of vegetative reproduction, transitions to obligate asex often involve sterility mutations (37). Here, genome dilution might depend on the exact mechanism by which sex is lost. If the male function is uncompromised, such mutations could theoretically be transferred to sexual populations. For example, loss of sex in clonal populations of the plant Decodon verticillatus is mainly caused by a defect in female function (38): Pistils of clonal plants do not support normal pollen tube growth, and thus propagation through seeds is minimal. However, these plants still produce considerable amounts of pollen, which could transfer the sterility mutation to sexual plants. Ultimately, however, ecological constraints are likely to prevent this kind of gene flow. First, because asexual plants often occupy marginal habitats (39), they might be spatially separated from sexual populations. Second, even in those cases where sexuals and asexuals co-occur in the same (marginal) habitat, adverse environmental conditions and/or lack of pollinator activity might suppress sexual activities in both reproductive types. A consequence of these violations is that the cost of genome dilution in such systems will be much lower than its theoretical expectation.
The Cost of Recombination.
Recombination is common to all sexual organisms. Mechanistically, chromosomal crossover allows for recombination among homologous loci between the paired chromosomes inherited from both parents. In population genetic terms, this process leads to a randomization of allele frequencies and brings their distributions closer to linkage equilibrium. Whether this process is beneficial or detrimental depends on the exact circumstances. For example, meiotic recombination can lead to a cost of sex if its net effect consists in breaking up favorable combinations of genes built by past selection. If an individual is highly adapted to its environment, some of its adaptedness will be due to epistatic gene interactions. Recombination will destroy these combinations to a greater extent than it will create new beneficial ones. This will lead to the reduction of mean fitness in sexually vs. asexually derived offspring. Although this issue is relevant to all sexual populations, it is even more severe in species that infrequently engage in sex, for example cyclical parthenogens, because intermittent phases of clonal propagation can greatly exaggerate genetic disequilibrum (40). Thus, in such situations, an apomictic asexual mutant should enjoy a benefit, because its gene combinations will be 100% transferred to the next generation.
One assumption in the cost of recombination model is that asexuals truly lack recombination. However, this assumption may be violated in those cases where asexuality involves automixis or gamete doubling. Both processes can increase homozygosity in offspring, which often results in reduced fitness of offspring owing to inbreeding depression—at least in the very first generations of a newly established asexual lineage. Another potential factor to consider is mitotic recombination (MR). MR can lead to a loss of heterozygosity and may thus affect evolutionary rates in diploid asexual lineages. It has been demonstrated in some asexuals (41), even though the net effect of MR on the fitness of asexual lineages is still to be elucidated (42, 43). A second set of assumptions is that the genotypes are adapted to their environment and that the environment of the offspring will be the same as that of the parents. These assumptions may also be violated, because many factors can be responsible for a lack of fit between an organism and its environment, such as environmental changes, migration, or coevolving parasites. In fact, these violations have given rise to a large number of theories on the benefits of sexual reproduction (8).
What is the net effect of recombination on fitness? The concept of “genetic slippage” holds that recombination may incur a short-term cost, via the decrease in mean fitness, and a long-term benefit, via the increase in variance of fitness (40, 44). However, it has been shown that recombination does not always decrease the mean and increase the variance (8). A major challenge in research on the maintenance of sex is to define how the main evolutionary forces (selection, drift, gene flow, and mutation) interact and affect linkage disequilibrium of real populations (8, 45). Another major question is whether these interactions are truly universal, as drift-based theories might suggest (8). Once these gaps in our knowledge are filled, we can tell whether recombination entails a cost of sex, or a universal benefit, or whether its effects are highly lineage-specific and depend strongly on the population genetic circumstances an organism faces.
Costs of Mating.
Several costs can arise due to engagement in mating activities (46). These costs might result in reductions of the total reproductive output, because resources spent for mating are traded off against other aspects of the life history. For example, resources invested in costly physical structures (e.g., showy flowers in plants to attract pollinators) might not be available for other fitness components. Engagement in mating activities may be costly in terms of resources or time, or it may expose females to survival risks. Sexual females may also face a higher likelihood of disease transmission. This is obvious in the case of venereal diseases, but it also applies to other diseases whose transmission is facilitated by proximity to other population members.
