Significance
Frontal pole cortex (FPC) refers to the most anterior part of prefrontal cortex, a region that is highly developed in anthropoid primates. However, because of technical difficulties in studying this area, its role in primate cognition had remained largely unknown. We studied effects of selective bilateral lesions within FPC on monkeys’ cognitive flexibility. FPC lesion did not impair the performance in well-learned cognitively demanding tasks. However, FPC-lesioned monkeys remained more focused than control monkeys in exploiting the current task when they faced newly introduced interruptions by a simple secondary task or free rewards. This unique pattern of behavioral changes in FPC-lesioned monkeys suggests that FPC is involved in redistribution of cognitive resources from the current task to novel opportunities.
Keywords: frontal pole cortex, posterior cingulate cortex, executive control
Abstract
Frontal pole cortex (FPC) and posterior cingulate cortex (PCC) have close neuroanatomical connections, and imaging studies have shown coactivation or codeactivation of these brain regions during performance of certain tasks. However, they are among the least well-understood regions of the primate brain. One reason for this is that the consequences of selective bilateral lesions to either structure have not previously been studied in any primate species. We studied the effects of circumscribed bilateral lesions to FPC or PCC on monkeys’ ability to perform an analog of Wisconsin Card Sorting Test (WCST) and related tasks. In contrast to lesions in other prefrontal regions, neither posttraining FPC nor PCC lesions impaired animals’ abilities to follow the rule switches that frequently occurred within the WCST task. However, FPC lesions were not without effect, because they augmented the ability of animals to adjust cognitive control after experiencing high levels of conflict (whereas PCC lesions did not have any effect). In addition, FPC-lesioned monkeys were more successful than controls or PCC-lesioned animals at remembering the relevant rule across experimentally imposed distractions involving either an intervening secondary task or a surprising delivery of free reward. Although prefrontal cortex posterior to FPC is specialized for mediating efficient goal-directed behavior to maximally exploit reward opportunities from ongoing tasks, our data led us to suggest that FPC is, instead, specialized for disengaging executive control from the current task and redistributing it to novel sources of reward to explore new opportunities/goals.
Frontal pole cortex (FPC) occupies the most anterior part of prefrontal cortex and is occupied by cytoarchitectural area 10. Technical difficulties in accessing FPC has made it a difficult target for lesion and recording studies, and therefore, the current theories regarding its function are mostly originating from imaging studies in humans. FPC activation has been seen across a wide range of different experimental settings and paradigms in human neuroimaging studies (1–23). Furthermore, in some studies, FPC activity in resting conditions is even higher than that observed during conditions where cognitively demanding tasks are required (8, 14). Neuropsychological examinations of patients with FPC damage have indicated that their abilities are within the normal range on many tests of intelligence and language (11, 12, 24). Nonetheless, such patients do have problems in organizing their daily life and are noticeably impaired in dealing with complicated, novel, or changing situations (11, 12, 24).
Posterior cingulate cortex (PCC) has a close neuroanatomical link with FPC (25–29), and these two brain regions also show coactivation or codeactivation while subjects perform various tasks; accordingly, FPC and PCC are considered to be core structures within the default mode network (7, 18). Recording studies in monkeys and imaging studies in humans suggest a role for PCC in focused attention, evaluation of task demands, change detection, and ensuing alteration of behavior (22, 30–39). Like FPC patients, those with PCC damage also showed impairments in multitasking and adapting to novel situations or changes in cognitive set, although the impairments might have resulted from deficits in other cognitive functions, such as assessing the saliency of task-relevant events or detecting changes in task context (11, 12, 35–37).
Overall, these studies suggest that FPC and PCC might both be involved in behavioral adaptation to changing task demands. However, the heterogeneity and inconsistency of lesion extent across patients mean that interpretation of which specific behavioral deficits are attributable to FPC or PCC damage per se is fraught with difficulty. Also, although imaging studies provide valuable information about the correlation of activation in a brain area with different aspects of cognitive task performance, they cannot necessarily indicate whether a brain area has an essential role in a particular cognitive function. Therefore, it has been difficult to formulate a unified theory for what the unique contributions of each one of these two regions, FPC and PCC, are to cognition that could explain the pattern of their activations in different experimental settings and paradigms in human neuroimaging studies.
The Wisconsin Card Sorting Test (WCST) is routinely used in neuropsychological assessment of patients with frontal cortex damage or mental diseases to assess cognitive flexibility in adapting to changing task demands (40–44). It is a multifaceted test that demands the coordination of multiple cognitive processes in addition to basic perceptual and motor processes and therefore, a suitable test to examine the contribution of FPC and PCC to cognition. We trained macaque monkeys to perform a close computerized analog of the WCST (Fig. 1A), in which they had to match a central sample on a touchscreen with one of three surrounding test items based on whether a color-matching or a shape-matching rule was currently reinforced. No cue was given to indicate the relevant rule or its frequent changes, and therefore, the monkeys had to find the relevant rule by trial and error by relying on the feedback to their behavioral response. To establish a causative link between FPC, PCC, and cognitive functions, we examined the pattern of spared and altered cognitive functions after highly circumscribed lesions to FPC or PCC in macaque monkeys in the context of WCST and related cognitive tasks.
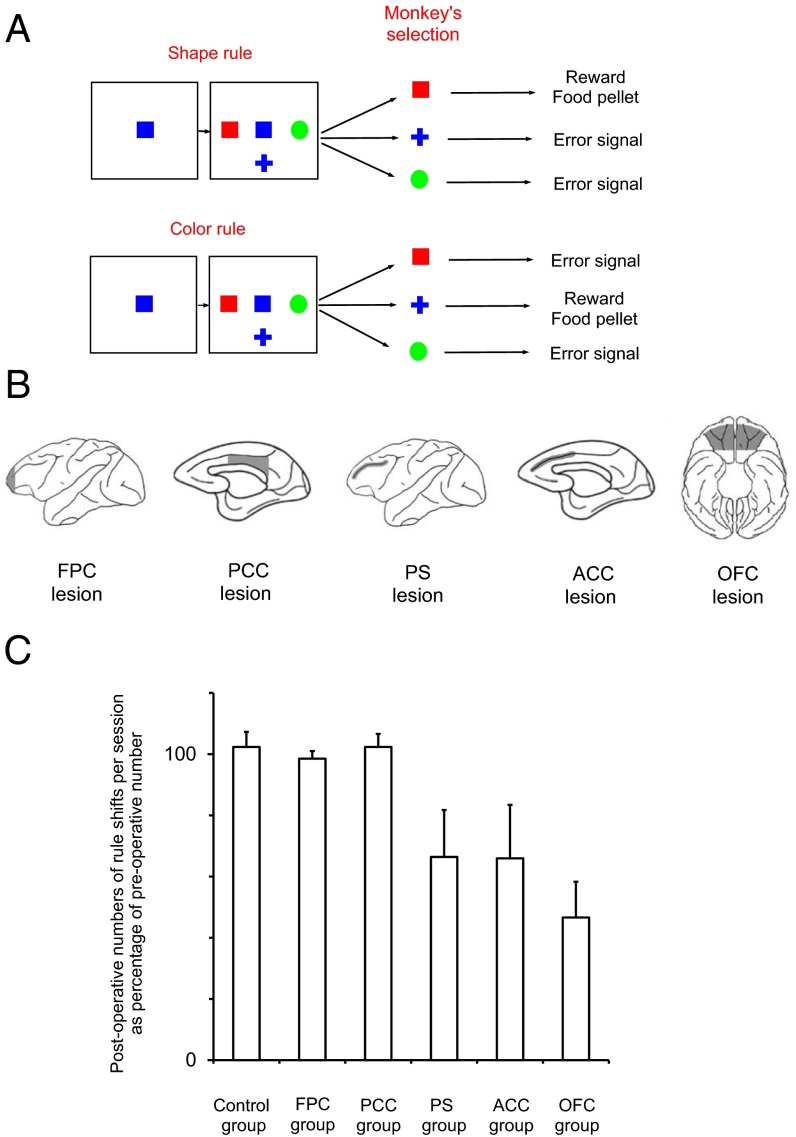
Fig. 1.
Intended lesion extend, task procedures, and overall performance in the standard WCST analog. (A) In each trial, a sample was presented, and when the monkey touched the sample, three test items appeared surrounding the sample. One test item matched the sample in color but not in shape, a second matched in shape but not in color, and the remaining one did not match in either color or shape. Sample and test items were randomly selected from a set of 36 stimuli made by combining six colors and six shapes. The monkeys had to touch the test item that matched the sample either in color or shape, depending on the currently relevant rule, within 3,000 ms to receive a food reward. If the monkey made an incorrect choice, a white circle appeared for 1,000 ms as an error signal, and no reward was provided. The intertrial interval was 6 s after correct responses and 12 s after erroneous responses. The matching rule changed every time that the animal attained 17 corrects in 20 consecutive trials (shift criterion). (B) The extents of cortical lesions indicated by the shaded areas in the brain diagrams. (C) The mean number of postoperative rule shifts achieved per daily session is expressed as a percentage of the mean number of preoperative rule shifts in each monkey for the control group (n = 3) and monkeys with lesions within the FPC (n = 4), PCC (n = 3), PS cortex (n = 4), ACC (n = 4), or OFC (n = 3). The data of PS, ACC, and OFC groups were obtained from our previous study with the same task (42). Error bars indicate the SEM across monkeys in each group.
We found that, in contrast to the consequences of lesions in dorsolateral prefrontal cortex, orbitofrontal cortex (OFC), or anterior cingulate cortex (ACC), lesions within FPC and PCC did not impair the cognitive ability in adapting to the rule changes required within the WCST. However, lesions within FPC but not within PCC made the monkeys less distractible by the salient extratask events and more focused on exploiting the current task. We conclude that neither FPC nor PCC has an indispensable role in adapting to rule changes in the WCST and propose that FPC has a unique role in redistributing executive control resources among potential goals existing in complex changing situations.
Results
All animal training, surgery, and experimental procedures were done in accordance with the guidelines of NIH and the Japanese Neuroscience Society, and also approved by RIKEN's Animal Experiment Committee. After about 1 y of training, the monkeys were all able to perform different versions of the WCST analog to a very high level as measured by their ability to achieve more than 12 rule shifts in each daily session (the maximum number of rule shift criterion achievable in 300 trials of each daily session was 15). Based on preoperative performance levels, seven monkeys (Macaca mulatta) were divided into two performance-matched groups so that the mean and range of the numbers of preoperative rule shifts were comparable between the groups; one group received bilateral aspiration lesions to FPC (Figs. 1B, FPC lesion and 2) (FPC group, n = 4), and the other group remained as unoperated controls (control group, n = 3). In the second stage of the study, the three monkeys in control group received bilateral lesions in PCC (Figs. 1B, PCC lesion and 3 and Fig. S1) (PCC group), and their pre- and postoperative performances were compared. The lesion extent was as intended in all of the FPC-lesioned animals and covered the dorsal, medial, and orbital parts of FPC (Fig. 2). The lesion extent was also as intended in the three PCC-lesioned monkeys and included cortex in the posterior cingulate gyrus and lower bank of posterior cingulate sulcus extending as far posteriorly as to include area 31 (33) (Fig. 3 and Fig. S1). There was some damage to the anterior portions of areas 29 and 30 (retrosplenial cortex) in the three PCC-lesioned monkeys (32).
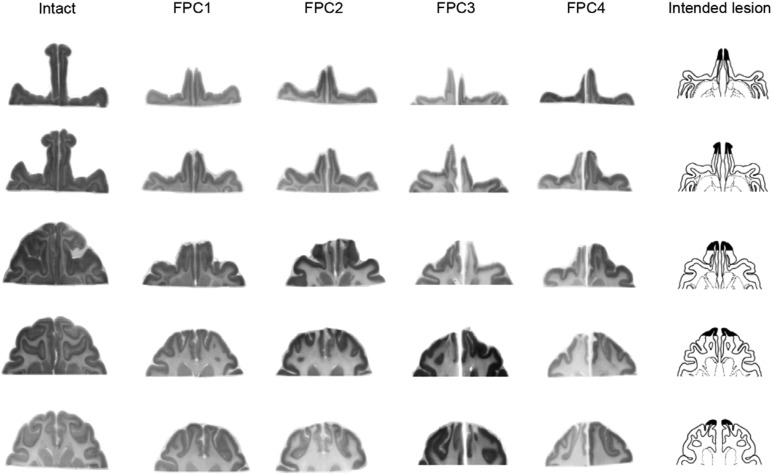
Fig. 2.
