Significance
Though the asymmetric distribution of proteins is a crucial first step in establishing polarity and guiding cell migration, the molecular mechanisms regulating many of these localizations are unknown. Our study reports on the novel protein Callipygian (CynA), which localizes to the rear of cells during symmetry breaking, thereby promoting polarity and increasing migration efficiency. Our data indicate that CynA localization is mediated by two distinct mechanisms, which may be important for segregating proteins in other polarized cell types including epithelial cells, neurons, and immune cells. Thus, our findings have implications for tissue formation during embryonic development, the migration of immune cells during wound healing and infection, and the aberrant migrations associated with arthritis, asthma, atherosclerosis, cancer metastasis, and other diseases.
Keywords: chemotaxis, polarity, asymmetry, Dictyostelium, protein localization
Abstract
Asymmetric protein localization is essential for cell polarity and migration. We report a novel protein, Callipygian (CynA), which localizes to the lagging edge before other proteins and becomes more tightly restricted as cells polarize; additionally, it accumulates in the cleavage furrow during cytokinesis. CynA protein that is tightly localized, or “clustered,” to the cell rear is immobile, but when polarity is disrupted, it disperses throughout the membrane and responds to uniform chemoattractant stimulation by transiently localizing to the cytosol. These behaviors require a pleckstrin homology-domain membrane tether and a WD40 clustering domain, which can also direct other membrane proteins to the back. Fragments of CynA lacking the pleckstrin homology domain, which are normally found in the cytosol, localize to the lagging edge membrane when coexpressed with full-length protein, showing that CynA clustering is mediated by oligomerization. Cells lacking CynA have aberrant lateral protrusions, altered leading-edge morphology, and decreased directional persistence, whereas those overexpressing the protein display exaggerated features of polarity. Consistently, actin polymerization is inhibited at sites of CynA accumulation, thereby restricting protrusions to the opposite edge. We suggest that the mutual antagonism between CynA and regions of responsiveness creates a positive feedback loop that restricts CynA to the rear and contributes to the establishment of the cell axis.
The directional migration of polarized cells toward a chemical gradient plays a critical role in many normal biological processes during development and homeostasis. In addition, polarity and chemotaxis are involved in the pathogenesis of many diseases, including asthma, atherosclerosis, and cancer metastasis. Despite its significance, investigators have only begun to fully appreciate the mechanisms of polarity and directed migration.
Many of the signaling molecules that mediate these events are localized or activated specifically at the leading or, more rarely, lagging edge of migrating cells (reviewed in ref. 1). Events that occur at the leading edge include the activation of Ras, TORC2, and AKT/PKB proteins, accumulation of phosphatidylinositol(3,4,5)-trisphosphate (PIP3), recruitment of several cytoskeletal proteins, and actin polymerization; proteins that accumulate at the lagging edge include the lipid phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and Myosin II. Furthermore, these leading- and lagging-edge proteins respond to uniformly applied chemoattractant by transiently relocalizing onto or off of the membrane, respectively. In polarized cells, the asymmetric localization patterns are accentuated and maintained even in the absence of a chemoattractant gradient. Many of these asymmetries are conserved in migratory cells, such as Dictyostelium discoideum, mammalian leukocytes, fibroblasts, and axonal growth cones (2–6). In addition, these signaling molecules display characteristic localization patterns that are important for the regulation of other cellular behaviors, such as cytokinesis and phagocytosis, as well as in nonmigratory cells, such as epithelia (7–13).
D. discoideum is an excellent model system for studying directional migration because of its genetic accessibility and the nature of its life cycle. Growing Dictyostelium cells spontaneously extend transient protrusions in alternating directions, which results in frequent directional changes and poor chemotaxis (14). Upon starvation, the cells differentiate, undergoing a program of gene-expression changes that lead to an increased sensitivity to the chemoattractant cAMP. In addition, differentiation causes cells to elongate, have a differential sensitivity to cAMP along their axis, and extend protrusions preferentially at the front, resulting in improved chemotactic ability (15).
Because many molecules involved in polarity and chemotaxis are localized to the front or back of cells, we designed a screen using Dictyostelium to identify novel regulators based on the spatial distributions of GFP-tagged proteins in migrating cells. This approach circumvents the pitfalls of traditional loss-of-function screens for defects in chemotaxis: some regulatory components may be essential for cytokinesis or phagocytosis, resulting in lethal mutations; other important components may be redundant, their loss causing only a partial phenotype (reviewed in ref. 1). Using our localization-based technique, we found a previously unidentified protein at the lagging edge that appears to be part of a positive feedback loop that brings about polarity by acting at the cell rear.
Results
Callipygian Localizes to the Rear of Migrating Dictyostelium Cells.
Because of their role in PIP3 signaling, pleckstrin homology (PH) domain-containing proteins are likely candidates for asymmetric localization and regulation of chemotaxis. However, PH domains have widely varied binding specificities, and there are more than 100 PH domain-containing proteins in Dictyostelium (16, 17). We focused on a group of 23 Dictyostelium PH domain-containing proteins that were predicted to bind specifically to PIP3 using an algorithm that was generated by comparing the sequences of PIP3-responsive and PIP3-nonresponsive domains (18). This subset of PH domain-containing proteins, as well as several random cDNAs, were tagged with GFP, expressed in Dictyostelium cells, and assessed for intracellular localization during migration. Unexpectedly, one of the PH domain-containing proteins, PH21, was identified at the lagging edge. We designated it Callipygian (CynA) (DictyBase gene ID DDB_G0284337).
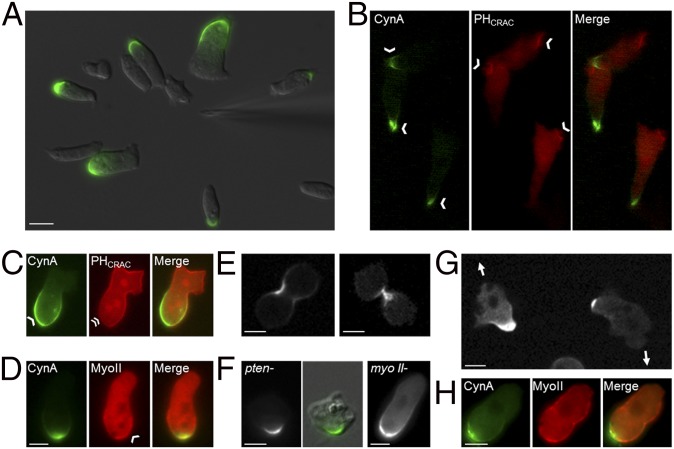
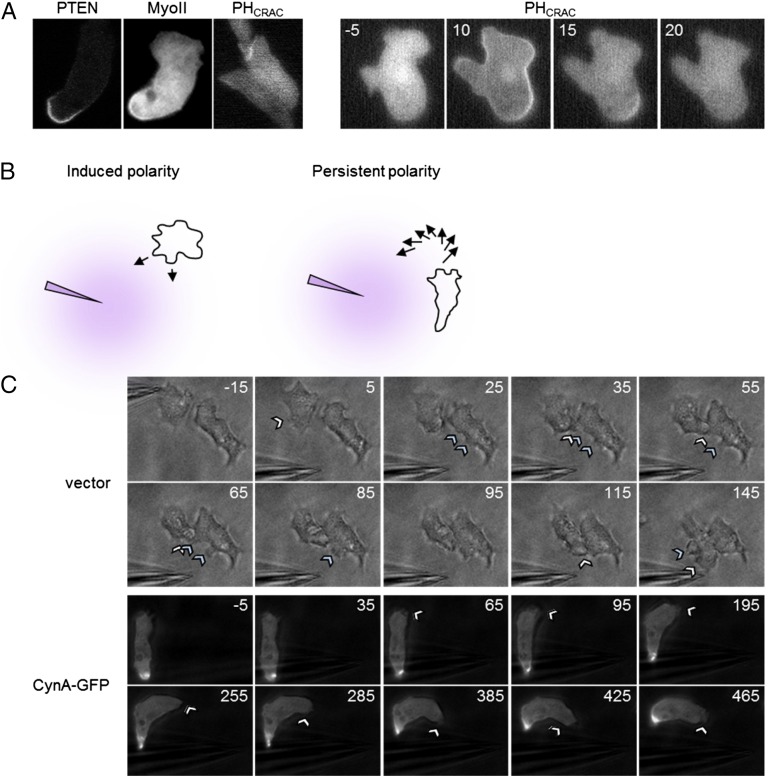
We further characterized the localization of CynA. Consistent with the original observation that CynA-GFP localized to the rear of randomly migrating cells, this protein was found at the lagging edge of differentiated cells migrating in a gradient of chemoattractant (Fig. 1A and Movie S1). Furthermore, CynA-GFP was excluded from sites of accumulation of the PIP3 biosensor, PHCRAC-RFP, a well-defined leading edge marker, in differentiated cells that were randomly migrating or uniformly stimulated with cAMP (Fig. 1 B and C) (19). Additionally, CynA-GFP and mCherry-Myosin II, a known lagging-edge protein, both localized to the rear of migrating cells (Fig. 1D) (20). Consistent with other proteins found at the lagging edge, CynA-GFP strongly accumulated at the cleavage furrow during both early and late stages of cytokinesis (Fig. 1E) (9, 20–23).
Fig. 1.
