Significance
Mitochondrial Ca2+ is a fundamental signal that allows for adaptation to physiological stress but a liability during ischemia-reperfusion injury in heart. On one hand, mitochondrial Ca2+ entry coordinates energy supply and demand in myocardium by increasing the activity of matrix dehydrogenases to augment ATP production by oxidative phosphorylation. On the other hand, inhibiting mitochondrial Ca2+ overload is promulgated as a therapeutic approach to preserve myocardial tissue following ischemia-reperfusion injury. We developed a new mouse model of myocardial-targeted transgenic dominant-negative mitochondrial Ca2+ uniporter (MCU) expression to test consequences of chronic loss of MCU-mediated Ca2+ entry in heart. Here we show that MCU inhibition has unanticipated consequences on extramitochondrial pathways affecting oxygen utilization, cytoplasmic Ca2+ homeostasis, physiologic responses to stress, and pathologic responses to ischemia-reperfusion injury.
Keywords: myocardium, mitochondrial calcium uniporter, ischemia-reperfusion injury
Abstract
Myocardial mitochondrial Ca2+ entry enables physiological stress responses but in excess promotes injury and death. However, tissue-specific in vivo systems for testing the role of mitochondrial Ca2+ are lacking. We developed a mouse model with myocardial delimited transgenic expression of a dominant negative (DN) form of the mitochondrial Ca2+ uniporter (MCU). DN-MCU mice lack MCU-mediated mitochondrial Ca2+ entry in myocardium, but, surprisingly, isolated perfused hearts exhibited higher O2 consumption rates (OCR) and impaired pacing induced mechanical performance compared with wild-type (WT) littermate controls. In contrast, OCR in DN-MCU–permeabilized myocardial fibers or isolated mitochondria in low Ca2+ were not increased compared with WT, suggesting that DN-MCU expression increased OCR by enhanced energetic demands related to extramitochondrial Ca2+ homeostasis. Consistent with this, we found that DN-MCU ventricular cardiomyocytes exhibited elevated cytoplasmic [Ca2+] that was partially reversed by ATP dialysis, suggesting that metabolic defects arising from loss of MCU function impaired physiological intracellular Ca2+ homeostasis. Mitochondrial Ca2+ overload is thought to dissipate the inner mitochondrial membrane potential (ΔΨm) and enhance formation of reactive oxygen species (ROS) as a consequence of ischemia-reperfusion injury. Our data show that DN-MCU hearts had preserved ΔΨm and reduced ROS during ischemia reperfusion but were not protected from myocardial death compared with WT. Taken together, our findings show that chronic myocardial MCU inhibition leads to previously unanticipated compensatory changes that affect cytoplasmic Ca2+ homeostasis, reprogram transcription, increase OCR, reduce performance, and prevent anticipated therapeutic responses to ischemia-reperfusion injury.
Entry of Ca2+ into the mitochondrial matrix is a central event for Ca2+ homeostasis in cardiomyocytes (1) as well as for coordinating fundamental and diverse responses to physiological (2) and pathological stress (3). The paradigm for Ca2+ as a physiological second messenger that enhances oxidative phosphorylation to enable fight-or-flight responses but in excess contributes to disease and dysfunction is well established in myocardium (4). The molecular identity of the mitochondrial Ca2+ uniporter (MCU) was recently discovered, enabling development of new genetic models to understand the role of MCU in vivo. MCU is an ion channel protein that acts as the primary pathway for Ca2+ entry into the mitochondrial matrix (5, 6). Recent findings in global Mcu−/− mice (7) suggest that the MCU pathway is dispensable for regulating cellular energy production, except under extreme physiological stress, and for activation of pathways leading to cell death; however, the effect of selective myocardial MCU inhibition is unknown. We developed a new transgenic mouse model with myocardial delimited dominant negative (DN)-MCU protein overexpression to test the role of MCU-mediated Ca2+ entry for myocardial physiology and pathological stress.
We tested whether loss of MCU-mediated Ca2+ entry substantially alters myocardial energetics. Surprisingly, we found that DN-MCU hearts had a higher oxygen consumption rate (OCR) due, at least in part, to secondary actions on cytoplasmic Ca2+ homeostasis. We also found that chronic MCU inhibition failed to protect against myocardial ischemia-reperfusion injury despite reducing generation of reactive oxygen species (ROS). We queried mRNA expression in adult hearts and identified diverse changes in multiple gene pathways induced by DN-MCU expression. Our findings reveal in vivo physiologic and pathological roles for cardiac MCU and suggest that loss of mitochondrial Ca2+ entry increases OCR, elevates cytoplasmic Ca2+, and sensitizes extramitochondrial cell death pathways.
Results
Increased Myocardial Oxygen Consumption and Reduced Performance in DN-MCU Hearts.
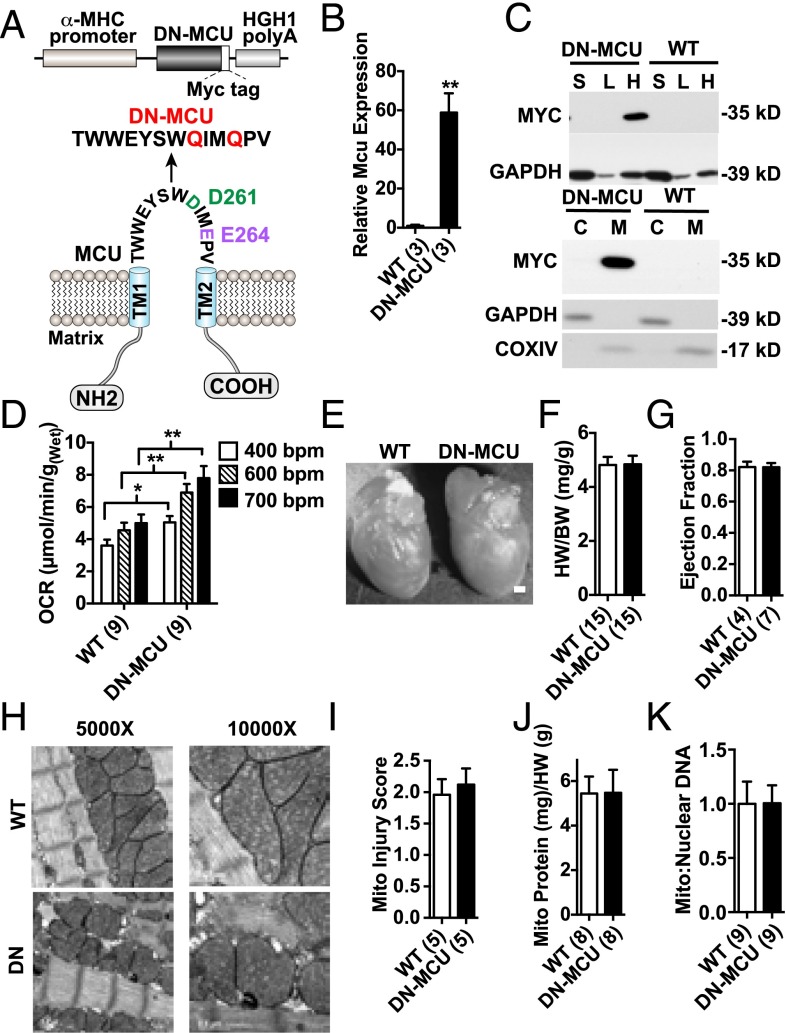
MCU, the pore-forming subunit of the mitochondrial Ca2+ uniporter, consists of two transmembrane domains that span the inner mitochondrial membrane and a linker-loop sequence (5, 6). The highly conserved aspartic acid-isoluecine-methionine-glutamic acid motif contains two negatively charged amino acids in the pore-forming linker-loop sequence. DN-MCU with D261Q/E264Q mutations inhibited mitochondrial Ca2+ uptake in HeLa cells (6). Based on this information, we developed a myocardial-selective in vivo model of MCU inhibition by transgenic expression of DN-MCU under control of the α-myosin heavy chain (αMHC) promoter (8) (Fig. 1A). DN-MCU mice were interbred into a CD1 background, based on evidence that CD1 background is permissive for loss of MCU current (9). DN-MCU mice were born in Mendelian ratios and survived into adulthood. We used a primer set to detect MCU and DN-MCU transcripts and found that the transcript level of Mcu was 60-fold higher in transgenic samples (Fig. 1B). The Myc-tagged DN-MCU protein was resident only in cardiac mitochondria from DN-MCU transgenic mice (Fig. 1C) and was detectable with an MCU antibody that showed markedly increased expression in DN-MCU compared with WT heart lysates (Fig. S1).
Fig. 1.
Increased myocardial oxygen consumption in DN-MCU hearts. (A) Schematic of the DN-MCU construct and expressed mutant channel in the transgenic mice. (B) Quantitative PCR measurement for WT and DN Mcu transcript expression. (C, Top) Western blot detection of MYC-tagged protein in heart (H), liver (L), and skeletal muscle (S) tissues from DN-MCU and WT mice. GAPDH was used as a loading control. (Bottom) Western blot detection of MYC-tagged protein in mitochondria (M) or cytosolic (C) isolates from DN-MCU and WT hearts. GAPDH confirmed purity of cytosolic isolates, and COXIV confirmed purity of mitochondrial isolates. (D) Oxygen consumption rates in Langendorff-perfused and paced hearts (beats/min). (E) Representative isolated hearts. (Scale bar, 200 μm.) (F) Summary of heart weight (HW) to body weight (BW) ratio measurements. (G) Left ventricular ejection fraction measured by echocardiography in unanesthetized mice. (H) Representative transmission electron microscopy images (5,000× and 10,000×). (I) Summary data of mitochondrial injury scores. (J) Mitochondrial protein (mg) measurements normalized to heart weight (g). (K) Mitochondrial:nuclear DNA. All error bars represent SEM. *P < 0.05, **P < 0.01, Student’s t test. Sample size (n) indicated for each group in parentheses.
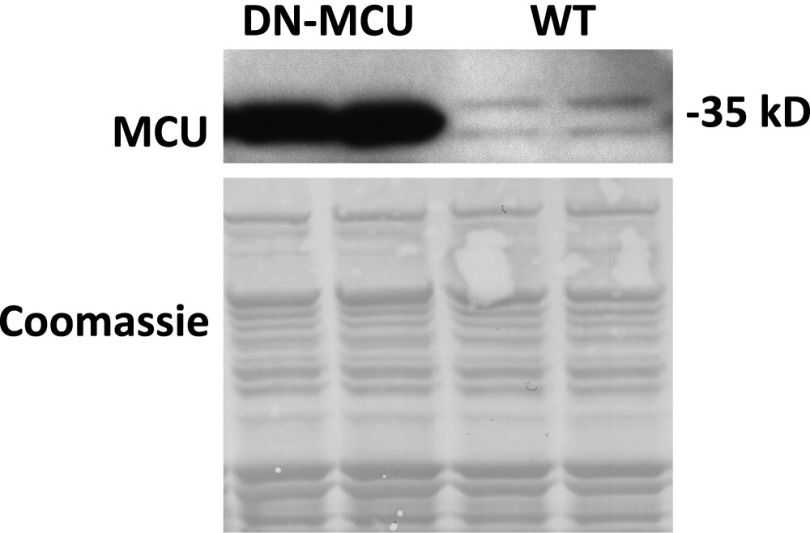
Fig. S1.
MCU protein is increased in DN-MCU hearts. Western blot detecting MCU in mitochondrial isolates from DN-MCU and WT hearts. Coomassie stain was used as a loading control.