Extent of FPC lesions confirmed in horizontal brain sections. Nissl-stained horizontal sections were taken from five dorsoventral levels where FPC had existed in each monkey. The column 1 is for an intact monkey (intact), columns 2–5 are for four FPC-lesioned monkeys (FPC1–FPC4), and column 6 shows the intended lesion extent on drawings of a representative brain. Note the absence of gray matter tissue at the most rostral part of the brain in each FPC-lesioned animal. Microscopic examination of the stained sections confirmed complete lesions of the entire extent of the cortex in the frontal pole, with no damage outside of the intended region.
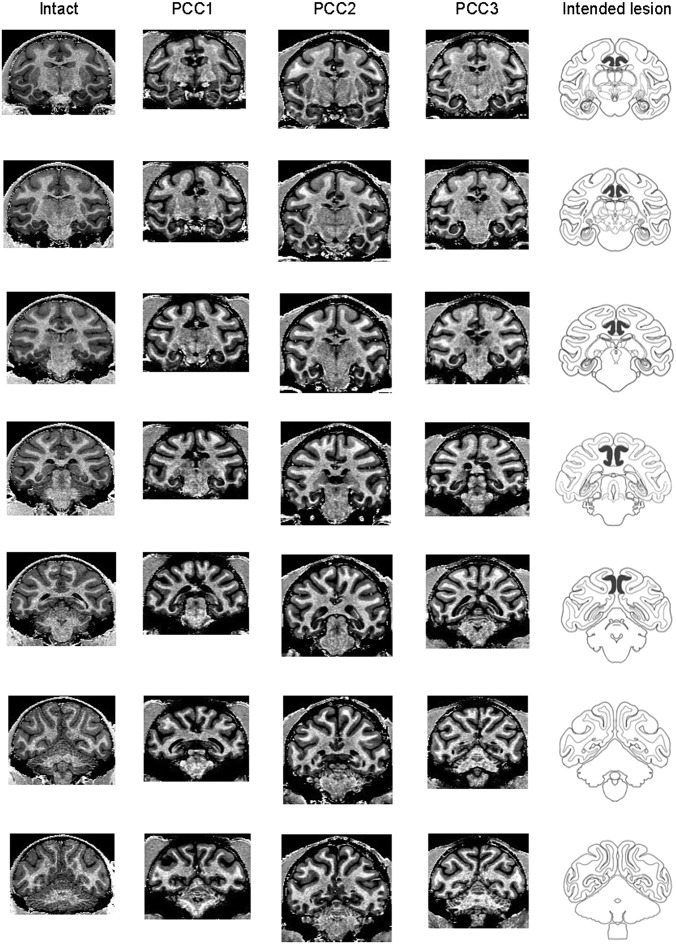
Fig. 3.
Extent of PCC lesions confirmed in MRI. MRI scans were taken after the end of postoperative data collection to verify the lesion extent. Column 1 is for an intact monkey (intact), and columns 2–4 are for three PCC-lesioned monkeys (PCC1–PCC3). Frontal sections are taken from seven anterior–posterior levels covering the PCC in each monkey. The sections in PCC-lesioned monkeys were selected so that their gyri and sulci shapes match those of the intact monkey as much as possible. The gray matter tissues are missing bilaterally in the ventral bank of cingulate sulcus and on the surface of posterior cingulate gyrus in each PCC-lesioned animal. The lesions of PCC were as intended, with no damage outside the target area (Fig. S1). The schematic diagrams showing the intended lesion extent (column 5) were adapted from the NIMH (National Institute of Mental Health) Rhesus Macaque Brain Atlas.
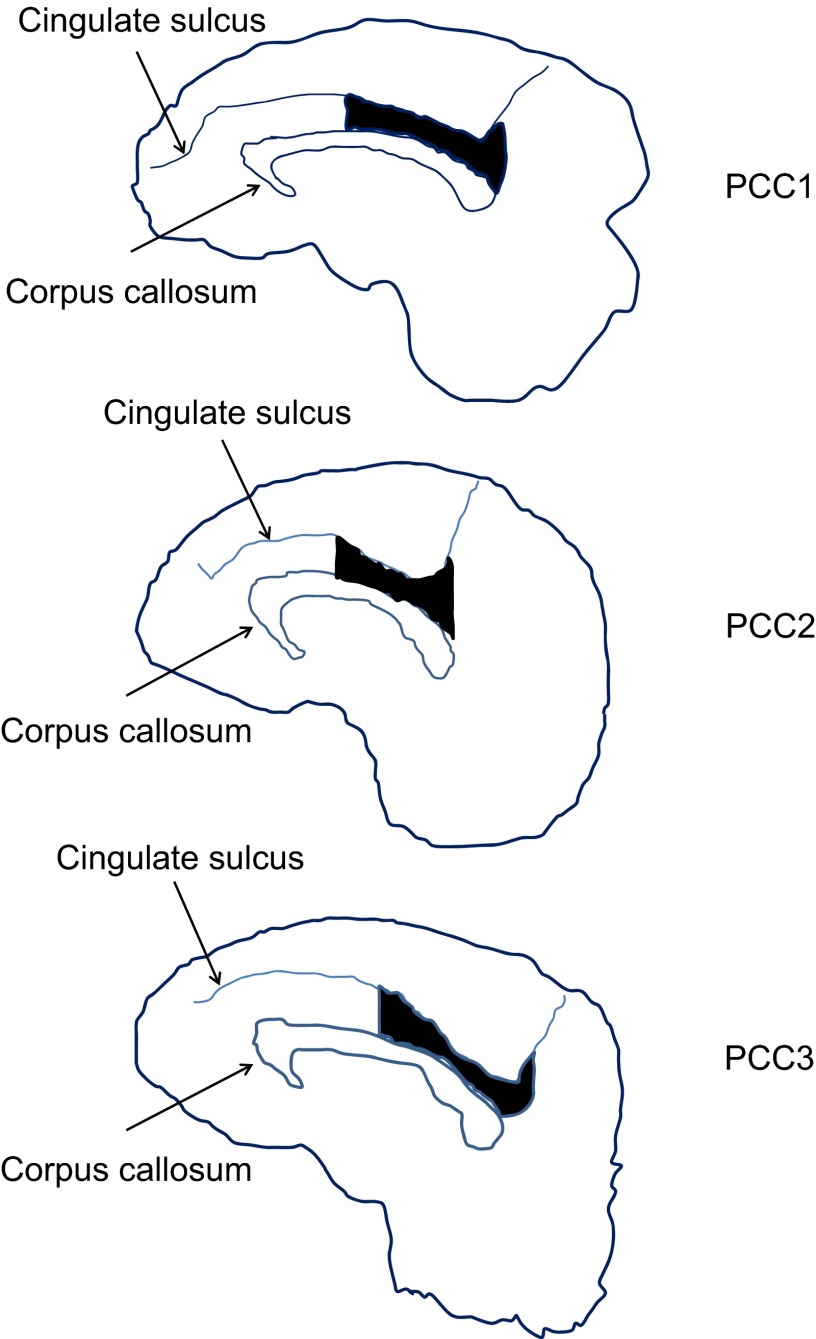
Fig. S1.
Extent of lesion in PCC. Extent of PCC lesion in the medial view of the brain. The shapes of the brain, corpus callosum, and cingulate sulcus together with the lesion extent were reconstructed from structural MRIs in each monkey. The black areas indicate the lesion extent. Scattered and partial damage to corpus callosum occurred in three monkeys.
Lesion Effects on Adapting to Abstract Rule Changes (Standard WCST Analog).
The general aspects of the cognitive ability in adapting to rule changes were analyzed in the data obtained with the standard version of WCST analog (Fig. 1A) consisting only of high-conflict trials. Neither the FPC lesion nor the PCC lesion affected the mean number of rule shifts per day achieved in postoperative sessions (Fig. 1C). In the control group, the mean pre- and postoperative rule shifts per session were 12.8 ± 0.8 (mean ± SE) and 13.0 ± 0.5, respectively. These values were 12.5 ± 0.6 and 12.3 ± 0.7, respectively, in the FPC group. The same values were 13.7 ± 0.03 and 13.7 ± 0.3, respectively, in the PCC group. We examined the changes in the number of rule shifts between pre- and postoperative performances by a two-way ANOVA [monkey (between-subject factor) × lesion (pre\post, within-subject factor)] in each group. No significant difference in the number of rule shifts between the pre- and postoperative performances was seen in the control [lesion effect (pre\post): F(1,42) = 1.046, P = 0.31] or FPC group [lesion effect (pre\post): F(1,56) = 0.58, P = 0.45]. A t test applied to the difference in the mean number of rule shifts between the pre- and postoperative performances of individual monkeys confirmed that there was no significant difference in the lesion-induced changes between control and FPC groups [one-tailed t test; t(3.1) = 0.62, P = 0.28]. There was also no significant difference between preoperative and postoperative performances in the PCC group [one-tailed paired t test; t(2) = 0.28, P = 0.41]. We also calculated postoperative rule shifts as a percentage of their preoperative number (Fig. 1C) in control, FPC, and PCC groups. A one-way ANOVA applied to these numbers of rule shifts did not show any significant difference [F(2,7) = 0.14, P = 0.87].
These results indicated that the cognitive ability in adapting to frequent rule shifts was unimpaired after either the FPC or PCC lesion; however, our previous studies (42) showed that lesions to the principal sulcus (PS) within dorsolateral prefrontal cortex or to OFC or ACC all significantly impaired animals’ performance on the same measure. Separate cohorts of animals were used in the current and previous lesion studies, and their performance was compared with two separate groups of control intact monkeys. We here pooled six and three control intact monkeys, respectively, from the two studies and considered them as a control group to assess the consequence of lesions within different areas in the same statistical analysis. In each control or lesion monkey, we first calculated the mean number of postoperative rule shifts as a percentage of their preoperative number of rule shifts. Then, a one-way ANOVA was applied to these percentages in control, PS, ACC, OFC, FPC, and PCC groups. The main effect was highly significant [F(5,21) = 5.18, P = 0.0030], and multiple comparison tests (Dunnett test) showed that there was a significant difference between control and PS, between control and ACC, and between control and OFC but not between control and FPC or between control and PCC. These findings indicated that ACC, PS, and OFC lesion groups but not FPC and PCC lesion groups were impaired compared with the control intact monkeys (Fig. 1C).
