Significance
Many viruses use a molecular motor to pump DNA into a preformed protein shell called the capsid, a process that is essential for the formation of infectious virus particles. The ATPase machine powering this process is the strongest known biological motor. However, the structure and mechanism of this motor are unknown. Here, we derive a structural model of the ATPase assembly using a combination of X-ray crystallography, small-angle X-ray scattering, molecular modeling, and biochemical data. We identify residues critical for ATP hydrolysis and DNA binding, and derive a mechanistic model for the translocation of DNA into the viral capsid. Our studies introduce a model for ATPase assembly and illustrate how DNA is pumped with high force.
Keywords: ASCE ATPase, thermophile, bacteriophage, translocase, motor protein
Abstract
Many viruses package their genomes into procapsids using an ATPase machine that is among the most powerful known biological motors. However, how this motor couples ATP hydrolysis to DNA translocation is still unknown. Here, we introduce a model system with unique properties for studying motor structure and mechanism. We describe crystal structures of the packaging motor ATPase domain that exhibit nucleotide-dependent conformational changes involving a large rotation of an entire subdomain. We also identify the arginine finger residue that catalyzes ATP hydrolysis in a neighboring motor subunit, illustrating that previous models for motor structure need revision. Our findings allow us to derive a structural model for the motor ring, which we validate using small-angle X-ray scattering and comparisons with previously published data. We illustrate the model’s predictive power by identifying the motor’s DNA-binding and assembly motifs. Finally, we integrate our results to propose a mechanistic model for DNA translocation by this molecular machine.
Double-stranded DNA (dsDNA) viruses ranging from bacteriophages to the human pathogens of the herpesvirus family form infectious virions by packaging their genomes into preformed procapsids using a powerful ATPase machine (1). The viral genome packaging motor is a multicomponent molecular machine that must complete several tasks in sequential order, the foremost of which is the ATP-dependent pumping of viral DNA into the procapsid (Fig. 1A). Because the DNA progresses from a flexible state to a semicrystalline state as it fills the capsid interior, the motor pumps against a tremendous force. The pressures inside the filled capsid are estimated to reach 60–70 atm (2, 3), equivalent to 10-fold higher than a bottle of champagne. Thus, the viral packaging motor represents one of the most powerful biological motors known (2, 4).
Fig. 1.
Genome packaging in bacteriophage and TerL constructs. (A) Maturation of dsDNA viruses is a five-step process: (1) the small terminase (TerS) subunit of the motor recognizes the concatemeric viral genome; (2) the motor binds to the portal complex on the capsid; (3) TerL hydrolyzes ATP to translocate DNA into the capsid; (4) after the genome is translocated into the capsid, TerL switches its enzymatic activity from translocation to DNA cleavage; and (5) the motor is finally released for maturation of another virus while portal binds to the tail proteins to complete a mature, infectious virion. (B) TerL constructs used in this study. Full-length TerLP74-26 is produced with an N-terminal T7-gp10 expression tag that is removed by prescission protease (PP) cleavage. The C-terminal His10 tag is noncleavable.
The central component of the packaging motor is the ATPase subunit, which drives DNA translocation. The ATPase subunit is a member of the additional strand, conserved glutamate (ASCE) superfamily of ATPases (5). In herpesviruses, as well as many bacteriophages, this ATPase is from a specific clade of the ASCE family called the terminase family (1, 6). In viruses that use a terminase-type motor for genome packaging, the motor consists of several proteins that assemble into homomeric rings (7) (Fig. 1A). The large terminase (TerL) protein harbors the motor’s two enzymatic activities (7): the ATPase activity that pumps DNA into the capsid and an endonuclease domain that cleaves packaged DNA from the remaining concatemeric DNA when the capsid is full. Cryoelectron microscopy (cryo-EM) studies indicate that a pentamer of TerL subunits attaches to the capsid by binding to a dodecameric assembly called portal (8). However, there are conflicting reports as to the orientation of TerL relative to portal during packaging (8–10).
A structural model for the bacteriophage T4 TerL ring has been previously proposed (8) with these salient features: (i) the nuclease domains assemble to constitute the ring distal to the capsid, with the ATPase domains protruding radially to interact with portal; (ii) consequently, it was proposed that the active site residues that catalyze ATP hydrolysis are derived from one subunit exclusively; (iii) the pore of the ring has a net negative charge and is largely unconserved; and (iv) DNA was proposed to be bound primarily by the nuclease domain. This model is substantially different from models observed for other nucleic acid translocases of the ASCE family, in which the pore is largely conserved and positively charged, and ATP hydrolysis is catalyzed in trans (11–15). Resolving these disparities between the proposed assembly and conserved features of the ASCE family is necessary to understand the molecular mechanisms of motor action.
Several issues have prevented elucidation of the terminase mechanism. First, the TerL proteins that have been studied in vitro thus far fail to form rings in isolation (16–19). Second, crystal structures of the TerL proteins from bacteriophages Sf6 and T4 (20, 21) (gp2 and gp17, respectively; hereafter known as Sf6-TerL and T4-TerL) do not show ATP-dependent conformational changes sufficient to power DNA translocation. Consequently, the structural mechanism coupling ATP hydrolysis to DNA translocation remains nebulous. Structural analysis of the WT TerL ATPase domain in different nucleotide states will be necessary to address this issue.
To answer these critical questions, we used a novel model system for understanding motor function. Because packaging motor ATPases are often insoluble (22–24), we first sought a packaging motor system that is highly soluble and forms rings in isolation to enable mechanistic analyses in a simplified system. We anticipated that TerL from a thermophilic phage would have higher stability, solubility, and propensity to form a pentamer. Moreover, we expected that the features necessary for DNA translocation would be more exaggerated in a thermophilic motor because packaging is expected to be more difficult at high temperature due to the increased entropic penalty of ordering DNA within the capsid (3).
Here, we describe the TerL protein from the phage P74-26 (hereafter known as TerLP74-26), a thermophilic siphovirus that infects Thermus thermophilus (25). We show that the TerLP74-26 assembles into a pentamer that has ATPase and DNA-binding activity. We report the structure of the TerLP74-26 ATPase domain in both the apo and ADP•BeF3-bound states, and observe a large conformational change in a subdomain in response to ATP hydrolysis and release. We also show that ATP hydrolysis is catalyzed by a conserved arginine finger residue that is provided in trans by a neighboring TerL subunit. Finally, a combination of biochemical, biophysical, structural, and computational data is used to build a structural model for the TerL ring and a mechanistic model for how ATP hydrolysis results in translocation of DNA.
Results
Characterization of TerLP74-26.
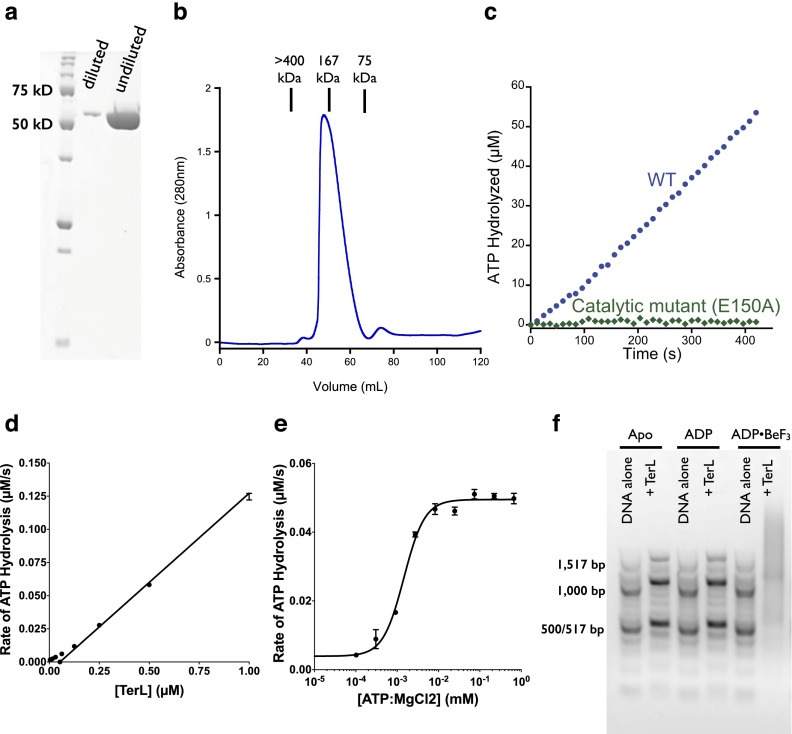
We recombinantly expressed and purified TerLP74-26 in Escherichia coli (Fig. S1A). TerLP74-26 predominantly elutes in size exclusion chromatography (SEC) at a volume consistent with a pentamer [molecular mass by SEC (MMSEC) of ∼270 kD, MMpentamer of 285 kD] with a small monomer peak (MMSEC of ∼57.3 kD, MMmonomer of 57.1 kD) (Fig. S1B). This observation is notable, because other TerL proteins are predominantly monomeric in isolation (16–19), only forming a pentameric ring when bound to the procapsid (8). TerLP74-26 is an active ATPase that exhibits apparent cooperativity in ATP hydrolysis, suggesting that the active sites are coupled (Fig. S1 C–E). TerLP74-26 tightly binds DNA when locked into an ATP-bound state by the ATP mimic ADP•beryllium trifluoride (BeF3) (26, 27) (Fig. S1F). Thus, because the isolated TerLP74-26 protein can assume a pentamer consistent with its functional form, we argue that TerLP74-26 is an excellent minimal model for understanding packaging motor structure.
Fig. S1.