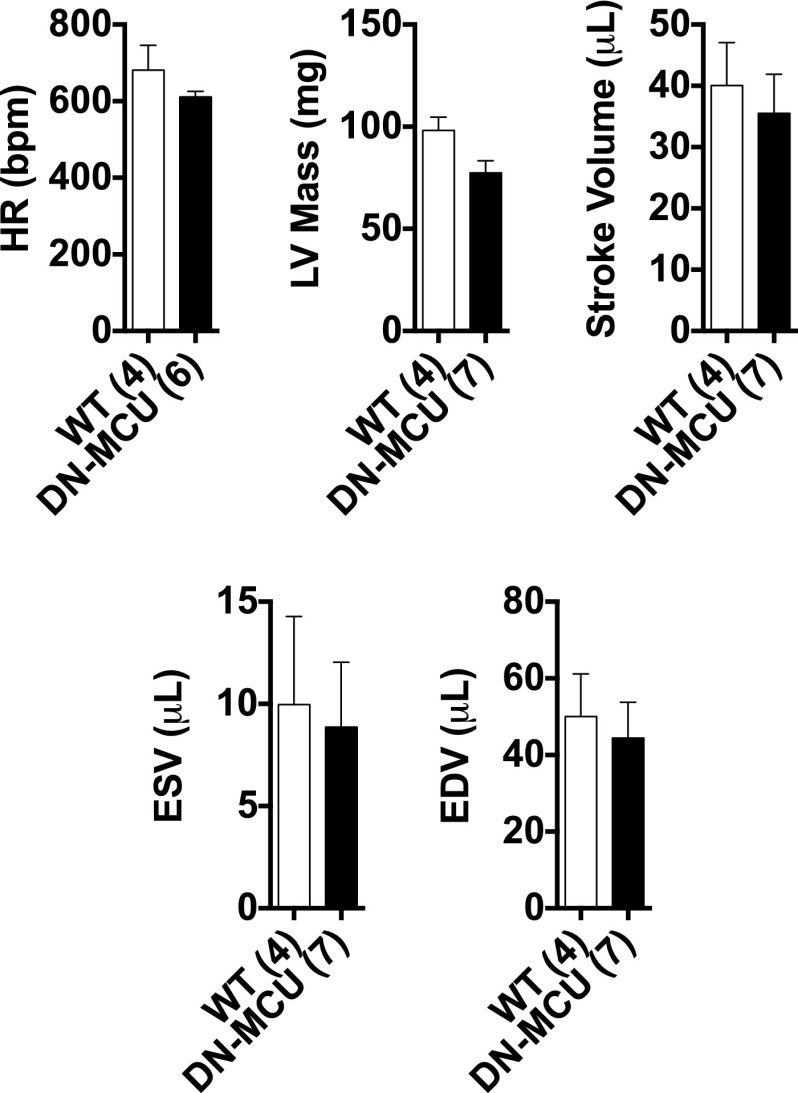
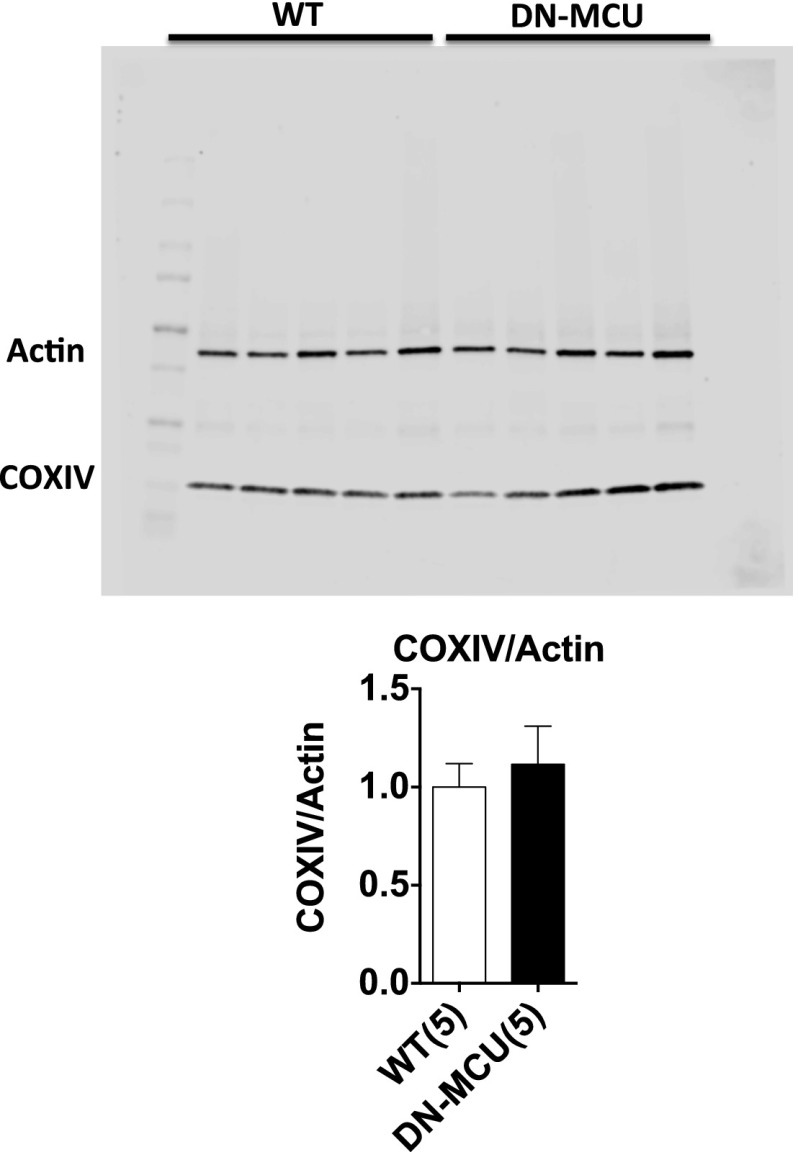
Increased mitochondrial Ca2+ can enhance oxidative phosphorylation (10). Based on the known relationship between mitochondrial Ca2+ and oxidative phosphorylation, we initially hypothesized that DN-MCU hearts lacking Ca2+ entry through MCU would have reduced O2 consumption rates (OCR) compared with WT. Contrary to our expectations, unloaded Langendorff-perfused and ventricular paced DN-MCU hearts consumed more O2 at 400 (P < 0.05), 600 (P < 0.01), and 750 beats/min (bpm) (P < 0.01) compared with WT (Fig. 1D). OCR was increased in WT between 400 and 600 bpm (P < 0.01) but not between 600 and 750 bpm. OCR was increased in DN-MCU between 400 and 600 bpm (P < 0.0001) as well as between 600 and 750 bpm (P < 0.05). No differences in cardiac morphology or baseline heart rate (Fig. 1E and Fig. S2) or in the heart weight:body weight ratios (Fig. 1F) were observed. We measured left ventricular (LV) ejection fraction in conscious, unsedated mice and found no difference between groups (Fig. 1G). No differences were detected between groups in the mitochondrial injury score (3) (Fig. 1 H and I), total mitochondrial protein content normalized to heart weight ratios (Fig. 1J), or mitochondrial-to-nuclear DNA content (Fig. 1K). Additionally, cyclooxygenase 4 (COXIV) protein levels were not different between groups (Fig. S3). These findings suggest that the increase in OCR in DN-MCU hearts was not related to alterations in myocardial or mitochondrial mass or structure but that DN-MCU hearts were less efficient than WT, based on higher OCR.
Fig. S2.
Echocardiographic analysis. Heart rate (HR), left ventricular (LV) mass, stroke volume, end systolic volume (ESV), and end diastolic volume (EDV), as measured by echocardiography. All error bars represent SEM. Sample size (n) is indicated for each group in parentheses.
Fig. S3.
COXIV protein expression is similar in WT and DN-MCU hearts. (Top) DN-MCU and WT whole-heart lysates were blotted for cyclooxygenase IV (COXIV) and Actin. (Bottom) The ratio of COXIV:Actin was used to compare mitochondrial protein content to cytosolic protein. Error bars represent SEM. Sample size (n) is indicated for each group in parentheses. No significant differences were detected.
DN-MCU Expression Decreases Inotropic and Lusitropic Responses to Stress.
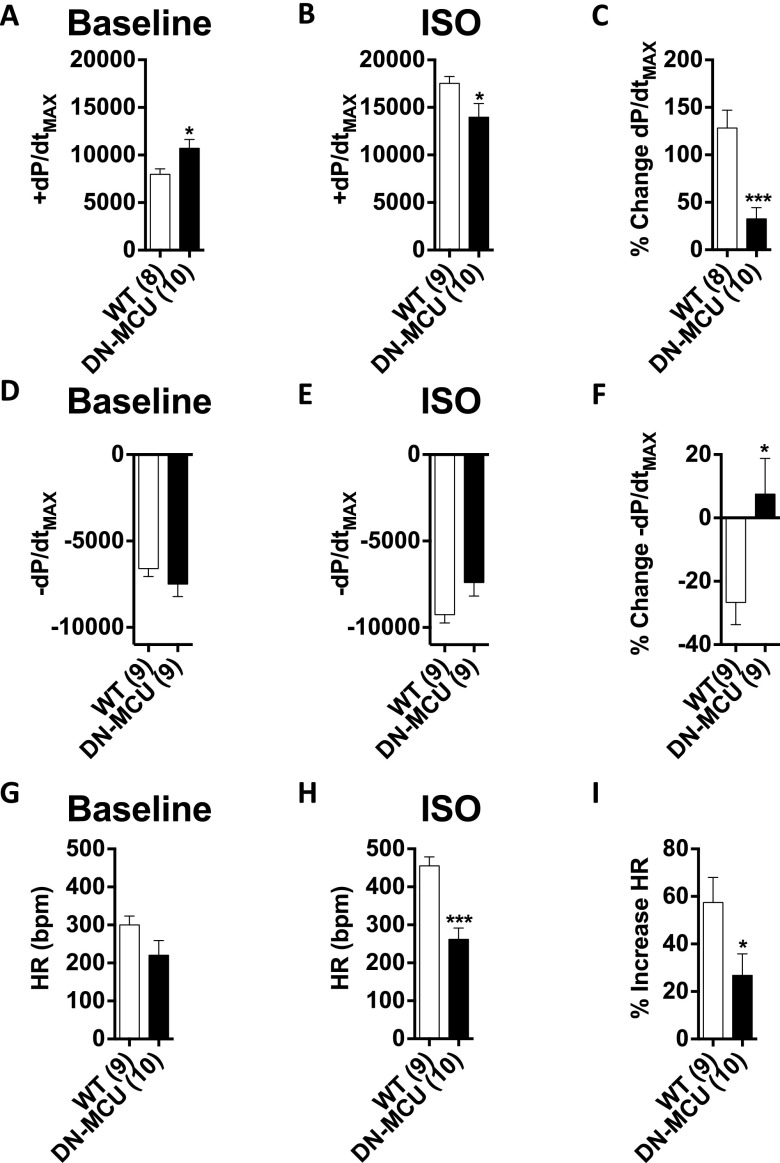
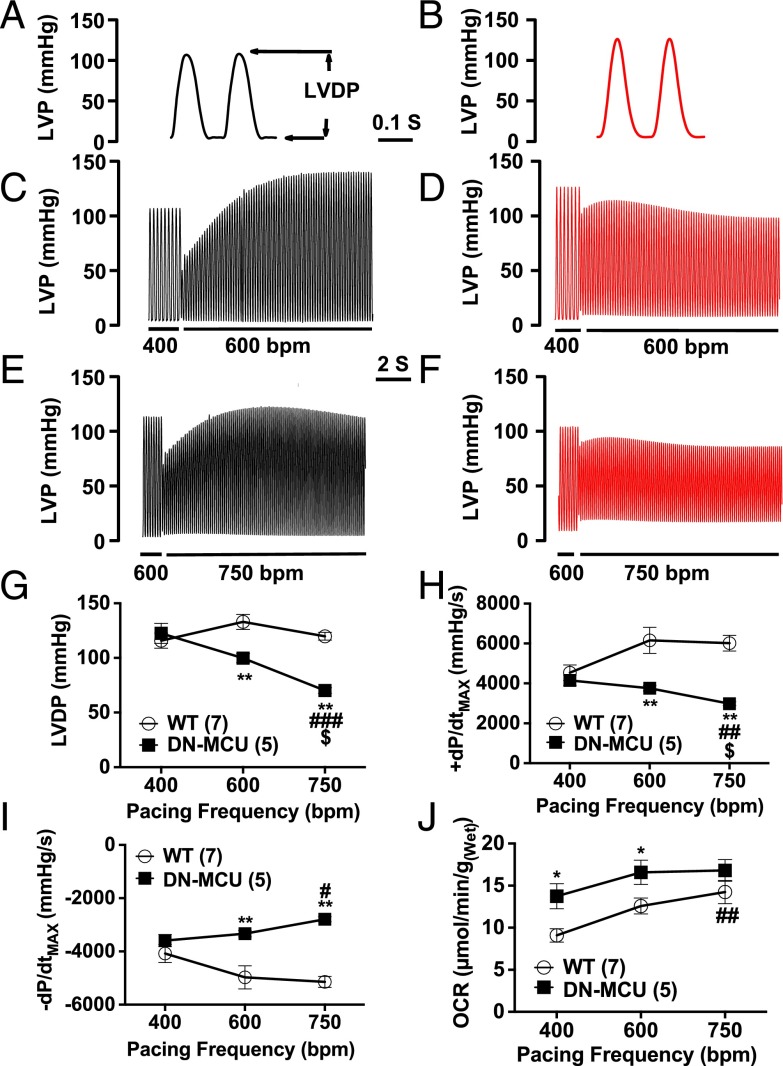
In vivo LV pressure measurements showed that DN-MCU mice had increased baseline +dP/dtMAX (the maximal LV pressure change rate during systole) and similar −dP/dtMAX compared with WT. DN-MCU mice showed reduced ±dP/dtMAX responses to isoproterenol (10 μg/kg) compared with WT (Fig. S4 A–F). Based on the defect in myocardial performance in DN-MCU mice in vivo, we repeated the Langendorff-perfused heart studies under conditions suitable for measuring LV pressure. We found that DN-MCU and WT hearts had equal LV-developed pressure (LVDP) at 400 bpm, but DN-MCU hearts had significantly reduced LVDP at 600 and 750 bpm compared with WT (P < 0.01) (Fig. 2 A–G). DN-MCU hearts had reduced LVDP between 400 and 750 bpm (P < 0.01) and 600 and 750 bpm (P < 0.05). +dP/dtMAX and –dP/dtMAX were not different at 400 bpm, but DN-MCU hearts had diminished ±dP/dtMAX responses at 600 bpm (P < 0.01) and 750 bpm (P < 0.01) (Fig. 2 G and H). The +dP/dtMAX was reduced in DN-MCU hearts between 400 and 750 bpm (P < 0.01) and 600 and 750 bpm (P < 0.05) and had significantly diminished –dP/dtMAX between 400 and 750 bpm (P < 0.05) (Fig. 2I). Under these conditions DN-MCU hearts had significantly higher OCR at 400 and 600 bpm (P < 0.05) (Fig. 2J). Within groups, OCR at 400 and 750 bpm was significantly different (P < 0.01) in WT, but not in DN-MCU, suggesting that DN-MCU hearts contracting against an afterload have a smaller pacing-induced OCR range than unloaded hearts.