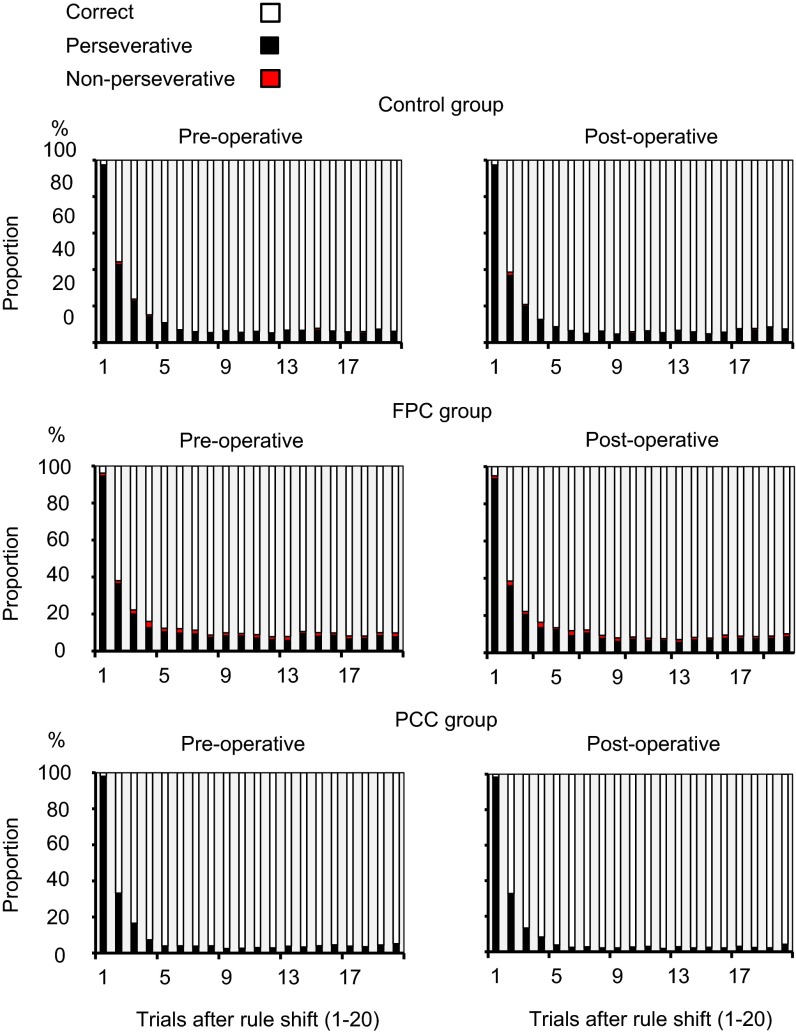
The speed of adaptation to a rule shift was also unaffected by FPC or PCC lesions. Fig. 4 depicts the mean pre- and postoperative distributions of response types for the first 20 trials after rule shift. Preoperatively, the majority of monkeys’ errors were perseverative errors, defined here as matching based on the currently irrelevant rule, and they were more frequently committed immediately after the rule shift. The proportion of perseverative errors in several trials immediately after the rule shift or the total number of perseverative errors throughout the block was unaffected by either the FPC or PCC lesion (Fig. 4 and SI Results, Frequency of Errors in WCST Analog).
Fig. 4.
No changes in the frequency of erroneous responses immediately after rule shifts in the WCST analog after FPC or PCC lesions. Proportions of three response types (perseverative errors, nonperseverative errors, and correct responses) are shown for each of the first 20 trials after rule shifts in Control, FPC, and PCC groups (SI Results).
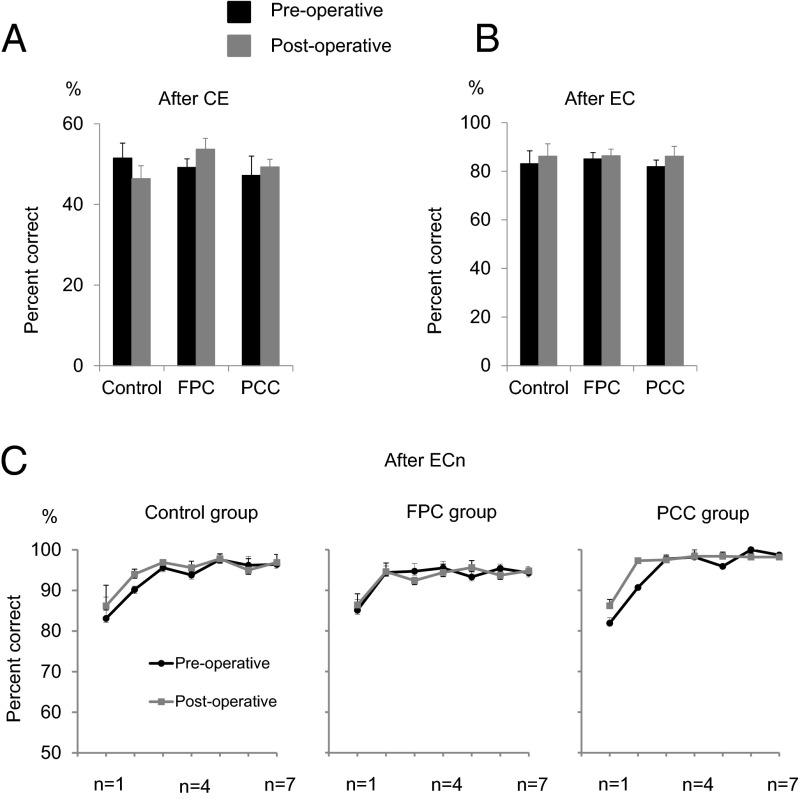
A crucial aspect of WCST is the necessity to assess the behavioral outcomes to maintain or revise the rule to apply. We first considered the performance of the monkeys after commission of a single error preceded by a correct trial (EC trials). We excluded the first three trials after rule shift from this analysis, because after the rule shift, there was a higher tendency to select the previously relevant rule. The mean percentages of correct responses in trials that followed EC trials were 51.5 ± 3.7 preoperatively and 46.4 ± 3.2 postoperatively in control group, 49.2 ± 2.1 preoperatively and 53.7 ± 2.7 postoperatively in FPC group, and 47.2 ± 4.8 preoperatively and 49.3 ± 1.9 postoperatively in the PCC group (Fig. 5A). A t test applied to pre\post differences in individual monkeys indicated that the FPC group was not impaired relative to the control group [one-tailed t test; t(4.9) = 0.83, P = 0.22]. Another t test showed that there was no significant difference between pre- and postoperative percentages in the PCC group [one-tailed paired t test; t(2) = 0.6, P = 0.3].
Fig. 5.
Comparable degrees of learning from the behavioral outcomes (rewards to correct responses and no rewards and error signals to erroneous responses) in Control, FPC, and PCC groups. (A) Mean preoperative (black bars) and postoperative (gray bars) percentages of correct responses in the trials immediately after a sequence of correct and error trials (after CE). (B) Mean percentages of correct responses in the trials immediately after an EC trial sequence (after EC). (C) Mean preoperative (black lines) and postoperative (gray lines) percentages of correct responses in the trials immediately after a sequence of n consecutive correct trials after an error trial (after ECn). The data at n = 1 are identical to those in B (SI Results).
We then examined the monkeys’ performance in the trials that followed EC (a correct trial that was preceded by an error) trials. The mean percentages of correct responses in these after EC trials were 83.1 ± 5.3 preoperatively and 86.2 ± 5.1 postoperatively in the control group, 85.1 ± 2.6 preoperatively and 86.4 ± 2.7 postoperatively in the FPC group, and 81.9 ± 2.7 preoperatively and 86.2 ± 4 postoperatively in the PCC group (Fig. 5B). A t test applied to pre\post differences in individual monkeys indicated that the FPC group was not impaired relative to the control group [one-tailed t test; t(4.1) = 0.67, P = 0.26]. Another t test showed that there was no significant difference between pre- and postoperative percentages in the PCC group [one-tailed paired t test; t(2) = 1.85, P = 0.103]. These results indicated that the ability of monkeys in rapidly improving their performance after a single success experience was not affected by the FPC or PCC lesions. Because our previous study showed that this aspect of cognitive control in the WCST analog was significantly degraded by the OFC lesion (42), these results showed the dissociation of functional roles of FPC and PCC from those of the OFC.
The monkeys’ performance further improved after multiple consecutive correct trials. To analyze the extent that the monkeys benefitted from accumulated success experiences, we scored the percentage of correct responses in the trial after a varying number (n) of consecutive correct responses after an error (after ECn; with n ranging from one to seven) (Fig. 5C). A three-way ANOVA was applied to the mean percentages at different n values in individual FPC and control monkeys ([group(FPC\control, between-subject factor) × n (within-subject factor)] × pre\post[within-subject factor]). There was no significance in the main effect of group [F(1,5) = 0.17, P = 0.70], the main effect of pre\post [F(1,5) = 2.6, P = 0.17], the interaction between group and n [F(6,30) = 1.02, P = 0.43], or interaction between pre\post and n [F(6,30) = 1.2, P = 0.36], although the main effect of n was significant [F(6,30) = 13.5, P < 10−6]. A two-way ANOVA [PCC lesion (pre\post, within-subject factor) × n (within-subject factor)] showed that the main effect of PCC lesion and the interaction between PCC lesion and n were not significant [F(1,2) = 6.1, P = 0.13 and F(6,12) = 2.6, P = 0.7, respectively], although the main effect of n was significant [F(6,12) = 15.5, P < 0.0001]. These results showed that the ability of monkeys in improving their performance after consecutive success experiences was not affected by FPC or PCC lesions.
These results confirmed that FPC- and PCC-lesioned monkeys could assess behavioral outcomes and rapidly modify their rule-based behavior depending on the history of recent behavioral outcome as efficiently as Controls or their preoperative performance level.
Lesion Effects on Context-Dependent Executive Control Adjustment (WCST Conflict).
We examined the role of FPC and PCC in context-dependent adjustment of executive control by using the data obtained with the WCST conflict (Fig. 6A), in which the conflict level between behavioral rules varied from trial to trial. Conflict in information processing or responses can trigger adaptive behavioral modulation supposedly through recruitment and allocation of executive control (42–47). Previously, we showed that lesions within PS or OFC impaired conflict-induced behavioral adjustment in the WCST conflict (43, 44).
Fig. 6.
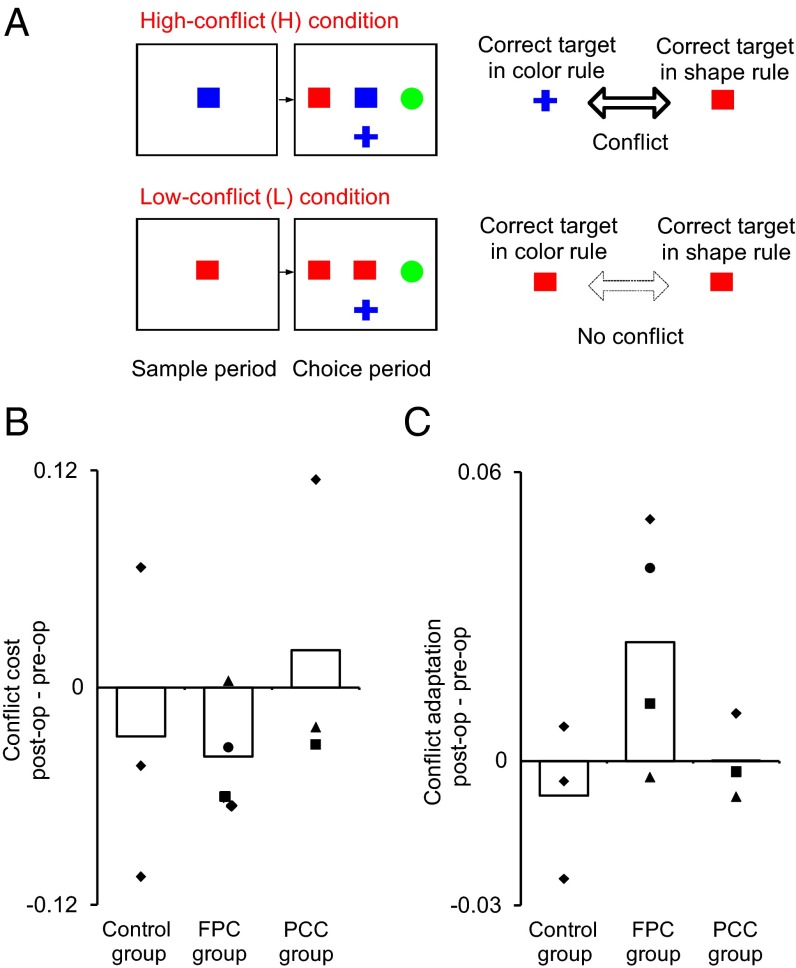
Effects of FPC and PCC lesions on conflict-induced behavioral modulation. (A) In the WCST conflict test, the level of conflict varied from trial to trial. In the high-conflict condition (H), one of the test items matched the sample in color, a second matched the sample in shape, and the last one matched neither color nor shape. Therefore, the monkeys had to resolve the competition\conflict between two potential matching rules to select the correct target. In the low-conflict condition (L), one of the test items matched the sample in both color and shape, and the other two matched in neither color nor shape. Thus, there was no conflict. The low- and high-conflict trials were intermingled, and the selection of low- or high-conflict trial was done according to a pseudorandom sequence that equated the numbers of trials between the two conditions. Trial events and feedbacks were similar between the two conditions. Before testing the monkeys with WCST conflict, the monkeys were trained with two consecutive sessions comprised only of low-conflict trials. All of the other aspects of the task were the same as those of the standard WCST analog. (B) No changes in conflict cost by either the FPC or PCC lesions. The conflict cost was calculated as the difference in STS between the L and H conditions (STS in L trials − STS in H trials), and then, the preoperative value was subtracted from the postoperative value. (C) The conflict adaptation was significantly augmented in FPC-lesioned but not PCC-lesioned monkeys. The conflict adaptation in preoperative as well as postoperative testing was calculated as a mean difference in STS between LH and HH trials, and then, the preoperative value was subtracted from the postoperative value in each monkey. The bars indicate the group mean in each group. The tilted squares indicate values of individual monkeys. The markers for individual data points in B and C indicate the monkeys (Figs. 2 and 3) from which the data were obtained: ●, FPC1; ▲, FPC2; ■, FPC3; and ◆, FPC4 for the FPC group and ■, PCC1; ◆, PCC2; and ▲, PCC3 for the PCC group.