There are no quantitative models on the costs of mating, because most of these costs are highly lineage-specific and depend on peculiarities of the mating system. However, some general considerations may allow identification of the presence/absence of significant mating costs. First, mating costs are only relevant as a cost of sex if they are incurred by the females. Risks and mating costs incurred by males are irrelevant, unless there is some feedback to the females (e.g., males monopolizing resources to build costly physical structures while leaving females to starvation). Certainly, time investments or risk exposures by males are inconsequential as a cost of sex as long as the sexual females still have a high chance to be fertilized. Second, a basic assumption for these costs to apply as “costs of sexual reproduction” is that mating activities are reduced or missing in asexual females and that the saved resources are reallocated to asexual fitness components. Like in the previous examples, there are cases where this assumption is violated. Physical structures such as flower size are rarely reduced in asexual plants (e.g., ref. 47). Evidence for increased vegetative growth (i.e., reallocation) in clonal vs. sexual plants is equally scarce (37). Finally, asexual females may still depend on fertilization (48). For example, in many vertebrate and invertebrate parthenogens egg development still needs to be triggered by sperm, which means that asexual females are dependent on finding a sexual male that performs this task (49). Dependency of this kind can reduce the costs of mating, or even lead to a disadvantage of asexuals, if sexual males learn to discriminate against asexual females (48).
The Cellular Mechanical Cost of Meiosis.
Lewis (50) introduced the cellular mechanical cost of meiosis, a cost that should be particularly relevant for small organisms such as unicellular algae or heterotrophic protists. Meiotic processes require a large amount of cellular mechanisms and they generally take longer time than mitotic processes (50). In addition, in unicellular organisms, there is always a conflict between meiotic processes and other cellular processes owing to the lack of physical separation. For example, a cell that currently engages in meiosis cannot maintain full function in other aspects, such maintaining transcriptional activity, which is the basis for normal cell metabolism. In conclusion, the additional time required for meiotic processes should ultimately constrain the rate at which protists can divide. Based on published data, Lewis (50) estimated this particular cost of sex to be 5- to 100-fold. Thus, a fully clonal protist would divide 5–100 times faster than its sexual counterpart—provided that the latter continuously engages in sex. It is unlikely that sex provides sufficient universal compensatory benefits in these protists to counter such an enormous cost. However, this is not necessary anyway: Because protists reproduce by mitotic division most of the time, the cost applies only to the short time periods toward the end of the growing season when sex is induced. During those periods, resources are scarce and the conditions will be suboptimal for asexual growth, which might further reduce the costs (see The Effect of Ecological Conditions on the Costs of Sex). Thus, the cellular mechanical cost provides an explanation for why protists reproduce clonally most of the time. However, in organisms where development time is much larger than the time needed for meiosis (i.e., most multicellular organisms) this cost is irrelevant.
Empirical Measurements of Costs
The combined cost of sex in a particular species consists of several, but usually not all, of the costs listed above. This is straightforward for the “cost of males” vs. “cost of genome dilution,” which are traded off against each other (35). Likewise, cellular mechanical costs of sex will be more important for very short-lived organisms, whereas costs associated with mating should be more important in species with elaborate mating behavior. A major challenge is to integrate these cost components into a realistic estimate of the combined cost of sex in a lineage, or in other words, the fitness gain of a potential transition to asexuality.
Ideally, to obtain a direct estimate of the combined cost of sex one would quantify the fitness ratio between clonal and sexual individuals of the same species in a common garden environment. This common garden should exclude universal conditions that would favor sex, which allows focusing exclusively on the costs of sex. One would then either measure the various life-history variables that are tightly connected to fitness, such as fecundity, survival, or the speed of development, or compare the performance of both reproductive types in direct competition experiments. Unfortunately, there are a number of caveats to be considered. First, empirical estimates are prevented if asexual counterparts do not exist. This may seem trivial; however, many species reproduce exclusively by sexual reproduction, so in those species we can only imagine what an asexual version would look like. Interestingly, in a few cases it has been possible to artificially generate an asexual version. For example, Goddard et al. (51) genetically engineered an apomictic yeast strain by deleting two genes required for normal recombination and meiosis. Second, in those species where sexual and clonal individuals co-occur in the same population, empirical estimates on the cost of sex are complicated by the fact that we usually do not know when these asexuals separated from their sexual ancestors. For example, asexuals might have been derived from a completely different sexual lineage from the one in which they are found to coexist in nature. Genetic markers and phylogenetical reconstruction might give some clues, but there is still the danger that the sample does not include the closest sexual relative. Thus, fitness estimates might be confounded, because asexuals have a different genetic background or because they already suffer from secondary effects of long-term asexuality (e.g., mutation accumulation) that would not be relevant for an assessment of short-term costs of sex. In some cases, a remedy may consist in using natural genetic variation underlying the different reproductive modes. In several organisms simple genetic mechanisms are responsible for transitions to asex (52). In those cases, it is possible to breed sexual and asexual variants with a similar genetic background (e.g., ref. 26). Third, it has been argued that using species where both sexual and asexual counterparts coexist in nature may be altogether unsuitable for testing whether there is a cost of sex, because the very fact that both forms coexist suggests that their fitness ratio should be close to one (4). However, such species are still useful, because they provide models for the lineage-specific biological mechanisms that reduce the cost of sex and thus prevent the asexual mutants from spreading to fixation. In addition, species where both sexual and asexual counterparts coexist can be suitable models for disentangling the effects of traits that sometimes accompany transitions to asex, such as elevated ploidy levels or increased heterozygosity (53).