Fig. S4.
In vivo left ventricular pressure recordings. (A) Baseline +dP/dtMAX (mmHg/s). (B) +dP/dtMAX (mmHg/s) 2 min after isoproterenol administration (10 μg/kg, i.p.). (C) Percentage increase in +dP/dtMAX from baseline to isoproterenol condition. (D) Baseline −dP/dtMAX (mmHg/s). (E) −dP/dtMAX (mmHg/s) 2 min after isoproterenol administration (10 μg/kg, i.p.). (F) Percentage decrease in −dP/dtMAX from baseline to isoproterenol condition. (G) Baseline heart rate (HR) (bpm) measured by pressure waveform. (H) HR 2 min after isoproterenol administration (10 μg/kg, i.p.). (I) Percentage increase in HR from baseline to isoproterenol condition. All error bars represent SEM. Sample size (n) is indicated for each group in parentheses. *P < 0.05, ***P < 0.001, Student’s t-test.
Fig. 2.
DN-MCU expression reduced left ventricular pressure responses to pacing. (A and B) Representative waveform of left ventricular pressure (LVP) (mmHg) in WT and DN-MCU at 400 bpm, respectively. LVDP indicated by arrows. (C and D) Representative LVP changes in WT and DN-MCU hearts when increasing pacing rate from 400 to 600 bpm. (E and F) Representative LVP changes in WT and DN-MCU hearts when increasing pacing rate from 600 to 750 bpm. (G) LVDP (mmHg) at 400, 600, and 750 bpm. (H) +dP/dtMAX (mmHg/s) at 400, 600, and 700 bpm. (I) −dP/dtMAX (mmHg/s) at 400, 600, and 700 bpm. (J) OCR [μmol/min/g(Wet)] in WT and DN-MCU hearts. All error bars represent SEM. Sample size (n) indicated for each group in parentheses. *P < 0.05, **P < 0.01, Student’s t test. #P < 0.05, ##P < 0.01, and ###P < 0.001 comparing 750 to 400 bpm; $P < 0.05 comparing 750 to 600 bpm, Tukey’s post hoc multiple comparison test.
DN-MCU Expression Alters Mitochondrial and Cytoplasmic Ca2+ Dynamics.
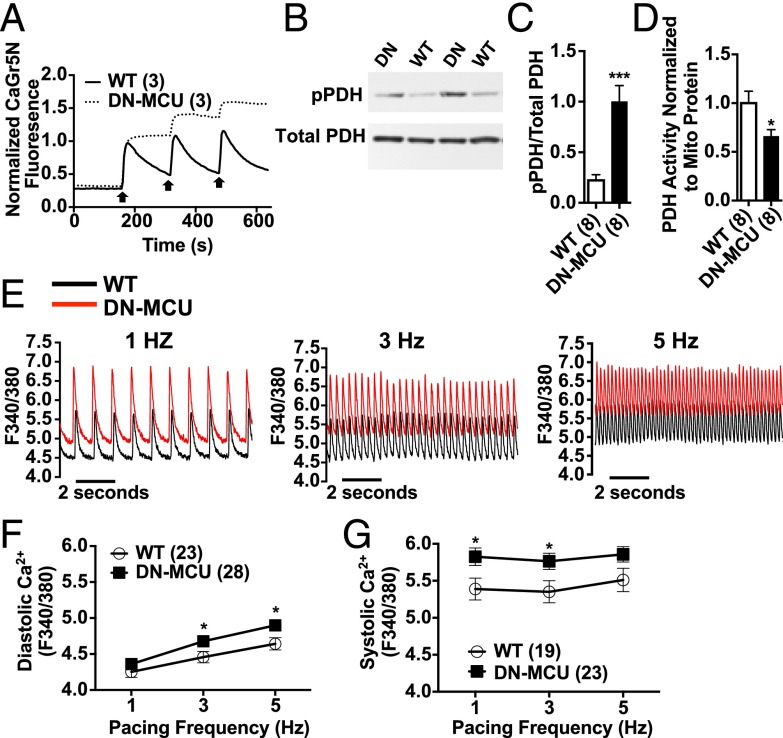
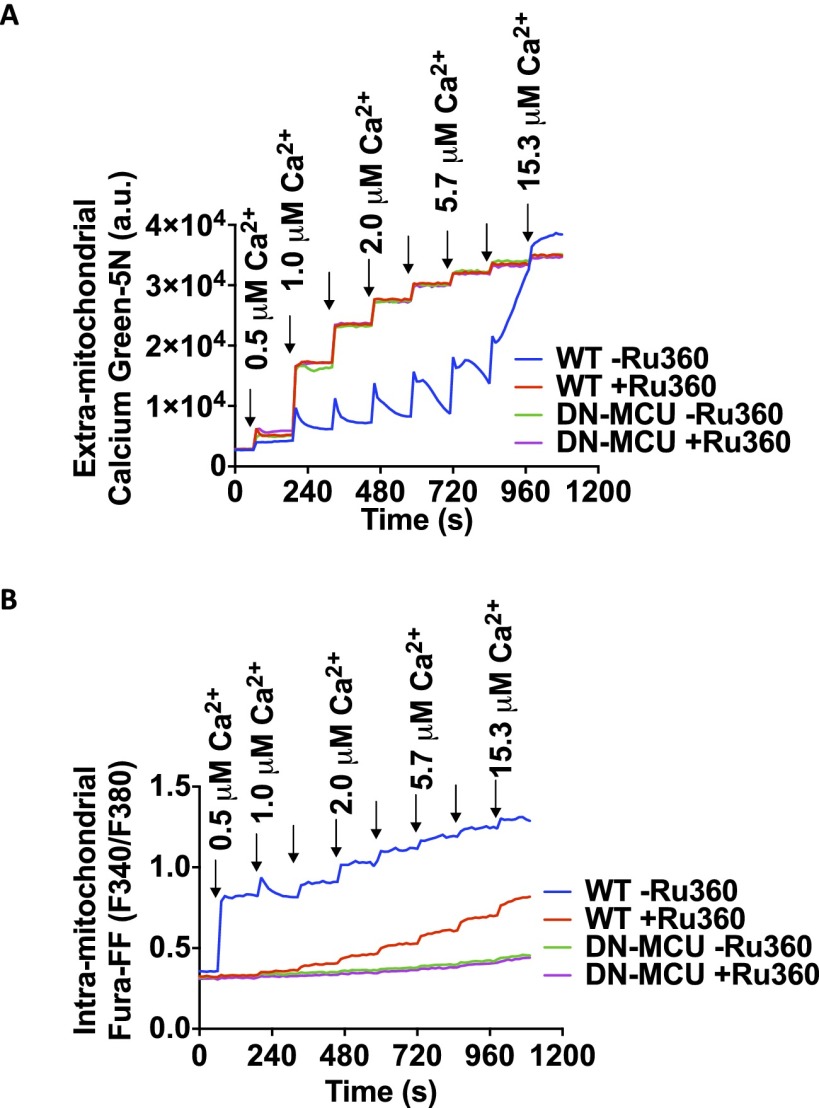
We next tested whether DN-MCU expression in ventricular myocytes prevented rapid mitochondrial Ca2+ entry. We used freshly isolated adult ventricular myocytes with permeabilized cell membranes and incubated them with Ca2+ green-5N (CaGr5N), a membrane-impermeable Ca2+ sensitive fluorescent dye (3). We confirmed that DN-MCU ventricular myocytes and isolated mitochondria had complete or nearly complete loss of mitochondrial Ca2+ uptake (Fig. 3A and Fig. S5A), similar to phenotypes observed in cells treated with the MCU antagonist Ru360 (11), lacking MCU expression (7) or expressing DN-MCU (12).
Fig. 3.
DN-MCU expression alters cytoplasmic Ca2+ dynamics. (A) Normalized kinetic tracings for CaGr5N-loaded, cell membrane-permeabilized ventricular myocytes. Arrows represent addition of 100 μM Ca2+. (B) Western blot detection of phosphorylated (pPDH) and total pyruvate dehydrogenase (PDH). (C) Summary data for the pPDH to total PDH ratio. (D) Summary data for PDH activity normalized to mitochondrial protein. (E) Representative Ca2+ transient traces from WT (black) and DN-MCU (red) ventricular myocytes stimulated by field stimulation. Summary data for (F) diastolic and (G) systolic [Ca2+] measurements made with Fura-2–loaded cells stimulated by field stimulation. All error bars represent SEM. *P < 0.05, ***P < 0.001, Student’s t test. Sample size (n) indicated for each group in parentheses.
Fig. S5.
Mitochondrial Ca2+ uptake measurement. (A) Assessment of Ca2+ uptake by isolated mitochondria using mitochondrial impermeant dye Calcium Green-5N. (B) Assessment of Ca2+ uptake by isolated mitochondria preloaded with intramitochondrial calcium indicator Fura-FF-AM.
To determine if mitochondrial Ca2+ ([Ca2+]mt) was different between DN-MCU and WT hearts, we tested [Ca2+]mt in isolated mitochondria loaded with Fura-4FF. We found that DN-MCU mitochondria had no observable step-wise increase in Fura-4FF signal in response to repetitive Ca2+ boluses (Fig. S5B). Furthermore, Ca2+ uptake in mitochondria isolated from DN-MCU hearts was unaffected by the MCU antagonist Ru360 (Fig. S5B), confirming that DN-MCU expression ablated the Ru360-sensitive Ca2+ influx. Taken together, these data show that DN-MCU myocardial mitochondria lack the MCU-mediated, rapid Ca2+ uptake pathway.