CynA is a novel protein that localizes asymmetrically independently of other lagging edge proteins. (A) Differentiated Dictyostelium cells expressing CynA-GFP were imaged by time-lapse fluorescence microscopy while migrating toward a micropipette filled with the chemoattractant cAMP. (B and C) Differentiated cells coexpressing CynA-GFP and PHCRAC-RFP were imaged during aggregation (B) or 5 s after uniform stimulation with 1 µM cAMP (C). Arrowheads indicate sites of protein accumulation; the double arrowhead indicates an absence of PHCRAC-RFP at the site of CynA-GFP localization. (D) Differentiated cells coexpressing CynA-GFP and mCherry-Myosin II were imaged. An arrowhead indicates mCherry-Myosin II accumulation. (E) Wild-type cells expressing CynA-GFP were imaged during early (Left) and late (Right) stages of cytokinesis. (F) Differentiated pten- (Left) and myosin II- (Right) cells expressing CynA-GFP were imaged during random migration. The center panel shows a merge of the DIC and GFP fluorescence images for the pten- cell to illustrate the localization of CynA-GFP relative to the cell morphology. (G) Undifferentiated wild-type cells expressing CynA-GFP were imaged during random migration. Arrows indicate the direction of migration for each cell. (H) Undifferentiated cells coexpressing CynA-GFP and mCherry-Myosin II were imaged. The direction of migration was toward the top of the panels unless noted. (Scale bars, 5 µm.)
To determine whether other lagging-edge proteins are required for the localization of CynA, we expressed CynA-GFP in both pten- and myo II- cells, induced differentiation, and assessed the CynA-GFP distribution pattern during random migration and chemotaxis. In both mutant cell lines, CynA-GFP localized to the rear of migrating cells as it did in wild-type cells, suggesting that CynA localization does not require either PTEN or Myosin II (Fig. 1F). However, in addition to its localization at the lagging edge, CynA-GFP showed several other distribution patterns in pten- cells; for example, CynA-GFP was often found on convex regions of curvature on the top surface rather than on the lateral surface, as in wild-type or most pten- cells, or in membrane-adjacent cytosolic patches (Fig. S1A). This finding suggests that CynA-GFP is targeted less efficiently to the lagging edge, or its localization at the rear is less stable, in pten- cells, likely because of the dynamic morphological changes observed in this mutant strain (24).
Fig. S1.
The relationship between CynA localization and other lagging edge proteins. (A) Differentiated pten- cells expressing CynA-GFP were imaged by time-lapse fluorescence microscopy during random migration or in the presence of a micropipette filled with cAMP. In addition to its wild-type localization as in Fig. 1F, CynA-GFP showed a variety of distribution patterns when expressed in pten- cells. (Top Left) CynA-GFP was often dispersed throughout the membrane, and occasionally had a punctate distribution, in these cells. (Middle Left) Frequently, CynA-GFP was found in a hazy, dispersed patch in the cytosol, often near the membrane, in pten- cells. (Bottom Left) CynA-GFP was sometimes targeted to concave or flat regions on the membrane, between protrusions, in pten- cells, as opposed to its normal enrichment at regions of convex membrane curvature at one pole in wild-type and most pten- cells. (Right) In pten- cells, CynA-GFP occasionally accumulated in regions of convex curvature that did not coincide with the cell periphery and were most likely sitting on the cell surface. The fluorescent signal is shown alone (Top), or merged with the corresponding brightfield image (Bottom). (B) Undifferentiated cells coexpressing CynA-GFP and mCherry-Myosin II were imaged by time-lapse fluorescence microscopy during random migration. The cell shown was migrating toward the top of the panel. (Magnification, 40×.)
CynA-GFP appeared to be more tightly limited to the cell rear than other lagging-edge proteins. Before differentiation, Myosin II, PakA, and PTEN are found uniformly around the cell periphery, with the exception of protrusions, and gradually become restricted to the lagging edge as differentiation progresses and cells polarize (20, 23, 24). In contrast, CynA-GFP was found only at the back of randomly migrating, undifferentiated cells, whereas other back markers were still uniformly localized (Fig. 1 G and H and Movie S2). This result suggests that the spatial targeting of CynA occurs before the polarization of other chemotactic signaling molecules, consistent with the observation that CynA does not require either PTEN or Myosin II to localize to the rear. In ∼80% of growing cells, the back-most region, where the accumulation of CynA-GFP was strongest, actually appeared to be depleted of mCherry-Myosin II relative to other portions of the cortex (Fig. S1B).
CynA Localization Is Regulated by Chemoattractant Stimulation.
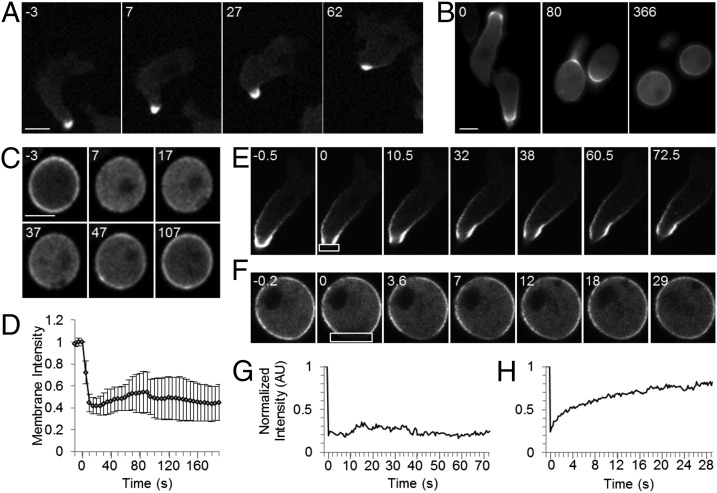
We next assessed the dynamics of CynA localization. To test whether CynA localization is responsive to chemoattractant, we expressed CynA-GFP in wild-type cells, allowed the cells to differentiate to induce chemotactic sensitivity, and stimulated them uniformly with 1 µM cAMP. In the vast majority of cells, CynA-GFP was strongly restricted to the lagging edge, and this distribution was largely unchanged upon the addition of cAMP (Fig. 2A). To test whether the polarized shape of differentiated cells influences the response of CynA to chemoattractant, we disrupted the actin cytoskeleton by treating with 1 µM Latrunculin A, which caused the cells to round up. This morphological change resulted in the dispersal of CynA-GFP from the lagging edge to the entire cell periphery (Fig. 2B). In Latrunculin-treated cells that were uniformly stimulated, CynA-GFP translocated into the cytosol within 10 s and began to slowly return to the membrane within 30 s, although about half of the cells show less than 50% recovery of the CynA-GFP membrane signal (Fig. 2 C and D and Movie S3). This translocation also occurred in the 10% of cells that were intrinsically rounded even in the absence of Latrunculin (Fig. S2 A and B). With slightly faster kinetics, PHCRAC-RFP transiently translocated onto the plasma membrane (Fig. S2 C and D). These transient redistributions in response to global stimulation are characteristic of other known “front” and “back” proteins (1, 24–30). Taken together, these observations suggest that the accumulation of CynA at the lagging edge is stabilized in polarized cells, preventing the protein from relocalizing in response to chemoattractant.
Fig. 2.
CynA accumulation at the cell periphery is stabilized by polarity. (A) Differentiated cells expressing CynA-GFP were stimulated uniformly with 1 µM cAMP and imaged by fluorescence microscopy at 5-s intervals. Time (s) after the addition of cAMP is indicated. (B) Differentiated cells expressing CynA-GFP were treated with 0.5 µM Latrunculin A and imaged every 3 s. Time (s) after the addition of Latrunculin A is indicated. (C and D) Differentiated cells expressing CynA-GFP were treated with 1 µM Latrunculin A, stimulated with 1 µM cAMP and imaged every 5 s (C). Time (s) after the addition of cAMP is indicated. The mean membrane intensity was calculated as described (see SI Materials and Methods) (D). Shown is the mean ± SD (n = 38 cells from eight movies). (E–H) Differentiated cells expressing CynA-GFP were assessed by FRAP with (F and H) or without (E and G) 1 µM Latrunculin A treatment. Time (s) after photobleaching the region indicated by the box is shown. For the cells shown in E and F, the normalized fluorescence intensity was measured as described (SI Materials and Methods) and plotted (G and H, respectively). (Scale bars, 5 µm.)
Fig. S2.
Polarity affects the localization and behavior of CynA. (A) Cells expressing CynA-GFP were stimulated uniformly with 1 µM cAMP at 0 s and imaged at 5-s intervals by time-lapse epifluorescence microscopy during early differentiation. Time (s) after cAMP addition is indicated. CynA-GFP fluorescence at the membrane is indicated by arrowheads. (B) The mean membrane to cytosolic fluorescence intensity ratio was calculated using membrane fluorescence values generated by the kymograph software as described (SI Materials and Methods) and plotted for the cell shown in A. (C and D) Differentiated wild-type cells coexpressing CynA-GFP and PHCRAC-RFP were pretreated with 1 µM Latrunculin A, stimulated uniformly with 1 µM cAMP at 0 s, and imaged at 5-s intervals using time-lapse confocal microscopy (C). Time (s) after the addition of cAMP is indicated. The mean membrane intensity was calculated as described (SI Materials and Methods) (D). Shown is the mean ± SD (n ≥ 14 cells from three separate movies). (E) Randomly migrating undifferentiated cells expressing CynA-GFP were imaged by confocal microscopy for 10 min at 20-s intervals. The frame number, which indicates the number of times that cells were exposed to laser light, is indicated. Within a few minutes of imaging (frames 10–15), cells retracted their pseudopods and became rounder and less motile. (F) Undifferentiated cells expressing CynA-GFP (Left) or coexpressing CynA-GFP and LimEΔcoil-RFP (Right) were imaged by confocal microscopy during random migration. Shown are images representative of the occasional cells that displayed limited physical displacement due to either reduced or excessive pseudopod extension. In these cases, polarity was reduced and CynA-GFP was not sharply localized to one pole of the cell. (G) Undifferentiated wild-type cells expressing CynA-GFP were imaged by time-lapse confocal microscopy for 10 min at 15-s intervals while migrating randomly. Shown is a cell changing its direction of migration and reversing its axis of polarity, resulting in the redistribution of CynA-GFP to the opposite pole. Red and green arrowheads designate retracting and newly extending pseudopods, respectively. Time (s) is indicated for each frame, and arrows indicate the original and final directions of migration. (Scale bars, 5 µm.)