An alternative to a direct estimation of the cost of sex is to compile biological information to make an informed guess on the various costs of sex in a particular organism. This has been done, for instance, in the case of dandelions (47). The most notable cost of sex in this system was a combination of a cost of males and genome dilution. However, the magnitude of this cost was much lower than the theoretical expectation, which was mainly caused by a higher incidence of male sterility in asexuals and low fertilization success of asexual pollen. Other costs of sex (e.g., mating costs) were either irrelevant or negligible (47). The authors concluded that weak ecological differentiation between sexuals and asexuals in combination with the low costs of sex were sufficient to explain coexistence of both reproductive modes.
The Effect of Ecological Conditions on the Costs of Sex
Some sexual costs, for example the cost of males, are problems of resource allocation. It is thus not surprising that they are strongly affected by ecological conditions related to resource distributions, resource supply, and sharing of resources between asexual and sexual competitors (i.e., niche overlap).
One implicit assumption in the cost of males model is that there is complete niche overlap between asexuals and their sexual relatives. Ecologists have pointed out that this assumption may often be violated. In fact, each individual of a sexual population may show some degree of genetically determined resource specialization (54, 55). Asexuals, which are only “snapshots” of such individual genotypes, produce genetically identical offspring and can thus only use a small proportion of resources, whereas sexuals as a whole can occupy a wider niche space due to variable offspring [“tangled bank hypothesis” (1)]. Models that incorporate such components, for example a highly diverse resource spectrum, asymmetrical competition coefficients between the two reproductive morphs, or negative feedbacks between resource use in the current vs. availability in the next generation, usually find that the invasive potential of asexuals strongly diminishes (e.g., refs. 56–58). This has led to the conclusion that sex is not paradoxical under realistic ecological conditions (57). However, it is not clear whether the tangled bank scenario is a truly universal condition, or whether it applies only for certain species or clades. Currently, examples of individual specialization have a strong taxonomic bias toward vertebrate animals, in particular fish (55). Thus, we do not know whether most generalist species are true generalists or a genetically diverse assembly of specialists. If the latter applies, the invasive potential of asexual mutants should be strongly reduced. Thus, the tangled bank mechanism might act at least as a lineage-specific mechanism to stabilize sexual populations against invasion by asexuals.
Another general observation is that externally caused growth suspension leads to a reduction in the cost of sex. Most costs of sex are related to reproductive rates and require that sexuals and asexuals have a significant offspring production. Thus, if an organism is deprived of critical resources, causing suspension of growth and reproduction, there can be no growth penalty. Facultative asexuality can provide a means of avoiding high costs of sex by adjusting the timing of sex into periods of slow growth. This is also suggested by the fact that environmental conditions that indicate deterioration often stimulate sex in facultative asexuals (e.g., high population density, low food, or transition from long to short day length).
Case Study: The Costs of Sex in Brachionus Rotifers
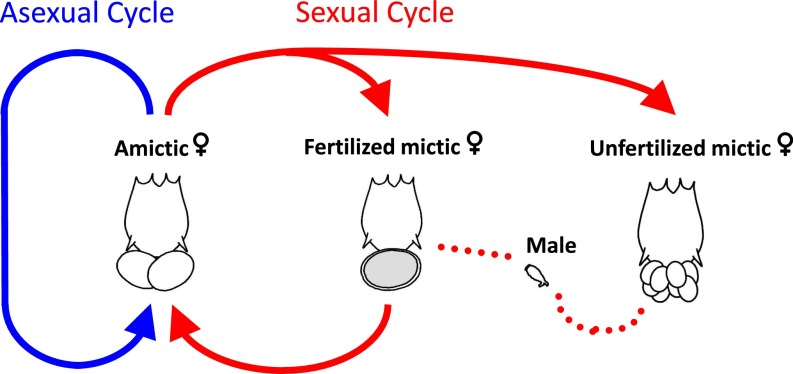
Monogonont rotifers of the genus Brachionus provide an interesting model system for examining the various costs of sexual reproduction. Normally, monogonont rotifers reproduce by cyclical parthenogenesis (i.e., they can alternate between ameiotic parthenogenesis and sexual reproduction) (Fig. 2). Transitions to obligate parthenogenesis have been observed by different research groups (59–61) and in one case the genetic mechanism has been elucidated (62, 63): obligate parthenogens are homozygous for a recessive loss-of-function allele op (for obligate parthenogenesis). Clones with the genotype op/op are unresponsive to the mixis-inducing protein, a quorum-sensing factor that normally induces sex at high population densities (64). Thus, the genotype op/op lacks any sexual stage of the rotifer life cycle (mictic females, males, diapausing eggs) and reproduces exclusively by ameiotic parthenogenesis. The simple Mendelian inheritance mechanism allows the generation of sexual lineages (CP clones) and asexual lineages (OP clones) with essentially the same genetic background. What are the costs of sex in such a system?