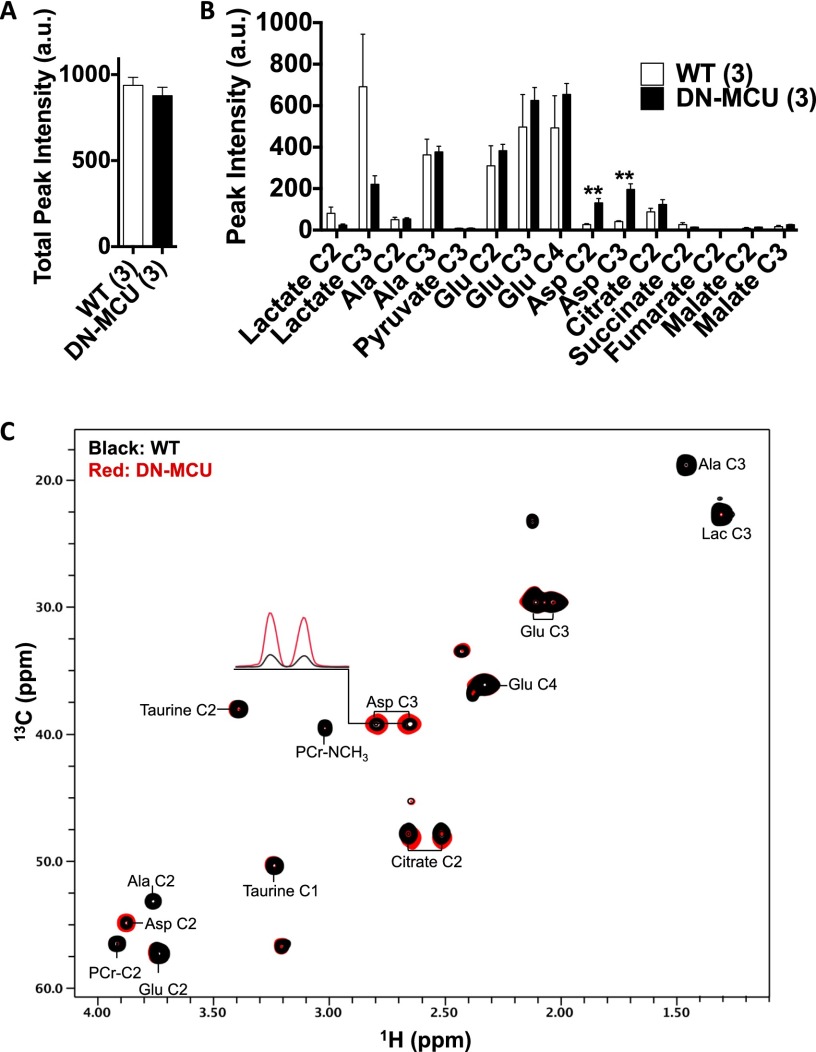
Mitochondrial Ca2+ stimulates pyruvate dehydrogenase phosphatase to dephosphorylate pyruvate dehydrogenase (PDH), increasing its enzymatic activity (13). We measured PDH phosphorylation in DN-MCU and WT hearts and found that PDH was significantly (P < 0.001) more phosphorylated (Fig. 3 B and C) and exhibited lower enzyme activity (Fig. 3D) in DN-MCU compared with WT cardiac mitochondria. To test whether glucose metabolism was altered in DN-MCU hearts, we used a Langendorff perfusion model with buffer containing [1,2-13C2]-glucose followed by NMR analysis. The 13C incorporation was not different between groups (Fig. S6A), and aspartate was the only significantly increased metabolite in DN-MCU compared with WT hearts (Fig. S6 B and C). These data indicate that the lack of MCU-mediated Ca2+ uptake in DN-MCU mitochondria is sufficient to impair activity of the Ca2+-sensitive enzyme PDH, but without widespread changes in myocardial glucose metabolism detectable by NMR.
Fig. S6.
DN-MCU expression does not alter glucose metabolism in the heart. (A) Total peak intensity (in arbitrary units), a measure of total 13C incorporation obtained by summation of the peak intensities present in the heteronuclear multiple quantum coherence (HMQC) spectra. (B) Measured NMR peak intensity (in arbitrary units) of glucose metabolites in the perfused heart extracts. The peak intensities were measured from the assigned HMQC spectra (**P < 0.01 compared with WT, Student’s t test). (C) Overlay of the HMQC spectra of glucose metabolites of WT (black) and DN-MCU (red) perfused heart extracts. Select cross-peaks are labeled: lactate (Lac), alanine (Ala), glutamate (Glu), aspartate (Asp), and phosphocreatine (PCr). The one-dimensional slices through the Asp C3 are shown to display significant differences in peak intensity between the WT and DN-MCU heart extracts.
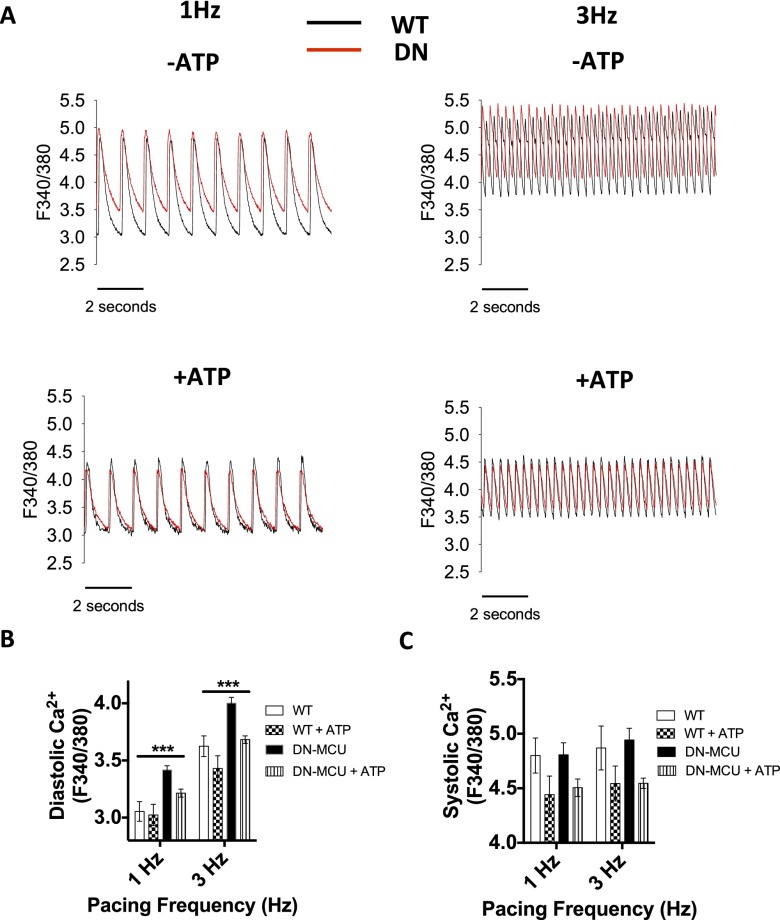
We next asked whether loss of mitochondrial Ca2+ uptake would increase cytosolic [Ca2+] in DN-MCU cells, potentially imposing an energy demand on the sarcoplasmic reticulum/endoplasmic reticulum Ca2+-ATPase (SERCA2a). We found significantly higher diastolic and systolic cytoplasmic [Ca2+] in DN-MCU cells (Fig. 3 E–G) compared with WT, suggesting that chronic MCU inhibition triggers a demand for increased SERCA activity. These findings show that chronic loss of MCU-mediated mitochondrial Ca2+ uptake in myocardium increased OCR, possibly by enhancing the metabolic cost of cytoplasmic Ca2+ homeostasis during excitation-contraction coupling.
We recently reported that cardiac pacemaker cells isolated from DN-MCU mice have impaired ATP production and defective cytosolic [Ca2+] homeostasis that was corrected by ATP dialysis (12). Based on these findings, we asked if ATP deficiency contributed to pacing-induced increases in cytoplasmic [Ca2+] in DN-MCU ventricular myocytes. Fortifying intracellular ATP (5 mM added to the pipette solution) significantly decreased cytosolic diastolic [Ca2+] only in DN-MCU ventricular myocytes (Fig. S7 A and B). In contrast, added ATP equally and slightly decreased systolic cytosolic [Ca2+] in WT and DN-MCU, but did not reach statistical significance (Fig. S7C). Taken together with findings in DN-MCU pacemaker cells (12), we interpreted these data to suggest that physiologic cytoplasmic [Ca2+] homeostasis requires MCU-mediated ATP production.
Fig. S7.
Reversal of elevated diastolic cytosolic [Ca2+] in voltage clamp-stimulated DN-MCU ventricular myocytes by ATP dialysis. (A) Representative [Ca2+] transients from isolated ventricular WT (black tracings) and DN-MCU (red tracings) myocytes stimulated at 1 and 3 Hz in the absence or presence of ATP (5 mM), which was added to the pipette (intracellular) solution. Summary data for diastolic (B) and systolic (C) cytosolic [Ca2+] in WT and DN-MCU ventricular myocytes in the presence and absence of pipette solution with 5 mM ATP. Cell number in each group is indicated in parentheses: (B) 1 Hz WT (7), WT + ATP (8), DN-MCU (16), DN-MCU + ATP (11); 3 Hz WT (8), WT + ATP (7), DN-MCU (13), DN-MCU + ATP (9). (C) 1 Hz WT (6), WT + ATP (6), DN-MCU (9), DN-MCU + ATP (6); 3 Hz WT (8), WT + ATP (7), DN-MCU (9), DN-MCU + ATP (10). ***P < 0.001, one-way ANOVA analysis of means. Error bars show SEM.
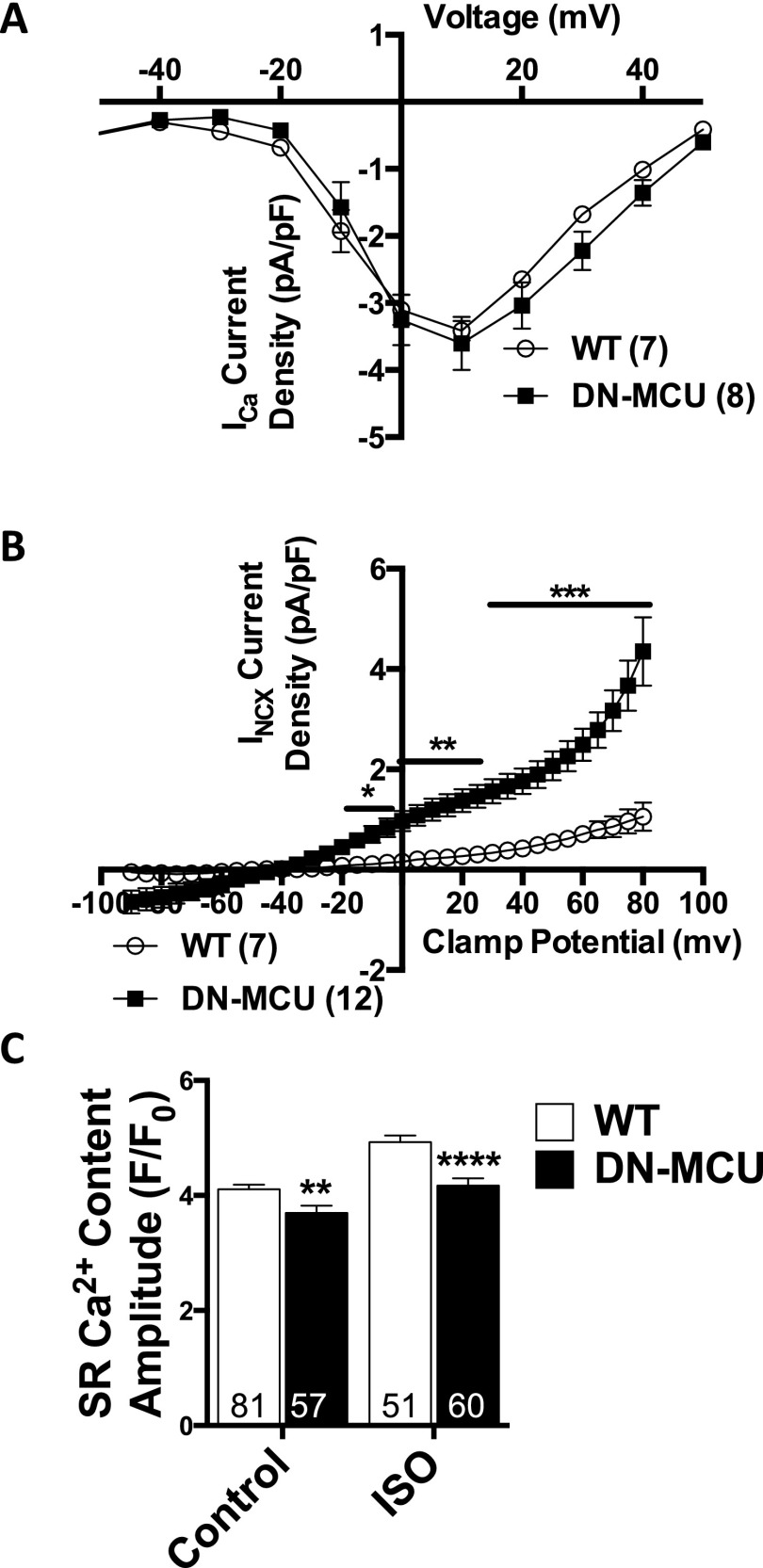
Multiple ionic conductances may contribute to differences in cytoplasmic [Ca2+] homeostasis between DN-MCU and WT ventricular myocytes. Therefore, we measured voltage-gated L-type Ca2+ current (ICa), Na+/Ca2+ exchanger current (INCX), and sarcoplasmic reticulum Ca2+ content (Fig. S8). We found no difference in ICa (Fig. S8A), but INCX density was increased (Fig. S8B) in DN-MCU cells, suggesting that DN-MCU cells partially rely on NCX to compensate for loss of MCU function. Interestingly, DN-MCU myocytes had reduced SR Ca2+ content at baseline (P < 0.01) and after isoproterenol treatment (P < 0.0001) compared with WT (Fig. S8C). We interpreted these data to suggest that MCU-mediated ATP production contributes to SR Ca2+ loading in ventricular myocytes and that MCU inhibition is accompanied by increases in INCX.