Other conditions that altered cell morphology also delocalized CynA-GFP. First, in the ∼10% of cells that did not elongate strongly during differentiation, CynA-GFP was more dispersed around the cell periphery than in highly elongated cells (Fig. S2A). Second, cells exposed to excessive light gradually rounded up and the distribution of CynA-GFP at the membrane became uniform (Fig. S2E). Third, growing cells were occasionally quiescent, with fewer productive protrusions and a rounder morphology than their actively migrating counterparts, because of day-to-day variations; the distribution of CynA-GFP at the membrane was more dispersed in these cells (Fig. S2F). Fourth, when randomly migrating growing cells reversed their direction, CynA-GFP became diffuse before relocalizing to the new rear within roughly 30 s (Fig. S2G). Interestingly, a predominant CynA-GFP signal remained on the membrane throughout this process. Taken together, these observations suggest that asymmetric cell shape regulates the intracellular distribution of CynA.
We next tested the effect of cell morphology on CynA protein mobility using fluorescence recovery after photobleaching (FRAP). In highly motile elongated cells, the fluorescent signal that was lost after CynA-GFP was photobleached at the lagging edge did not recover over the course of 72 s (Fig. 2 E and G and Movie S4). Thus, the diffusion of CynA in polarized cells occurs more slowly than would be expected for a typical membrane protein. However, in cells pretreated with Latrunculin A, roughly 80% of the CynA-GFP fluorescence recovered from cytosolic pools, because the bleached region filled in uniformly. The recovery was biphasic, with 20% recovering with a half-time of 1.5 s, and 80% recovering with a half-time of 40 s (Fig. 2 F and H and Movie S4). Furthermore, undifferentiated cells, which are highly photosensitive, often acquired a rounded morphology because of light damage during the course of FRAP analysis, causing CynA-GFP to spread more uniformly around the cell periphery. In these cells, the fluorescence recovered similarly to that of Latrunculin-treated cells (10% recovering with a half-time of 100 ms, and 90% recovering with a half-time of 20 s). Taken together, these results suggest that membrane-bound CynA can exchange rapidly with cytosolic pools, but this exchange no longer occurs as CynA becomes stably localized to the cell rear in polarized cells.
Localization of CynA to the Lagging Edge Depends on a WD40 Clustering Domain and a Membrane Tether.
We further characterized the domain composition of CynA. In addition to its N-terminal PH domain (amino acids 10–144), CynA contains a putative WD40 domain and a long C-terminal extension (Fig. 3A). The WDSP WD40-repeat protein structure predictor found a full seven-bladed propeller in the CynA sequence (amino acids 278–616) (31). A seven-bladed propeller structure was also supported by a model of CynA generated by the threading software RaptorX, which found seven adjacent four-strand–containing β-sheets, although these sheets were separated into two distinct clusters in this model (32). Although CynA lacks sequence homology to proteins in higher eukaryotes, it may have functional homologs because several mammalian proteins share a similar domain composition. For example, the Rho GEF ARHGEF10 and several BEACH domain family proteins, including neurobeachin (NBEA), lipopolysaccharide-responsive, beige-like anchor protein (LRBA), lysosomal trafficking regulator (LYST), and neutral sphingomyelinase activation-associated factor (NSMAF), contain both WD40 and PH domains (33).
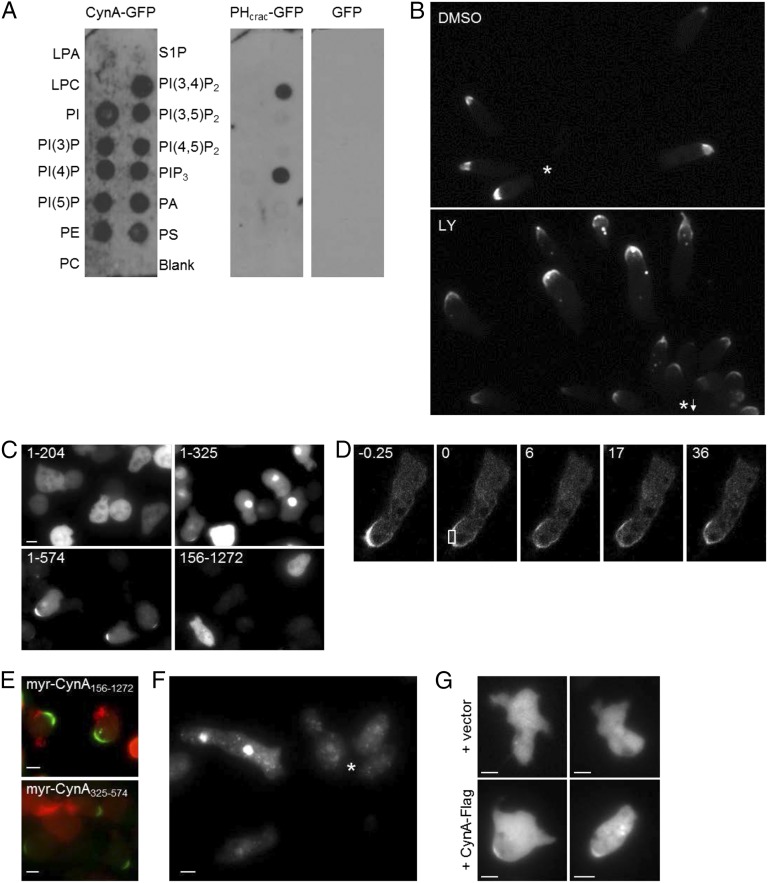
Fig. 3.
CynA localization is specified by its PH and WD40 domains. (A) A schematic representation of the GFP-tagged CynA protein (Top) and a series of GFP-tagged CynA truncation constructs. (B) Differentiated cells expressing the indicated GFP-tagged truncation constructs, depicted in A, were imaged by time-lapse fluorescence microscopy while migrating toward a micropipette filled with cAMP. Asterisks indicate the position of the micropipette. (C) Differentiated cells expressing the indicated GFP-tagged truncations of CynA were treated with 1 µM Latrunculin A and imaged. (D) Differentiated cells expressing CynA1–325-GFP were imaged at 30-s intervals while migrating toward a micropipette filled with cAMP. Time (s) after the cAMP gradient was established is indicated. Asterisks indicate the position of the micropipette. (E) Differentiated cells expressing CynA1–204+1–204-GFP were imaged while migrating toward a micropipette filled with cAMP, indicated by an asterisk. (F) Differentiated cells coexpressing the indicated myristoylated, GFP-tagged truncations of CynA and LimEΔcoil-RFP were imaged while migrating toward a micropipette filled with cAMP. The images shown are a merge of the RFP and GFP channels. The asterisk marks the position of the micropipette. (G–I) Differentiated cells coexpressing CynA156–1272-GFP and either a vector control or CynA-Flag, as indicated, were imaged while migrating toward a micropipette filled with cAMP (toward the top of each panel). The images shown were acquired using the GFP channel (G). Western blot analysis indicates the expression of CynA-Flag in the cell population (H). Coexpressing cells were treated with 5 µM Latrunculin A after two frames and imaged using GFP illumination every 10 s. Time (s) after the addition of Latrunculin A is indicated (I). (Scale bars, 5 µm.)
The PH domain of CynA was predicted to bind PIP3; however, CynA-GFP in cell lysates bound to many of a series of lipids spotted on hydrophobic membranes (Fig. S3A), with a slight preference for PI(3,4)P2 and no preference for PIP3. This finding suggests that binding of a specific lipid is not the key regulator of CynA localization. Furthermore, the localization of CynA-GFP was unaffected by the addition of the PI3K inhibitor LY294002, which has been shown to greatly reduce PIP3 levels in vivo (Fig. S3B) (34). This finding is consistent with the observation that CynA-GFP and PIP3 biosensors were not colocalized (Fig. 1 B and C).
Fig. S3.