Characterization of TerLP74-26. (A) SDS/PAGE analysis of TerLP74-26 purity. Diluted TerLP74-26 shows that the protein runs as a single band and that the undiluted protein shows no significant protein contaminants. Precision Plus Protein Unstained Standards (BioRad) are used as molecular mass markers. (B) TerLP74-26 elutes in SEC (S200) at a volume consistent with a pentamer. (C) TerLP74-26 shows significant ATPase activity as measured by a coupled-enzyme assay (50), whereas a variant that removes the catalytic glutamate (E150A-TerLP74-26) is completely inactive, suggesting that the observed ATPase activity in WT TerL is not due to contaminants. The E150A variant’s active site is disabled in cis but can still donate an intact arginine finger to an adjacent subunit in the assembly. (D) Steady-state ATPase activity is shown for different concentrations of TerL. Error bars are the SD from at least three replicates. (E) Michaelis–Menten kinetic analysis of TerLP74-26. Titration of ATP indicates that TerLP74-26 has high affinity for ATP•MgCl2 (concentration for half-maximal activity of ∼1.5 ± 0.2 μM) and robust turnover [apparent catalytic rate constant (kcat,app) of ∼0.091 ± 0.003 s−1]. ATP hydrolysis is sigmoidal with respect to the concentration of ATP•MgCl2, with an observed Hill coefficient of 1.7 ± 0.3. This apparent cooperativity suggests that there is coupling between ATPase active sites within the TerL assembly. (F) TerLP74-26 binds DNA when incubated with an ATP mimic. One hundred twenty-five nanograms of 100-bp ladder (New England BioLabs) was incubated with either TerLP74-26 or a buffer control for 30 min at room temperature and run on a 1.5% (wt/vol) agarose gel at 80 V. Tight binding to DNA is only observed for TerLP74-26 that is preincubated with ADP•BeF3. Slight shifts in the DNA migration are observed for TerLP74-26 in the apo state or incubated with ADP or ATP, which may indicate weak binding.
Structure of the TerL ATPase Domain.
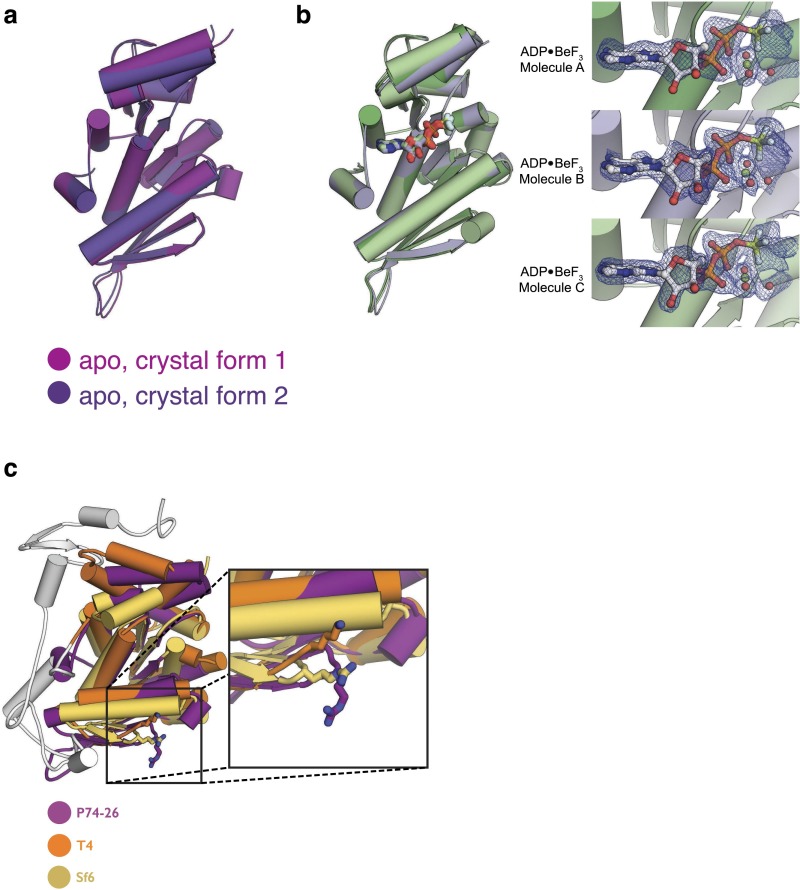
We next sought to elucidate the structural mechanism of genome packaging by TerLP74-26. Because DNA translocation is ATP-dependent, considerable insight can be obtained from the structure of the isolated ATPase domain. Therefore, we expressed, purified, and crystallized a construct that corresponds to the N-terminal ATPase domain of TerLP74-26 (residues 1–256; Fig. 1B). The structure in the absence of nucleotide was determined by single-wavelength anomalous dispersion (SAD) (28) using selenomethionine (SeMet)-labeled protein, and phases were extended to 2.1 Å using diffraction data from native protein (Table S1). A second crystal form yielded another structure of the apo TerLP74-26 ATPase domain to 1.9 Å. The structures from each crystal form are very similar (Cα rmsd of 0.8Å; Fig. S2A and Table S2). Unlike the full-length TerLP74-26, the isolated ATPase domain does not form a pentameric arrangement in either crystal form.
Table S1.
Crystallographic statistics
| Parameter | Apo-1 | Apo-1 R139A | ADP•BeF3 | Apo-2 |
| Data collection | ||||
| Wavelength, Å | 0.9774 | 1.54 | 1.100 | 1.033 |
| Resolution range, Å | 45.81–2.097 (2.171–2.097) | 28.98–2.532 (2.622–2.532) | 38.46–2.068 (2.142–2.068) | 34.4–1.931 (2–1.931) |
| Space group | I 2 3 | I 2 3 | P 32 | P 32 2 1 |
| Unit cell a, b, c | 129.578 129.578 129.578 | 129.578 129.578 129.578 | 76.92 76.92 131.12 | 62.89 62.89 133.1 |
| Unit cell α, β, γ | 90 90 90 | 90 90 90 | 90 90 120 | 90 90 120 |
| Total reflections | 298,214 (29,811) | 527,226 (52,149) | 247,951 (24,350) | 113,291 (10,868) |
| Unique reflections | 21,390 (2,138) | 11,395 (1,232) | 51,948 (5,228) | 23,556 (2,255) |
| Multiplicity | 13.9 (13.9) | 43.1 (42.3) | 4.8 (4.7) | 4.8 (4.8) |
| Completeness, % | 99.99 (100.00) | 100.00 (100.00) | 98.02 (98.03) | 99.68 (97.75) |
| Mean I/σ(I) | 15.80 (2.48) | 45.99 (2.59) | 14.24 (6.39) | 10.71 (2.96) |
| Wilson B-factor | 46.87 | 62.63 | 23.19 | 24.32 |
| R-merge | 0.087 (1.275) | 0.074 (2.214) | 0.058 (0.2278) | 0.090 (0.504) |
| R-meas | 0.090 (1.323) | 0.075 (2.240) | 0.066 (0.258) | 0.102 (0.569) |
| R-pim | 0.024 (0.353) | 0.011 (0.343) | 0.031 (0.120) | 0.045 (0.256) |
| CC1/2 | 0.999 (0.759) | 1.000 (0.952) | 0.997 (0.988) | 0.997 (0.851) |
| CC* | 1.000 (0.929) | 1.000 (0.988) | 0.999 (0.997) | 0.999 (0.959) |
| Refinement | ||||
| R-work | 0.182 (0.263) | 0.201 (0.386) | 0.201 (0.246) | 0.163 (0.214) |
| R-free | 0.211 (0.292) | 0.244 (0.440) | 0.243 (0.301) | 0.206 (0.263) |
| No. of nonhydrogen atoms | 2,221 | 2,126 | 6,872 | 2,351 |
| Macromolecules | 2,085 | 2,044 | 6,273 | 2,122 |
| Ligands | 35 | 30 | 111 | 20 |
| Water | 101 | 52 | 490 | 209 |
| Protein residues | 251 | 250 | 764 | 258 |
| rms, bonds | 0.009 | 0.007 | 0.007 | 0.009 |
| rms, angles | 1.13 | 0.92 | 0.99 | 1.17 |
| Ramachandran favored, % | 99 | 96 | 98 | 98 |
| Ramachandran outliers, % | 0 | 0 | 0.13 | 0 |
| Clash score | 2.88 | 2.21 | 3.19 | 0.47 |
| Average B-factor | 57.8 | 71.4 | 34.9 | 29.4 |
| Macromolecules | 57.3 | 70.6 | 34.1 | 28.2 |
| Solvent | 56.2 | 68.4 | 43.7 | 39.5 |
CC, correlation coefficient; I/σ(I), intensity divided by error of intensity; R, residual factor; R-meas, redundancy independent merging R factor; R-pim, precision indicating merging R factor.
Fig. S2.
Comparison of TerLP74-26 structures. (A) Similarity between the two apo structures of the TerLP74-26 ATPase domain (Cα rmsd of 0.8 Å). The structures from both crystal forms are superposed with small deviations at the top of the lid and beta-hairpin loop. These regions make different lattice contacts in the two crystal forms. (B, Left) Superposition of the three individual ADP•BeF3-bound ATPase domains shows that there are only minor differences between each monomer. The loop linking beta-strands 4 and 5 displays significant (>1 Å) differences between all three conformers. This loop is longer than seen in the structures of the Sf6-TerL and T4-TerL proteins (20, 21), and it is in different packing environments in each monomer in the asymmetric unit. Thus, we conclude that this loop is relatively flexible in TerLP74-26. (B, Right) Omit map density for the ADP•BeF3 ligands, magnesium ions, and waters in each active site. Omit maps contoured at 3σ (0.34 e−/Å2) are shown for the active sites of all three ATPase domains in the asymmetric unit. Although molecule A has a slightly different electron density in the active site, its overall conformation is essentially the same as in molecules B and C, with a Cα rmsd within active site residues of 0.17 Å and 0.16 Å, respectively. (C) Comparison of the ATPase domains of TerL orthologs. TerL ATPase domains from Sf6 (tan) and T4 (orange) are superposed onto P74-26 (purple), with Cα rmsd values of ∼2.1 Å and 1.7 Å, respectively. Note that the gray region in T4-TerL is unique to T4 and closely related phages, and was not used for superposition. (Inset) Conservation of the TerLP74-26 basic patch. Lys223 in T4-TerL is at the same position as DNA-binding residue Arg101 of TerLP74-26. Arg82 in Sf6-TerL is at the same position as Arg101 of TerLP74-26.