The monkeys in the control, FPC, and PCC groups all performed the WCST conflict task efficiently, attaining more than 10 rule shifts per daily session. For all groups, we observed that the level of conflict influenced monkeys’ behavior in current as well as subsequent trials. For each trial, we calculated speed of target selection (STS) defined as the reciprocal of the time between the test item onset and the first screen touch. With regard to current trials, we found that, in control, FPC, and PCC groups, the STS was slower in high-conflict trials than in low-conflict trials (SI Results, Conflict Cost and Conflict Adaptation in WCST Conflict Test), indicating that the presence of conflict adversely affected the monkeys’ performance (i.e., conflict cost). There was no significant difference in post/pre change of conflict cost (i.e., conflict cost in the postoperative session minus that in the preoperative session) between control and FPC groups [one-tailed t test; t(2.4) = 0.21, P = 0.43] (Fig. 6B). There was also no significant difference in conflict cost between pre- and postoperative performances in the PCC group [one-tailed paired t test; t(2) = 0.44, P = 0.35] (Fig. 6B).
However, we did find significant differences in monkeys’ STSs in subsequent trials between the monkey groups. The conflict hypothesis (45, 46) posits that the assessment of conflict and the ensuing adjustment in control should result in enhanced resolution of conflict and improved behavioral choices when conflict arises subsequently, referred to as a conflict adaptation effect (43–46). To estimate this effect, we compared STS between the high-conflict trials that followed low-conflict trials (LH condition) and the high-conflict trials that followed high-conflict trials (HH condition). STS was calculated in the second trial of each LH or HH pairing. Preoperatively, STS in the HH condition was significantly faster than that in the LH condition in all monkey groups (SI Results). We examined the changes in conflict adaptation between pre- and postoperative performances by two-way ANOVA [monkey (between-subject factor) × lesion (pre\post, within-subject factor)] in each monkey group. Session means in individual monkeys from pre- and postoperative data collection sessions were used in this analysis. We observed a significant main effect of lesion [F(1,55) = 4.8, P = 0.032] without significant interaction between lesion and monkey factors [F(3,55) = 1.23, P = 0.31] in the FPC group. The observed augmentation of conflict adaptation in FPC-lesioned monkeys was related to a significant increase of STS in HH trials, because a two-way ANOVA [monkey (between-subject factor) × lesion (pre\post, within-subject factor)] applied to the STS in HH trials showed that the main effect of lesion was significant [F(1,55) = 22.85, P = 0.000014], but no significant main effect of lesion was seen when the ANOVA was applied to LH trials [F(1,55) = 0.88, P = 0.35].
No significant difference in the magnitude of conflict adaptation between the pre- and postoperative performances was seen in the control group [main effect of lesion (pre\post): F(1,41) = 0.22, P = 0.64; interaction effect: F(2,41) = 0.36, P = 0.7] (Fig. 6C). Having confirmed the significant increase in conflict adaptation in the FPC but not the control group, we compared the post/pre change of conflict adaptation between FPC and control groups by a t test [one-tailed, t(4.96) = 2.06, P = 0.047], which showed a significant difference between the FPC and control groups. These results indicate that the FPC lesion augmented conflict adaptation. In contrast to the FPC group, there was no significant difference in the magnitude of conflict adaptation between the pre- and postoperative performances in the PCC group [one-tailed paired t test; t(2) = 0.021, P = 0.49] (Fig. 6C).
The significantly augmented conflict adaptation in FPC-lesioned monkeys suggests that the conflict-induced recruitment of executive control was significantly enhanced in these monkeys. This finding of enhanced conflict adaptation in FPC-lesioned monkeys but not in PCC-lesioned monkeys indicates a functional dissociation between FPC and PCC. It also distinguishes the effects of FPC lesion from the behavioral consequences of lesions in more posterior parts of prefrontal and medial frontal cortexes, because we previously found that conflict adaptation was impaired after PS or OFC lesions (43, 44).
Lesion Effects on the Monkeys’ Ability to Maintain Information of a Pending Task During Performance of an Interrupting Task (WCST Interruption).
The intact ability of FPC-lesioned monkeys to adapt to frequent rule shifts indicates that FPC is not involved in the execution or coordination of cognitive processes for performing the current task. Rather, their enhanced conflict-induced recruitment of executive control indicates that the allocation of control to the ongoing task could be easily triggered by conflict and appear as an augmented conflict adaptation. These findings suggest that FPC is involved in reallocating parts of cognitive control resources to the alternative tasks/goals, thereby limiting the allocation of cognitive control to the ongoing task. To further evaluate this emerging hypothesis about FPC function, we next conducted a version of WCST analog, which included an interrupting task. The monkeys started their daily session with our standard WCST analog, and in some blocks, when their performance with one of the rules (color matching or shape matching) reached the shift criterion, we required the monkeys to perform two trials of a face detection task (where they were required to select a monkey face over another nonface object), after which they returned to the WCST and continued with the same rule that was relevant before the initiation of the face detection trials. Our control redistribution hypothesis predicts that the monkeys in the FPC group will be less affected by the intervening task performance, because in the absence of FPC-driven exploratory pressure to divert cognitive control from the current task (WCST) toward the new source of reward (face discrimination), the monkeys’ cognitive resources will remain more focused on maintaining the information necessary for performing the WCST analog.
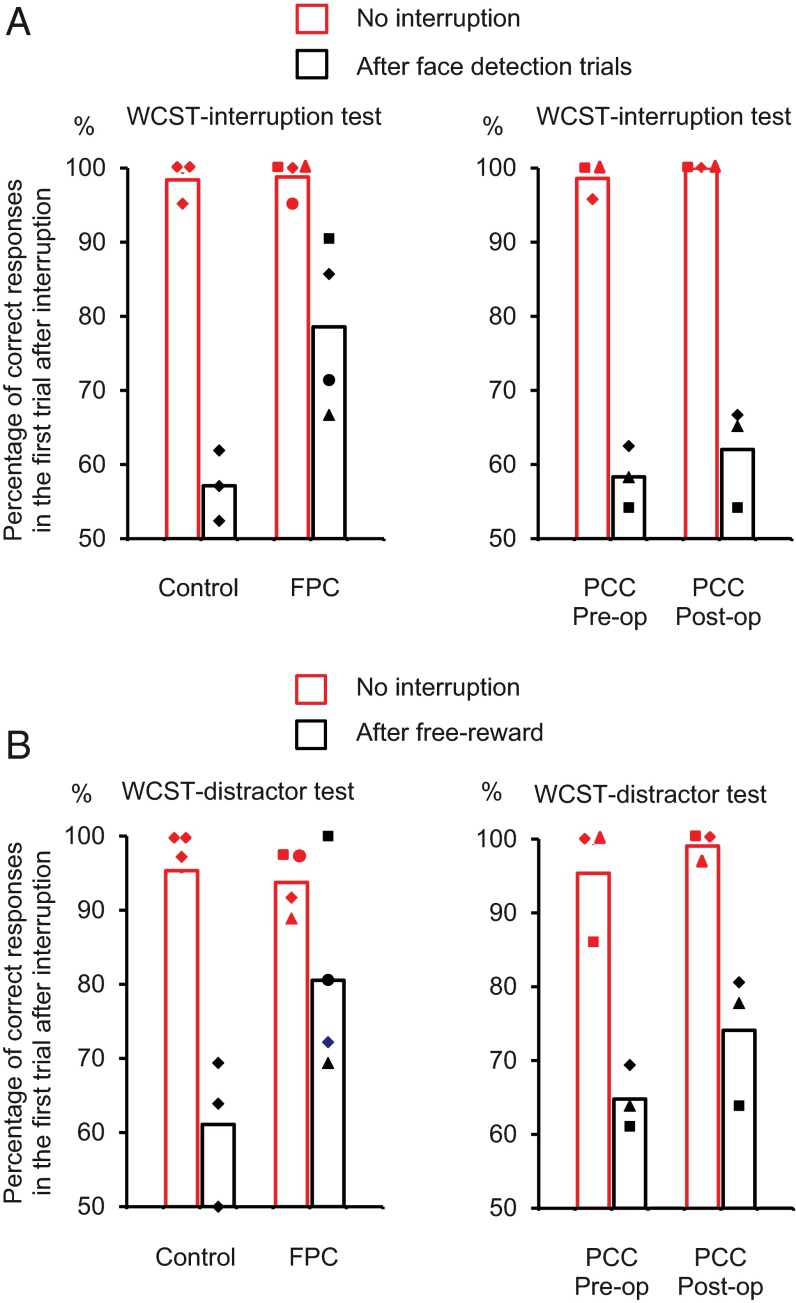
We found a significant difference among groups when the monkeys returned to the WCST task, whereas all of the monkeys in the control, FPC, and PCC groups performed the face detection task very well (more than 90% correct) and took and consumed the obtained reward. In those blocks in which there were no intervening face detection trials, the mean performance in the following WCST trial was very high (∼98%) (Fig. 7A, no interruption), with no significant difference between control and FPC groups [one-tailed t test; t(4) = 0.20, P = 0.85] or between pre- and postoperative performances in PCC group [one-tailed paired t test; t(2) = 1.0, P = 0.42]. The percentage of correct responses in the first trial after resuming WCST was significantly different between after face detection and after no interruption trials (paired t test; P < 0.05 in all of the groups), indicating that imposing the face detection trials adversely affected the performance of all of three groups of monkeys. However, the interruption decreased the performance to different degrees [mean percentage of correct trials in the first trial after the interruption; control group: 57.0 ± 2.7% (mean ± SE); FPC group: 78.6 ± 5.7%; PCC group (preoperative): 58.3 ± 2.4%; PCC group (postoperative): 62 ± 3.9%] (Fig. 7A, after face detection trials and Fig. S2A). The performance of control and PCC-lesioned monkeys after resumption of the pending WCST task was no longer different from the chance level [50%; one-sample t test; control group: t(2) = 2.6, P = 0.12; PCC group (preoperative): t(2) = 3.5, P = 0.07; PCC group (postoperative): t(2) = 3.1, P = 0.09], whereas the performance of monkeys with FPC lesions remained significantly above chance level [one-sample t test; t(3) = 5.04, P = 0.015]. The difference in performance between the control and FPC-lesioned groups was also significant, because a two-way ANOVA [group (control\FPC; between-subject factor) × interrupt (face\no face; within-subject factor)] showed a significant interaction between group and interrupt factors [F(1,5) =10.9, P = 0.022]. There was no significant difference in performance of WCST interruption tests between pre- and postoperative performances in the PCC group, because a paired t test applied to the differences between the interrupted (face) and noninterrupted (no face) trials of each monkey in the PCC group showed that there was no significant difference between the pre- and postoperative performances [t(2) = 1.0, P = 0.42]. A t test applied to the differences between the interrupted (face) and noninterrupted (no face) trials of each monkey in the postoperative performance showed that there was a significant difference between the FPC and PCC groups [unpaired two-tailed t test; t(4.96) = 2.70, P = 0.043]. These results indicate that FPC-lesioned monkeys but not control or PCC-lesioned monkeys maintained information of a pending task during the performance of an interrupting task and then, successfully reinstated the pending task using the maintained information. The failure of control monkeys in remembering the currently relevant rule after performing the face detection task could not be caused by difficulty of face detection trials, because all of the groups of monkeys rarely made errors in face detection trials. Then, the attention of control and PCC-lesioned monkeys might have been diverted to explore the significance of the new task, and this exploration interfered with the maintenance of the currently relevant rule over the interruption. The tendency to explore the significance of the alternative task was diminished in FPC-lesioned animals, and therefore, they remained more focused on maintaining information of the ongoing task (WCST).