The regulation of CynA localization by phosphoinositides and protein domains. (A) Wild-type cells expressing CynA-GFP, PHCRAC-GFP, or cytosolic GFP were starved for 3 h and filter-lysed. Echelon PIP strips, prespotted with the indicated lipids, were incubated with the resulting supernatants and then probed using an anti-GFP antibody. (B) Differentiated cells expressing CynA-GFP were pretreated with DMSO or 50 µM LY294002 for 50 min, then imaged by time-lapse microscopy for 30 min at 30-s intervals while migrating toward a micropipette filled with cAMP. The fluorescence images shown were acquired roughly 30 min after the cAMP gradient was established. An asterisk indicates the position of the micropipette; an asterisk with an arrow indicates the direction of the micropipette, which was placed outside of the frame shown. (C) Undifferentiated cells expressing GFP-tagged truncations of CynA were imaged by epifluorescence microscopy. The amino acid residues included in each fragment are indicated. (D) Differentiated cells expressing CynA1–325-GFP were assessed by FRAP. Time (s) after photobleaching the region indicated by the box is shown. (E) Undifferentiated cells coexpressing LimEΔcoil-RFP and the indicated GFP-tagged myristoylated fragment of CynA were imaged by epifluorescence microscopy. The images shown are a merge of the GFP and RFP channels. (F) Differentiated cells expressing CynA325–574-GFP were imaged while migrating toward a micropipette filled with cAMP. The asterisk indicates the position of the micropipette. (G) Differentiated cells coexpressing CynA156–574-GFP and either a vector control or CynA-Flag, as indicated, were imaged while migrating toward a micropipette filled with cAMP (toward the top of each panel). The images shown were acquired using the GFP channel. (Scale bars, 5 µm.)
To determine which region of the CynA protein controls localization to the lagging edge, we generated a series of GFP-tagged C- and N-terminal truncation constructs and expressed them in undifferentiated cells and in polarized cells undergoing chemotaxis (Fig. 3A). CynA1–204 localized mostly to the cytosol, but appeared weakly on the plasma membrane, with enrichment at the rear, in a small percentage of actively migrating cells (Fig. 3B). CynA1–325 displayed greatly increased localization to the lagging edge, but compared with the full-length protein there was an increased cytoplasmic pool of fluorescent protein, fewer cells showed strong membrane localization after treatment with Latrunculin A, strong accumulation at the back was not apparent in less motile cells compared with chemotaxing cells, and the signal at the back could recover rapidly after photobleaching (Fig. 3 B–D and Fig. S3 C and D). CynA1–574, like the full-length protein, was strongly bound to the membrane, even after Latrunculin A treatment, and tightly accumulated at the rear even in relatively unpolarized cells (Fig. 3 B and C and Fig. S3C). Longer N-terminal fragments of CynA were also enriched at the lagging edge, although the extent of membrane accumulation varied between the different truncated proteins. CynA156–1272 and other N-terminal truncations localized to the cytosol, although protein expression was low (Fig. 3B). The results of the truncation analyses suggest that the PH domain of CynA is required for membrane binding and that the N-terminal 574 amino acids are sufficient for tight accumulation at the lagging edge.
We next wanted to test whether the PH domain was also sufficient for membrane binding. The PH domain alone, CynA1–204, localized weakly to the plasma membrane (Fig. 3B); therefore, we artificially fused two copies of the PH domain in a single construct to increase membrane affinity. This GFP-tagged tandem PH domain protein localized strongly to the rear of highly motile elongated cells but localized more globally to the plasma membrane in undifferentiated cells, similarly to CynA1–325 (Fig. 3E). Furthermore, when cells were pretreated with Latrunculin A and stimulated uniformly, the tandem PH domain protein transiently relocalized from the membrane to the cytosol (Movie S5). This finding suggests that the PH domain is sufficient to bind to and sense the properties of the plasma membrane that determine the front versus back state.
To test whether the membrane binding and strong back-targeting functions of CynA are separable, we fused CynA156–1272-GFP, which is located in the cytosol, to the myristoylated N-terminal portion (amino acids 1–128) of PKBR1, which is distributed uniformly on the membrane (35). Remarkably, this chimeric protein localized tightly to the lagging edge, essentially identically to the full-length CynA-GFP (Fig. 3F and Fig. S3E). Furthermore, regions containing just the WD40 region of CynA (CynA156–574 and CynA325–574), which lack membrane localization on their own, were sufficient to localize strongly to the plasma membrane at the cell rear when fused to the exogenous myristoylation motif (Fig. 3F and Fig. S3 E and F). The data shown in Fig. 3 E and F suggest that the localization of CynA is regulated by two distinct mechanisms. First, the PH domain controls binding to the membrane and senses external cues. Second, a “clustering” motif, located between amino acids 325 and 574, causes the accumulation of membrane-bound CynA at the rear of cells; this region corresponds to the putative WD40 domain.
Several pieces of evidence suggested that the WD40 region of CynA may be mediating oligomerization events, including the increased membrane binding observed for the dimerized PH domain, the tightly clustered localization of the WD40-containing region, which correlates with reduced protein mobility as observed by FRAP, and the observation that a smaller WD40 region, CynA325–574, localized to small intracellular puncta (Fig. S3F). To test whether CynA can self-oligomerize, we coexpressed a GFP-tagged, cytosol-localized portion of CynA, CynA156–1272-GFP, with either empty vector or a full-length Flag-tagged CynA protein (Fig. 3 G and H). Coexpression with empty vector resulted in a cytosolic and occasionally (about 20% of cells) punctate GFP signal, as observed for CynA156–1272-GFP alone. However, when this protein was coexpressed with full-length CynA-Flag, the GFP-tagged protein was detected at the lagging edge of 95% of cells that had a detectable GFP signal (Fig. 3 G and H). CynA156–574-GFP also localized to the lagging edge when coexpressed with CynA-Flag in a small percentage of cells (∼5%) (Fig. S3G). This finding indicates that full-length CynA-Flag could bind to and mediate the proper lagging edge membrane localization of the portion of CynA that includes the WD40 domain. Treatment with Latrunculin A led to uniform localization but did not reverse the membrane association of CynA156–1272-GFP in these coexpressing cells, indicating that the actin cytoskeleton and polarity are not required for CynA oligomerization (Fig. 3I).
CynA Mediates Cell Morphology and Migration.
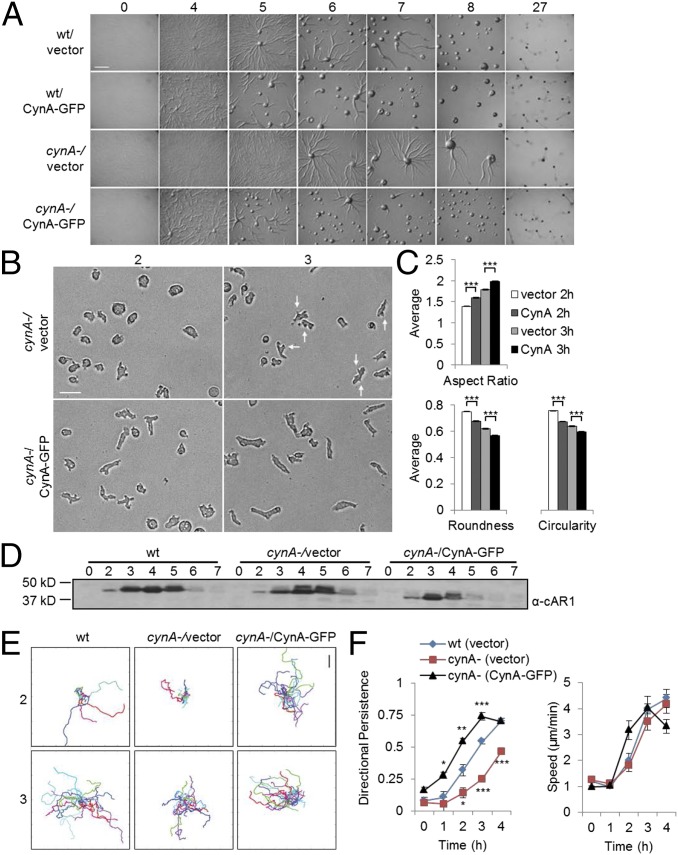
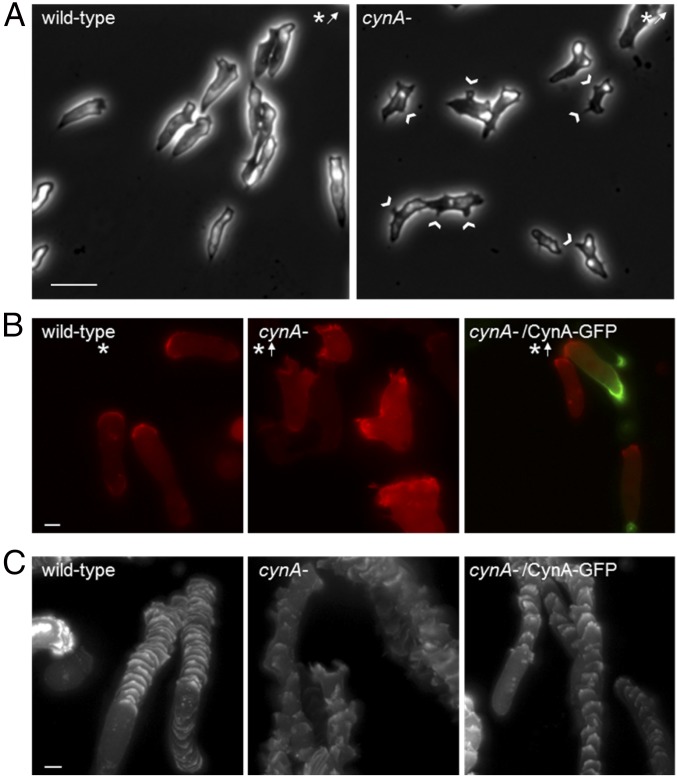
To study the function of CynA, we generated several independent null strains using homologous recombination (Fig. S4A). The resulting mutants were plated as a monolayer on nonnutrient agar and assessed for differentiation and chemotaxis. Under these conditions, wild-type cells differentiated and formed streams of migrating cells by the 5-h stage (36). The cynA- cells also formed streams and aggregated, but this process was delayed by about 2 h for two independently disrupted cell lines (Fig. 4A). This delay in early differentiation was reversed by expressing CynA-GFP in the cynA- cells, suggesting that the GFP-tagged protein is functional (Fig. 4A). Furthermore, exogenous expression of CynA-GFP in wild-type cells caused streaming and aggregation behaviors to be accelerated by about 2 h (Fig. 4A).