Table S2.
Cα rmsd
| Apo-1 | Apo-2 | BeF3-A | BeF3-B | BeF3-C | Apo-1 R139A | |
| Apo-1 | 0.778 | 1.225 | 1.197 | 1.056 | 0.165 | |
| Apo-2 | 1.055 | 1.127 | 0.897 | 0.784 | ||
| BeF3-A | 0.526 | 0.461 | 1.231 | |||
| BeF3-B | 0.641 | 1.119 | ||||
| BeF3-C | 1.053 | |||||
| Apo-1 R139A |
Values are measured in angstroms.
We note three prominent features of the TerLP74-26 ATPase domain (Fig. 2). First, TerLP74-26, as in other terminase ATPase domains (20, 21), contains a C-terminal subdomain (residues 221–251 in TerLP74-26) that sits above the ATPase active site. We call this region the “lid” subdomain with reference to the structurally unrelated but analogous lid subdomains found in other ASCE ATPases (29). Lid subdomains are often used in contacts between adjacent subunits in ASCE oligomers (11, 30), and their conformation can be modulated by ATP hydrolysis (11, 31). Second, we observe a large patch of six basic residues (R100, R101, R102, R104, R128, and K130) in the turns and strands of the antiparallel section of the beta-sheet, a region that is specific to the terminase ASCE subfamily (6). A basic residue is conserved at the Arg101 position in the other large terminase structures [Lys223 and Arg82 in T4 (8) and Sf6 (20), respectively] (Fig. S2C). We hypothesize that this region binds to the DNA backbone during packaging. In support of this hypothesis, we observe a sulfate ion bound to Arg101 in all of our crystal forms. The third region of note is a conserved arginine that is on the opposite face from the active site. We propose that this residue is used to activate ATP hydrolysis in a neighboring subunit in the assembly (discussed below).
Fig. 2.
Structure of the TerLP74-26 ATPase domain. Characteristics of the TerL ATPase domain are color-coded. The Walker A and Walker B motifs form the active site. The lid subdomain (residues 221–251) is adjacent to the ATP-binding site and contains large hydrophobic residues that are positioned away from the hydrophobic core of the Rossmann fold. The hydrophobic residues Trp231 and Tyr238 are solvent-exposed, suggesting that they could participate in protein–protein interactions. Arg100, Arg101, Arg102, Arg104, Arg128, and Lys130 form a continuous, solvent-exposed basic patch. Arg101 is identified as critical for DNA binding. Arg139, positioned ∼25 Å from the active site, is established as necessary for catalysis in trans.
To determine the conformational changes induced by ATP binding, we solved a 2.0-Å crystal structure of the TerLP74-26 ATPase domain cocrystallized with the ATP mimic, ADP•BeF3. The packing of the ADP•BeF3 cocrystal is unrelated to the apo crystals, with three ADP•BeF3-bound TerLP74-26 ATPase domains in the asymmetric unit (Fig. S2B). The residues of the Walker A and B motifs are in the active conformation (Fig. 3A). The largest conformational change between the apo and ADP•BeF3 states occurs in the lid subdomain. Upon binding the ATP analog, the lid rotates ∼13° in a rigid body motion (Fig. 3B and Movie S1). Upon binding the ATP mimic, the P-loop residues Arg39 and Gln40 rotate to contact the gamma-phosphate mimic directly. The P-loop’s interactions with several lid residues (Ser221, Trp225, Arg228, and Tyr232) result in a commensurate rotation of the lid toward the ATP adenine ring (Fig. 3C). Thus, the motion appears to be induced by the P-loop, which bridges the gamma-phosphate mimic and residues at the base of the lid. The rotation of the lid upon ATP binding results in a reorientation of the lid’s hydrophobic residues such that they are now less solvent-exposed, potentially altering TerL’s interactions within the motor (Fig. 3D).
Fig. 3.
Structure of the TerLP74-26 ATPase domain in complex with an ATP analog. (A) ADP•BeF3 is bound in the TerL active site. An omit map density (blue mesh) contoured to 3σ (0.34 e−/Å2) shows ADP•BeF3, a magnesium ion (green), and waters (red) bound in the TerL active site. Ser44, an ADP beta-phosphate oxygen, fluoride, and three waters coordinate the magnesium ion. Glu150 is pointed toward the BeF3 moiety to catalyze ATP hydrolysis. (B) Conformational changes upon ATP hydrolysis and release. The ATPase domain of the ADP•BeF3-bound structure (light purple, with ADP•BeF3 in green) was superposed onto the Rossmann fold of the apo structure (dark purple). The lid was not used for superposition. Vectors (orange arrows) show alpha-carbon position differences of 2.5 Å or greater between the two structures. The teal bar marks the lid’s axis of rotation, which lies just off the ATP gamma-phosphate mimic. (C) P-loop interactions with the lid (green) appear to drive conformational changes. Hydrogen bonds are shown as yellow dashes. (Left) Backbone carbonyls of P-loop residues Arg39 and Gln40 (dark purple) interact with side chains of lid residues Ser221 and Trp225. (Right) Arg39 and Gln40 change position to bind ADP•BeF3, pulling at the base of the lid. The new Gln40 position binds Tyr232, stabilizing the new lid conformation. The Trp225 indole rearranges to stack with the adenine base (purple disks), and Arg39 and Gln40 side chains engage the beryllium fluoride (purple dashes). (D) Schematic illustration depicting the conformational change of the lid subdomain in response to nucleotide hydrolysis and release. The lid is held fixed such that the Rossmann fold rotates by ∼13° outward.
Identification of the Trans-acting Arginine Finger.
The structures of TerL ATPases reported here and elsewhere (20, 21) display incomplete active sites, presumably because all were crystallized without formation of an active ring. These monomeric TerL structures have only two positively charged residues within the P-loop that contact the gamma-phosphate of ATP to stabilize the transition state for hydrolysis. However, other ASCE family members have at least three positive residues that contact the gamma-phosphate (11–15, 31, 32). Indeed, our ATPase domain construct lacks ATPase activity (Fig. S3A), confirming that the active site in our structure is incomplete. In ASCE-type ATPases, the third positive charge is provided in trans by a residue known as the “arginine finger,” which is most often arginine, although it can also be lysine (29). The location of the arginine finger within the Rossmann fold varies widely across the ASCE family, which means that ASCE subfamilies often have different relative orientations of their ATPase domains within the oligomer (33). Thus, the identification of the trans-acting arginine finger in TerL is necessary both for elucidation of the ATPase mechanism and for determining the architecture of the TerL ring assembly.
Fig. S3.
Identification of the trans-acting arginine finger. (A) TerLP74-26 ATPase domain is not catalytically active. Steady-state ATPase activity is shown for 0.5 μM TerL. Error bars are the SD from at least three replicates. (B) Mixture of the R139A and E150A single mutants has optimal ATP hydrolysis at a 1:1 ratio. TerL-R139A and TerL-E150A are mixed at the indicated ratios, with a final concentration of 1 μM total TerL, and steady-state ATPase activity is measured. (C) R139A mutation does not cause conformational changes in the ATPase domain. The 2.5-Å crystal structure of the R139A-TerLP74-26 ATPase domain (yellow) is superposed onto the 2.0-Å structure of the WT structure (dark purple). Position 139 is shown in a ball-and-stick representation for both variants. (D) Arginine finger residue is conserved in other TerL ortholog structures. T4-TerL and Sf6-TerL (PDB ID codes 3CPE and 4IEE, respectively) were superposed onto TerLP74-26, with the arginine finger residue shown in stick representation.
We used a two-step strategy to identify the arginine finger of TerLP74-26. First, we screened mutants of surface-exposed arginines in full-length TerLP74-26 for ATPase activity. For mutants that showed no ATPase activity in this initial scan, we tested whether an inactive TerLP74-26 protein (mutated Walker B motif) could restore activity by donating its intact arginine finger.
We measured steady-state ATPase activity for 11 different arginine mutants in full-length TerLP74-26 (Fig. 4A). The R39A, R139A, and R235A variants completely abrogate ATPase activity, making them candidates for the arginine finger. Of these candidate residues, Arg39 and Arg235 reside within or near the active site, suggesting that they may affect catalysis in cis. Conversely, Arg139 is located ∼25 Å away from the gamma-phosphate of ATP (Fig. 2).
Fig. 4.
Identification of the TerL arginine finger residue. (A) ATP hydrolysis rates of TerLP74-26 arginine mutants. The only arginine mutants that abolish ATPase activity are R39A, R139A, and R235A (compare activity with activity of the inactive E150A mutant). The final TerL concentration is 0.5 μM. For all relevant panels in this figure, error bars are the SD from at least three replicates. R39 is a conserved P-loop residue proposed by Sun et al. (8) to be a cis-acting arginine finger. (B) Arginine finger complementation assay. Different TerL variants at 0.5 μM each are mixed either with an alternate TerL mutant in a 1:1 ratio (as indicated by the “+” sign) or with buffer, and their steady-state ATPase activity is measured. Note that the R139A/E150A double mutant is a single variant and not a mixture. Only a mixture of the R139A and E150A mutants significantly restores activity. Schematic illustrations illustrate that the Walker B mutant (E150A) has a debilitated active site but can donate its intact arginine finger (shown as an arrow), whereas the arginine finger mutant has an intact active site but lacks ATPase activity due to loss of the trans-acting residue. (C) Arginine finger residue is conserved in other TerL ortholog sequences. A logo diagram (46) was made from a sequence alignment of 70 TerL proteins. The residue numbering is shown for TerLP74-26.