Fig. 7.
The performance of monkeys in control, FPC, and PCC groups in (A) WCST interruption and (B) WCST distractor tests. (A) Black bars show percentages of correct responses in the first trial of WCST analog after interruption by two face detection trials. Red bars indicate percentages of correct responses in the same context but without interruption by face detection trials. (B) The same as in A but with the interruption by two free rewards. The markers for individual data points indicate the monkeys (Figs. 2 and 3) from which the data were obtained: ●, FPC1; ▲, FPC2; ■, FPC3; and ◆, FPC4 for the FPC group and ■, PCC1; ◆, PCC2; and ▲, PCC3 for the PCC group (Fig. S2).
Fig. S2.
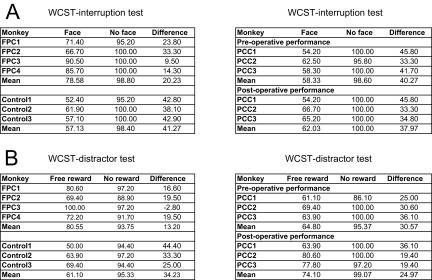
The performance of monkeys in WCST interruption and WCST distractor tests: individual monkey data. (A) The values in face columns are the percentages of correct responses in the first trial of WCST after two face detection trials. Those in no face columns are the percentages of correct responses in the same context but without the interruption by face detection trials. Those in difference columns are the differences between the two values (no face − face). (B) The same as in A but with the interruption by two free rewards. FPC1–FPC4, Control1–Control3, and PCC 1–PCC3 refer to FPC-lesioned, control intact, and PCC-lesioned monkeys.
Lesion Effects on Resistance to Interference by Extratask Salient Events (WCST Distractor).
Our hypothesis also predicts that monkeys with FPC lesions would be less concerned about extratask events, even when they have salience. We tested this in a new postoperatively conducted test that the animals had no previous experience with (WCST distractor). In this test, the monkeys performed the WCST analog; however, in some blocks, after the monkeys reached the shift criterion, two free rewards (food pellets) were given during the following intertrial interval, and the relevant rule remained the same in the following trials. We assessed the monkeys’ performance in the first trial after the administration of the free reward. The arrival of two free rewards in the intertrial interval period was intended to be an unexpected extratask event that might divert the monkeys’ attention/control resources away from the ongoing task, such that performance would be adversely affected in the upcoming WCST trial when they should continue applying the rule that was relevant before the free reward. All monkeys in control, FPC, and PCC groups took and consumed all of the free rewards in our WCST distractor.
Arrival of free reward adversely affected the performance of the monkeys in both control and FPC groups but to different degrees (Fig. 7B and Fig. S2B). When there was no free reward, the mean percentages of correct trials in the first subsequent trial were 95.3 ± 1.0 (mean ± SE) and 93.8 ± 2.1 in control and FPC groups, respectively, and no significant difference was seen between the two groups [one-tailed t test; t(4.08) = 0. 69, P = 0.26]. The mean percentages of correct trials after the free reward dropped to 61.1 ± 5.8 and 80.6 ± 6.9 in control and FPC groups, respectively. Although the performance of control group monkeys was no longer different from the chance level [one-sample t test; t(2) = 1.9, P = 0.19], the performance of FPC-lesioned monkeys remained significantly above the chance level [one-sample t test; t(3) = 4.4, P = 0.021]. A two-way ANOVA [group (control\FPC; between-subject factor) × free reward (delivered\not-delivered; within-subject factor)] showed a significant interaction between group and free reward factors [F(1,5) = 7.19, P = 0.045]. This significant difference between the control and FPC-lesioned groups indicated that the behavior of monkeys with FPC lesions was much less affected by the free reward.
The monkeys in the PCC group had been exposed to WCST distractor when they were previously part of the control group. Their performance was better when data were collected with WCST distractor before (preoperative) and after (postoperative) PCC lesion, possibly because reexposure to the free reward had decreased its effect as a surprising\unexpected extratask event. However, the difference was not statistically significant (paired t test applied to the differences in the percentage of correct responses between the free reward and no free reward showed no significant difference [t(2) = 0.66, P = 0.58, two tailed] between the preoperative testing of the first and second stages of testing). To assess the lesion effect, we calculated the differences in the percentages of correct responses between the free reward and no free reward trials of each monkey in pre- and postoperative testing. A paired t test applied to these differences showed no significant difference between the pre- and postoperative performances [t(2) = 0.66, P = 0.58, two tailed] (Fig. 7B and Fig. S2B), indicating that PCC lesion did not affect the ability of monkeys in performing WCST over extratask distracting salient events.
The WCST distractor test examined the effect of salient events on monkeys’ performance, whereas all other task requirements remained the same, and the monkeys were not required to perform any additional task. The intriguing finding that FPC-lesioned monkeys performed significantly better than Controls cannot be explained by the difference in interest or motivation to obtain the free reward, because all of the FPC monkeys obtained and ate all of the given free reward; also, their performance in no free reward blocks was not different from that of control group monkeys. The results of this WCST distractor test suggest that, although monkeys in both control and FPC groups were aware of the free reward arrival and consumed the reward, its consequences were different between the two groups. The control monkeys’ attention was diverted to explore the new source of reward, and therefore, they temporarily failed to maintain the necessary information to exploit the ongoing task, whereas FPC-lesioned monkeys remained focused and could better exploit the current task. This finding strongly supports our proposed hypothesis that FPC is involved in exploring and reorienting the cognitive control resources from the current task toward the salient events and potential reward sources unrelated to the current task.
Discussion
Our findings show that FPC- and PCC-lesioned monkeys were unimpaired in integrating and coordinating the cognitive processes required to perform the WCST analog (Figs. 1, 4, and 5). The absence of impairment in any behavioral measures related to working memory of rule, selective attention to the current rule, inhibition of a previously relevant rule, and assessment of the behavioral outcome to detect and adapt to the unannounced rule changes is intriguing and indicates functional dissociation between these two areas (FPC and PCC) and other prefrontal and medial frontal cortical areas, such as PS, OFC, and ACC, which comprise a network of areas involved in organizing ongoing task performance (48–51). However, we found significant behavioral differences between FPC-lesioned monkeys and intact monkeys. First, the conflict adaptation effect was significantly augmented in FPC-lesioned monkeys (Fig. 6). Second, the FPC-lesioned monkeys performed significantly better than the intact group monkeys when they returned to the WCST analog after performing an interruption task (Fig. 7A) or facing salient distracting events (Fig. 7B). These results cannot be explained by any of the hypotheses previously raised for FPC function (1–23).
Our findings indicated that FPC lesions improved the monkeys’ performance at exploiting the current task as if the FPC-lesioned monkeys had a more robust working memory to maintain information of the relevant rule while they faced interruptions or salient events. A better than intact performance after FPC damage has also been reported for humans who performed spatial or verbal working memory tasks in individual trials during functional MRI scanning (16). The patients performed significantly better than the intact control subjects when they repeated the same task. These findings together with our findings in this study indicate that a better than intact performance in routine well-learned tasks is common to humans and monkeys after damage to FPC. Although FPC-damaged humans better performed when the same task was repeated, they were impaired when shifting between tasks was required. The rule changes in WCST might be among such task switches; however, FPC-lesioned monkeys did not show impairment in adapting to rule changes in WCST in this study, probably because the long-term training of monkeys with WCST had made the rule shifts a component of the current task.
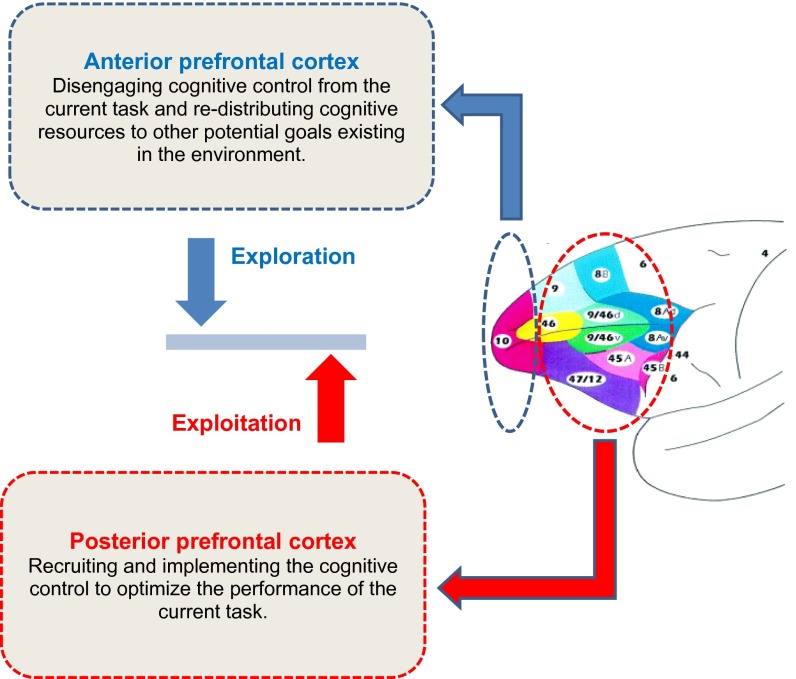
Here, we propose a functional hypothesis of FPC that can explain the unique pattern of behavior that we observed after circumscribed FPC lesions in monkeys and the results of recent human imaging and monkey lesion studies (9, 19, 52). In this hypothesis, FPC is not directly involved in the execution or coordination of cognitive processes for performing a particular task. Instead, we suggest that FPC plays a key role in redistribution of cognitive resources away from the current task to other potential tasks and goals. In complex changing situations, cognitive control sometimes need to be focused on the task at hand (51), but it also needs to be disengaged from the current task and distributed to other potential tasks and goals when focusing on the current goal is no longer behaviorally advantageous (49). Consider an animal engaged in a socially important task, such as grooming a conspecific; it would be maladaptive to devote all available cognitive resources to the current task and perform it the best at the expense of missing cues to other important events and opportunities (for example, those related to obtaining access to mating and food resources). Therefore, the amount of control that ought to be allocated to the current task should be continually regulated and remain amenable to disengagement. We maintain that more posterior parts of prefrontal and medial frontal cortexes, such as PS, area 8, OFC, and ACC, are essential for the cognitive control of a particular task (42, 43, 48–50). Thus, we posit a balance between an “exploitatory” drive from the posterior parts of prefrontal cortex trying to allocate the control to the current task and an “exploratory” drive from FPC that redistributes the control away from the current task when other potential goals are deemed to be behaviorally more important (Fig. 8).
Fig. 8.
Hypothetical functional role of FPC in cognition. Here, we propose that FPC and posterior parts of prefrontal cortex have complementary but dissociable roles in adjusting the distribution of cognitive control. In this model, there is a balance between the exploitation drive from the posterior parts of prefrontal cortex trying to allocate the control to the current task and the exploratory drive from FPC that tries to limit the focus on the current task and directs parts of cognitive resources to other potential goals. The labeling of the cytoarchitectonic areas follows the classification by Petrides and Pandya (25, 26).
In line with this hypothesis, imaging studies in humans have revealed FPC activations associated with the explorative selection of an action target for which smaller outcomes were expected (9) and also, activations associated with the estimated value of unselected targets, which could lead to an actual switch in the following trial (19). The hypothesis also explains the results of the only single-cell recording study ever to be conducted in FPC to date, because unlike neuronal activity in the posterior parts of prefrontal cortex, the FPC cell activity did not represent the task events or the strategy used by the monkeys, and the modulation appeared only around the time of reward delivery (53).