Fig. 2.
Life cycle of the rotifer Brachionus sp. Cyclical parthenogens exhibit the full life cycle (asexual and sexual cycle). Obligate parthenogens lack all sexual stages (mictic females, males, and diapausing eggs) and are thus constrained to the asexual cycle (blue arrow).
The cost of male production has been studied in detail using comparisons of lifetime reproduction between CP and OP clones and by invasion experiments, where a small proportion of each reproductive type was introduced into a resident population of its counterpart (26). In most cases, OP clones were competitively superior and their fitness advantage depended on the propensity for sex in the CP clones. Two caveats apply. First, the cost of males included a cost of diapause. This is caused by the fact that males are haploid in these rotifers and develop from unfertilized oocytes. If oocytes are fertilized the zygote develops into a resting egg, which does not immediately hatch. Thus, both processes, male production and resting egg production, slow down immediate population growth in CP clones. Second, the fact that OP clones do not produce resting eggs contributes to their higher population growth, but it also means that OP clones are more vulnerable to extended periods of adverse conditions. A variant of the cost of males is the cost of sexual conflict, which can apply in mating systems where the reproductive interests of males and females diverge. In Brachionus rotifers, male mating behavior can sometimes be interpreted as harassment and there is female behavior, called foot-flipping, which is interpreted as a response of females to evade unwanted mating attempts of males (65). In addition, Brachionus rotifers have traumatic insemination, because males will inseminate females at any body part that is not covered by a thick cuticula. Altogether this indicates that females might suffer from having too many male encounters in their lifetime. However, there is almost no empirical data on the fitness effects of such behavior, and likewise we do not know whether such behaviors would also be displayed toward females of an OP clone. In conclusion, it is questionable whether sexual conflict would enlarge the cost of males in rotifers. The cost of genome dilution does not apply to this system. Because OP clones do not produce males at all, there is no mechanism by which op alleles could reenter into the gene pool of CP clones and generate new asexual lineages. As discussed in Common Costs of Sex, the cost of recombination depends on various factors and can be expected to be high in populations that are highly adapted to their environment. There is currently one study that demonstrated a cost of recombination in rotifers. Becks and Agrawal (66) found that, in benign laboratory environments, sexually produced offspring in Brachionus rotifers were on average less fit than asexually produced offspring. However, this comparison was done within cyclical parthenogenetic clones, so the authors compared the two reproductive modes within the same strains. The costs of mating have not been directly measured in Brachionus. Monogonont rotifers have male mate choice and depend on chance encounters between males and females. The actual process of mate recognition is mediated by glycoproteins on the body surface of females, for which the males have receptors (67, 68). Simulation of encounter rates have shown that at moderate to low population densities, the ability to locate mates can become limiting and females might not be fertilized within their lifetime (69). However, several features of the rotifer life cycle prevent such costs from becoming high: (i) Unfertilized females produce males (these have a chance to fertilize females as the population growth continues) and (ii) sex is induced only at high population densities (which means encounter rates are also high). It is unknown whether there is a significant cellular mechanical cost of meiosis in rotifers. Lewis (70) suggested so, because rotifers have short egg-to-egg intervals. The time required for meiosis might prolong the embryonic/juvenile development of amictic vs. mictic females, because meiotic divisions only occur in the latter. In addition, a cost of syngamy and karyogamy might result in a longer duration of embryonic development of fertilized sexual eggs. However, because fertilized eggs usually enter a diapausing stage, the added time delay due to syngamy or karyogamy seems negligible. Nevertheless, this cost might be visible in exceptional cases, for instance in rotifer clones that produce spontaneously developing resting eggs (71, 72). To summarize, the combined cost of sex in Brachionus seems to consist mainly of the cost of male and diapausing egg production. Selective sweeps from populations composed of mainly sexuals to populations of mainly asexuals have been observed in laboratory experiments (26) and in the field (73), which confirms that low or no sex can be selectively advantageous, at least for a limited time.