Fig. S4.
Overexpression or disruption of CynA affects cell polarity. (A) The gene encoding CynA was replaced with a Blasticidin S-resistance cassette by homologous recombination (Left), resulting in several independent cynA- strains. The mutant genotype was confirmed for each strain by Southern blotting, using a piece of flanking DNA at the 3′ end (3′ arm) as a probe (Right). (B) The morphology of cynA- cells, expressing either the KF2 vector control or CynA-GFP and imaged for 30 min after either 2 or 3 h of differentiation on nonnutrient agar, was quantified using ImageJ. The data shown here is the same dataset as in Fig. 4C, but presented as histograms normalized to the number of cells in each sample (Left), or as cumulative percentages (Right), to show the distribution of data. Each dataset is comprised of between 1,304 and 1,591 cells from a total of nine movies taken from three separate experiments per sample. Cell morphology was quantified using the frame acquired after 30 min of imaging. The data shown do not include the most highly polarized cells, mostly from the population of CynA-GFP–expressing cells at 3 h of differentiation, because these cells were already aggregating and could not be properly segmented.
Fig. 4.
CynA affects aggregation and polarity during early differentiation. (A) Undifferentiated wild-type or cynA- cells expressing either CynA-GFP or a vector control were plated onto nonnutrient agar at a density of 5 × 105 cells/cm2 and imaged as they proceeded through differentiation. Time (h) of starvation is indicated. (Scale bar, 1 mm.) (B–D) Cells were prepared on sets of nonnutrient agar plates as in A and collected for further analysis over the course of differentiation. A fraction of the cells was imaged during random migration for 30 min at 30-s intervals by multipoint time-lapse microscopy (B); cells were also lysed and analyzed by Western blotting, using antibodies against the endogenous cAR1 protein (D). Time (h) of differentiation is indicated. The images shown in B were acquired after 30 min of imaging and include arrows to indicate some of the lateral protrusions. (Scale bar, 25 µm.) The morphology of cynA- cells, expressing either the KF2 vector control or CynA-GFP and imaged after either 2 or 3 h of differentiation as in B, were quantified using ImageJ (C). Each bar on the graph represents the mean ± SEM (n > 1,300 cells, taken from three separate experiments per sample). ***P < 0.001. (E and F) Cells were differentiated on nonnutrient agar and imaged during random migration as in B, and cell centroids were tracked manually to generate trajectories (E) and measure motility parameters (F) as described in SI Materials and Methods. Tracks in E show 25–35 cells per sample. (Scale bar, 100 µm.) Each data point in the graphs in F represent the average of the means ± SEM (n = 4 movies, with 25 cells each, per sample). *P < 0.05, **P < 0.02, ***P < 0.01 (all comparisons to wild-type with a Bonferroni correction).
To further characterize the developmental delay of cynA- cells, we imaged cells that were washed off of nonnutrient agar plates at various time points throughout differentiation. Cells expressing CynA-GFP became elongated at earlier time points in differentiation than the cynA- cells (Fig. 4 B and C and Fig. S4B). In fact, the difference in morphology across cell types is even more pronounced than the data suggest, because many of the CynA-GFP–expressing cells aggregated during imaging and could not be included in the analyses. Additionally, 50% of cynA- cells retained protrusions around their lateral edges even after becoming elongated (Fig. 4B). Endogenous cAR1 protein, a well-characterized marker for cell differentiation, was up-regulated on a similar timescale for wild-type and cynA- cells, as well as for cells exogenously expressing CynA-GFP (Fig. 4D) (37, 38). Interestingly, endogenous cAR1 levels began to decline at earlier time points in cells expressing CynA-GFP compared with wild-type or cynA- strains. The precocious down-regulation of cAR1 may be caused by the earlier aggregation of these cells because it was not observed when cells were differentiated in suspension.
The differences in morphology observed for cynA- cells also translated to defects in random migration. Tracks of cynA- cell motility were shorter and had a jagged trajectory compared with wild-type, whereas tracks for cells overexpressing CynA-GFP were longer and straighter on average (Fig. 4E). Furthermore, cynA- cells moved with decreased directional persistence and similar speeds compared with wild-type cells throughout differentiation. Conversely, cells expressing CynA-GFP migrated with increased persistence and became faster at an earlier time point. After about 3 h of differentiation, cells expressing CynA-GFP began to aggregate immediately upon plating for imaging; the added component of gradient sensing and chemotaxis appeared to decrease cell speed, resulting in shorter tracks, while further increasing directional persistence (Fig. 4 E and F).
CynA Affects the Morphology and Behavior of Cells in Response to Chemoattractant Gradients.
Using a micropipette assay to test chemotaxis, cynA- cells sensed the gradient of chemoattractant and migrated toward the cAMP source (Fig. 5A and Movies S6 and S7). We observed similar behavior in myo II- cells, which elongated and aligned in the direction of a chemoattractant source despite their known polarity defects (Fig. 1F) (39–43). Although cynA- cells could chemotax with wild-type–like motility speeds (3.70 ± 0.11 µm/min for cynA- vs. 3.45 ± 0.19 µm/min for wild-type; P = 0.25; data are the mean ± SEM, n = 80 or 180 cells for wild-type or cynA-, respectively), these cells showed slight decreases in directional persistence (0.48 ± 0.02 for cynA- vs. 0.65 ± 0.03 for wild-type; P < 0.01), chemotactic index (0.35 ± 0.02 for cynA- vs. 0.53 ± 0.03 for wild-type; P < 0.01) and chemotactic speed (1.17 ± 0.08 µm/min for cynA- vs. 1.91 ± 0.18 µm/min for wild-type; P < 0.01). Expression of CynA-GFP, but not an empty vector, in the cynA- cells was able to reverse these defects in directional persistence (0.51 ± 0.02 for CynA-GFP vs. 0.42 ± 0.02 for vector; P < 0.01; data are the mean ± SEM, n = 152 or 183 cells for CynA-GFP or vector expression, respectively), chemotactic index (0.37 ± 0.03 for CynA-GFP vs. 0.30 ± 0.02 for vector; P = 0.02), and chemotactic speed (1.89 ± 0.19 µm/min for CynA-GFP vs. 1.11± 0.10 µm/min for vector; P < 0.01). As with cells that were differentiated on nonnutrient agar, the cynA- cells appeared to have altered morphologies compared with wild-type controls during chemotaxis. Although they were still elongated, cynA- cells frequently had lateral pseudopodia that were not observed in wild-type cells (Fig. 5A). These aberrant protrusions could potentially explain the decreased speed and persistence observed during chemotaxis. Despite these defects, PTEN, Myosin II, and PIP3 markers were still able to localize asymmetrically during late stages of differentiation in cynA- cells (Fig. S5A).
Fig. 5.
Disruption of CynA leads to an increase in lateral protrusions and an altered leading edge morphology. (A) Differentiated wild-type and cynA- cells were imaged by time-lapse microscopy for 30 min at 30-s intervals while migrating toward a micropipette filled with cAMP. Images are shown for the last frame. Asterisks indicate the position of the micropipette; arrows indicate that the micropipette was placed outside of the frame shown. Arrowheads indicate lateral protrusions. (Scale bar, 20 µm.) (B and C) Differentiated wild-type and cynA- cells coexpressing LimEΔcoil-RFP and either CynA-GFP or empty vector were imaged by fluorescence microscopy at 30-s intervals while chemotaxing toward a micropipette filled with cAMP (B). The images shown in B are a merge of the GFP and RFP channels. Tracks of the RFP fluorescence intensities for the cells shown in B were generated using ImageJ, such that each pixel shown in the overlaid image corresponds to the maximum pixel intensity for that location over the course of the movie (C). The first 5 min of each movie, during which cells were turning to orient toward the cAMP gradient, were excluded from analysis in C for simplification. Asterisks indicate the position of the micropipette; arrows indicate that the micropipette was placed outside of the frame shown. (Scale bars, 5 µm.)
Fig. S5.
(A) Differentiated cynA- cells expressing PTEN-GFP, mCherry-Myosin II, or the PIP3 biosensor PHCRAC-RFP were imaged by time-lapse fluorescence microscopy during random migration (Left) or after global stimulation with 1 µM cAMP at 0 s (Right). For each of the cells shown in the panels on the left, the leading edge is positioned toward the top of the frame. Frames shown in the panels on the right were acquired at 5-s intervals, and time (s) after the addition of cAMP is indicated. The translocation of PHCRAC-RFP to the membrane was similar to that of wild-type cells. (B) A schematic to illustrate how cell polarity affects the response of cells to changes in chemoattractant gradients. Weakly polarized cells form new protrusions to rapidly reorient themselves with respect to the repositioned cAMP source. Highly polarized cells do not form new protrusions but instead maintain their existing leading edge and slowly turn to face the new gradient. (C) Partially differentiated wild-type cells expressing CynA-GFP or a vector control were imaged by fluorescence or brightfield time-lapse microscopy for 10 min at 10-s intervals while migrating toward a micropipette filled with cAMP. Once the cells began migrating toward the cAMP source, the micropipette was repositioned such that the direction of the gradient was approximately reversed. Time (s) is indicated for each frame, such that 0 s is the first frame acquired after the micropipette was repositioned. Arrowheads denote the formation of new protrusions. (Magnification, 40×.)