Fig. S8.
ICa and INCX recordings and SR Ca2+ content. (A) ICa recording in ventricular myocytes. Sample size (n) noted in parentheses. Error bars represent SEM. (B) INCX recording in patched ventricular myocytes. *P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test. Sample size (n) noted in parentheses. Error bars represent SEM. (C) Summary SR Ca2+ content data from isolated ventricular myocytes loaded with Fluo-4 AM at baseline before and after addition of isoproterenol (ISO) (100 nM). Maximum SR Ca2+ release was triggered by a caffeine spritz (SI Materials and Methods). Sample size (n) is indicated for each group. **P < 0.01 and ****P < 0.0001, Student’s t test. Error bars show SEM.
No Effect of DN-MCU on OCR in Isolated Mitochondria.
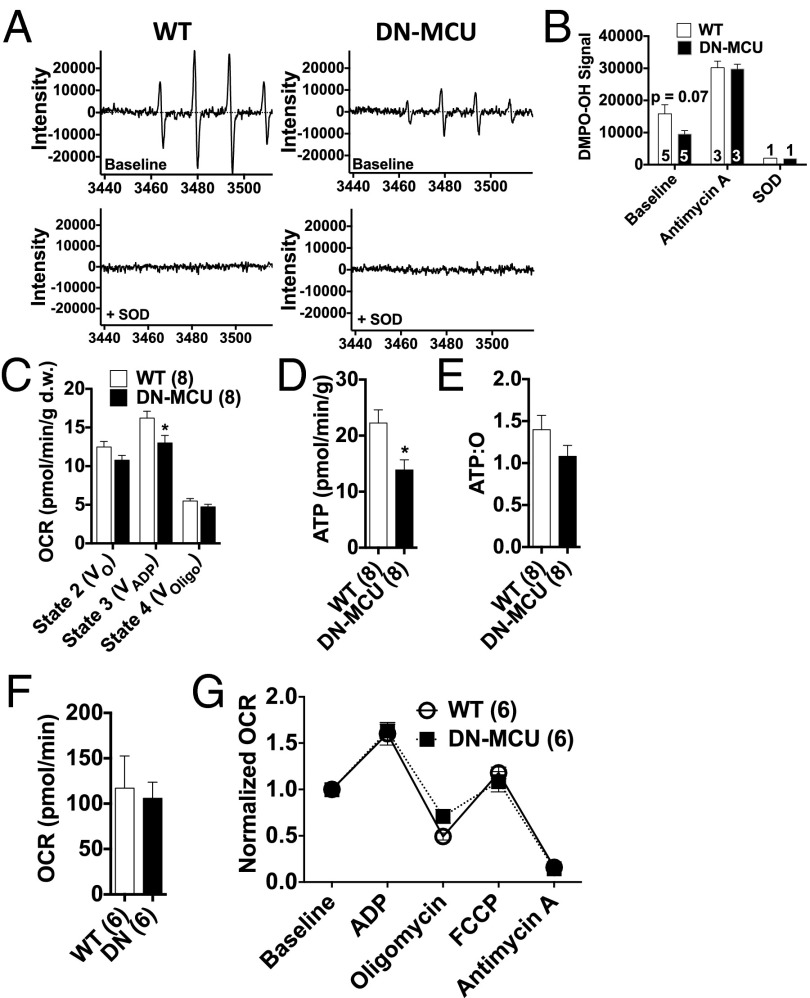
Mitochondria are an important source of O2•- in cardiomyocytes (14) and may induce mitochondrial uncoupling to increase oxygen consumption. Therefore, we used electron paramagnetic resonance (EPR) spin trapping with the cyclic nitrone 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (15) to measure O2•- levels from isolated mitochondria. We found a trend (P = 0.07) toward less DMPO-OH signal in DN-MCU mitochondria (Fig. 4 A and B), suggesting reduced O2•- production. Importantly, when antimycin A, a complex III inhibitor and agent known to increase one-electron reductions of O2 to form O2•-, was included in the reaction mixture the signals from WT and DN-MCU mitochondria increased to similar peak values (Fig. 4B), indicating that DN-MCU and WT mitochondria have a similar capacity to produce O2•-. Addition of superoxide dismutase (SOD) completely quenched the DMPO-OH signal in both DN-MCU and WT mitochondria, indicating that DMPO-OH was reporting on O2•- levels with high fidelity. These EPR data demonstrated that loss of MCU-mediated Ca2+ entry may modestly reduce O2•- production in isolated mitochondria.
Fig. 4.
DN-MCU mitochondria have normal OCR. (A) Representative electron paramagnetic resonance spectra of the DMPO-OH spin adduct produced by isolated cardiac mitochondria. (B) Summary showing the signal intensity of DMPO-OH baseline (P = 0.07, Student’s t test) and after addition of antimycin A (1 μM) or SOD (100 U/0.5 mL). (C) OCR in permeabilized myocardial fibers with 156 nM Ca2+ and 10 mM succinate in different states V0 (no secondary substrate), VADP (1 mM ADP), and VOligo (1 mM oligomycin). Units are pmol oxygen/min/mg of dry fiber weight. (*P < 0.05 from WT in same state, Student’s t test). (D) Bioluminescent quantification of ATP (*P < 0.05 Student’s t test). (E) Calculated ATP:O ratios in permeabilized fibers. (F) Baseline OCR from isolated mitochondria without added ADP. (G) OCR normalized to baseline after addition of ADP (4 mM), oligomycin (2.5 μg/mL), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (4 μM), and antimycin A (4 μM). All error bars represent SEM. Sample size (n) indicated for each group in parentheses.
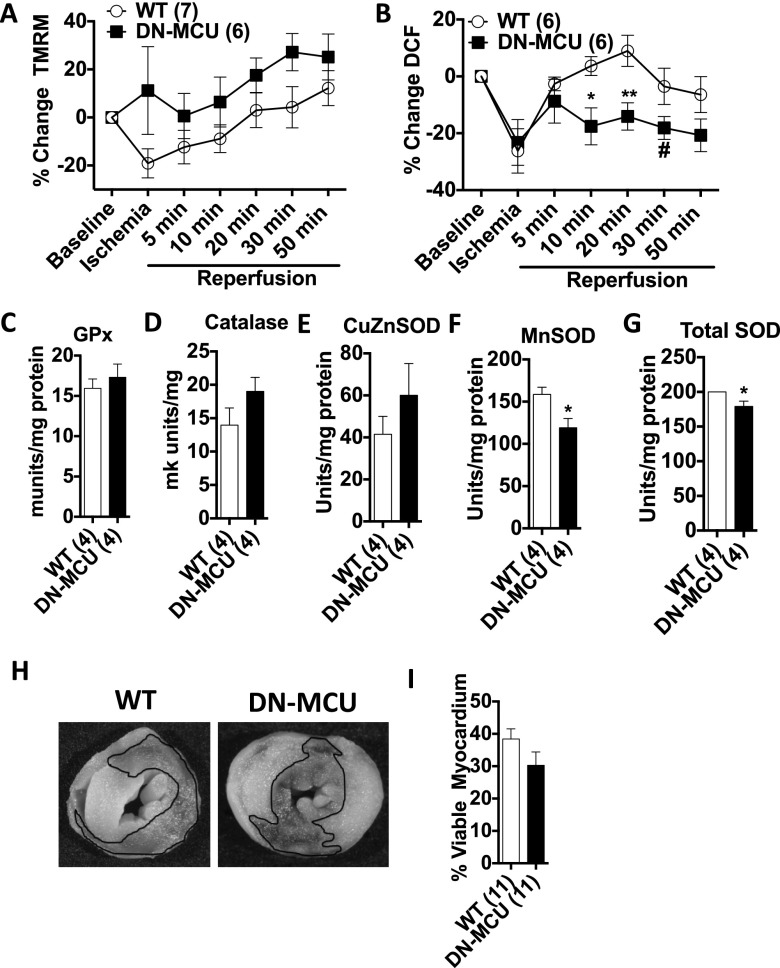
Excessive mitochondrial Ca2+ entry promotes opening of the mitochondrial permeability transition pore, loss of ΔΨm (16), and release of mitochondrial reactive oxygen species (ROS) (17), which are consequences of ischemia-reperfusion injury that lead to myocardial death (18). We adapted our ischemia-reperfusion model to simultaneously measure ΔΨm and ROS in Langendorff-perfused hearts using confocal microscopy. The DN-MCU hearts maintained ΔΨm at baseline values during ischemic stress, whereas WT hearts showed a 19 ± 6% decrease in ΔΨm during ischemia compared with baseline (Fig. S9A), although the difference in ΔΨm between genotypes was not significantly different (P = 0.12). In the reperfusion interval, WT hearts showed increased ROS compared with DN-MCU hearts (Fig. S9B). Toward the end of the reperfusion interval the DN-MCU hearts had significantly reduced ROS compared with baseline (P < 0.05), whereas WT hearts did not show a decline in ROS relative to baseline (Fig. S9B). Additionally, two-way ANOVA revealed that changes in ROS during ischemia reperfusion were significantly different between DN-MCU and WT hearts (P < 0.05). We considered the possibility that reduced ROS in DN-MCU mitochondria could be related to increased reductive enzyme activity. To test this concept, we measured glutathione peroxidase, catalase, Cu/ZnSOD, and MnSOD in freshly isolated whole hearts and found decreased activity of MnSOD and total SOD activity (Fig. S9 C–G) in DN-MCU compared with WT, suggesting that the reduced ROS signal in DN-MCU hearts during ischemia reperfusion was not due to increased antioxidant enzymes. Based on an extensive body of work (16, 19, 20), we initially hypothesized that lower levels of ROS would result in myocardial protection. However, despite reduction in ROS, the DN-MCU hearts were not protected from cell death following ischemia-reperfusion injury (Fig. S9 H and I).
Fig. S9.
DN-MCU hearts are not protected from ischemia-reperfusion injury. (A) Percentage change in TMRM signal from baseline in heart tissue during ischemia reperfusion. Two-way ANOVA analysis shows that the genotype was not a significant source of variation. (B) Percentage change in DCF signal from baseline in heart tissue during ischemia reperfusion, (*P < 0.05, **P < 0.01) compared with WT at same time point, Student’s t test; (#P < 0.05) compared with baseline value within genotype, Dunnett’s multiple comparison post test; (P < 0.05) Two-way ANOVA testing genotype as a source of variation. (C) Glutathione peroxidase activity. (D) Catalase activity. (E) Copper/zinc SOD-specific activity. (F) Manganese SOD-specific activity. (G) Total SOD activity. Sample size (n) is indicated for each group in parentheses. *P < 0.05, Student’s t test. Error bars show SEM. (H) Representative TTC-stained heart sections after ischemia reperfusion. The black line outlines viable tissue. (I) Quantification of viable myocardium from TTC staining. All error bars represent SEM. Sample size (n) is indicated for each group in parentheses.
Given the increased OCR in DN-MCU hearts, we next asked whether intrinsic properties of DN-MCU mitochondria contribute to high O2 consumption. First, we studied isolated permeabilized myocardial fibers (21) (bath [Ca2+] = 156 nM) to assess OCR. We found that, in contrast to myocardium with intact cell membranes, DN-MCU fibers consumed significantly less O2 than WT controls under state 3 conditions (P < 0.05), but had similar OCR relative to WT under state 2 and 4 conditions (Fig. 4C). ATP concentrations (Fig. 4D) were significantly lower (P < 0.05) in DN-MCU compared with WT samples, suggesting blunted ATP production, higher ATP hydrolysis, or both. The ratio of ATP content:O2 consumption was not significantly different between DN-MCU and WT fibers (Fig. 4E). These findings suggest that DN-MCU mitochondria have reduced oxidative capacity compared with WT controls and are not intrinsically uncoupled, at least in the setting of membrane permeabilization and low extramitochondrial Ca2+.