Does the lack of a diapause stage ultimately prevent asexual invasions? In Brachionus this seems to be the case. OP clones are competitively superior under benign conditions but have a low (or zero) probability of surviving through harsh periods. This would correspond to the case of “transient asexuality” (Fig. 1). However, there are some observations in Brachionus and in other cyclical parthenogens that suggest that other outcomes might be possible. In particular, these observations challenge the tight links in the causal chain asex → no diapausing stage → no long-term survival. First, it has been shown that sexual eggs are not always diapausing. Some authors have reported that sexual eggs of Brachionus hatched spontaneously after 1–3 d (71, 72). Second, there are monogonont rotifers with asexual diapausing eggs. For example, some strains of Synchaeta pectinata lay thick-shelled asexual eggs, which hatch after a few days to several months (74). Third, diapausing eggs may not be strictly required to survive harsh periods. For example, in other cyclical parthenogens the occurrence of “overwintering clones” suggests that a diapausing stage is not the only way to survive through harsh periods (75, 76). Theoretical models have shown that relaxing the assumption of diapause as a requirement for survival makes asexual invasions more likely (75). In conclusion, these observations suggest that the lack of a diapause stage per se will not always prevent an asexual invasion, but it may do so in some cases.
Conclusions
The costs of sex are a central component in the “paradox of sex.” It is important to get better empirical estimates on the magnitude of such costs, because this has implications for theories that try to explain the maintenance of sex in multicellular eukaryotes. Most of these theories claim to provide a universal mechanism. For example, mutation accumulation, coevolving parasites, or certain types of linkage disequilibria generated by drift have been proposed to be universal “problems” that apply to sexual and asexual organisms alike, and that may be best solved by sex. The benchmark for such universal theories is often a twofold cost of sex, even though this can be relaxed by allowing multiple mechanisms and synergistic interactions (77). However, it remains to be empirically determined whether these proposed mechanisms are truly universal. For example, the Red Queen hypothesis predicts that sexual reproduction is stabilized against asexual invasions because it provides an advantage in organisms that are challenged by an ever-changing environment (such as a coevolving parasite). The freshwater snail Potamopyrgus antipodarum provides a compelling case study of how the Red Queen mechanism might aid in preventing clonal genotypes from spreading to fixation (78). In addition, parasites have been found in almost any extant species. Nevertheless, it remains to be determined how often parasites elicit a coevolutionary arms race that is resolved by the host engaging in high rates of sex, or whether it is resolved by alternative mechanisms (e.g., ref. 17). Another empirical challenge is to get a better estimate of the real costs of sex across the many eukaryotic lineages. This would allow identification of those lineages with the highest costs, the ones for which the maintenance of sex is most difficult to explain.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution IX: Clonal Reproduction: Alternatives to Sex,” held January 9–10, 2015, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/ILE_IX_Clonal_Reproduction.
This article is a PNAS Direct Submission.
References
- 1.Bell G. The Masterpiece of Nature. Univ of California Press; San Francisco: 1982. [Google Scholar]
- 2.Maynard Smith J. The Evolution of Sex. Cambridge Univ Press; Cambridge, UK: 1978. [Google Scholar]
- 3.Williams GC. Sex and Evolution. Princeton Univ Press; Princeton: 1975. [Google Scholar]
- 4.Lehtonen J, Jennions MD, Kokko H. The many costs of sex. Trends Ecol Evol. 2012;27(3):172–178. doi: 10.1016/j.tree.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Hill WG, Robertson A. Linkage disequilibrium in finite populations. Theor Appl Genet. 1968;38(6):226–231. doi: 10.1007/BF01245622. [DOI] [PubMed] [Google Scholar]