We next tested the effect of CynA-GFP overexpression in cells responding to shifting chemoattractant gradients. These cells were more elongated and had fewer lateral protrusions than control cells. Following repositioning of the micropipette, the control cells extended protrusions toward the new chemoattractant source, whereas the CynA-GFP–expressing cells maintained their existing fronts and turned (Fig. S5 B and C). Furthermore, CynA-GFP was consistently localized to the lagging edge, even when the micropipette was positioned directly behind the cells (Fig. S5C).
To better observe the morphology defects of the cynA- strain, we expressed LimEΔcoil-RFP, a marker for newly polymerized actin, and assessed chemotaxis (44). Consistent with previous results, cynA- cells displayed a broader distribution of LimEΔcoil-RFP at the leading edge than wild-type cells or cynA- cells expressing CynA-GFP (Fig. 5 B and C and Movies S8 and S9). Furthermore, cynA- cells, but not those expressing CynA-GFP, showed the accumulation of LimEΔcoil-RFP at multiple additional sites along the cell perimeter, indicating excessive pseudopod formation (Fig. 5 B and C and Movies S8 and S9). Expression of LimEΔcoil-RFP also highlighted differences across cell lines in the shape of pseudopodia. Whereas the edges of protrusions in cynA- cells were jagged and dynamic, moving rapidly from side to side, those in wild-type cells had smooth edges and extended evenly (Fig. 5 B and C and Movies S8 and S9).
CynA Spatially Restricts Actin Polymerization.
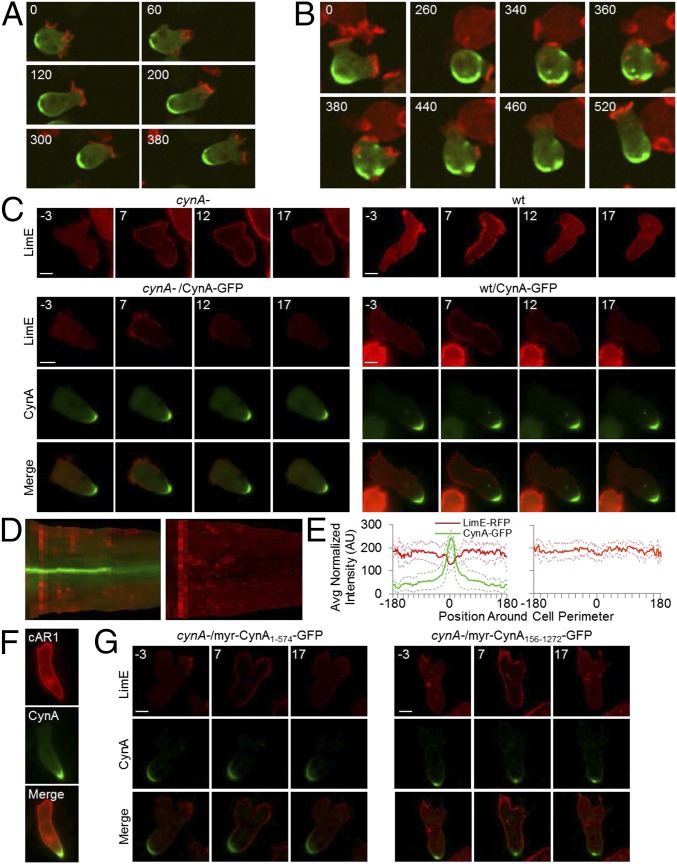
We next compared the localizations of CynA-GFP and LimEΔcoil-RFP. In growing cells, which have reduced polarity but greater spontaneous actin polymerization than differentiated cells, CynA-GFP and LimEΔcoil-RFP had opposing distributions (Fig. 6A). This was true even for the most active cells, which extended actin protrusions all around the cell periphery, occasionally resulting in a broader distribution or multiple sites of CynA-GFP accumulation (Fig. 6B and Fig. S2F). In these cells, an actin protrusion extended near sites of CynA-GFP accumulation about 15% of the time, resulting in the retraction of that protrusion and cell movement in the original direction. Rarely, an actin protrusion broke through the patch of CynA-GFP accumulation; when this occurred, the CynA-GFP cluster dispersed such that the two proteins did not colocalize (Fig. 6B and Fig. S2G). These protrusions were most often rapidly retracted, but also occasionally resulted in a directional change.
Fig. 6.
CynA locally inhibits actin polymerization. (A and B) Undifferentiated cynA- cells coexpressing CynA-GFP and LimEΔcoil-RFP were imaged for 10 min at 20-s intervals while migrating. The images shown are a merge of the GFP and RFP channels. Time (s) is indicated. (C–E) Differentiated wild-type and cynA- cells coexpressing LimEΔcoil-RFP and either CynA-GFP or the KF2 vector control were stimulated with 1 µM cAMP and imaged for 2.5 min at 5-s intervals (C). Time (s) after the addition of cAMP is indicated. The fluorescence intensities of cynA- cells, coexpressing LimEΔcoil-RFP and either CynA-GFP (Left) or the KF2 vector control (Right), stimulated with cAMP as in C, were converted into kymographs (D), such that each vertical bar in the kymograph represents the intensities of GFP and RFP (Left) or RFP alone (Right) along the perimeter of the cell for a given point in time. cAMP was added after the second frame. The fluorescence intensities along the perimeter of cynA- cells coexpressing LimEΔcoil-RFP and either CynA-GFP (Left) or the KF2 vector control (Right), stimulated with cAMP as in C, were plotted for the time point corresponding to the maximal chemoattractant response, which occurred 7 s after stimulation (E). Solid lines represent the mean (n ≥ 11 cells), and dotted lines represent the mean ± SD. Before averaging, the fluorescence intensities for each cell were aligned such that 0° corresponds to the morphological back of each cell. In cells expressing CynA-GFP, the LimEΔcoil-RFP intensities of the pixels centered around the cell rear were significantly lower than those at the other regions of the cell boundary when compared by a Student’s t test (mean ± SEM: 134.16 ± 2.14 at the rear vs. 180.14 ± 0.86 at the nonrear, P < 0.01); LimEΔcoil-RFP pixel intensities at the cell rear were also significantly lower in CynA-GFP-expressing cells than in cynA- cells (mean ± SEM: 134.16 ± 2.14 for CynA-GFP vs. 151.69 ± 2.39 for cynA-, P < 0.01). (F) Differentiated cells coexpressing cAR1-RFP and CynA-GFP were imaged as in C. (G) Differentiated cynA- cells coexpressing LimEΔcoil-RFP and either myrPKBR1-CynA1–574-GFP or myrPKBR1-CynA156–1272-GFP were stimulated with 1 µM cAMP and imaged for 2.5 min at 5-s intervals. Time (s) after the addition of cAMP is indicated. (Scale bars, 5 µm.)
The mutually exclusive localizations of CynA-GFP and LimEΔcoil-RFP, as well as the increase in cortical LimEΔcoil-RFP in cynA- cells, suggest an antagonistic relationship between CynA and actin polymerization. We tested this connection by observing the response in globally stimulated cells. Following uniform chemoattractant addition, LimEΔcoil-RFP was recruited to the cortex within 10 s of cAMP stimulation and returned to the cytosol by 20 s (Fig. 6C and Movie S10). Interestingly, although the cells were stimulated uniformly, LimEΔcoil-RFP was not recruited to the cortex at the rear of wild-type or CynA-GFP-expressing cells (Fig. 6 C–E and Movie S10). In cells exogenously expressing CynA-GFP, the patch of cortex without LimEΔcoil-RFP recruitment corresponded to sites of CynA-GFP accumulation (Fig. 6 C–E and Movie S10). In contrast, LimEΔcoil-RFP was recruited uniformly around the cell periphery, including the back, in cynA- cells that were differentiated for equivalent amounts of time (Fig. 6 C–E and Movie S10). Furthermore, the cynA- cells appeared to have a slightly prolonged and elevated actin response compared with cells expressing CynA. Accumulation of CynA-GFP did not prevent the localization of cAR1-RFP to the plasma membrane, indicating that the absence of LimEΔcoil-RFP at these sites was not an imaging artifact (Fig. 6F). Together, these results suggest that the presence of CynA locally inhibits actin polymerization in response to cAMP stimulation and during random migration in unstimulated cells. Furthermore, GFP-tagged truncations of CynA that localized to the lagging edge also blocked the actin response in globally stimulated cells. Specifically, fusions of CynA1–574-GFP or CynA156–1272-GFP to the myristoylation motif of PKBR1 both localized to the lagging edge and restricted actin polymerization, suggesting that the clustering activity of the CynA WD40 domain is sufficient for this inhibition (Fig. 6G and Movie S10).
CynA Clusters Chemoattractant Receptors at the Lagging Edge and Locally Inhibits Their Signaling.