Next, we extended our findings in disrupted myocardial fibers by determining OCR in isolated mitochondria in a buffer with nominally absent Ca2+. At baseline, we found that isolated DN-MCU mitochondria had similar OCR to WT (Fig. 4F). DN-MCU and WT mitochondria responded similarly to ADP, oligomycin, FCCP, and antimycin A (Fig. 4G). Thus, the reduction in mitochondrial respiration in permeabilized DN-MCU fibers and isolated mitochondria in low [Ca2+] compared with isolated hearts suggests that extramitochondrial [Ca2+] contributes to the increased OCR in intact DN-MCU hearts.
Broad Transcriptional Reprogramming in DN-MCU Hearts.
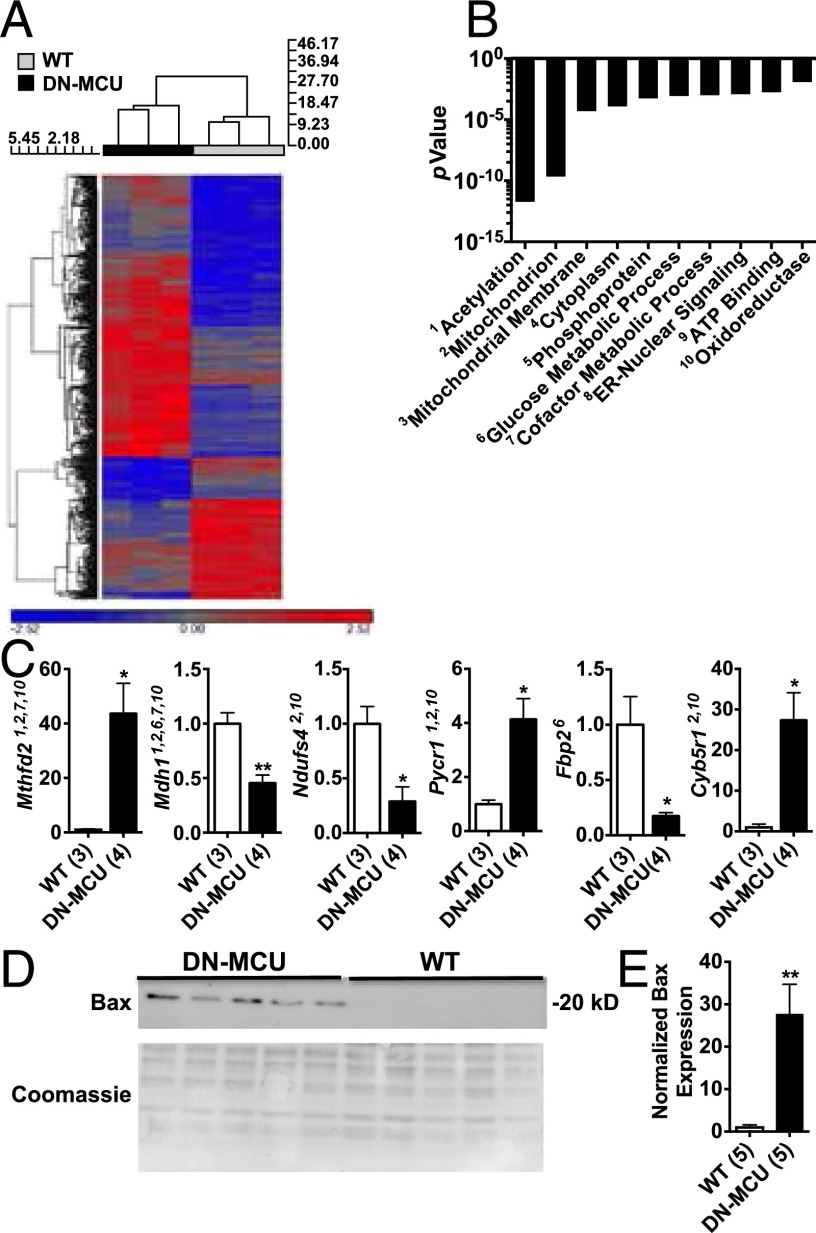
Our findings showed that inhibition of MCU has important consequences for cytoplasmic Ca2+ homeostasis and physiological and pathological stress responses in heart. As a first step toward identifying potential transcriptional foundations for these effects, we profiled transcriptional changes induced by inhibition of MCU by performing microarray analysis to measure mRNA expression levels. We detected 636 genes with more than twofold expression change (false discovery rate < 0.05) in adult DN-MCU mice, relative to WT littermates (Fig. 5A and Table S1, GSE62049). Functional gene annotation clustering revealed that these genes are significantly enriched for a variety of biological processes, including acetylation, redox biochemistry, and endoplasmic nuclear signaling (Fig. 5B). We used qRT-PCR to measure mRNA targets from selected functional gene annotation terms and validated these targets with working primer sets (Fig. 5C). These data support a concept that MCU-mediated mitochondrial Ca2+ entry has the potential to regulate transcriptional programs controlling diverse cellular pathways in myocardium. Our ANOVA analysis of mRNA expression levels revealed B-cell lymphoma 2 (Bcl2) to be elevated in DN-MCU samples (P = 0.00036). Therefore, we queried other members of the Bcl2 family and found markedly increased expression of the Bcl2-associated X protein (Bax) transcript (up-regulated ninefold, P = 0.006, Student’s t test) and significantly (P < 0.01) elevated Bax protein expression in DN-MCU heart lysates (Fig. 5 D and E). Bax is a Bcl-2 family member implicated in mitochondrial-dependent and -independent cell death pathways (22, 23) and has been shown to impact Ca2+ homeostasis (23), suggesting that DN-MCU hearts may have increased susceptibility to Bax-mediated death during ischemia-reperfusion injury.
Fig. 5.
Broad transcriptional reprogramming in DN-MCU hearts. (A) Hierarchical clustering of 700 differentially expressed genes (WT, black; DN-MCU, gray). (B) Graph shows P values for 10 functional terms enriched in the DN-MCU gene set. (C) qRT-PCR data showing validation of selected functional annotation terms listed in B. Superscript numbers indicate the functional annotation cluster being queried from B. (D) Western blot detecting Bax protein in whole-heart homogenates. Coomassie-stained blot shows gel loading for each sample. (E) Quantification of Bax Western blot (*P < 0.05, **P < 0.01, Student’s t test). All error bars represent SEM. Sample size (n) indicated for each group in parentheses.
Discussion
We found that loss of MCU causes unanticipated cellular responses that increase OCR, disrupt cytoplasmic Ca2+ homeostasis, and trigger transcriptional reprogramming. The ability of mitochondria to buffer cytosolic Ca2+ is controversial (24–26). Our findings show that inhibition of MCU increases cytosolic [Ca2+], potentially consistent with a loss of mitochondrial Ca2+-buffering capacity and/or ATP deficiency. We recently found that MCU inhibition limited fight-or-flight heart rate increases by lowering ATP below a critical threshold and that dialysis of 4 mM ATP was sufficient to rescue fight-or-flight responses in cardiac pacemaking cells (12). Thus, it is possible that elevated [Ca2+] in DN-MCU ventricular myocytes is at least partly due to inadequate ATP for physiological Ca2+ buffering.
We observed an abrogation of OCR differences using low [Ca2+] buffers and when mitochondria were tested in buffers nominally lacking Ca2+, suggesting that elevation of cytosolic [Ca2+] in DN-MCU hearts contributed to elevated OCR in situ. An elevated or depressed heart rate could increase or decrease OCR (27), but we controlled for this by measuring OCR at equal pacing intervals. Our in vivo data showed that DN-MCU mice had similar heart rates to WT at baseline, but an inability to increase heart rate after isoproterenol administration, consistent with recent evidence that MCU is necessary for heart rate increases during physiological stress (12). Rapid pacing in DN-MCU hearts may increase cytosolic [Ca2+] to a greater extent than in WT hearts due to loss of mitochondrial Ca2+ buffering and/or inadequate ATP to sustain intracellular Ca2+ homeostasis.
Our NMR glucose metabolite measurements did not reveal major differences in metabolites between DN-MCU and WT hearts. However, we cannot exclude the possibility that 13C was lost as CO2 through the tricarboxylic acid (TCA) cycle because we measured labeled metabolites in clamp-frozen hearts and not effluents (28). The amount of glucose uptake in paced hearts is low (29) and was below the limit of detection in our NMR studies. Thus, it is possible that DN-MCU and WT hearts had differences in glucose uptake that were undetected under these experimental conditions.
By selectively eliminating MCU activity in myocardium, our studies revealed an unanticipated feature of the interdependence of cytosolic [Ca2+] and oxidative phosphorylation. Elevation in cytosolic [Ca2+] was substantially a consequence of impaired Ca2+-sensitive metabolism in the mitochondrial matrix because total ATP was reduced in DN-MCU hearts, compared with WT littermate controls, and because addition of exogenous ATP through a patch pipette improved cytoplasmic [Ca2+] in DN-MCU cardiomyocytes. To quantify the role of mitochondrial Ca2+ buffering in sculpting cytosolic [Ca2+] independently of metabolic actions, it would be necessary to develop a model with matrix resident Ca2+-activated dehydrogenases engineered for Ca2+ insensitivity.
Our study suggests that chronic manipulation of MCU is not a viable strategy to protect cardiomyocytes from ischemia-reperfusion injury. Acute MCU inhibition has shown promise as a therapeutic target to protect against cell death (30). We only tested for cell death in cardiomyocytes and cannot exclude the possibility that chronic MCU inhibition in different cell types could protect from pathologic stimuli. Our findings suggest that further advances in understanding mitochondrial mechanisms governing cell survival and cellular responses to loss of MCU-mediated mitochondrial Ca2+ entry are required before developing therapies designed to prevent mitochondrial Ca2+ overload.
Materials and Methods
A complete description can be found in SI Materials and Methods.
Mice Lacking Functional Myocardial MCU.
DN-MCU transgenic mice were recently described (12) and generated by αMHC promoter-driven expression of cDNA encoding the dominant negative form of MCU.
In Vivo and ex Vivo Measurements.
Hemodynamic and LV pressure measurements were made in anesthetized mice with a 1-F Millar catheter and in isolated, Langendorff-perfused hearts in the presence and absence of a LV pressure transducer.
Mitochondrial and Cytoplasmic Ca2+, OCR, and ROS Generation.
Extramitochondrial Ca2+ uptake was measured in cell membrane-permeabilized ventricular myocytes with CaGr5N, intramitochondrial Ca2+ measured with Fura-FF and cytoplasmic Ca2+ measured with Fura 2. OCR was measured in ex vivo hearts, cell membrane-permeabilized muscle fibers, and isolated mitochondria using SeaHorse Biosciences extracullular flux analyzer. O2•- was measured as SOD quenchable signal from isolated mitochondrial using EPR.
SI Materials and Methods
Ca2+ Green-5N Measurements.
We measured mitochondrial Ca2+ uptake using permeabilized ventricular myocytes as previously described (3).
Transmission Electron Microscopy.
LV free-wall tissue was isolated and processed as previously described (3).
Echocardiography.
Unanesthetized mice were used for echocardiography. The anterior chest was shaved and warmed gel was applied. The mouse was grasped gently by the nape of the neck and positioned in the left lateral position. A 30-MHz linear array transducer was applied to the chest to obtain cardiac images. The transducer was coupled to a Vevo 2100 imager (FUJIFILM VisualSonics). Images of the short and long axis were obtained with a frame rate of ∼180–250 Hz. All image analysis was performed offline using Vevo 2100 analysis software (Version 1.5). Endocardial and epicardial borders were traced on the short axis view in diastole and systole. The left ventricular length was measured from endocardial and epicardial borders to the left ventricular outflow tract in systole and diastole. The biplane area-length method was then performed to calculate left ventricular mass and ejection fraction.
In Situ Tetramethylrhodamine Methyl Ester and DCF Measurements.