Recent hypotheses have considered the crucial role for PCC in attention and adaptation to changing task demands and suggest its involvement in cognitive deficits associated with various neuropsychological disorders (37, 38). Our findings indicated that, even without a functional PCC selective attention to the current rule, the assessment of behavioral outcome and adaptation to changes in behavioral rule could still be fully realized. Although PCC and ACC have close reciprocal connections (33, 54, 55), our previous (42, 55) and current findings showed that lesions within ACC impaired cognitive flexibility in adapting to rule changes (42) but that PCC lesions had no effect (Fig. 1). The dissociation of behavioral effects of lesions within FPC and PCC indicates that, despite coactivation and coinactivation of FPC and PCC in some imaging studies (7, 18, 56, 57), these brain regions play dissociated roles in primates’ cognition.
Multitasking and branching paradigms (2–4, 12) require maintenance of crucial information of a main task when subjects engage in performing other intervening tasks. The maintained information would allow resumption of the pending task and achieve its goals in a complex situation when attending to other subgoals or tasks is required. Previous studies suggest that FPC is involved in multitasking and cognitive branching (2–4, 8, 12); however, our findings showed that, in the context of WCST, the FPC-lesioned monkeys could maintain information of an ongoing task (WCST) while they performed an interrupting task (face detection) and successfully resume performance of the pending WCST task. The branching or multitasking tasks used in humans included phonological information and required performing intervening tasks, which were more difficult and demanding than the face detection task. Also, in the previous branching paradigms, the subjects continued to switch between the main and intervening tasks. This paradigm was quite different from our case, in which the switches between WCST and face detection tasks occurred only a few times per day. The face detection task at the middle of the WCST might have kept its novelty for the monkeys throughout the data acquisition. This novelty might be a necessary condition to recruit FPC to disengage attention from the main task to explore the significance of a new source of reward. Therefore, considering the differences between the branching paradigms used in human studies and the WCST interruption task used in our studies with monkeys, our findings do not necessarily rule out the involvement of FPC in multitasking or branching.
In humans, macaque monkeys, and marmosets, the most rostral part of the frontal lobe is occupied by area 10; however, the size of human area 10 is much larger than the corresponding areas in the other primates (26–29, 58) and might include several functionally distinct compartments (59). Therefore, human rostral frontal cortex might support some unique cognitive functions that are inaccessible in nonhuman primates and could not be simply explained by models proposed based on studies in nonhuman subjects. In addition, some caution needs to be taken in generalizing the results of our study in macaque monkeys and the functional hypothesis to the human case. Long-term training required for monkey studies might influence the functional organization of the neural network that supports cognitive set shifting and multitasking. That said, understanding the crucial contribution of FPC in nonhuman primates would mark a major advance in understanding of FPC function in humans, because one would presume that human FPC function would quite likely be an elaboration on or more sophisticated use of some basic underlying cognitive processes mediated by this region in nonhuman primates (25, 27–29).
Our hypothesis assumes that FPC is not just acting like a switch to promote shifting between exploitation and exploration; instead, FPC enables an efficient context-dependent allocation of cognitive resources by adjusting redistribution of executive control. To redistribute the cognitive resources to other potential tasks and goals, FPC has to collect highly processed information regarding the cost and benefit of each task and also, the internal state of the subject and constructs estimated values of potential tasks\goals in complex changing situations. FPC has one of the highest spine densities and numbers of spines per neuron (60) and receives the majority of its input from multimodal association areas (8, 26–29), which suggest that FPC has a high capacity for integrating highly processed information. Our hypothesis predicts that FPC lesions would impair the overall flexibility of an animal to properly distribute cognitive resources to salient events and potential opportunities and that the chance of success in social life and survival in a complex environment may be compromised in the long term.
Our findings for the first time, to our knowledge, show that selective and bilateral lesions within primate FPC did not impair performance in highly demanding cognitive tasks and indeed, made the animals more focused on current task performance. An important message of our findings is that, when tasks are well-practiced, posterior regions of lateral, orbital, and medial frontal cortices support execution of these well-learned tasks. Our findings suggest that there is a functional dissociation between the anterior and posterior parts of prefrontal cortex for supporting the execution of well-learned tasks. Additional studies to examine defective effects of lesions within FPC need to evaluate the contribution of FPC during the learning period or when the subjects are introduced to a novel situation with multiple tasks/goals. In these situations, FPC might be involved in assessing the significance of each task/goal and adjust the allocation of cognitive control for learning and pursuing the most advantageous task/goal.
Conclusion
The emergence of executive control (43, 51) for organizing cognitive processes has been an evolutionary gift that has increased the efficiency and flexibility of implementing cognitive resources to achieve the current goal. However, the complexity of the behavioral context might have provided strong evolutionary pressure for developing another complementary system for disengagement and redistribution of the executive control. The behavior of monkeys with FPC lesions suggests that the latter functional system depends on the integrity of FPC neurocircuitry. By providing this capacity to cognition, FPC might have supported the emergence of some of the most complex and flexible behavior in primates.
SI Text
Adequate measures were taken to minimize pain or discomfort. Water was always available ad libitum in the home enclosure; each monkey’s daily food ration was delivered in the test box at the end of each behavioral session and supplemented with fruit in the home enclosure. Seven monkeys (males; 5–7 kg; age between 3 and 5 y at the start of training) without previous training experience with any experimental task were used in this study.
Surgery.
The lesion operations were performed in sterile conditions with the aid of an operating microscope. The monkeys were first sedated with both ketamine (10 mg/kg) and xylazine (0.25–0.5 mg/kg) i.m. and intubated, and then, they were artificially respirated and anesthetized throughout surgery with isoflurane (1.0–2.0% to effect). Steroids (dexamethasone, 2 mg/kg) were given the night before surgery i.m., and three doses were given 4–6 h apart (i.v. or i.m.) on the day of surgery to protect against intraoperative edema and postoperative inflammation. The monkey was given atropine (0.05 mg/kg) to reduce secretions, antibiotic (pentocillin, 8.75 mg/kg) for prophylaxis of infection, opioid (buprenorphine 0.01 mg/kg i.v., repeated two times at 4- to 6-h intervals on the day of surgery i.v. or i.m.) and nonsteroidal antiinflammatory (meloxicam, 0.2 mg/kg i.v.) agents for analgesia, and an H2 receptor antagonist (ranitidine, 1 mg/kg i.v.) to protect against gastric ulceration as a side effect of the combination of steroid and nonsteroidal antiinflammatory treatment. Heart rate, oxygen saturation of hemoglobin, mean arterial blood pressure, end tidal CO2, body temperature, and respiration rate were monitored continuously throughout surgery.
For the FPC lesions, a bone flap was raised over the right and left anterior prefrontal cortexes, the dura mater was cut and reflected, and the craniotomy was extended anteriorly by rongeurs. The cortex within intended lesion extent in both hemispheres was removed by aspiration using a small-gauge metal aspirator. The caudal limit of the FPC lesions was an imaginary line drawn at 2 mm posterior to the rostral tip of the PS. All cortex anterior to this line was removed (Fig. 2). The white matter was spared, except in the most rostral part of the lesion. For the PCC lesions, a semicircular bone flap was raised largely over one hemisphere but slightly extending over midline to the other hemisphere, and the dura mater was cut and reflected to provide access to the interhemispheric region. The lesion was made in one hemisphere first, and then, the falx was cut to provide access to the cortex in the lesion site in the other hemisphere. The dura mater was subsequently sewn back, the bone flap was replaced, and the wound was closed in layers. The operated animals rested for 2 wk after surgery before beginning postoperative testing. Unoperated control animals rested for the same period between preoperative and postoperative testing. The rostral limit of the PCC lesions was an imaginary vertical line passing through the most posterior level of the central sulcus, and the caudal limit was another imaginary line passing from the most posterior aspect of the splenium of the corpus callosum and extending up to the posterior end of the cingulate sulcus; to avoid making a lesion in area 7m, we also considered another imaginary line that continued horizontally at about the level of cingulate sulcus before it turns superiorly. Therefore, the posterior border was limited by the crossing of these two imaginary lines. The cortex on the ventral bank of cingulate sulcus and the surface of cingulate gyrus (dorsally limited by the cingulate sulcus and ventrally limited by the corpus callosum) was removed. The PCC lesion aimed at removing much of the cortex in areas 23 and 31 (Fig. 3 and Fig. S1) (32, 33). The lesion was made in one hemisphere first, and then, the falx was cut to provide access to the cortex in the lesion site in the other hemisphere.
Histology.
At the conclusion of the experiments, the FPC-lesioned animals were deeply anesthetized and then, perfused through the heart with saline followed by formol saline solution. Their brains were blocked in the stereotaxic plane, removed from the skull, put in sucrose formalin solution until the block sank, and subsequently, cut in 50-μm horizontal sections on a freezing microtome. Every 5th or 10th section was retained and stained with cresyl violet.
Apparatus, Stimuli, and WCST Analog Task.
The monkeys were trained to perform standard WCST analog, WCST conflict, and several related behavioral tasks. The tasks were provided in automated test apparatuses as described in our previous study (42, 43, 48). The subject sat unrestrained in a wheeled transport cage fixed in position in front of a touch-sensitive screen, on which the stimuli were displayed. An IR camera monitored the general status of the monkey, especially pellet uptake and consumption. The versions of the WCST used in this lesion study used 36 different stimuli comprising of six colors (red, yellow, green, cyan, blue, and magenta) and six shapes (square, triangle, circle, hexagon, cross, and ellipse). The sample in each trial was selected at random (without replacement until the entire set had been used) from 36 stimuli. In each trial, the test items were also selected from the same set of 36 stimuli and at random (with the restrictions imposed by the necessity to generate either a low- or high-conflict trial). The locations of the three test items (i.e., to the left, right, or bottom of the sample) were also chosen at random. The size of the stimuli was 5–6 cm on the screen. The center to center distance between the test items and sample was 15 cm on the screen.
Cognitive Tasks.
Standard WCST analog (comprised only of high-conflict condition trials).
To facilitate comparison between results of this study on FPC and those of our previous studies, we used the same task paradigm and the same analytical approaches that were used in our previous studies (42) (Fig. 1A). The total number of trials in a daily session was fixed at 300, and the uncued rule shifts occurred whenever the animal attained 85% correct in 20 consecutive trials. The first rule of the day alternated between days. The correction trial procedure was not used. For the WCST analog, our data were collected from 15 preoperative sessions and 15 postoperative consecutive sessions.
WCST conflict (comprised of low- and high-conflict condition trials randomly intermixed).
The task paradigm was the same as that we used previously (43) and is illustrated in Fig. 6A.
The repetition of an identical stimulus is problematic in studies of conflict (61): priming caused by the stimulus repetition would occur in addition to adaptation caused by conflict experience. However, in our study, the probability that an identical sample appeared in succeeding two trials was very low, because samples were taken from a set without replacement: all stimuli in the set (36 samples) had to be exhausted before the next set started. At the start of a new set only, there was a possibility (0.03) for the repetition of the same sample as that shown in the previous trial. Moreover, because the correct test item was randomly selected from five stimuli, the probability of repetition of an identical pair of sample and correct test item was even smaller.
For the WCST conflict test, the data were collected from 15 preoperative and 15 postoperative sessions. If the monkeys performed markedly less well than normal in any given session (fewer than seven rule shifts in a daily session), then that daily session was excluded from the analyses; as a result, one monkey from the control group and one monkey from the FPC group only contributed 14 sessions to the overall data. In a few daily sessions, the monkeys exhibited fatigue toward the end of the daily sessions so that their STS significantly decreased toward the end of daily sessions. We applied a three-way ANOVA [early/late × conflict (high/low) × response direction (left/right/bottom)] to STS in every daily session for each monkey. The early/late factor had two levels of the first 100 trials and the last 100 trials of each session. A few sessions with a significant (P < 0.01) main effect of early/late or its interaction with conflict or overall interaction of early/late and conflict and response direction were selected. A common observation was that, in these daily sessions, the STS in the last 100 trials was slower than that of the first 100 trials. In these selected daily sessions, the analyses were confined to the first 200 trials.