To assess which of our candidates is the arginine finger, we developed a biochemical complementation assay. We mixed each of our three candidate arginine finger mutants with the E150A variant whose active site is disabled in cis (Fig. S1C) but can still donate an intact arginine finger to an adjacent subunit in the assembly. Thus, E150A should restore activity to an arginine finger mutant, but not to a mutant whose ATPase activity is disabled in cis. Mixing E150A with R139A results in restoration of ATPase activity, whereas mixtures of E150A with R39A or R235A exhibit no significant ATP hydrolysis (Fig. 4B). ATPase activity is maximal at a 1:1 ratio of E150A to R139A (Fig. S3B), as expected for complementation of an arginine finger mutant. In contrast, a double mutant combining both the R139A and E150A mutations in the same protein has no activity, and cannot be complemented by either the E150A or R139A single mutant. Our complementation results mirror the results of similar experiments from Cox et al. (34) definitively establishing that the ASCE family member RecA uses trans-interactions to catalyze ATP hydrolysis.
We verified that the R139A mutation does not perturb ATPase domain structure by solving the 2.5-Å resolution structure of the isolated ATPase domain with the R139A mutation. This structure is essentially identical to the WT structure (Cα rmsd = 0.2 Å; Fig. S3C), suggesting the observed deficit is not due to structural changes in cis. In support of this finding, we observe that arginine or lysine is conserved at this position in the large terminase family (Fig. 4C and Fig. S3D). Hence, we expect that the arginine finger is playing a similar role throughout the family of large terminases. Taken together, our results indicate that Arg139 is the arginine finger, which mediates ATP hydrolysis in TerL in trans. Our identification of the arginine finger illustrates that (i) ATP hydrolysis is catalyzed in trans and (ii) the previously proposed structural model (8) must be substantially refined to account for these trans-interactions within the TerL ring.
Modeling of the TerL Ring.
Our two key results, the TerLP74-26 ATPase domain structure and identification of the arginine finger residue, allow us to create a model for the TerL ring assembly. The structure provides a basis for molecular docking, and the arginine finger provides a spatial restraint that must be satisfied for the TerL assembly. We used the program M-ZDOCK (35, 36) to model the TerL ATPase ring. Fivefold symmetry was the only constraint applied. We used the trans-arginine finger interaction to validate the docking results independently. Although many ATPase rings are asymmetrical during function (12, 37), we assume fivefold symmetry for simplicity. Regardless, most ringed ATPase structures were first modeled as symmetrical assemblies (32, 38), and these models were refined later to show asymmetry during function (9, 10, 12, 37). After docking of the ATPase ring, we used the full-length crystal structure of T4-TerL (8) to orient a homology model of the P74-26 nuclease domain, which results in minimal steric clashes between the two domains. The soft energetic potential used in M-ZDOCK results in slightly shorter interatomic distances (35), and therefore a constricted ring; thus, we treat the molecular docking results in a qualitative fashion.
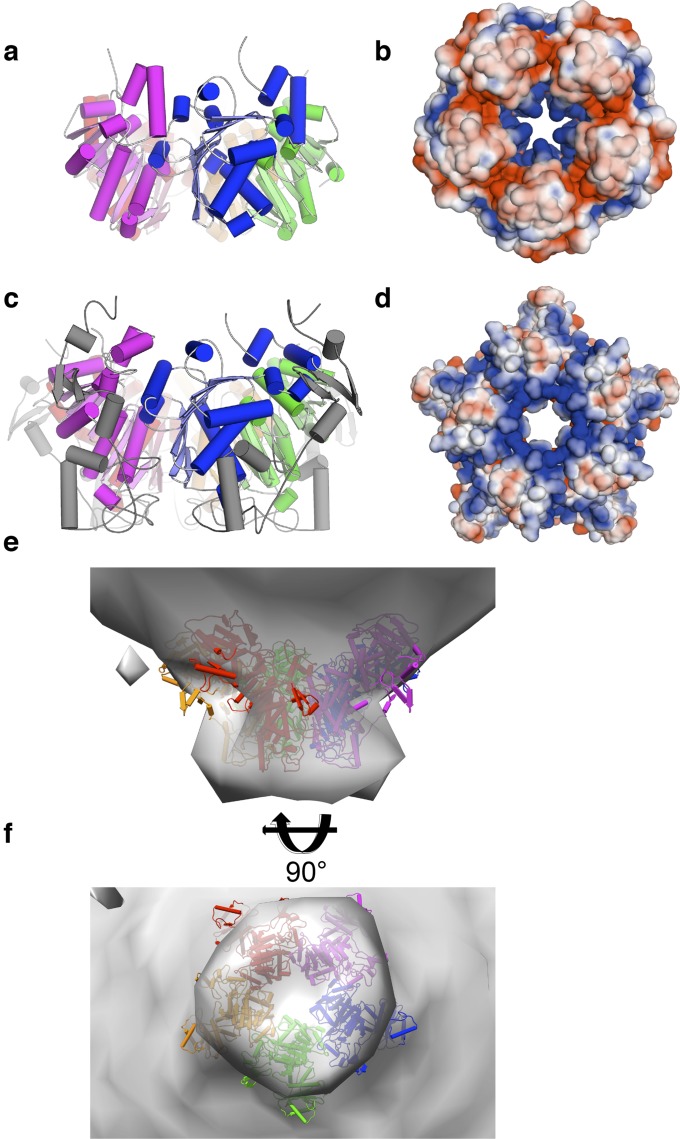
Molecular docking produced a TerL assembly that is consistent with our identification of the arginine finger. Arg139 contacts the ATP gamma-phosphate in a neighboring subunit, despite no restraint for this interaction imposed during the calculation (Fig. 5 A and B). Thus, these models satisfy a critical spatial interaction identified by our biochemical results. The lid subdomains interact with an adjacent subunit, with the nuclease domains arranged radially (Fig. 5C).
Fig. 5.
Derivation of the TerLP74-26 ring assembly structure. (A) Model of the TerLP74-26 ATPase ring derived from symmetry-constrained docking. A bottom-up view shows each of the five subunits in different colors, with the ATP ligand in green. The arginine finger and the conserved basic site (Arg101) are shown as colored spheres. (B) Side view of the TerLP74-26 ATPase ring derived from symmetry-constrained docking. Colors and the image are produced as in A. (C) Schematic depiction of the TerL ring. The TerL ring is shown from the perspective of the pore, with the ring artificially “broken” and flattened on the page so that all subunits can be viewed simultaneously. Each subunit is colored as shown in other panels in this figure. The nuclease domains are depicted as translucent squares to represent the ambiguity of the placement of the nuclease domain within the TerL ring. The loops protruding from the sides of each subunit represent the conserved basic patch (Arg101 in TerLP74-26). The gamma-phosphate of ATP is contacted by the arginine finger from a neighboring subunit.
We tested our model’s plausibility by comparing our TerL assembly with other TerL crystal structures and a cryo-EM reconstruction. First, orthologous TerL ATPase crystal structures (8, 20) can be identically positioned without significant steric clashes of the ATPase domains (Fig. S4 A and C). In fact, the N-terminal extension that is unique to T4 is placed on the outside of the ring rather than lining the pore of the ring as in the previous proposal (8). Moreover, the pores of our Sf6-TerL and T4-TerL ATPase models are positively charged (Fig. S4 B and D). Second, our structural model predicts that for other TerL proteins that are monomeric in isolation, ring assembly would be enhanced by ATP mediating cross-subunit interactions. Indeed, this dependency has been observed for T4 (39), T3 (40), and lambda (41). Third, our T4-TerL ring model is a bowl-like assembly that reasonably fits the cryo-EM map (8) for an actively packaging T4 phage (Fig. S4 E and F). However, this fitting positions the ATPase domain ring distal to the capsid, whereas the nuclease domains interact with portal (Fig. S4E), an interaction that has been observed recently (10). Thus, our revised TerL ring model is a different arrangement than that previously proposed.
Fig. S4.
Superposition of TerL orthologs onto the TerLP74-26 ATPase ring model. (A) Model of the Sf6-TerL ATPase ring. The TerL ATPase domain from Sf6 was superposed onto the docked model of the TerLP74-26 ATPase ring. (Left) Each subunit is shown in a different shade, and the ring is viewed from the side. There are no significant clashes in this model. (B) View of the electrostatic surface potential of the Sf6-TerL ring from the bottom shows a net positively charged pore as would be expected from the DNA-binding region. (C) Model of the T4-TerL ATPase ring. The TerL ATPase domain from T4 was superposed onto the docked model of the TerLP74-26 ATPase ring. The region specific to the T4 phages is shown in gray, the main ATPase domain is shown in various colors, and the ring is viewed from the side proximal to the nuclease domains. There are no significant clashes in this model, and the T4-specific region is present on the outside of the ring, away from the core machinery. (D) Electrostatic surface potential of the T4-TerL ring pore, as viewed from the bottom of the ring. Again, the residues within the pore have a net positive charge, as would be expected for a region that binds DNA. (E and F) Model of the T4-TerL ring fits a cryo-EM structure of the intact motor (E is viewed from the side and F from the bottom). The revised model of the T4-TerL ring is shown as a colored cartoon, with the cryo-EM envelope of the T4 capsid and packaging motor, which was calculated without fivefold averaging (gray surface).