Excised hearts were perfused with tetramethylrhodamine methyl ester (TMRM, 2 μM) and CM-H2DCFDA (DCF, 10 μM, Molecular Probes) containing Kreb–Henseleit’s (KH) solution (in mM: 120 NaCl, 24 NaHCO3, 11.1 glucose, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.42 NaH2PO4, 10 taurine, 5 creatine, oxygenated with 95% O2 and 5% CO2) at room temperature for 30 min via the retrograde Langendorff perfusion system. Hearts were later transferred to another Langendorff apparatus (37 °C) attached to the confocal microscope system after TMRM and DCF loading was completed. The heart was placed onto a recording chamber for in situ confocal imaging of TMRM and DCF from epicardial myocytes under sinus rhythm. To avoid motion artifacts during imaging, Blebbistatin (10 μM, Sigma) and 2, 3-butandion-monoxim, 10 mM (Sigma) were added to the perfusion solution. Imaging of TMRM or DCF was achieved by tandem excitation at 488 and 561 nm, and the emission was collected at 500–545 and >575 nm, respectively. The heart was allowed to stabilize (10 min) before taking control images; the perfusion solution was switched to a mock ischemia solution (in mM: 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 Hepes) without 95% O2 and 5% CO2 for 30 min. The confocal frame images were acquired with confocal microscope (LSM510, Carl Zeiss MicroImaging Inc.) at 0, 5, 10, 15, 20, 30, and 40 min after reperfusion with KH solution. The microscope was equipped with 63× (N.A. = 1.4) oil immersion lens. The optical pinhole was set to 1 airy disk (<1 μm axial resolution) during confocal imaging. Each image frame contains 202 × 202 μm2 area of myocytes. Five images from different locations of each left ventricular free wall were acquired, and intensity values from each ventricle were averaged to represent the global TMRM and DCF fluorescence intensity of each ventricle.
qRT-PCR.
RNA was isolated using TRIzol reagent (Invitrogen) and purified using a RNeasy MinElute Cleanup Kit (Qiagen). RNA concentrations were measured using a Nanodrop 2000 Spectrophotometer (Thermo Scientific). iScript cDNA Synthesis Kit (Bio-Rad) was used to generate cDNA from RNA using oligo(dT) primers. Validated PrimePCR primers (Bio-Rad) were used for qPCR on a ViiA 7 Real-Time PCR system (Applied Biosystems). Transcript levels were quantified by the ΔΔCt method.
In Situ O2 and LV Pressure Measurements.
Isolated hearts were retrograde-perfused at 90 mm Hg with Krebs–Henseleit buffer containing glucose (10 mM) bubbled with 95% O2/5% CO2 at 37 °C and pH 7.4 (31, 32). The atrioventricular node was mechanically dissociated and hearts were paced (Bloom Electrophysiology) using a platinum pacing catheter (NuMed) positioned in the right ventricular apex. LVP was measured by inserting a balloon into the LV chamber through the mitral valve. The balloon was filled with degassed water and was connected to an APT-300 pressure transducer (World Precision Instruments). The signals were acquired with Axon Clampex software (Molecular Devices). The left ventricular end diastolic (LVEDP) was adjusted to 4–10 mmHg. The following parameters were measured: LV contractility parameters, the LVDP (the pressure difference between the peak LV systolic pressure and LVEDP) and the maximal LV pressure change rate during systole (+dP/dtMAX), and the lusitropic parameter-minimum LV pressure change rate during diastole (−dP/dtMAX). Cardiac O2 consumption was calculated based on Henry’s Law as the difference between the O2 partial tension measured in the perfusate prior (PO2pre) and after (PO2post) heart passage (Model NeoFox Instech Laboratories). The rate of O2 consumption (VOs) was calculated as: VO2 = (PO2pre – PO2post)⋅α⋅v/mheart, where v is the rate of coronary flow (T402, Transonic System Inc.), α = 0.024 mL O2 per mL H2O/760 mm Hg denotes the Brunsen’s solubility coefficient (31, 33), and mheart is the wet heart weight. After 15 min of stabilization, only hearts with coronary flow rate between 2.5 and 5.0 mL⋅s−1 and with LVDP greater than 80 mmHg were included for experimentation. The pacing protocol for O2 consumption and LVP calculations was 9 min of continuous pacing consisting of 3 min at each of the following sequential cycle lengths: 150, 100, and 80 ms.
Permeabilized Fiber O2 and ATP Measurements.
Mitochondrial function was studied using the saponin-permeabilized fiber technique (21) on heart muscle. State 2 respiration was measured after addition of 10 mM succinate. State 3 respiration was assessed by adding 1 mM ADP, and state 4 respiration was measured after addition of 1 mM oligomycin. Mitochondrial ATP measurements were performed by bioluminescence, as described (21).
Mitochondrial O2 Measurements.
Hearts were excised and finely minced at 4 °C in a mitochondrial isolation buffer (70 mM sucrose, 210 mM mannitol, 5 mM Hepes, 1 mM EGTA, 0.5% BSA, pH 7.2). The minced heart was dounce-homogenized and then transferred to chilled Eppendorf tubes. Tubes were centrifuged at 4 °C twice at 600 × g, and supernatant was collected. A final spin of 10,000 × g at 4 °C was performed. The pellet was resuspended in mitochondrial assay buffer (70 mM sucrose, 210 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM Hepes, 1 mM EGTA, 0.2% BSA, 10 mM succinic acid, 2 μM rotenone, pH 7.2). Mitochondria were plated on a 96-well Seahorse Extracellular Flux Analyzer plate (Seahorse Biosciences) at a density of 0.5 μg/well. The plate centrifuged at 770 × g at 4 °C to ensure mitochondria stably present in the bottom of each well. The mitochondria assay buffer was replaced with prewarmed assay buffer. The addition of ADP (4 mM), oligomycin (2.5 μg/mL), FCCP (4 μM), and antimycin A (4 μM) were added sequentially with OCR measurements taken between each substrate addition.
Western Blotting.
Twenty micrograms of mitochondrial or heart lysate was loaded per well. Samples were in 4× LDS buffer (Life Technologies) containing RIPA, phosphatase (p0044, Sigma-Aldrich), and protease (Roche) inhibitors, and sample reducing agent (Life Technologies). Samples were run on 4–12% SDS/PAGE gels at room temperature and transferred to PVDF membrane overnight at 4 °C. pPDH (1:1,000, Cell Signaling), total PDH (1:1,000, Cell Signaling), MYC (1:1,000, Rockland), Bax (1:1,000, Cell Signaling), and MCU (1:500, Yenzyme) were incubated at room temperature for 2 h in TBST containing 5% milk. HRP-conjugated secondary antibodies were incubated at room temperature for 1 h.
Fura-2 Ca2+ Measurements.
Cytosolic Ca2+ levels were recorded from Fura-2–loaded cells, excited at wavelengths of 340 and 380 nm and imaged with a 510-nm long-pass filter. Single isolated ventricular myocytes were loaded with 4 µM Fura-2 acetoxymethyl (AM) for 30 min and then perfused for 30 min to de-esterfy the Fura-2 AM in normal Tyrode’s solution. After placement on a recording chamber, the cells were perfused in normal Tyrode’s solution at 33 ± 0.5 °C. Myocytes were stimulated with 1-, 3-, and 5-Hz bipolar pulse from SIU-102 (Warner Instrument).
L-Type Ca2+ Current Density Recordings.
As described (34), ICa was measured using whole-cell patch clamp technique at room temperature. ICa was confirmed by its sensitivity to Nifedipine 5 µM. Depolarizing voltage pulses (300 ms in duration) to various potentials (−70 to 60 mV in 10-mV steps) were applied from a holding potential of −80 mV. A prepulse to −40 mV of 50 ms was applied before each step to inactivate Na+ currents. The pipette (intracellular) solution comprised (mM): 120 CsCl, 10 Hepes, 20 tetraethylammonium (TEA) chloride, 1.0 MgCl2, 0.05 CaCl2, 10 glucose; pH was adjusted to 7.2 with 1.0 N CsOH. The bath (extracellular) solution comprised (mM): 137 NaCl, 10 Hepes, 10 glucose, 1.8 CaCl2, 0.5 MgCl2, and 25 CsCl; pH was adjusted to 7.4 with NaOH.
Na+/Ca2+ Exchanger Current Density Recordings.
INCX was recorded using whole-cell patch clamp technique at room temperature. The external solution contained the following (mM): 140 NaCl, 1.0 MgCl2, 5.0 Hepes, 2.5 CaCl2, 1.0 BaCl2, 10 glucose, and 10 µM Nitrendipine, and 10 µM Strophanthidin (pH 7.4, adjusted with NaOH). The internal solution contained the following (mM): 110 CsCl, 10 NaCl, 5.0 glucose, 1.0 CaCl2, 0.4 MgCl2, 10 Hepes, and 20 TEA chloride, 5.0 EGTA (pH 7.2, adjusted with CsOH). Membrane currents were elicited by a command ramp of 2 s from +80 mV to −120 mV. Holding potential was −80 mV. The protocol was applied every 10 s. Recordings were taken when five consecutive ramp recordings showed nearly identical currents. INCX was measured as the Ni-sensitive current. NiCl2 (5 mM) was added to define the fraction of current from NCX (the difference currents between total currents and post-Ni2+ currents from the same cell).
Ca2+ Transients Recording with Voltage Clamp Pacing.
Cytosolic Ca2+ levels were recorded from Fura-2–loaded ventricular cells, excited at wavelengths of 340 and 380 nm, and imaged with a 510-nm long-pass filter. Single isolated ventricular myocytes were loaded with 4 μM Fura-2 AM for 30 min and then perfused for 30 min to de-esterfy the Fura-2 AM in normal Tyrode’s solution. After placement on a recording chamber, the cells were perfused in bath solution comprised (mM): 137 NaCl, 10 Hepes, 10 glucose, 1.8 CaCl2, 0.5 MgCl2, and 25 CsCl; pH was adjusted to 7.4 with NaOH, at 35 ± 0.5 °C. Whole-cell patch clamp technique was used to dialyze cells. The pipette (intracellular) solution comprised (mM): 120 CsCl, 10 Hepes, 20 TEA chloride, 1.0 MgCl2, 0.05 CaCl2, 10 glucose; the pH was adjusted to 7.2 with 1.0 N CsOH. Adenosine 5′-triphosphate disodium salt hydrate 5 mM was added to pipette solution when needed. Myocytes were stimulated at 1 and 3 Hz using voltage protocol holding at −80 mV and step to 0 mV for 100 ms from a prepulse of 50 ms at −40 mV.
NMR Spectroscopy.
Hearts from 61-d-old male mice were perfused with KH solution containing 10 mM [1,2-13C2]-glucose for 15 min and paced at 600 bpm. The hearts were ground in a precooled mortar, extracted with 4% perchloric acid, and centrifuged at 47,800 × g for 20 min at 4 °C. The supernatant was then neutralized using 1 M KOH and centrifuged again at 13,000 × g for 10 min at 4 °C. The resulting supernatant was lyophilized and dissolved in 50 mM sodium phosphate, pH 7.4, in 100% D2O for NMR studies. 1H homonuclear 2D DQF-COSY, TOCSY, and ROESY experiments, 1H/13C heteronuclear 2D HMQC, HMBC, HSQC, and H2BC experiments, and 12C-filtered/13C-selected 1H/1H 2D COSY and TOCSY experiments were collected on the heart extract samples using a Bruker Avance II 800 MHz NMR spectrometer equipped with a sensitive cryoprobe for metabolite assignments. One-dimensional 13C spectra and 1H/13C 2D ultra-high-resolution HSQC spectra with 1J13C-13C resolution were also acquired on a Bruker Avance II 500 MHz NMR spectrometer for 13C isotopomer quantification. NMR spectra of the heart extract samples were recorded at 25 °C. The 1H and 13C chemical shifts were referenced to 2,2-dimethyl-2-silapentane-5-sulfonate. All NMR spectra were processed using NMRPipe package (35) and analyzed using NMRView (36).
Ischemia-Reperfusion Studies.
Excised hearts were perfused through the aorta with an oxygenated KH solution for 10 min. Thereafter, 30 min of no-flow global ischemia was induced. Following ischemia, hearts were reperfused with KH solution for 50 min. Hearts were flash-frozen for 2,3,5-triphenyltetrazolium chloride (TTC) staining. Briefly, hearts were serially sectioned and stained with 1% TTC in phosphate-buffered solution at 37 °C. Sections were then placed in 10% neutral buffered formalin and imaged on a stereoscope. Viable myocardium was compared with total tissue surface area using Image J.
Electron Paramagnetic Resonance.