To further explore the relationship between CynA and the response to cAMP stimuli, we fused either full-length CynA-GFP or various truncations to the chemoattractant receptor cAR1, a GPCR with seven transmembrane domains that localizes uniformly throughout the plasma and internal membranes (Fig. 6F) (45). Remarkably, each of the resulting cAR1 fusions localized to the cell rear in both growing and differentiated cells (Fig. 7 A and B). We next coexpressed the cAR1–CynA fusions with LimEΔcoil-RFP in car1-/3- cells, so that cAMP receptor activity originated only at sites of CynA-GFP localization, and tested the actin response. Following global stimulation with cAMP, LimEΔcoil-RFP was transiently recruited to the entire cortex, except in regions of cAR1-CynA accumulation (Fig. 7C and Movie S11). This finding suggests that clusters of CynA protein or fragments thereof are capable of blocking actin polymerization even when the majority of detectable signaling is initiated in that spot. The recruitment of LimEΔcoil-RFP to the remainder of the cortex suggests that there may be low levels of cAR1–CynA fusion protein around the perimeter of the cell. These proteins would be capable of initiating a response at the front but not concentrated enough for the inhibitory function of CynA. Alternatively, it is possible that activation signals generated by localized receptors can rapidly become global.
Fig. 7.
CynA clusters proteins at the lagging edge and locally blocks the chemoattractant response. (A) Undifferentiated car1-/3- cells expressing the indicated GFP-tagged proteins were imaged by fluorescence microscopy. (B) Differentiated car1-/3- cells coexpressing LimEΔcoil-RFP and the indicated GFP-tagged proteins were imaged while migrating in the presence of a micropipette filled with cAMP, which was oriented toward the top of each panel. For each cell line, the GFP image is shown alone (Left) and merged with the RFP image (Right). (C) Differentiated car1-/3- cells coexpressing LimEΔcoil-RFP and the indicated GFP-tagged proteins were stimulated with 1 µM cAMP and imaged for 2.5 min at 5-s intervals. Time (s) after the addition of cAMP is indicated. (Scale bars, 5 µm.)
Discussion
CynA Is a Novel Lagging-Edge Protein with Membrane-Binding and “Clustering” Domains.
Although CynA shares many similarities to other lagging edge proteins, it also has several unique properties. Like CynA, other back proteins translocate transiently into the cytosol upon chemoattractant stimulation, accumulate in the cleavage furrow during cytokinesis, and have spatial distributions that are tightly coupled to cell polarity. However, CynA localizes asymmetrically in growing cells, whereas the other lagging-edge proteins target to the back only as the cells differentiate (20, 23, 24). Furthermore, CynA accumulation at the rear is more stable and tightly localized than that of other lagging-edge proteins, such as PTEN. In fact, the lagging-edge localization of CynA is strong enough to override the endogenous localizations of other membrane proteins, forcing them to cluster at the back when fused to CynA.
We explored the extrinsic factors that regulate the localization of CynA. First, although the stable asymmetric localization of CynA requires cell polarity or an intact actin cytoskeleton, newly polymerized actin can cause local patches of accumulated CynA to disperse. Second, the localization of CynA does not require PTEN or Myosin II and clustering occurs in growing cells before other proteins become restricted to the lagging edge. Third, the binding site for CynA appears to be up-regulated at the cell rear with differentiation, either by increases in total amount (i.e., via transcriptional regulation) or by increases in local concentrations (i.e., via asymmetric distribution). Finally, although there is speculation that PI(4,5)P2 is important for the localization of PTEN, neither Dictyostelium PTEN nor CynA binds to PI(4,5)P2 specifically in vitro (46, 47). In fact, CynA showed a slight preference for PI(3,4)P2 on lipid strips, which indicates either that PI(3,4)P2 is enriched at the lagging edge or that lipid interactions are not important for conferring asymmetric localization.
CynA contains a PH domain required for membrane binding and a WD40 domain that likely regulates oligomerization; these domains mediate binding to the lagging edge via two distinct mechanisms. Like PTEN, the PH domain senses changes in the membrane that cause dissociation both from protrusions and following chemoattractant stimulation, indicating that the PH domain accumulates at the lagging edge by dissociating from the front. In contrast, myristoylated versions of CynA lacking the PH domain remain bound to the membrane during stimulation and thus accumulate at the lagging edge by a different mechanism. The WD40 domain controls this behavior, likely by mediating the formation of higher-order oligomers. It has been reported that high-order oligomerization and membrane-binding motifs artificially target cytosolic proteins to membrane patches, which accumulate at the lagging edge in polarized cells (48–52). Oligomerizing membrane microdomain components also enrich at the rear of polarized neutrophils or T cells (53–57). The WD40 domain enhances the stability and immobility of CynA at the lagging edge, either through oligomerization alone or by interaction with other proteins, as evidenced by the differences between CynA1–574 and either CynA1–325 or the tandem PH domain, CynA1–204+1–204. The formation of large stable complexes with the actomyosin network could explain the dispersal of CynA throughout the membrane upon disruption of the actin cytoskeleton. Moreover, the mechanisms of localization to the rear mediated by the domains of CynA synergize because the WD40 domain requires a membrane anchor for targeting, whereas WD40-mediated oligomerization of the PH domain is expected to enhance its affinity for the membrane.
CynA Can Be Used as a Tool for Targeting Other Proteins to the Lagging Edge.
The tightly clustered distribution of CynA allows it to serve as a useful tool for targeting other proteins to the lagging edge, opening the way for a multitude of additional studies examining the role of protein localization in migration and many other contexts. We were surprised to find that fusing CynA to several diverse membrane anchors did not affect CynA localization, but rather caused clustering of proteins that are normally distributed uniformly. Thus, we could not test the effects of distributing CynA uniformly throughout the membrane. The fact that CynA recruited cAR1 to the rear allowed us to determine how CynA regulates a localized chemoattractant response. In the future, cAR1–CynA fusions can be used to answer a number of questions related to cAR1 function in migration and adaptation; for example, can cells properly sense a chemoattractant gradient and initiate an appropriate response if receptors are clustered at the cell rear? These and many other potential studies are now possible using CynA as a tool for recruitment to the lagging edge.
CynA Defines the Axis of Symmetry and Promotes Polarity.
The behavior and localization of CynA suggest a connection with cell polarity. Migrating cells display different degrees of polarity. On one end of the polarity spectrum, a cell extending spontaneous protrusions displays a “transient” polarity. At an intermediate level, cells extending protrusions in the direction of an external cue demonstrate an “induced” polarity. Finally, cells with stable front and back regions, even in the absence of directional cues, exhibit a “persistent” polarity that can be augmented by chemoattractants. Such persistent polarity enhances directionality during migration, allowing cells to chemotax more efficiently in steady gradients, but impairs responsiveness to shifting gradients (15, 58, 59). Undoubtedly, overlapping networks of components are involved in these various events. Our observations indicate that clustered CynA promotes a shift toward the persistent end of the polarity spectrum.
Furthermore, our results suggest that CynA promotes polarity by regulating the localization of actin polymerization and therefore protrusions. Briefly, we found that overexpression of CynA decreases the ability of the lateral or lagging edges of cells to form new extensions toward a source of cAMP. Furthermore, cynA- cells display multiple spontaneous protrusions along the lateral edges, and actin polymerization almost never occurs near sites of CynA accumulation, either spontaneously or in response to a global stimulus, even if receptor-mediated signaling is initiated by receptors clustered at these regions. Experiments using truncated forms of CynA that lack either the N terminus or C terminus of the protein suggest that the clustering domain, CynA156–574, is sufficient for these inhibitions of actin polymerization. Of course, CynA may not inhibit actin polymerization directly, but may potentially alter the mechanical properties of the cytoskeleton or act as a negative regulator of signaling events. Because CynA impacts the leading edge morphology, it may regulate a global inhibitory mechanism; alternatively, low levels of CynA protein at the cell front may locally fine-tune the cytoskeletal dynamics.
Combined, these results suggest that the asymmetric localization of CynA is an early step in symmetry breaking and helps establish the axis of polarity. When exogenous CynA-GFP is expressed in growing cells, it accumulates in a patch on the membrane, locally repressing pseudopod formation. This contributes to the creation of a functional lagging edge and increases directional persistence in these cells. Accordingly, this accelerates polarization, but not the expression of stage-specific markers, such as cAR1, during the course of differentiation. Other lagging-edge proteins do not localize asymmetrically in growing cells or noticeably increase polarity when overexpressed (11, 21, 24, 60). Consistent with its role in enhancing polarity, CynA transcription is up-regulated during differentiation, with low levels in growing cells and a peak after 8 h of starvation, when cells acquire maximal polarity and aggregate into mound structures (61, 62) (data are accessible at dictybase.org) (36, 63, 64). Although our data indicate that CynA is important for establishing polarity, other factors also mediate this process, as expected given the redundancy of the signaling and cytoskeletal networks.
Although the underlying mechanisms are still unclear, many models for the establishment of persistent polarity have been proposed. Most have focused on positive feedback loops at the front of cells involving a number of specific signaling molecules, such as RhoGTPases and PI3K, and a global inhibitory component that prevents activity at multiple sites (65–77). An increasing number of studies have implicated events at the cell rear, such as the local inhibition of signaling activities and force generation through actin depolymerization or Myosin II (78–82). The behavior of CynA shows how positive feedback at the lagging edge can contribute to polarity. CynA clusters act as negative regulators of protrusive activity and, in turn, CynA is forced away from sites of protrusions, resulting in an increased concentration of CynA in surrounding regions. This appears to further restrict the regions that are permissive for protrusions. As the cell becomes increasingly polarized, CynA becomes more tightly clustered at the lagging edge, further reinforcing the restriction of protrusive activity to the leading edge, thereby generating a positive feedback mechanism. It is possible that this combination of both CynA-mediated inhibition of protrusions and the polarity-mediated spatial restriction of CynA clusters is what drives the polarity that is characteristic of differentiated, migrating cells.