- 6.Barton NH, Otto SP. Evolution of recombination due to random drift. Genetics. 2005;169(4):2353–2370. doi: 10.1534/genetics.104.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keightley PD, Otto SP. Interference among deleterious mutations favours sex and recombination in finite populations. Nature. 2006;443(7107):89–92. doi: 10.1038/nature05049. [DOI] [PubMed] [Google Scholar]
- 8.Otto SP. The evolutionary enigma of sex. Am Nat. 2009;174(Suppl 1):S1–S14. doi: 10.1086/599084. [DOI] [PubMed] [Google Scholar]
- 9.Ramesh MA, Malik SB, Logsdon JM., Jr A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol. 2005;15(2):185–191. doi: 10.1016/j.cub.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Simon JC, Delmotte F, Rispe C, Crease TJ. Phylogenetic relationships between parthenogens and their sexual relatives: The possible routes to parthenogenesis in animals. Biol J Linn Soc Lond. 2003;79(1):151–163. [Google Scholar]
- 11.Engelstädter J. Constraints on the evolution of asexual reproduction. BioEssays. 2008;30(11-12):1138–1150. doi: 10.1002/bies.20833. [DOI] [PubMed] [Google Scholar]
- 12.Nunney L. The maintenance of sex by group selection. Evolution. 1989;43(2):245–257. doi: 10.1111/j.1558-5646.1989.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 13.de Vienne DM, Giraud T, Gouyon PH. Lineage selection and the maintenance of sex. PLoS ONE. 2013;8(6):e66906. doi: 10.1371/journal.pone.0066906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladyshev EA, Meselson M, Arkhipova IR. Massive horizontal gene transfer in bdelloid rotifers. Science. 2008;320(5880):1210–1213. doi: 10.1126/science.1156407. [DOI] [PubMed] [Google Scholar]
- 15.Boschetti C, et al. Biochemical diversification through foreign gene expression in bdelloid rotifers. PLoS Genet. 2012;8(11):e1003035. doi: 10.1371/journal.pgen.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gladyshev E, Meselson M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc Natl Acad Sci USA. 2008;105(13):5139–5144. doi: 10.1073/pnas.0800966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson CG, Sherman PW. Anciently asexual bdelloid rotifers escape lethal fungal parasites by drying up and blowing away. Science. 2010;327(5965):574–576. doi: 10.1126/science.1179252. [DOI] [PubMed] [Google Scholar]
- 18.Michiels N, Beukeboom L, Greeff J, Pemberton A. Individual control over reproduction: An underestimated element in the maintenance of sex? J Evol Biol. 1999;12(6):1036–1039. [Google Scholar]
- 19.Arnqvist G, Rowe L. Sexual Conflict. Princeton Univ Press; Princeton: 2005. [Google Scholar]
- 20.Rankin DJ, Dieckmann U, Kokko H. Sexual conflict and the tragedy of the commons. Am Nat. 2011;177(6):780–791. doi: 10.1086/659947. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal AF. Sexual selection and the maintenance of sexual reproduction. Nature. 2001;411(6838):692–695. doi: 10.1038/35079590. [DOI] [PubMed] [Google Scholar]
- 22.Rice WR, Chippindale AK. Intersexual ontogenetic conflict. J Evol Biol. 2001;14(5):685–693. [Google Scholar]
- 23.Lively CM. Male allocation and the cost of biparental sex in a parasitic worm. Lect Math Life Sci. 1990;22:93–107. [Google Scholar]
- 24.Jokela J, Lively CM, Dybdahl MF, Fox JA. Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology. 1997;78(2):452–460. [Google Scholar]
- 25.Wolinska J, Lively CM. The cost of males in Daphnia pulex. Oikos. 2008;117(11):1637–1646. [Google Scholar]
- 26.Stelzer CP. The cost of sex and competition between cyclical and obligate parthenogenetic rotifers. Am Nat. 2011;177(2):E43–E53. doi: 10.1086/657685. [DOI] [PubMed] [Google Scholar]
- 27.Corley LS, Moore AJ. Fitness of alternative modes of reproduction: developmental constraints and the evolutionary maintenance of sex. Proc Roy Soc B Biol Sci. 1999;266(1418):471–476. [Google Scholar]
- 28.Kramer MG, Templeton AR. Life-history changes that accompany the transition from sexual to parthenogenetic reproduction in Drosophila mercatorum. Evolution. 2001;55(4):748–761. doi: 10.1554/0014-3820(2001)055[0748:lhctat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Kearney M, Shine R. Lower fecundity in parthenogenetic geckos than sexual relatives in the Australian arid zone. J Evol Biol. 2005;18(3):609–618. doi: 10.1111/j.1420-9101.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 30.Wetherington JD, Kotora KE, Vrijenhoek RC. A test of the spontaneous heterosis hypothesis for unisexual vertebrates. Evolution. 1987;41(4):721–731. doi: 10.1111/j.1558-5646.1987.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 31.Kearney M. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol Evol. 2005;20(9):495–502. doi: 10.1016/j.tree.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Brash DP. What does sex really cost? Am Nat. 1976;110(975):894–897. [Google Scholar]