We examined whether our structural model is globally consistent with a molecular envelope, as calculated from small-angle X-ray scattering (SAXS) data. Whereas WT TerLP74-26 exhibits slight aggregation, we obtained high-quality SAXS data by exploiting the R104E-TerLP74-26 mutant, a more soluble variant than the WT protein (Fig. S5 A and B). R104E-TerLP74-26 is well folded and has a radius of gyration of 48.0 ± 2.0 Å (Fig. S5 B and C), consistent with a pentameric assembly. We performed an ab initio calculation of the TerLP74-26 molecular envelope using our SAXS data and imposing fivefold symmetry. The calculated molecular envelope fits the scattering data well (χ2 = 0.79) and is roughly shaped like a bowl, with a pore in the center and a V-shaped cross-section. The overall bowl-like shape of the TerLP74-26 ring model fits the SAXS reconstruction well; the ATPase domains form the V-shaped base, and the nuclease domains form the bowl’s lip (Fig. 6A). We note that extra density is present between the nuclease domains, possibly indicating conformational heterogeneity in the bowl’s lip or a poor fit of our nuclease domain homology model. Regardless, our model is broadly consistent with reconstructions from both SAXS and cryo-EM.
Fig. S5.
Functional and biophysical analysis of the TerLP74-26 pentameric assembly. (A) SAXS profile of a highly soluble variant of TerLP74-26 (R104E). Scattering intensity is shown as a semilog plot vs. the scattering angle (q). (B) Guinier plot of low scattering angles shows no aggregation, and a radius of gyration of 48.0 ± 2.0 Å. (C) TerLP74-26 construct is well-folded. A Kratky plot of the SAXS data is shown. The peak at low scattering angles and the relatively flat and low profile at high scattering angles are hallmarks of well-folded protein. (D) TerLP74-26 R101E variant is catalytically active. Steady-state ATPase activity is shown for 0.5 μM TerL. E150A and WT are shown as points of comparison. Error bars are the SD from at least three replicates. (E) Key functional residues mapped onto the previous model for the TerL assembly. The view is as seen from the perspective of the capsid/portal. The TerLP74-26 ATPase domain crystal structure and nuclease domain homology model were superposed onto the previous model for the T4 TerL ring (PDB ID code 3EZK). Key residues for testing each model are shown as colored spheres: The arginine finger (Arg139) is orange, the DNA-binding residue (Arg101) is blue, and the lid hydrophobic patch residues (W231 and Y238) are shown in rusty red. The nuclease domain homology model is semitransparent. (F) Same as in E, but viewed from the side. The procapsid and portal would be above the TerL ring.
Fig. 6.
Validation of the TerL ring assembly model. (A) Model of TerLP74-26 fits the ab initio SAXS envelope. The modeled TerLP74-26 ring structure (colored cartoon) is superposed onto the SAXS envelope calculated using GASBOR (47) (imposing fivefold symmetry). Dummy atoms for the SAXS envelope are shown as gray spheres. (B) Hydrophobic patch on the lid subdomain mediates critical intersubunit interactions. The structural model suggests that Trp231 and Tyr238 of the lid hydrophobic patch (ball-and-stick representation) mediate critical interactions with a neighboring subunit for positioning the arginine finger. (C) TerLP74-26 lid hydrophobic patch is critical for TerL function. Steady-state ATPase activity is shown for 0.5 μM TerL. Mutation of either W231 or Y238 results in a large decrease in ATPase activity. Error bars are the SD from at least three replicates. (D) Electrostatic map of the docked TerLP74-26 ATPase ring, with positive and negative surface potentials shown in blue and red, respectively. Note the positive charge lining the pore of the TerLP74-26 ATPase ring. The electrostatic surface was calculated using the APBS plug-in for PyMOL (DeLano Scientific) (48). Figure is colored by electrostatic potential (kbT/ec) as indicated. (E) DNA binding for multiple arginine mutants. EMSA was carried out as in Fig. S1F, including several arginine mutants. Arg101 is required for DNA binding, whereas Arg39 and Arg58 are dispensable. (F) Mutation of the conserved pore arginine abrogates DNA binding. The ability of three different arginine mutants to bind DNA tightly in the EMSA assay was mapped onto the model of the TerLP74-26 ring. As predicted by the structural model, Arg101 is necessary for DNA binding and mutations distal to the pore (R39A and R58A) have no measurable effect on DNA binding. A 100-bp DNA ladder (New England BioLabs) is used as a standard.
We tested various aspects of our structural model to determine whether it has predictive power. Our model predicts that the lid’s hydrophobic patch (Trp231 and Tyr238) contacts an adjacent subunit for arginine finger positioning (Fig. 6B). To test the hypothesis that these residues are important for ATPase activity, we individually mutated Trp231 and Tyr238 to alanine. Mutation at each of these residues results in severe loss of ATPase activity (Fig. 6C). Thus, the lid’s hydrophobic patch is critical for TerL activity.
As an additional test of our model’s predictive power, we used our model to identify the DNA-binding motif. Basic residues line the pore of the TerL ring, with a central pore residue (Arg101) that is conserved (Fig. 6D and Fig. S2C). A positive electrostatic environment in the pore is similar to other ASCE nucleic acid translocases (12, 13, 15). We hypothesize that Arg101 binds DNA during packaging. To test this hypothesis, we mutated several surface arginines in full-length TerLP74-26 and measured the ability of these mutants to interact with DNA in the presence of ADP•BeF3 (Fig. 6E). Mutation of Arg101 causes loss of DNA binding, whereas mutation of residues distal to the pore (Arg39 and Arg58) has no measurable effect (Fig. 6F). Although a defect in binding ATP could cause loss of DNA binding, the R101E mutant retains WT levels of ATPase activity (Fig. S5D), indicating that the DNA-binding defect is not due to loss of ATP binding. Thus, we have identified the critical DNA-binding motif in TerL, supporting our structural model for the TerL ring.
Discussion
Our results suggest a substantially different organization of the TerL ring than the initial model that was proposed previously in a ground-breaking study of the T4 terminase. The previous model, which is based on fitting the T4-TerL crystal structure to a cryo-EM reconstruction with a resolution of 34 Å, proposed that the TerL ATPase domains form a ring that contacts portal through the lid subdomains (8). The pore of the ATPase ring has a net negative charge and is lined by a portion of TerL that is unique to T4. The nuclease domain was proposed to form a ring distal to portal that grips and translocates DNA through the pore. Because the ATPase active sites do not contact a neighboring subunit, Sun et al. (8) proposed that an invariant arginine in the P-loop (Arg162 in T4 TerL, Arg39 in TerLP74-26) acts as the arginine finger to catalyze ATP hydrolysis.
Our identification of the conserved trans-acting arginine finger indicates that the previous model requires significant modification. Although the P-loop arginine previously proposed as the cis-acting arginine finger is necessary for packaging (42) and ATPase activity (8) (Fig. 4A), our data do not support the hypothesis that it is the arginine finger. Instead, we propose that this residue, Arg39 in TerLP74-26, is conceptually analogous to the sensor II arginine found in the family of ATPases known as the ATPases associated with diverse cellular activities (AAA+), which aids ATP hydrolysis in cis and confers movement of the AAA+ lid subdomain upon ATP hydrolysis (11, 31). In order for the previous model to accommodate the trans-acting arginine finger, a rotation of the ATPase domain of ∼110° is necessary. Although formally possible, this rotation of the ATPase domain does not fit the cryo-EM density for the actively translocating motor (8) and would disrupt portal interactions. Therefore, we disfavor this model.
Our docked model for the TerL ring satisfies the distance constraint imposed by the arginine finger and revises the orientation of TerL relative to portal. We propose that the nuclease domains form a radially arranged ring that is proximal to portal, whereas the ATPase ring is distal. Our model positions the lid subdomains at the interface between adjacent ATPase subunits, where they assist in positioning the arginine finger, as supported by our mutagenesis data (Fig. 6C). In the previously proposed model (8), the lid residues interact with portal (Fig. S5 E and F). However, because our sample lacks portal, the observed ATPase defects in lid mutants are not due to a disruption of portal interactions, but are consistent with our proposed role in TerL assembly.
Our updated model accounts for functional conservation across the TerL family. First, our model contains a largely basic patch lining the pore. Within this patch, we identify Arg101 as a critical component of the DNA-binding motif. With conservation of a basic residue at this position, our model is congruent with the terminase family. Notably, in the previously proposed structural model, the equivalent residue in T4-TerL is positioned distal to the pore and the nuclease domain was hypothesized to bind DNA (8) (Fig. S5 E and F), both of which are inconsistent with our observations. Second, our identification of the arginine finger in TerL brings the mechanism of ATP hydrolysis into accord not only with the terminase family but also with the rest of the ASCE family. Conservation of the DNA-binding and catalytic mechanism therefore supports our updated structural model.
Although our model allows accurate predictions for regions of function, the overall model is qualitative in nature. As previously mentioned, the ring dimensions are slightly constricted due to the docking algorithm, with the pore’s smallest inner diameter measuring ∼16 Å, as calculated from the Cβ positions of surface-exposed arginines lining the pore (we use the Cβ position because it is rigidly fixed). Thus, the modeled pore is too small to accommodate dsDNA. We propose that the TerL ring is expanded relative to the docked model because the docked model exhibits contacts that are clearly too close with several overlapping atoms, which would artificially constrict the ring. Furthermore, our model positions the nuclease domain based on the full-length T4-TerL structure, which crystallized as a monomer (8). The nuclease domain position may be ortholog-dependent or may be altered upon ring formation and/or upon portal binding. Nuclease position is important because the nuclease domain may be playing a key role in pentamer assembly, considering that the isolated TerLP74-26 ATPase domain crystallizes as a monomer. Although ongoing and future investigations will refine the details of our structural model, we have established that it captures essential aspects of the TerL ring through its consistency with prior data, as well as its predictive power. Our structural model, combined with our nucleotide-dependent structural changes, allows us to derive a preliminary mechanistic model for DNA translocation in this family.