Mitochondria were isolated from whole hearts in an MSE buffer (110 mM mannitol, 20 mM sucrose, 2 mM Tris⋅HCl, 1 mM DPTA, pH 7.2) by fine chopping with a razor and homogenization in a 5-mL Teflon dounce homogenizer. Mitochondria were pelleted and resuspended in a mitochondrial respiratory buffer (100 mM K-Aspartate, 20 mM KCl, 10 mM Hepes, 5 mM pyruvate, 1 mM glutamate, 1 mM malate, 0.3% fatty-acid-free BSA) at a concentration of 1 μg/1 μL. Mitochondrial samples (500 μL with 100 μg total protein) containing 100 mM of the spin trap DMPO (Dojindo Labs) were loaded into a standard quartz TM-flat cell (Wilmad LabGlass) and centered in a 4103 TM cavity (Bruker Instruments). Antimycin A (Sigma) and bovine erythrocyte Cu/Zn superoxide dismutase (Sigma), when added, were at concentrations of 1 μM and 100 units, respectively, with total reaction volume of 500 μL. ESR spectra were obtained while at room temperature with a Bruker EMX spectrometer at a frequency of 9.78 GHz and a modulation frequency of 100 kHz. Seven additive scans (1,024 points per x-scan) were signal-averaged with the microwave power at 39.85 mW, modulation amplitude 1.0 G, time constant 81.9 ms, scanning 80G/41.9 s, with a receiver gain of 5.02 × 105. Signal heights were measured using the second low-field peak of the four-line DMPO spin adduct and are reported as arbitrary signal heights.
PDH Enzyme Assay.
The dipstick assay was performed in accordance with kit ab109882 (AbCam) using isolated mitochondria. Optical density was measured using Image J.
Gene-Expression Profiling Analysis.
Gene expression profiles for mouse heart tissues were performed using the Affymetrix Mouse 430A_2 chip as described (37). Partek Genomics Suite (Partek GS) was used for microarray data analysis. Affymetrix raw fluorescence intensity was quantified and normalized using multiarray analysis. Neonatal and adult sample data were used to perform a one-way ANOVA to detect differentially expressed genes in adult mice, but neonatal samples were not considered further. Functional annotation clustering was performed using The Database for Annotation, Visualization and Integrated Discovery (NIH).
Mitochondrial:Nuclear DNA Content Measurement.
DNA was extracted from apical ventricular myocardium using a DNEasy Blood and Tissue Kit (Qiagen). Mitochondrial (CCCATTCCACTTCTGATTACC, ATGATAGTAGAGTTGAGTAGCG) and nuclear (GTACCCACCTGTCGTCC, GTCCACGAGACCAATGACTG) targeted primers were used with SYBR Green mastermix (Bio-Rad) on an ABI StepOne Plus (Applied Biosystems) to compare mitochondrial to nuclear DNA ratio with the ΔΔCt method, as previously described (38).
In Vivo dP/dt Measurements.
Mice were anesthetized with ketamine/xylazine (87.5/12.5 mg/kg, i.p.) and placed supine on a sterile heating pad to maintain body temperature. A small incision was made in the anterior cervical region, and dissection was performed to expose the carotid artery. A 1.4-F Millar catheter was inserted into the artery and advanced into the left ventricle. Pressure was continuously recorded. ±dP/dtMAX were analyzed just before isoproterenol treatment and 2 min after treatment with isoproterenol (10 μg/kg, i.p.) (LabChart, ADInstruments). Heart rates were calculated based on the characteristic pressure waves of the left ventricle.
Fura-FF Mitochondrial Ca2+ Uptake Measurements.
Mitochondria were isolated as described (25). Briefly, ventricles were homogenized in isolation buffer containing 75 mM sucrose, 225 mM mannitol, 1 mM Hepes, 1 mM EGTA, pH7.4, with 0.1 mg/mL bacterial proteinase type XXIV (Sigma-Aldrich). After addition of fatty-acid-free BSA (Calbiochem) to a final concentration of 0.2% (wt/vol), the homogenates were centrifuged at 500 × g for 5 min, and the supernatant was then centrifuged at 10,000 × g for 5 min. The mitochondrial pellet was then washed once with isolation buffer with 0.2% fatty-acid-free BSA and once without BSA. The mitochondria were then resuspended in resuspension solution (RS) with 75 mM sucrose, 225 mM mannitol, 1 mM Hepes, 20 µM EGTA, pH7.4, to 10–20 mg mitochondrial protein/mL as determined by Bradford protein assay (Bio-Rad).
Ca2+ uptake assays were carried out on a BioTek Synergy MX plate reader. For calcium uptake assay with extramitochondrial calcium indicator Calcium Green-5N (Life Technologies), 120 µg of mitochondria was added to each well of the 96-well assay plates containing assay solution with 1 µM Calcium Green-5N. The assay solution consists of 137 mM KCl, 20 µM EGTA, 20 mM Hepes, 5 mM glutamic acid, 5 mM disodium malate, 2 mM of potassium phosphate, pH 7.15. Free [Ca2+] was calculated by WEBMAXC STANDARD (web.stanford.edu/∼cpatton/webmaxcS.htm). Addition of 15.0, 17.6, 20.3, 25.0, and 35.0 µM of total [Ca2+] corresponded to 0.5, 1.0, 2.0, 5.7, and 15.3 µM increase of free [Ca2+], respectively. For calcium uptake assay with intramitochondrial calcium indicator Fura-FF-AM (Life Technologies), the mitochondria were first loaded in RS containing 20 µM Fura-FF-AM at room temperature for 30 min. After loading, mitochondria were washed twice with RS and then resuspended in RS and used for assay. When indicated, 5 µM Ru360 (Calbiochem) was added.
Laser-Scanning Confocal Imaging of Single Ventricular Myocytes.
Mouse ventricular myocytes isolated from WT or DN-MCU mice were loaded with Fluo-4 AM (5 μM) for 30 min at room temperature. After 20 min of de-esterification, the cell were placed in a recording chamber and perfused with normal Tyrode’s solution (1.8 mM Ca2+) at 36 ± 1 °C (Temperature Controller, TC2BIP, Cell MicroControls). Confocal Ca2+ imaging was performed in line-scan mode with a laser-scanning confocal microscope (LSM 510, Carl Zeiss) equipped with a N.A. of 1.35, 63× lenses. Images of Ca2+ transients and caffeine-induced Ca2+ transients (SR Ca2+ contents) were acquired at a sampling rate of 1.93 ms per line along the longitudinal axis of the cells. Steady-state Ca2+ transients were achieved by a 30-s pacing at 1 Hz. SR Ca2+ content was determined by measuring the amplitude of Ca2+ release induced by local delivery of 20 mM caffeine at baseline and after addition of 100 nM isoproterenol. All digital images were processed with IDL 6.0 program (Research System Inc.).
Measurement of Antioxidant Enzymes.
Mice were fully anesthetized and then euthanized. Hearts were rapidly excised and washed in ice-cold PBS and then flash-frozen in liquid nitrogen.
Catalase activity assay.
Catalase activity was determined on whole-mouse hearts, homogenized in 50 mM potassium phosphate buffer (pH 7.8, with 1.34 mM diethylenetriaminepentaacetic acid). Activity was measured spectrophotometrically. The disappearance of 10 mM hydrogen peroxide (240 nm) was measured as described (39). The equation A60s = A0 e-kt was used to fit the exponential decay of H2O2 at 240 nm where k = the rate constant for the catalase-dependent disappearance of H2O2. Activity was normalized per mg protein (40) and expressed as mk units/mg protein as described.
SOD activity assay.
SOD activity was determined on mouse heart homogenates (as above) using an indirect competitive inhibition assay (41). The inhibition of superoxide-mediated nitroblue tetrazolium reduction (from xanthine/xanthine oxidase) was measured spectrophotometrically at 560 nm with one unit defined as the amount of protein capable of causing 50% of maximal inhibition. In this assay, configuration 1 unit corresponds to ∼6–10 ng for pure CuZnSOD/mL. MnSOD activity was determined by inhibition of CuZnSOD activity with 5 mM sodium cyanide. Activity is expressed as units/mg protein as determined by the Lowry method as described (42).
Glutathione peroxidase activity assay.
Selenium-dependent glutathione peroxidase activity (predominantly GPx1) was measured spectrophotometrically using 0.25 mM H2O2 as substrate with the method of Lawrence and Burk (43). One unit of enzymatic activity was defined as the amount of protein needed to oxidize 1 μmol NADPH/min and expressed as milliunits/mg protein as described (40, 42).
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth Murphy for advice and initial studies on 13C glucose metabolism; Chantal Allamargot and the University of Iowa Central Microscopy Facility for technical assistance with electron microscopy; the University of Iowa Gene Transfer Vector Core and Mouse Transgenic Facility for technical assistance; Jinying Yang for technical support; and Kathy Zimmerman, Dr. Nathan Funk, and Dr. Tariq Hameed for echocardiographic analysis. This work was supported by National Institutes of Health Grants F30 HL114258-02 (to T.P.R.), R01 HL079031 (to M.E.A.), R01 HL070250 (to M.E.A.), R01 HL096652 (to M.E.A.), R01 HL113001 (to M.E.A.), T32 GM007337 (to University of Iowa Medical Scientist Training Program), S10 RR026293-01 (to R.M.W.), R01 DK092412 (to L.V.Z.), and R01 CA182804 (to D.R.S.). This work was supported by Veterans Administration Merit Review Program Grant 1I0BX000718 (to L.V.Z.). The Electron Spin Resonance Facility was supported in part by Holden Comprehensive Cancer Center Grant P30 CA086862.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE62049).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504705112/-/DCSupplemental.
References
- 1.Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci USA. 2012;109(32):12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopper RK, et al. Mitochondrial matrix phosphoproteome: Effect of extra mitochondrial calcium. Biochemistry. 2006;45(8):2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joiner ML, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491(7423):269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51(14):2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan X, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15(12):1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramaniam A, et al. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991;266(36):24613–24620. [PubMed] [Google Scholar]
- 9.Murphy E, et al. Unresolved questions from the analysis of mice lacking MCU expression. Biochem Biophys Res Commun. 2014;449(4):384–385. doi: 10.1016/j.bbrc.2014.04.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013;52(16):2793–2809. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajnóczky G, et al. Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40(5-6):553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015;6:6081. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denton RM, McCormack JG, Edgell NJ. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111(8):1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 15.Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 16.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 17.Hou T, et al. Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal reactive oxygen species elevation. J Biol Chem. 2013;288(7):4602–4612. doi: 10.1074/jbc.M112.398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, et al. Superoxide flashes in single mitochondria. Cell. 2008;134(2):279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piot C, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 21.Boudina S, et al. Mitochondrial energetics in the heart in obesity-related diabetes: Direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56(10):2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 22.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 24.Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci USA. 2013;110(26):10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei AC, Liu T, Winslow RL, O’Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: Two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol. 2012;139(6):465–478. doi: 10.1085/jgp.201210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca(2)(+) homeostasis: Mechanism, role, and tissue specificities. Pflugers Arch. 2012;464(1):3–17. doi: 10.1007/s00424-012-1122-y. [DOI] [PubMed] [Google Scholar]
- 27.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88(3):1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 28.Lopaschuk GD, Barr RL. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem. 1997;172(1-2):137–147. [PubMed] [Google Scholar]
- 29.Tada H, et al. Myocardial glucose uptake is regulated by nitric oxide via endothelial nitric oxide synthase in Langendorff mouse heart. Circ Res. 2000;86(3):270–274. doi: 10.1161/01.res.86.3.270. [DOI] [PubMed] [Google Scholar]
- 30.de Jesús García-Rivas G, Guerrero-Hernández A, Guerrero-Serna G, Rodríguez-Zavala JS, Zazueta C. Inhibition of the mitochondrial calcium uniporter by the oxo-bridged dinuclear ruthenium amine complex (Ru360) prevents from irreversible injury in postischemic rat heart. FEBS J. 2005;272(13):3477–3488. doi: 10.1111/j.1742-4658.2005.04771.x. [DOI] [PubMed] [Google Scholar]
- 31.Alekseev AE, et al. Sarcolemmal ATP-sensitive K(+) channels control energy expenditure determining body weight. Cell Metab. 2010;11(1):58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zingman LV, et al. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J Mol Cell Cardiol. 2011;51(1):72–81. doi: 10.1016/j.yjmcc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinaasappel M, Donkersloot C, van Bommel J, Ince C. PO2 measurements in the rat intestinal microcirculation. Am J Physiol. 1999;276(6 Pt 1):G1515–G1520. doi: 10.1152/ajpgi.1999.276.6.G1515. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci USA. 2009;106(14):5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaglio F, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 36.Johnson BA, Blevins RA. NMR View: A computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4(5):603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 37.Zhan F, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99(5):1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 38.Puente BN, et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad IM, et al. Mitochondrial O2•- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280(6):4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 40.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 41.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179(1):8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 42.Spitz DR, et al. Oxygen toxicity in control and H2O2-resistant Chinese hamster fibroblast cell lines. Arch Biochem Biophys. 1990;279(2):249–260. doi: 10.1016/0003-9861(90)90489-l. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.