WCST Interruption Test.
Before conducting the WCST interruption task, all of the monkeys in the control and FPC groups performed three daily refresher sessions of our standard WCST analog (which contains only high-conflict trials) and then, performed three consecutive daily sessions that included only face detection trials so as to offer familiarity with that tasks’ requirements. The three daily refresher sessions of our standard WCST analog were also given in the second stage of testing before running the WCST interruption test for PCC-lesioned monkeys. In each face detection trial, a monkey face and another clipart object appeared on the left and right sides of the screen. The monkey face and clipart objects were randomly chosen from a large set of pictures. The side on which the face appeared was random between left and right sides of the screen. A touch to the monkey face was rewarded with the same reward that is normally given for a correct response in a WCST trial. The monkeys in both groups mastered the face detection task rapidly, and on the third daily session, they reached more than 85% correct performance. Then, the monkeys were tested in seven consecutive sessions with the WCST interruption test. Each daily session was comprised of six blocks. In three branching blocks (blocks 2, 4, and 6), after the monkeys reached a criterion of 11 corrects in 13 consecutive trials (i.e., shift criterion set at ∼85% correct), two face detection trials were introduced, and after resumption of the WCST, the monkeys had to attain the shift criterion again with the same rule (color or shape), which was reinforced before introducing the face detection trials. Each intertrial interval between the last WCST trial and the first face detection trial, between the two face detection trials, and between the second face detection and the following WCST trials was 4 s. In the three other no branching blocks (blocks 1, 3, and 5), after the monkeys reached the shift criterion, no face detection trial was introduced; however, the monkeys still had to attain the criterion again as they did in the branching blocks to enable control data in this noninterrupt condition to be acquired after the same performance levels on WCST were achieved. The starting WCST rule (in the first block) in each daily session alternated between color and shape. The monkeys rarely made errors in face detection, and those blocks with errors in the face detection trials were excluded from the analyses.
WCST Distractor Test.
After completion of the testing with the WCST interruption task, the monkeys in both control and FPC groups performed three refresher sessions of our standard WCST analog (i.e., all trials were high conflict, and no face detection trials intervened). Then, the monkeys were tested with the WCST distractor version. The three daily refresher sessions of our standard WCST analog were also given in the second stage of testing before running the WCST distractor test for PCC-lesioned monkeys. Because the monkeys had no previous experience with free reward given between trials while in the midst of the WCST performance, we required the monkeys to perform four consecutive daily sessions with the WCST free reward test before data were collected in nine consecutive daily sessions. Each daily session was comprised of six blocks composed of three distractor blocks (blocks 2, 4, and 6) and three no distractor blocks (blocks 1, 3, and 5). In distractor blocks, when the monkeys had reached a criterion of 11 corrects in 13 consecutive trials (the shift criterion for this task), two free rewards (food pellets) were given during the intertrial interval period (before the sample onset of the following trial); then, the monkey had to attain the shift criterion again with the same rule (color or shape) that was reinforced before introducing the free rewards. In no distractor blocks, after the monkeys reached the shift criterion, no free reward was delivered, but the monkeys had to attain the shift criterion again as they did in the distractor blocks. The averaged performance at the first trial immediately after the free rewards (for distractor blocks) or after attaining of the shift criterion (for no distractor blocks) was analyzed. The starting WCST rule (i.e., the rule reinforced in the first block) in each daily session alternated between color and shape across days.
In the FPC group, for all of the behavioral measures in WCST and WCST conflict tasks, we had pre- and postoperative data, and therefore, within-group comparisons were conducted. However, WCST interruption and WCST distractor tests were designed and examined postoperatively to evaluate our hypothesis about FPC, which emerged from behavior of FPC-lesioned monkeys in WCST and WCST conflict tasks, and therefore, the performances in these tasks were compared between FPC and control groups.
Speed of Target Selection in WCST Conflict Task.
We measured the time from the onset of the test items to the monkey’s first touch on the screen as the response time and took its reciprocal (1/response time) to obtain the STS in individual trials. We then averaged the STS across trials separately for each conflict condition [either low (L) /high (H) or LH/HH] and response direction (left/right/bottom) in each daily session of each monkey and normalized the average STS by dividing it by its mean between the conflict conditions in each response direction. We, thus, obtained a pair of STS values (either L/H or LH/HH) for each response direction in each daily session of each monkey. Finally, the normalized mean STSs were averaged across response directions and daily sessions to obtain a grand average STS for each conflict condition in each monkey (but separately for pre- and postoperative sessions). To assess the presence of the conflict cost (H vs. L) and conflict adaptation (HH vs. LH), the grand average STSs were compared between two conflict conditions by a one-sample Wilcoxon signed rank test. The effects of FPC lesions were evaluated by comparing the pre\post differences in conflict cost (L − H) or conflict adaptation (HH − LH) by t test between the FPC-lesioned and control monkeys. Note that only one grand average for each conflict condition in each monkey was used in the statistical tests. Session means for each conflict condition were used in the two-way ANOVA [monkey × lesion (pre\post)]. Only correct trials preceded by correct trials were used for these analyses.
SI Results
Frequency of Errors in WCST Analog.
More quantitative data and statistical results in relation to Fig. 4 are described here. The monkeys in control, FPC, and PCC groups rarely selected the test item that did not match the sample in either dimension (nonperseverative errors). The mean percentages of nonperseverative errors were 0.63 ± 0.29 (mean ± SEM) preoperatively and 0.61 ± 0.2 postoperatively in the control group, 1.75 ± 0.69 preoperatively and 1.65 ± 0.52 postoperatively in the FPC group, and 0.27 ± 0.09 preoperatively and 0.2 ± 0.06 postoperatively in the PCC group. A t test applied to the pre\post differences in individual monkeys indicated that the FPC group was not impaired relative to the control group [one-tailed t test; t(3.3) = 0.29, P = 0.39]. Another t test showed that there was no significant difference between pre- and postoperative percentages in the PCC group [one-tailed paired t test; t(2) = 2.0, P = 0.092].
Perseverative errors (defined here as matching based on the other currently irrelevant rule) comprised the majority of the total errors: 95.9 ± 1.2% preoperatively and 95.8 ± 0.9% postoperatively in the control group, 90.2 ± 3.6% preoperatively and 90.9 ± 2.7% postoperatively in the FPC group, and 97.8 ± 0.8% preoperatively and 97.8 ± 0.4% postoperatively in the PCC group. The monkeys committed more perseverative errors in the early trials after rule shift. The percentages of perseverative errors in seven trials after the rule shift were 33.6 ± 2.8 preoperatively and 31.07 ± 3.6 postoperatively in the control group, 32.7 ± 4.5 preoperatively and 33.08 ± 5.9 postoperatively in the FPC group, and 23.8 ± 1.15 preoperatively and 23.0 ± 1.2 postoperatively in the PCC group, after excluding the first block of each daily session. A t test applied to the pre\post differences of seven-trial mean percentages in individual monkeys indicated that the FPC group was not impaired relative to the control group [one-tailed t test; t(4.7) = 1.35, P = 0.12]. Another t test showed that there was no significant difference between pre- and postoperative percentages in the PCC group [one-tailed paired t test; t(2) = 1.1, P = 0. 19].
The total number of perseverative errors also was not different between pre- and postoperative levels in the control, FPC, or PCC group. The mean percentage of perseverative errors was 13.6 ± 2.1 preoperatively and 13.1 ± 1.84 postoperatively in the control group, 14.38 ± 2.1 preoperatively and 14.3 ± 2.7 postoperatively in the FPC group, and 10.7 ± 0.6 preoperatively and 9.7 ± 0.97 postoperatively in the PCC group. A t test applied to the pre\post differences in individual monkeys indicates that the FPC group was not impaired relative to the control group [one-tailed t test; t(4.3) = 0.34, P = 0.37]. Another t test showed that there was no significant difference between pre- and postoperative percentages in the PCC group [one-tailed paired t test; t(2) = 1.6, P = 0.12].
Conflict Cost and Conflict Adaptation in WCST Conflict Test.
More statistical results in relation to Fig. 6 are described here. A grand average of the difference between normalized STS in L and H conditions was calculated for each monkey. A one-sample Wilcoxon signed rank test (one tailed) applied to the grand averages in three monkeys of the control group, four monkeys of the FPC group, or three monkeys of PCC group showed that the conflict between behavioral rules adversely affected the performance of the monkeys in the current trial (conflict cost) in the preoperative tests in all groups (Z = −1.6, P = 0.05 in the control group; Z = −1.8, P = 0.03 in the FPC group; and Z = −1.6, P = 0.05 in the PCC group) as well as in the postoperative tests in all groups (Z = −1.6, P = 0.05 in the control group; Z = −1.8, P = 0.03 in the FPC group; and Z = −1.6, P = 0.05 in the PCC group). Results of the comparison of pre\post changes in the conflict cost are described in the text.
A grand average of the difference between normalized STS in HH and LH trials was calculated for each monkey. A one-sample Wilcoxon signed rank test (one tailed) applied to the grand averages in three monkeys of the control group, four monkeys of the FPC group, or three monkeys of the PCC group showed that the conflict between behavioral rules resulted in a faster STS in the following trial (conflict adaptation) in the preoperative tests in all groups (Z = −1.6, P = 0.05 in the control group; Z = −1.8, P = 0.03 in the FPC group; and Z = −1.6, P = 0.05 in the PCC group) as well as in the postoperative tests in all groups (Z = −1.6, P = 0.05 in the control group; Z = −1.8, P = 0.03 in the FPC group; and Z = −1.6, P = 0.05 in the PCC group). Results of the comparison of pre\post changes in the conflict adaptation are described in the text.
In the FPC group, for all of the behavioral measures in WCST and WCST conflict tasks, we had pre- and postoperative data, and therefore, within-group comparisons were conducted. However, WCST interruption and WCST distractor tests were designed and examined postoperatively to evaluate our hypothesis about FPC that emerged from behavior of FPC-lesioned monkeys in WCST and WCST face tasks, and therefore, the performances in these tasks were compared between FPC and control groups. In the PCC group, for all of the tests, we had pre- and postoperative data, and therefore, within-group comparisons were conducted.
SI Discussion
Difference in Lesion Extent Between the Lesion Groups.
The FPC lesion included the lateral, orbital, and medial areas of the frontal pole and therefore, was comparable in size to our previous lesions in ACC and OFC. The lesions in both banks of PS and PCC might have been slightly larger in extent. However, we found that lesions in ACC, OFC, and PS impaired the performance in WCST but that lesions in FPC and frontal cingulate cortex had no effect. Therefore, it is unlikely that the differences in the absolute extent of lesion led to the differences in consequences of lesions on performing the cognitive tasks.
Relevance of Our Findings to the Default Mode Network Defined in Human Imaging Studies.