Based on our TerL ring model and the observed conformational changes upon ATP hydrolysis and release, we propose a mechanistic model for DNA translocation. During genome packaging, DNA is gripped in the center of the TerL ring. In this model, it is assumed that the TerL ring hydrolyzes ATP one subunit at a time, and not in a concerted all-or-none mechanism. This assumption is consistent with the mechanism of ATP hydrolysis in other ringed ASCE ATPases (12, 13, 15, 43). When one subunit hydrolyzes ATP, it undergoes a conformational rearrangement such that the lid pivots 13° around the Rossmann fold (Fig. 3B). If we further assume that the lid remains bound to the neighboring subunit throughout packaging, as seen in homologs (44), the result would be the Rossmann fold pivoting outward and upward toward the capsid (Fig. 7 and Movie S2).
Fig. 7.
Proposed model for the mechanism of DNA translocation by TerL. (A) TerL ring is shown as in Fig. 6F. The nuclease domains interact with the portal complex (translucent gray rectangle) and possibly the procapsid (black curve). DNA is not shown but interacts with TerL through the DNA interaction motif (Arg101). Each subunit’s lid is bound tightly to the Rossmann fold of the adjacent subunit. (B) Upon ATP hydrolysis and release by the magenta subunit, the lid stays bound to the blue subunit and the Rossmann fold rotates 13° upward. To allow for this movement, the adjacent red subunit must also move in concert with the magenta subunit. To represent that the second site of symmetry breaking is unknown, the other three ATPase domains are faded. After hydrolyzing ATP, the magenta subunit releases DNA to the red subunit to translocate DNA upward through the pore; into the pore of the portal complex; and, ultimately, inside the procapsid. The release of DNA at each cycle by the ATP-hydrolyzing subunit allows for unidirectional DNA translocation.
We propose that the conformational change from one ATP hydrolysis event propagates to an adjacent subunit, sterically exerting force on the adjacent subunit such that the two subunits move in concert. Because TerL only binds DNA tightly in the ATP-bound form (Fig. S1F), ATP hydrolysis at one subunit will lead to that individual subunit releasing DNA. During a hydrolysis event, the ATP-hydrolyzing subunit loses its grip on DNA but initiates motion that is propagated to the adjacent ATP-bound subunit, which is still gripping DNA through interaction with Arg101. The conformational change of the ATP-hydrolyzing subunit results in an upward translocation motion of DNA at the adjacent subunit and resets the motor for unidirectional translocation. We estimate that this motion would translocate DNA perpendicular to the plane of the ring by ∼8 Å per hydrolysis event, or about 2.4 bp. In addition, we predict that DNA would rotate in the plane of the ring by ∼2.3° for each step. Because our model is qualitative, our estimated step rotation and size should be viewed with reservation. However, these values compare favorably with the 2.5-bp translocation (43) and ∼3.5° rotation per step (45) measured for the phi29 motor at low packaging force. Our proposed “lever-like” mechanism for force generation is in contrast to the previous model, wherein a “spring-like” motion of DNA-bound nuclease domains translocates DNA through the pore (8). Because several other studies have evaluated DNA packaging in the context of the previous TerL structural and mechanistic models, our work illustrates that these studies should be reinterpreted within the context of the updated TerL model.
It is unclear how this conformational change will affect subunits further downstream than the two moving subunits. The breakage of symmetry at the ATP hydrolysis site necessitates at least one other site of symmetry breaking to maintain a closed ring structure. Alternatively, the TerL assembly may form an open lock washer shape that dynamically alternates which subunits cap the ends of the lock washer, as has been proposed for the ASCE helicase DnaB (12). Although our current model is fivefold symmetrical, recent studies of ASCE family members illustrate that significant asymmetry exists during motor function (12, 13, 37). Future refinement of our structural model will identify how TerL asymmetry drives DNA translocation.
Materials and Methods
Cloning, Expression, and Purification of TerLP74-26.
The TerLP74-26 gene was synthesized by Genscript Corporation and subcloned into a modified pET24a vector using standard procedures. TerLP74-26 was recombinantly expressed in BLR-DE3 E. coli cells and purified by nickel affinity, ion exchange, and SEC (details are provided in SI Materials and Methods).
ATPase and DNA-Binding Assays.
ATPase activity was measured using a standard coupled enzyme assay. DNA binding was measured by EMSA (details are provided in SI Materials and Methods).
Crystallization, Diffraction Data Collection, SAXS, and Docking.
SeMet-labeled, apo, and ADP•BeF3-bound TerLP74-26 ATPase domain were crystallized with the hanging drop vapor diffusion method in solutions containing buffered ammonium sulfate. Crystals were frozen in a cryogenic buffer containing elevated respective precipitant concentrations supplemented with 30% (vol/vol) ethylene glycol. Diffraction data were collected at Advanced Light Source (ALS) beamline 5.0.1, at Advanced Photon Source beamline 23-ID-B, at Brookhaven National Laboratory beamline X25, or using a home source. SAXS data were obtained on filtered R104E-TerLP74-26 at the Structurally Integrated Biology for Life Sciences (SIBYLS) beamline at the ALS. The TerLP74-26 ATPase ring was modeled using M-ZDOCK (35) (details are provided in SI Materials and Methods).
SI Materials and Methods
Cloning.
The gene for TerLP74-26 was synthesized with codon optimization for expression in E. coli by Genscript Corporation. This gene was cloned into the BamHI and XhoI sites of a modified pET24a vector with an N-terminal T7-gp10 expression tag and prescission protease cut site, and a C-terminal noncleavable His10 tag (31). Site-directed mutagenesis was performed using a mutagenesis protocol similar to QuikChange (49). Enzymes were purchased from New England BioLabs. Oligonucleotides were obtained from IDT. The ATPase domain was constructed by deleting the codons encoding the C-terminal 229 residues using the aforementioned mutagenesis protocol (49). This deletion site was chosen based on limited proteolysis and bioinformatic analysis (data not shown).
Expression and Purification of TerLP74-26.
All proteins were expressed in E. coli BLR-DE3 cells containing the pET24a-TerLP74-26 plasmid. Bacterial cultures were grown in Terrific Broth (Research Products International) supplemented with 30 μg/μL kanamycin at 37 °C until an OD600 of 0.7 was reached. Cells were then placed at 4 °C for 20 min, after which overnight expression at 18 °C was induced by the addition of isopropyl-β-d-thiogalactopyranoside to 1 mM. Cells were pelleted by centrifugation and resuspended in buffer A [500 mM NaCl, 20 mM imidazole, 50 mM Tris (pH 8.5), 5 mM β-Mercaptoethanol (βME), 10% (vol/vol) glycerol] before being flash-frozen in liquid nitrogen for storage. BLR-DE3 cells with SeMet-labeled ATPase domain (residues 1–256) were grown in minimal media. When cultures reached an OD600 of 0.5, they were supplemented with amino acids, except for Met, and grown to an OD600 of 0.8. Cultures were then supplemented with SeMet. Induction and harvesting were performed as above.
For full-length constructs, thawed cells were lysed in a cell disruptor and pelleted to clear the lysate. Lysate was loaded onto a 10-mL centrifuge spin-column (Thermo Scientific) containing Ni-affinity beads (Thermo Scientific) preequilibrated with buffer A. Protein was eluted with buffer B [500 mM NaCl, 500 mM imidazole, 50 mM Tris (pH 8.5), 5 mM βME, 10% (vol/vol) glycerol] and dialyzed overnight at room temperature into buffer QA [25 mM Tris (pH 8.5), 125 mM NaCl, 2 mM DTT, 10% (vol/vol) glycerol]. Before dialysis, prescission protease was mixed with eluate to cleave the T7 expression tag. The sample was then loaded onto spin-columns containing Q resin (GE Healthcare) preequilibrated with buffer QA. After protein loading, the Q resin was washed with buffer QA and contaminants were eluted with buffer QA1 [25 mM Tris (pH 8.5), 200 mM NaCl, 2 mM DTT, 10% (vol/vol) glycerol]. Protein was eluted with buffer QB [25 mM Tris (pH 8.5), 450 mM NaCl, 2 mM DTT, 10% (vol/vol) glycerol] and dialyzed overnight at room temperature into buffer QA before concentrating.
For purification of the TerL-ATPase domain, cells were lysed and pelleted as above. After pelleting, all purification steps proceeded at room temperature. Lysate was loaded onto a preequilibrated 5-mL His-Trap column (GE Healthcare). The column was washed with buffer A and eluted with buffer B. Eluate was diluted fourfold with buffer A, and prescission protease was added. The protein was dialyzed overnight in buffer QA. The protein was then filtered and flowed over a 5-mL HiTrap Q-HP column (GE Healthcare) preequilibrated in buffer QA. The flow-through was collected, concentrated, and flash-frozen.
Coupled Enzyme ATPase Assays.
Coupled enzyme assays were performed at room temperature (50) using the following concentrations (unless otherwise noted): 6 U/mL pyruvate kinase, 6 U/mL lactate dehydrogenase, 1 mM phosphoenolpyruvate, 340 μM NADH, 50 mM Tris (pH 7.5), 500 μM Tris(2-carboxyethyl)phosphine, 100 mM potassium chloride, 5 mM magnesium chloride, 1 mM ATP, and 0.5 μM TerL. Absorbance was measured in a 96-well format with a Perkin–Elmer Victor3 1420 multichannel counter using an excitation filter centered at 355 nm, with a bandpass of 40 nm. In this assay, every NADH oxidized to NAD+ corresponds to one ATP hydrolyzed. To convert the measured absorbance directly to ATP concentration, we performed a standard curve so as to measure the NADH extinction coefficient directly under our experimental conditions.