- 33.Treisman M, Dawkins R. The “cost of meiosis”: Is there any? J Theor Biol. 1976;63(2):479–484. doi: 10.1016/0022-5193(76)90047-3. [DOI] [PubMed] [Google Scholar]
- 34.Charlesworth B. The cost of sex in relation to mating system. J Theor Biol. 1980;84(4):655–671. doi: 10.1016/s0022-5193(80)80026-9. [DOI] [PubMed] [Google Scholar]
- 35.Joshi A, Moody ME. The cost of sex revisited: Effects of male gamete output of hermaphrodites that are asexual in their female capacity. J Theor Biol. 1998;195(4):533–542. doi: 10.1006/jtbi.1998.0811. [DOI] [PubMed] [Google Scholar]
- 36.Mogie M. Is there a cost of sex in hermaphrodites? Naturwissenschaften. 1996;83(5):225–226. [Google Scholar]
- 37.Eckert CG. The loss of sex in clonal plants. In: Stuefer JF, Erschbamer B, Huber H, Suzuki JI, editors. Ecology and Evolutionary Biology of Clonal Plants. Springer; Berlin: 2002. pp. 279–298. [Google Scholar]
- 38.Eckert CG, Dorken ME, Mitchell SA. Loss of sex in clonal populations of a flowering plant, Decodon verticillatus (Lythraceae) Evolution. 1999;53(4):1079–1092. doi: 10.1111/j.1558-5646.1999.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 39.Silvertown J. The evolutionary maintenance of sexual reproduction: Evidence from the ecological distribution of asexual reproduction in clonal plants. Int J Plant Sci. 2008;169(1):157–168. [Google Scholar]
- 40.Lynch M, Deng HW. Genetic slippage in response to sex. Am Nat. 1994;144(2):242–261. [Google Scholar]
- 41.Omilian AR, Cristescu ME, Dudycha JL, Lynch M. Ameiotic recombination in asexual lineages of Daphnia. Proc Natl Acad Sci USA. 2006;103(49):18638–18643. doi: 10.1073/pnas.0606435103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandegar MA, Otto SP. Mitotic recombination counteracts the benefits of genetic segregation. Proc Roy Soc B Biol Sci. 2007;274(1615):1301–1307. doi: 10.1098/rspb.2007.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roze D, Michod RE. Deleterious mutations and selection for sex in finite diploid populations. Genetics. 2010;184(4):1095–1112. doi: 10.1534/genetics.109.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen DE, Lynch M. Both costs and benefits of sex correlate with relative frequency of asexual reproduction in cyclically parthenogenic Daphnia pulicaria populations. Genetics. 2008;179(3):1497–1502. doi: 10.1534/genetics.107.082479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barton NH, Charlesworth B. Why sex and recombination? Science. 1998;281(5385):1986–1990. [PubMed] [Google Scholar]
- 46.Daly M. The cost of mating. Am Nat. 1978;112(986):771–774. [Google Scholar]
- 47.Meirmans S, Meirmans PG, Kirkendall LR. The costs of sex: Facing real-world complexities. Q Rev Biol. 2012;87(1):19–40. doi: 10.1086/663945. [DOI] [PubMed] [Google Scholar]
- 48.Neiman M. Physiological dependence on copulation in parthenogenetic females can reduce the cost of sex. Anim Behav. 2004;67(5):811–822. [Google Scholar]
- 49.Beukeboom LW, Vrijenhoek RC. Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J Evol Biol. 1998;11(6):755–782. [Google Scholar]
- 50.Lewis WM. Interruption of synthesis as a cost of sex in small organisms. Am Nat. 1983;121(6):825–834. [Google Scholar]
- 51.Goddard MR, Godfray HC, Burt A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 2005;434(7033):636–640. doi: 10.1038/nature03405. [DOI] [PubMed] [Google Scholar]
- 52.Neiman M, Sharbel TF, Schwander T. Genetic causes of transitions from sexual reproduction to asexuality in plants and animals. J Evol Biol. 2014;27(7):1346–1359. doi: 10.1111/jeb.12357. [DOI] [PubMed] [Google Scholar]
- 53.Neiman M, Schwander T. Using parthenogenetic lineages to identify advantages of sex. Evol Biol. 2011;38(2):115–123. [Google Scholar]
- 54.Bolnick DI, et al. The ecology of individuals: Incidence and implications of individual specialization. Am Nat. 2003;161(1):1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- 55.Araújo MS, Bolnick DI, Layman CA. The ecological causes of individual specialisation. Ecol Lett. 2011;14(9):948–958. doi: 10.1111/j.1461-0248.2011.01662.x. [DOI] [PubMed] [Google Scholar]