In human imaging studies, FPC and PCC show coactivation or codeactivation while subjects perform various tasks or are at rest, respectively. Therefore, FPC and PCC have been considered to be core structures within the brain’s default mode network (7). Close neuroanatomical connections between these two brain regions is confirmed in primate species and suggest that these brain regions might be functionally related (25, 26–29). Electrophysiological recording in the PCC of monkeys also suggests its involvement in default mode processing (36–38). Vincent et al. (62) studied the functional connectivity in anesthetized monkeys by a seed-based approach, where the seed for finding the time-dependent correlations was placed at the PCC. Vincent et al. (62) did not find a correlation between PCC and FPC. However, the study by Vincent et al. (62) was conducted in anesthetized monkeys, and therefore, the differences in correlation maps between humans (who were in the awake resting state during scanning) and monkeys (under isoflurane anesthesia) might have resulted from differences in state. Vincent et al. (62) concluded that the PCC was more extensively correlated with the medial prefrontal cortex in humans than in monkeys and that these differences might reflect either species-specific functional differences or effects attributable to behavioral state. Therefore, these findings do not necessarily indicate that rostral prefrontal cortex is not part of default mode network in monkeys. In a follow-up study, Margulies et al. (63) examined resting-state functional connectivity in humans and macaque monkeys and showed that PCC and dorsolateral prefrontal cortex (including area 10) showed functional connectivity with the precuneus. Mantini et al. (64) performed a meta-analysis of imaging studies in monkeys and compared the resting-state connectivity patterns between humans and monkeys across different studies. An important aspect of the study by Mantini et al. (64) was that they also conducted an imaging study, in which during brain imaging, the monkeys were awake, but eye fixation without any additional task was required to simulate resting state in human imaging studies. Using different analytical approaches, Mantini et al. (64) defined a constellation of neural areas that could be the neural substrate of the default mode network in monkeys. These areas included the PCC but differed to some extent from the functional connectivity map originating in the PCC, which was reported by Vincent et al. (62). Mantini et al. (64) suggested that PCC and areas on the dorsal anterior aspect of monkeys’ brains (including areas 9 and 46) could be the main hubs of the default mode network in macaque monkeys; their defined areas extended to the most anterior parts of the dorsal prefrontal cortex. Kojima et al. (65) more directly addressed this issue in a PET study in awake behaving monkeys and compared the task performance with resting condition. Kojima et al. (64) reported that medial prefrontal cortex (area 10) and PCC showed higher activity during rest than during performance of a spatial or nonspatial delayed response task (Fig. 5) [supplementary tables 1–3 in the work by Kojima et al. (65) and the work by Watanabe (57)]. Furthermore, activation of FPC and PCC in resting state has been reported in chimpanzees (56). Therefore, previous studies suggest similarities in neural components of the default mode network between humans and nonhuman primates, but investigating the exact correspondence between the neural substrates in monkeys and humans requires imaging in a similar state (resting) in both species.
Relevance of Our Hypothesis to Activation Pattern in Imaging Studies.
In human imaging studies, the activation in FPC occurs in a wide range of task situations. However, many of these situations require browsing and exploring the representations of externally or internally generated information. Examples include internal generation of information and its maintenance during mind wandering, planning, relational or analogical integration in reasoning, retrieval of episodic or prospective memory, multitasking and branching paradigms, and integration of the outcome of multiple processes (8, 12). The activation of FPC in these studies could be attributed to the role of FPC in exploring the value of different tasks, events, or internal representations of stored information and disengaging and reallocating the cognitive resources among them.
Relevance of Our Hypothesis to the Conflict-Monitoring Hypothesis.
Theories that describe the recruitment and allocation of executive control in changing environments, such as the influential conflict-monitoring hypothesis (45), try to explain how the executive control is recruited\allocated to a particular task; however, it is not clear how the magnitude and duration of allocated control are adjusted in an environment with several potential goals\tasks and how the “disengagement of executive control from the current task” is regulated (49). Our proposed functional hypothesis (Fig. 8), which is a parsimonious explanation of the behavioral manifestation of FPC lesions in this study, also suggests answers to these important questions and embraces findings from recent imaging studies (9, 19).
Relevance of Our Hypothesis to Neuropsychological Disorders.
A distortion in the balance between exploitation and exploration drives (Fig. 8), which could originate from damage to either posterior or anterior parts of prefrontal areas, might lead to an inability to prioritize a goal and properly distribute the necessary amount of cognitive resources to particular goals in the environment. An inability to focus attention to a particular task\goal and increased distractibility by events outside the current task have been frequently reported in schizophrenic patients (66, 67). In these patients, attention is abnormally drawn by task-irrelevant stimuli. A functional MRI study found a hyperactivation in the FPC and a hypoactivation in the posterior–lateral prefrontal areas in schizophrenic patients (68). These findings in the context of our proposed hypothesis suggest that, in schizophrenic patients, a possible bias toward the exploration drive caused by enhanced activity in the FPC and a weakened exploitation drive caused by a hypoactivation in the posterior prefrontal areas might lead to a difficulty in focusing on a particular task and increased distractibility by task-irrelevant events. Obsessive–compulsive symptoms are characterized by recurring and repetitive thoughts and behaviors accompanied by excessive focus on the details of particular tasks. A functional MRI study found a decrease in FPC activity and an increase in the inferior frontal cortex activity in subjects with high obsessive–compulsive symptom scores (69). These findings suggest that a possible bias toward the exploitation drive and an attenuated exploratory drive might have contributed to the symptoms of obsessive–compulsive disorder.
Relevance of Our Hypothesis to the Hierarchical Hypothesis.
Influential hypotheses have assumed the presence of hierarchical anterior–posterior organization in the frontal lobe based on the level of abstractness of representation on which the control exerted (17) or the temporal structure of the information required for action selection (2, 4, 70); the more anterior areas work with more abstract representations or temporally more dispersed events. Our proposed model of functional organization of frontal cortex (Fig. 8) does consider a functional dissociation between posterior and anterior parts of the prefrontal cortex. However, our hypothesis emphasizes differences in the contribution of FPC and more posterior prefrontal areas to information processing in relation to the allocation of cognitive control rather than the hierarchy of information. Therefore, the contributions of FPC and posterior prefrontal areas to cognitive control are complementary. However, these distinct contributions may require handling of different kinds of information: posterior areas process information related to control of the current task, whereas FPC collects information about the relative values (cost and benefit) of various potential tasks and goals. The latter tends to be more abstract and temporally distant than the former. Thus, our hypothesis is not inconsistent with the hierarchy hypothesis.
Acknowledgments
We thank T. Hashikawa for histology. This work was supported by Grant-in-Aid for Scientific Research A19200027 and the Funding Program for World-Leading Innovative R&D on Science and Technology from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422629112/-/DCSupplemental.
References
- 1.Bludau S, et al. Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage. 2014;93(Pt 2):260–275. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399(6732):148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 3.Pollmann S. Anterior prefrontal contributions to implicit attention control. Brain Sci. 2012;2(2):254–266. doi: 10.3390/brainsci2020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donoso M, Collins AG, Koechlin E. Human cognition. Foundations of human reasoning in the prefrontal cortex. Science. 2014;344(6191):1481–1486. doi: 10.1126/science.1252254. [DOI] [PubMed] [Google Scholar]
- 5.Christoff K, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14(5):1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 6.Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117(6):1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- 7.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 8.Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 9.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441(7095):876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner G, Koch K, Reichenbach JR, Sauer H, Schlösser RG. The special involvement of the rostrolateral prefrontal cortex in planning abilities: An event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44(12):2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38(6):848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 12.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11(7):290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuda J, et al. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int J Psychophysiol. 2007;64(3):233–246. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Rowe JB, et al. Is the prefrontal cortex necessary for establishing cognitive sets? J Neurosci. 2007;27(48):13303–13310. doi: 10.1523/JNEUROSCI.2349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 19.Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62(5):733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Wendelken C, Bunge SA. Transitive inference: Distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J Cogn Neurosci. 2010;22(5):837–847. doi: 10.1162/jocn.2009.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volle E, Gilbert SJ, Benoit RG, Burgess PW. Specialization of the rostral prefrontal cortex for distinct analogy processes. Cereb Cortex. 2010;20(11):2647–2659. doi: 10.1093/cercor/bhq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci. 2011;15(4):143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumontheil I, Gilbert SJ, Frith CD, Burgess PW. Recruitment of lateral rostral prefrontal cortex in spontaneous and task-related thoughts. Q J Exp Psychol (Hove) 2010;63(9):1740–1756. doi: 10.1080/17470210903538114. [DOI] [PubMed] [Google Scholar]
- 24.Dreher JC, Koechlin E, Tierney M, Grafman J. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3:e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114(3):224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Burman KJ, et al. Subcortical projections to the frontal pole in the marmoset monkey. Eur J Neurosci. 2011;34(2):303–319. doi: 10.1111/j.1460-9568.2011.07744.x. [DOI] [PubMed] [Google Scholar]
- 29.Burman KJ, Reser DH, Yu HH, Rosa MG. Cortical input to the frontal pole of the marmoset monkey. Cereb Cortex. 2011;21(8):1712–1737. doi: 10.1093/cercor/bhq239. [DOI] [PubMed] [Google Scholar]
- 30.Small DM, et al. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 31.McCoy AN, Platt ML. Risk-sensitive neurons in macaque posterior cingulate cortex. Nat Neurosci. 2005;8(9):1220–1227. doi: 10.1038/nn1523. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: I. Three-dimensional and cytoarchitectonic organization. J Comp Neurol. 2000;426(3):339–365. doi: 10.1002/1096-9861(20001023)426:3<339::aid-cne1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol. 2005;485(3):218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60(1):19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heilbronner SR, Hayden BY, Platt ML. Decision salience signals in posterior cingulate cortex. Front Neurosci. 2011;5(2011):55. doi: 10.3389/fnins.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr Biol. 2009;19(18):1532–1537. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol. 1991;13(6):909–922. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- 41.Stuss DT, et al. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: Effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38(4):388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 42.Buckley MJ, et al. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325(5936):52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- 43.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318(5852):987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 44.Mansouri FA, Buckley MJ, Tanaka K. The essential role of primate orbitofrontal cortex in conflict-induced executive control adjustment. J Neurosci. 2014;34(33):11016–11031. doi: 10.1523/JNEUROSCI.1637-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 46.Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7(4):380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- 47.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 48.Kuwabara M, Mansouri FA, Buckley MJ, Tanaka K. Cognitive control functions of anterior cingulate cortex in macaque monkeys performing a Wisconsin Card Sorting Test analog. J Neurosci. 2014;34(22):7531–7547. doi: 10.1523/JNEUROSCI.3405-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioral adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 50.Mansouri FA, Matsumoto K, Tanaka K. Prefrontal cell activities related to monkeys’ success and failure in adapting to rule changes in a Wisconsin Card Sorting Test (WCST) analog. J Neurosci. 2006;26:2745–2756. doi: 10.1523/JNEUROSCI.5238-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 52.Boschin EA, Piekema C, Buckley MJ. Essential functions of primate frontopolar cortex in cognition. Proc Natl Acad Sci USA. 2015;112(9):E1020–E1027. doi: 10.1073/pnas.1419649112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsujimoto S, Genovesio A, Wise SP. Frontal pole cortex: encoding ends at the end of the endbrain. Trends Cogn Sci. 2011;15:169–176. doi: 10.1016/j.tics.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Morecraft RJ, Van Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. J Comp Neurol. 1993;337(4):669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- 55.Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469(1):37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- 56.Rilling JK, et al. A comparison of resting-state brain activity in humans and chimpanzees. Proc Natl Acad Sci USA. 2007;104(43):17146–17151. doi: 10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe M. Are there internal thought processes in the monkey?—default brain activity in humans and nonhuman primates. Behav Brain Res. 2011;221(1):295–303. doi: 10.1016/j.bbr.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 58.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 59.Moayedi M, Salomons TV, Dunlop KA, Downar J, Davis KD. Connectivity-based parcellation of the human frontal polar cortex. Brain Struct Funct. 2014;10:1–14. doi: 10.1007/s00429-014-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobs B, et al. Regional dendritic and spine variation in human cerebral cortex: A quantitative golgi study. Cereb Cortex. 2001;11(6):558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 61.Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6(5):450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- 62.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 63.Margulies DS, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106(47):20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantini D, et al. Default mode of brain function in monkeys. J Neurosci. 2011;31(36):12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kojima T, et al. Default mode of brain activity demonstrated by positron emission tomography imaging in awake monkeys: Higher rest-related than working memory-related activity in medial cortical areas. J Neurosci. 2009;29(46):14463–14471. doi: 10.1523/JNEUROSCI.1786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Arch Gen Psychiatry. 1990;47(2):171–179. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- 67.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64(1):34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbalat G, Chambon V, Franck N, Koechlin E, Farrer C. Organization of cognitive control within the lateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 2009;66(4):377–386. doi: 10.1001/archgenpsychiatry.2009.10. [DOI] [PubMed] [Google Scholar]
- 69.den Braber A, et al. Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain. 2010;133(10):3123–3140. doi: 10.1093/brain/awq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuster JM. Upper processing stages of the perception-action cycle. Trends Cogn Sci. 2004;8(4):143–145. doi: 10.1016/j.tics.2004.02.004. [DOI] [PubMed] [Google Scholar]