To understand how TerL concentration affects ATPase activity, we measured ATPase activity over a wide concentration range of TerL. The rise in ATPase rate is slow and nonlinear at low TerL concentrations, but it increases linearly at higher concentrations (Fig. S1D). The nonlinear activity at low TerL concentrations is not due to the limits of our assay, because we are able to measure ATPase rates reliably below the rate observed here using a different ATPase. The slow increase in ATPase rate at low concentration is most likely due to assembly of a TerL pentamer. It is noteworthy that the gp16 ATPase from the packaging motor of the phi29 phage shows a similar nonlinear increase in ATPase rate (51), suggesting that an assembly of the ATPase subunits may be a necessary step in full ATPase activity for other viral motors. Regardless, the experiments that follow were performed at a concentration of TerL (0.5–1 μM) that is well within the linear range. Data comparing the rate of ATP hydrolysis to [ATP•MgCl2] were fit using the Hill equation: v = Vmax * [ATP•MgCl2]n/(Khalf-maximaln + [ATP•MgCl2]n), where Vmax is the maximum rate, n is the Hill coefficient, and Khalf-maximal is the substrate concentration that yields half-maximal activity.
DNA-Binding Assays.
Full-length WT (0.13 nmol) or mutant TerLP74-26 (0.13 nmol) was incubated with 125 ng of 100-bp ladder (New England BioLabs) for 30 min at room temperature in apo, ADP, ADP•BeF3, or ATP buffer as indicated. Apo buffer was composed of 50 mM Tris (pH 7.5), 150 mM KCl, and 1 mM DTT. ADP buffer was composed of 50 mM Tris (pH 7.5), 150 mM KCl, 1 mM DTT, 1 mM ADP, and 10 mM MgCl2. ADP•BeF3 buffer was composed of 50 mM Tris (pH 7.5), 150 mM KCl, 1 mM DTT, 1 mM ADP, 10 mM NaF, 4 mM BeCl2, and 10 mM MgCl2. ATP buffer was composed of 50 mM Tris (pH 7.5), 150 mM KCl, 1 mM DTT, 1 mM ATP, and 10 mM MgCl2. Samples were then loaded onto a 1.5% (wt/vol) agarose gel with a 1:14,000 dilution of GelRed dye (Phenix Research) and run at 80 V for ∼2 h.
Crystallization.
SeMet-labeled, apo, and ADP•BeF3 -bound TerLP74-26 ATPase domain were crystallized with the hanging drop vapor diffusion method. Drops were set with final protein concentrations of 3–8 mg/mL apo, SeMet-labeled, and ADP•BeF3-bound samples were premixed with 10 mM magnesium chloride and 10 mM DTT before crystallization. The R139A variant was premixed only with DTT. ADP•BeF3 crystals were premixed with 2 mM ADP, 10 mM sodium fluoride, and 4 mM beryllium fluoride. ADP (2 mM) was premixed with SeMet-labeled protein. SeMet-labeled crystals formed in 0.9 M ammonium sulfate and 0.1 M trisodium citrate (pH 5.0). Cubic apo crystals formed in 0.7 M ammonium sulfate and 0.1 M trisodium citrate (pH 5.0). Apo R139A cubic crystals formed in 0.5 M ammonium sulfate and 0.1 M trisodium citrate (pH 4.5). Hexagonal apo crystals formed in 11% (wt/vol) PEG 2000, 0.15 M ammonium sulfate, and 0.1 M trisodium citrate (pH 5.0). ADP•BeF3-bound crystals formed in 19% (wt/vol) PEG 3350, 0.05 M ammonium sulfate, and 0.1 M trisodium citrate (pH 5.0). Single crystals formed only after microseeding. Cryogenic buffer conditions for each crystal type consisted of elevated respective precipitant concentrations supplemented with 30% (vol/vol) ethylene glycol. Cryogenic conditions also contained all reagents that were premixed with each respective protein sample before crystallization.
Data Collection and Structure Solution.
SeMet and apo ATPase domain datasets were collected at ALS beamline 5.0.1 at a wavelength of 0.9774 Å. SeMet structure experimental phases were calculated by SAD (28) using the PHENIX Autosol pipeline (52). The SeMet model was used for molecular replacement for the apo structure. The second apo ATPase crystal data were collected at Advanced Photon Source beamline 23-ID-B at a wavelength of 1.033 Å. ADP•BeF3 crystal data were collected at Brookhaven National Laboratory beamline X25 at a wavelength of 1.100 Å. ADP•BeF3 data were truncated due to anisotropy in the diffraction. After scaling, data were processed using the University of California, Los Angeles Molecular Biology Institute Diffraction Anisotropy Server (53). The apo R139A mutant crystal data were collected on a home source MicroMax007-HF/Saturn 944 CCD detector X-ray diffraction system at a wavelength of 1.54 Å. A C-terminal–truncated apo structure was used for molecular replacement. All datasets were processed with HKL3000 (54). Model building and refinement were performed with Coot (55) and PHENIX (56).
SAXS Data Collection and Analysis.
Samples were dialyzed into buffer [25 mM Tris (pH 8.5), 125 mM NaCl, 4 mM DTT, 2% (vol/vol) glycerol] using dialysis buttons (Hampton Research) and filtered through a 0.45-μm membrane (Millipore). WT TerLP74-26 exhibited low levels of aggregation, which hampered interpretation of data. We hypothesized that the basic patch (Fig. 2) contributes to the aggregation of TerLP74-26. To avoid aggregation, we made the R104E mutant. Indeed, this mutant shows no signs of aggregation (Fig. S5 A–C). Samples were shipped at 4 °C to the ALS, Lawrence Berkeley National Laboratory, for data collection at the SIBYLS beamline (57). Data were collected at 10 °C with exposures of 0.5 s, 1 s, and 6 s and at several concentrations of TerL, and buffer blanks were subtracted to give protein scattering. Due to radiation sensitivity at longer exposures, only the 1-s exposure was used for analysis. Data were processed using the ATSAS software package (58). The SAXS reconstruction was calculated using GASBOR (47) in real-space mode using fivefold symmetry. The docked model was optimally fit to the SAXS envelope by converting the GASBOR dummy atoms to a map using the “molmap” and “fit to map” commands in UCSF Chimera (59).
Molecular Docking and Modeling of the TerLP74-26 Ring.
The model of the TerLP74-26 ATPase ring was calculated with the ADP•BeF3-bound structure of the TerLP74-26 ATPase domain using M-ZDOCK (35). Fivefold symmetry was applied, but no other restraints were imposed during the docking. To model the TerLP74-26 nuclease domain, we used I-TASSER (60) to generate a homology model with the nuclease domains of Sf6-TerL and T4-TerL as structural templates. To model the full-length ring, we aligned the full-length T4 structure (8) [Protein Data Bank (PDB) ID code 3CPE] onto the ATPase domains of our model and then superposed the TerLP74-26 nuclease homology model onto the T4 nuclease domains.
To model the potential conformational changes that drive DNA translocation within the ring upon ATP hydrolysis, we first positioned the apo ATPase structure onto a subunit within the modeled ring, using only the lid subdomain for alignment. To model the effect of this change on downstream subunits in the ring assembly, a second ATPase domain (bound to ADP•BeF3) was placed adjacent to the apo subunit in an orientation identical to the orientation in the ring model. To calculate the approximate movement of individual subunits that may be associated with ATP hydrolysis, we took advantage of the convenient output format of M-ZDOCK, in which all symmetry-related atoms are in the same x–y plane. Therefore, overall translation of a DNA-binding residue upon ATP hydrolysis and release (in this case, Cα of Arg101) can be simply calculated from the change in the z coordinate. To calculate the in-plane rotation angle per step, we translated the Cα of Arg101 of the “translocating” subunit back into the original plane. We then calculated the in-plane angle swept out by the motion of this subunit by measuring the angle between these three points: the original position of Arg101 Cα within the ring, the center of mass of all Arg101 Cα atoms, and the new (back-projected along the z axis) Arg101 Cα position.
Modeling of the T4 TerL Ring and Fitting to Cryo-EM Reconstruction.
To make the model of the T4 TerL ring, we aligned the full-length T4-TerL structure (PDB ID code 3CPE) (8) onto the ATPase domains of our docked pentameric model using PyMOL (DeLano Scientific). The resulting model was then fit into the cryo-EM reconstruction of the T4 capsid with the TerL ring bound (EMDB-1572) (8) in UCSF Chimera using the fit to map command (59).
Supplementary Material
Acknowledgments
We thank the Rhind, Rando, Schiffer, Royer, Ryder, and Bolon laboratories for discussions and use of instrumentation. We thank beamline scientists at ALS sector 5 (Lawrence Berkeley National Laboratory), APS 23-ID-B (Argonne National Laboratory), and NSLS X25 (Brookhaven National Laboratory) for technical support with X-ray diffraction data collection, and Z. Maben and Dr. J. Birtley for assistance with data collection of the ADP•BeF3 cocrystals. This work was initiated by a grant from the Worcester Foundation. B.A.K. is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trusts.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4ZNI, 4ZNJ, 4ZNK, and 4ZNL).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506951112/-/DCSupplemental.