- 56.Doncaster CP, Pound GE, Cox SJ. The ecological cost of sex. Nature. 2000;404(6775):281–285. doi: 10.1038/35005078. [DOI] [PubMed] [Google Scholar]
- 57.Olofsson H, Lundberg P. The twofold cost of sex unfolded. Evol Ecol Res. 2007;9(7):1119–1129. [Google Scholar]
- 58.Scheu S, Drossel B. Sexual reproduction prevails in a world of structured resources in short supply. Proc Roy Soc B Biol Sci. 2007;274(1614):1225–1231. doi: 10.1098/rspb.2007.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett WN, Boraas ME. Isolation of a fast-growing strain of the rotifer Brachionus calyciflorus Pallas using turbidostat culture. Aquaculture. 1988;73(1–4):27–36. [Google Scholar]
- 60.Fussmann GF, Ellner SP, Hairston NG., Jr Evolution as a critical component of plankton dynamics. Proc Roy Soc B Biol Sci. 2003;270(1519):1015–1022. doi: 10.1098/rspb.2003.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stelzer CP. Obligate asex in a rotifer and the role of sexual signals. J Evol Biol. 2008;21(1):287–293. doi: 10.1111/j.1420-9101.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 62.Scheuerl T, Riss S, Stelzer CP. Phenotypic effects of an allele causing obligate parthenogenesis in a rotifer. J Hered. 2011;102(4):409–415. doi: 10.1093/jhered/esr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stelzer CP, Schmidt J, Wiedlroither A, Riss S. Loss of sexual reproduction and dwarfing in a small metazoan. PLoS One. 2010;5(9):e12854. doi: 10.1371/journal.pone.0012854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snell TW, et al. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera) Mar Biol. 2006;149(4):763–773. [Google Scholar]
- 65.Snell TW, Kim J, Zelaya E, Resop R. Mate choice and sexual conflict in Brachionus plicatilis (Rotifera) Hydrobiologia. 2007;593(1):151–157. [Google Scholar]
- 66.Becks L, Agrawal AF. The effect of sex on the mean and variance of fitness in facultatively sexual rotifers. J Evol Biol. 2011;24(3):656–664. doi: 10.1111/j.1420-9101.2010.02199.x. [DOI] [PubMed] [Google Scholar]
- 67.Snell TW, et al. Genetic determinants of mate recognition in Brachionus manjavacas (Rotifera) BMC Biol. 2009;7:60. doi: 10.1186/1741-7007-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snell TW, Stelzer C-P. Removal of surface glycoproteins and transfer among Brachionus species. Hydrobiologia. 2005;546:267–274. [Google Scholar]
- 69.Snell TW, Garman BL. Encounter probabilities between male and female rotifers. J Exp Mar Biol Ecol. 1986;97(3):221–230. [Google Scholar]
- 70.Lewis WM., Jr . The Cost of Sex: The Evolution of Sex and Its Consequences. Springer; Berlin: 1987. pp. 33–57. [Google Scholar]
- 71.Becks L, Agrawal AF. Higher rates of sex evolve in spatially heterogeneous environments. Nature. 2010;468(7320):89–92. doi: 10.1038/nature09449. [DOI] [PubMed] [Google Scholar]
- 72.Gilbert JJ, Dieguez MC. Low crowding threshold for induction of sexual reproduction and diapause in a Patagonian rotifer. Freshw Biol. 2010;55(8):1705–1718. [Google Scholar]
- 73.Carmona MJ, Dimas-Flores N, García-Roger EM, Serra M. Selection of low investment in sex in a cyclically parthenogenetic rotifer. J Evol Biol. 2009;22(10):1975–1983. doi: 10.1111/j.1420-9101.2009.01811.x. [DOI] [PubMed] [Google Scholar]
- 74.Gilbert JJ. Timing of diapause in monogonont rotifers: Mechanisms and strategies. In: Alekseev VR, De Stasio B, Gilbert JJ, editors. Diapause in Aquatic Invertebrates: Theory and Human Use. Springer; Berlin: 2007. pp. 11–27. [Google Scholar]
- 75.Rispe C, Pierre JS, Simon JC, Gouyon PH. Models of sexual and asexual coexistence in aphids based on constraints. J Evol Biol. 1998;11(6):685–701. [Google Scholar]
- 76.Hamrová E, Mergeay J, Petrusek A. Strong differences in the clonal variation of two Daphnia species from mountain lakes affected by overwintering strategy. BMC Evol Biol. 2011;11(1):231. doi: 10.1186/1471-2148-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West SA, Lively CM, Read AF. A pluralist approach to sex and recombination. J Evol Biol. 1999;12(6):1003–1012. [Google Scholar]
- 78.Jokela J, Dybdahl MF, Lively CM. The maintenance of sex, clonal dynamics, and host-parasite coevolution in a mixed population of sexual and asexual snails. Am Nat. 2009;174(Suppl 1):S43–53. doi: 10.1086/599080. [DOI] [PubMed] [Google Scholar]