References
- 1.Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. doi: 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- 2.Smith DE, et al. The bacteriophage straight phi29 portal motor can package DNA against a large internal force. Nature. 2001;413(6857):748–752. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- 3.Evilevitch A, Castelnovo M, Knobler CM, Gelbart WM. Measuring the force ejecting DNA from phage †. J Phys Chem B. 2004;108(21):6838–6843. [Google Scholar]
- 4.Fuller DN, Raymer DM, Kottadiel VI, Rao VB, Smith DE. Single phage T4 DNA packaging motors exhibit large force generation, high velocity, and dynamic variability. Proc Natl Acad Sci USA. 2007;104(43):16868–16873. doi: 10.1073/pnas.0704008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer LM, Makarova KS, Koonin EV, Aravind L. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: Implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 2004;32(17):5260–5279. doi: 10.1093/nar/gkh828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burroughs AM, Iyer LM, Aravind L. Comparative genomics and evolutionary trajectories of viral ATP dependent DNA-packaging systems. Genome Dyn. 2007;3:48–65. doi: 10.1159/000107603. [DOI] [PubMed] [Google Scholar]
- 7.Feiss M, Rao VB. The bacteriophage DNA packaging machine. Adv Exp Med Biol. 2012;726:489–509. doi: 10.1007/978-1-4614-0980-9_22. [DOI] [PubMed] [Google Scholar]
- 8.Sun S, et al. The structure of the phage T4 DNA packaging motor suggests a mechanism dependent on electrostatic forces. Cell. 2008;135(7):1251–1262. doi: 10.1016/j.cell.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Hegde S, Padilla-Sanchez V, Draper B, Rao VB. Portal-large terminase interactions of the bacteriophage T4 DNA packaging machine implicate a molecular lever mechanism for coupling ATPase to DNA translocation. J Virol. 2012;86(8):4046–4057. doi: 10.1128/JVI.07197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixit AB, Ray K, Thomas JA, Black LW. The C-terminal domain of the bacteriophage T4 terminase docks on the prohead portal clip region during DNA packaging. Virology. 2013;446(1-2):293–302. doi: 10.1016/j.virol.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erzberger JP, Mott ML, Berger JM. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat Struct Mol Biol. 2006;13(8):676–683. doi: 10.1038/nsmb1115. [DOI] [PubMed] [Google Scholar]
- 12.Itsathitphaisarn O, Wing RA, Eliason WK, Wang J, Steitz TA. The hexameric helicase DnaB adopts a nonplanar conformation during translocation. Cell. 2012;151(2):267–277. doi: 10.1016/j.cell.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomsen ND, Berger JM. Running in reverse: The structural basis for translocation polarity in hexameric helicases. Cell. 2009;139(3):523–534. doi: 10.1016/j.cell.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Yang H, Pavletich NP. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature. 2008;453(7194):489–494. doi: 10.1038/nature06971. [DOI] [PubMed] [Google Scholar]
- 15.Enemark EJ, Joshua-Tor L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature. 2006;442(7100):270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- 16.Leffers G, Rao VB. Biochemical characterization of an ATPase activity associated with the large packaging subunit gp17 from bacteriophage T4. J Biol Chem. 2000;275(47):37127–37136. doi: 10.1074/jbc.M003357200. [DOI] [PubMed] [Google Scholar]
- 17.Gual A, Camacho AG, Alonso JC. Functional analysis of the terminase large subunit, G2P, of Bacillus subtilis bacteriophage SPP1. J Biol Chem. 2000;275(45):35311–35319. doi: 10.1074/jbc.M004309200. [DOI] [PubMed] [Google Scholar]
- 18.Baumann RG, Black LW. Isolation and characterization of T4 bacteriophage gp17 terminase, a large subunit multimer with enhanced ATPase activity. J Biol Chem. 2003;278(7):4618–4627. doi: 10.1074/jbc.M208574200. [DOI] [PubMed] [Google Scholar]
- 19.White JH, Richardson CC. Gene 19 of bacteriophage T7. Overexpression, purification, and characterization of its product. J Biol Chem. 1988;263(5):2469–2476. [PubMed] [Google Scholar]
- 20.Zhao H, Christensen TE, Kamau YN, Tang L. Structures of the phage Sf6 large terminase provide new insights into DNA translocation and cleavage. Proc Natl Acad Sci USA. 2013;110(20):8075–8080. doi: 10.1073/pnas.1301133110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun S, Kondabagil K, Gentz PM, Rossmann MG, Rao VB. The structure of the ATPase that powers DNA packaging into bacteriophage T4 procapsids. Mol Cell. 2007;25(6):943–949. doi: 10.1016/j.molcel.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Guo P, Grimes S, Anderson D. A defined system for in vitro packaging of DNA-gp3 of the Bacillus subtilis bacteriophage phi 29. Proc Natl Acad Sci USA. 1986;83(10):3505–3509. doi: 10.1073/pnas.83.10.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibarra B, Valpuesta JM, Carrascosa JL. Purification and functional characterization of p16, the ATPase of the bacteriophage Phi29 packaging machinery. Nucleic Acids Res. 2001;29(21):4264–4273. doi: 10.1093/nar/29.21.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadal M, et al. Structure and inhibition of herpesvirus DNA packaging terminase nuclease domain. Proc Natl Acad Sci USA. 2010;107(37):16078–16083. doi: 10.1073/pnas.1007144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu MX, Slater MR, Ackermann H-W. Isolation and characterization of Thermus bacteriophages. Arch Virol. 2006;151(4):663–679. doi: 10.1007/s00705-005-0667-x. [DOI] [PubMed] [Google Scholar]
- 26.Bigay J, Deterre P, Pfister C, Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987;6(10):2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagawa R, Montgomery MG, Braig K, Leslie AG, Walker JE. The structure of bovine F1-ATPase inhibited by ADP and beryllium fluoride. EMBO J. 2004;23(14):2734–2744. doi: 10.1038/sj.emboj.7600293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendrickson WA, Teeter MM. Structure of the hydrophobic protein crambin determined directly from the anomalous scattering of sulphur. Nature. 1981;290(5802):107–113. doi: 10.1038/290107a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 30.Glynn SE, Martin A, Nager AR, Baker TA, Sauer RT. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell. 2009;139(4):744–756. doi: 10.1016/j.cell.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelch BA, Makino DL, O’Donnell M, Kuriyan J. How a DNA polymerase clamp loader opens a sliding clamp. Science. 2011;334(6063):1675–1680. doi: 10.1126/science.1211884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLaBarre B, Brunger AT. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat Struct Biol. 2003;10(10):856–863. doi: 10.1038/nsb972. [DOI] [PubMed] [Google Scholar]
- 33.Wang J. Nucleotide-dependent domain motions within rings of the RecA/AAA(+) superfamily. J Struct Biol. 2004;148(3):259–267. doi: 10.1016/j.jsb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Cox JM, Abbott SN, Chitteni-Pattu S, Inman RB, Cox MM. Complementation of one RecA protein point mutation by another. Evidence for trans catalysis of ATP hydrolysis. J Biol Chem. 2006;281(18):12968–12975. doi: 10.1074/jbc.M513736200. [DOI] [PubMed] [Google Scholar]
- 35.Pierce B, Tong W, Weng Z. M-ZDOCK: A grid-based approach for Cn symmetric multimer docking. Bioinformatics. 2005;21(8):1472–1478. doi: 10.1093/bioinformatics/bti229. [DOI] [PubMed] [Google Scholar]
- 36.Pierce BG, et al. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30(12):1771–1773. doi: 10.1093/bioinformatics/btu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M, et al. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015;518(7537):61–67. doi: 10.1038/nature14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey S, Eliason WK, Steitz TA. Structure of hexameric DnaB helicase and its complex with a domain of DnaG primase. Science. 2007;318(5849):459–463. doi: 10.1126/science.1147353. [DOI] [PubMed] [Google Scholar]
- 39.Vafabakhsh R, et al. Single-molecule packaging initiation in real time by a viral DNA packaging machine from bacteriophage T4. Proc Natl Acad Sci USA. 2014;111(42):15096–15101. doi: 10.1073/pnas.1407235111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujisawa H, Shibata H, Kato H. Analysis of interactions among factors involved in the bacteriophage T3 DNA packaging reaction in a defined in vitro system. Virology. 1991;185(2):788–794. doi: 10.1016/0042-6822(91)90550-u. [DOI] [PubMed] [Google Scholar]
- 41.Yang Q, Catalano CE, Maluf NK. Kinetic analysis of the genome packaging reaction in bacteriophage lambda. Biochemistry. 2009;48(45):10705–10715. doi: 10.1021/bi901016n. [DOI] [PubMed] [Google Scholar]
- 42.Rao VB, Mitchell MS. The N-terminal ATPase site in the large terminase protein gp17 is critically required for DNA packaging in bacteriophage T4. J Mol Biol. 2001;314(3):401–411. doi: 10.1006/jmbi.2001.5169. [DOI] [PubMed] [Google Scholar]
- 43.Moffitt JR, et al. Intersubunit coordination in a homomeric ring ATPase. Nature. 2009;457(7228):446–450. doi: 10.1038/nature07637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stinson BM, et al. Nucleotide binding and conformational switching in the hexameric ring of a AAA+ machine. Cell. 2013;153(3):628–639. doi: 10.1016/j.cell.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, et al. A viral packaging motor varies its DNA rotation and step size to preserve subunit coordination as the capsid fills. Cell. 2014;157(3):702–713. doi: 10.1016/j.cell.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider TD, Stephens RM. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990;18(20):6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svergun DI, Petoukhov MV, Koch MH. Determination of domain structure of proteins from X-ray solution scattering. Biophys J. 2001;80(6):2946–2953. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98(18):10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Naismith JH. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008;8:91. doi: 10.1186/1472-6750-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kornberg A, Pricer WE., Jr Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. J Biol Chem. 1951;193(2):481–495. [PubMed] [Google Scholar]
- 51.Lee TJ, Zhang H, Liang D, Guo P. Strand and nucleotide-dependent ATPase activity of gp16 of bacterial virus phi29 DNA packaging motor. Virology. 2008;380(1):69–74. doi: 10.1016/j.virol.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwart PH, et al. Automated structure solution with the PHENIX suite. Methods Mol Biol. 2008;426:419–435. doi: 10.1007/978-1-60327-058-8_28. [DOI] [PubMed] [Google Scholar]
- 53.Strong M, et al. Toward the structural genomics of complexes: Crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103(21):8060–8065. doi: 10.1073/pnas.0602606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otwinowski Z, Minor W. Processing of X-ray diffraction data. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 55.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 56.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hura GL, et al. Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS) Nat Methods. 2009;6(8):606–612. doi: 10.1038/nmeth.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petoukhov MV, Konarev PV, Kikhney AG, Svergun DI. ATSAS2.1—Towards automated and web-supported small-angle scattering data analysis. J Appl Cryst. 2007;40(Suppl):s223–s228. [Google Scholar]
- 59.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.