Materials and Methods
A detailed description of the materials and methods used can be found in SI Materials and Methods. Plasmids were generated by standard cloning procedures and expressed in Dicytostelium cells grown in HL5 media. CynA-null cells were generated by homologous recombination, such that the entire coding sequence was replaced by a drug-resistance cassette. Cells were differentiated in suspension culture with pulses of cAMP or by growing on nonnutrient agar plates as needed and imaged with a 40×/1.3 oil objective and DIC, GFP, and RFP (where applicable) illumination using either a Zeiss Observer.Z1 inverted microscope or Leica DMI6000 inverted microscope equipped with a Yokogawa CSU10 spinning disk. Global stimulation assays were performed using 1 µM cAMP. Chemotaxis assays were performed using a micropipette filled with 10 µM cAMP. Image processing and quantification were done using ImageJ or customized Matlab scripts.
Fig. S1 illustrates in more detail the relationship between CynA localization and other proteins at the lagging edge. Fig. S2 elaborates further on the influence of polarity on the localization, as well as responsiveness to cAMP, of CynA demonstrated in Fig. 2. Fig. S3 shows the phosphoinositide binding of CynA and the localization of CynA truncations and fusions in undifferentiated cells. Fig. S4 depicts the generation of cynA- cells and contains additional quantification of the polarity defects associated with disruption or overexpression of CynA as shown in Fig. 4. Fig. S5 shows the localization of front and back proteins in cynA- cells and the effects of CynA overexpression on polarity.
SI Materials and Methods
Cell Growth and Differentiation.
The majority of experiments were performed using a subclone of the AX3 wild-type strain, termed AX3.1, chosen for its ability to form fruiting bodies upon starvation and its morphology and behavior during chemotactic assays. Some experiments were done in the pten- or myo II- backgrounds (24, 83). All cells were grown at 22 °C in HL5 media on tissue culture dishes for cell line maintenance or in suspension to obtain high cell densities in preparation for experiments, as described previously (37). Cells expressing PTEN-GFP, mCherry-Myosin II, LimEΔcoil-RFP, PHCRAC-RFP, cAR1-GFP, CynA-Flag, or various CynA-GFP constructs were generated by electroporation of the appropriate plasmid and were selected and maintained in HL5 media containing 20 µg/mL G418, 30–50 µg/mL Hygromycin, or both, as needed.
To induce differentiation, cells growing exponentially in HL5 medium were washed twice in development buffer (DB: 5 mM Na2HPO4, 5 mM KH2PO4, 2 mM MgSO4, and 0.2 mM CaCl2) and starved in suspension in DB at 2 × 107 cells/mL for 1 h. Cells were then pulsed with 50 nM cAMP every 6 min for 3–5 h, as described previously (37). Alternatively, the cells shown in Fig. 4 A–D were differentiated on nonnutrient agar (see below). For all differentiation assays, the differentiation state was assessed for each cell line by lysing 1 × 108 cells/mL in SDS sample buffer and Western blotting for endogenous cAR1 protein (see below), as well as seeding 3.5 × 106 cells in six-well tissue-culture plates filled with DB and monitoring for streaming and aggregation behaviors as described previously (37). Differentiation and all imaging experiments were conducted at room temperature. For experiments using pharmacological inhibitors, either Latrunculin A or LY294002 was diluted in DB from a 1-mM stock solution in DMSO.
Gene Disruption.
To generate CynA-null cells, the CynA coding region between +31 and +3,915, in which +1 is the first and +3,945 is the last nucleotide of the ORF, was replaced by homologous recombination with a Blasticidin S-resistance (BSR) cassette. To generate this knock-out construct, regions of genomic DNA corresponding to the 5′ and 3′ CynA UTRs were amplified by PCR and ligated into the pBS vector, such that they flanked the BSR coding region excised from the pLPBLP plasmid (84). The entire genomic DNA–BSR construct was amplified by PCR or excised from the pBS vector by restriction digest; the resulting linear DNAs were electroporated into AX3.1 wild-type cells, which were then selected with 10 µg/mL Blasticidin S sulfate and grown clonally. Several individual clones from independent electroporations were screened for successful gene disruption by colony PCR, using eight separate primer pairs, and confirmed by Southern blotting using two different enzyme digestions and three different DNA probes. All experiments were conducted using at least two independent cynA- strains.
Plasmid Construction.
For exogenous expression of CynA constructs, the pKF2 plasmid was created for C-terminal tagging with GFP. To create pKF2, the GFP ORF from pEGFP (Clontech) was amplified by PCR using a 5′ primer designed to eliminate the ATG start codon from the GFP sequence, incorporate a flexible polylinker (GSSGG) to connect the sequence of interest to GFP, and generate a BglII/XhoI cloning site. Furthermore, the 3′ PCR primer was designed to introduce a NotI site following the GFP stop codon to allow for possible excision of the GFP sequence if needed. The resulting GFP PCR product was digested with BglII and BamHI and cloned into the BglII site of pJK1, such that the pKF2 plasmid has only one remaining BglII site for cloning purposes (85).
To generate full-length CynA-GFP, Dictyostelium discoideum chromosome 4 coordinates 1851676–1855617 were amplified by PCR from genomic DNA and cloned into the BglII and XhoI sites of pKF2, described above. For truncation studies, N- and C-terminal fragments of CynA were amplified by PCR, using CynA-GFP as a template, and cloned into the BglII and XhoI sites of pKF2. To generate CynA-GFP that was tagged N terminally with either cAR1 or the myristoylation motif of PKBR1, both full-length and fragments of CynA were amplified by PCR from CynA-GFP using a 5′ primer that removed the ATG start codon from the CynA ORF and incorporated an NheI site and flexible polylinker (GSGGSG) before the CynA sequence. This PCR product was cloned into the BglII and XhoI sites of KF2. Next, either full-length cAR1, minus the stop codon, or the region corresponding to amino acids 1–128 of PKBR1 was amplified by PCR and cloned into the BglII and NheI sites of the CynA-containing pKF2 plasmid. CynA-Flag was generated by PCR, using CynA-GFP as a template and a 3′ primer that incorporated the Flag sequence. The resulting product was subsequently cloned into both pKF2 and pDRHyg for alternative drug selection markers.
Microscopy.
To image fluorescent protein localizations in growing, undifferentiated cells, cells were plated on eight-well chambered coverglasses (Lab-Tek, Thermo Scientific, Nunc) and allowed to adhere for a minimum of 10 min. The HL5 growth media was aspirated from the chambers and DB was added to reduce the autofluorescence associated with HL5 and the photosensitivity of growing cells. Cells were imaged within an hour of DB addition to ensure that they remained in the undifferentiated state. To image cytokinesis or random migration in undifferentiated cells, as in Fig. 1 E and G, the cells were grown for several hours or overnight in chambered coverglasses and imaged either in HL5 media or washed into low-fluorescence media before imaging (86). Cells were imaged with a 40×/1.3 oil objective on a Zeiss Observer.Z1 inverted microscope. Time-lapse images were acquired with DIC, GFP, and RFP (where applicable) illumination at 3- to 30-s intervals, as indicated in the figure legends, using AxioVision software. Alternatively, confocal imaging was performed using a 40×/0.3 oil objective on a Leica DMI6000 inverted microscope equipped with a Yokogawa CSU10 spinning disk. Time-lapse images were acquired with brightfield, GFP, and RFP (where applicable) illumination at 5- to 20-s intervals, as indicated in the figure legends, using Slidebook or MetaMorph software.
To image randomly migrating polarized cells, cells were first differentiated by starvation with periodic pulses of cAMP as described above. Differentiated cells were then diluted in DB to ∼2.5 × 105 cells/mL, and 500 µL of this dilution was seeded into eight-well chambered coverglasses (Lab-Tek, Thermo Scientific, Nunc) and allowed to adhere for a minimum of 5–10 min before imaging. Cells were imaged with a 40×/1.3 oil objective on a Zeiss Observer.Z1 inverted microscope. Time-lapse images were acquired with DIC, GFP, and RFP (where applicable) illumination at 3- to 30-s intervals, as indicated in the figure legends, using AxioVision software. Cells shown in Figs. 2B and 3I were treated with 0.5 µM or 5 µM Latrunculin A, respectively, after two frames were acquired. Cells shown in Fig. 3C were pretreated with 1 µM Latrunculin A for 5–10 min before images were acquired with brightfield and GFP illumination using Slidebook software and a 40×/0.3 oil objective on a Leica DMI6000 inverted microscope equipped with a Yokogawa CSU10 spinning disk. Alternatively, cells were imaged at 5-s intervals with a 40×/1.3 oil objective on an Olympus IX81 inverted microscope using DIC, GFP, and RFP illumination and Slidebook software.
Global Stimulation Assays.
Stimulation assays using a uniform concentration of chemoattractant were performed as described previously (37). Cells were differentiated for 4 h with cAMP pulses in DB as above, diluted in DB to ∼2 × 105 cells/mL, plated on replicate eight-well chambered coverglasses (Lab-Tek, Thermo Scientific, Nunc), and allowed to adhere for a minimum of 5 min. Time-lapse images were acquired using DIC, GFP, and RFP (where applicable) illumination at 5-s intervals for 2.5 min using a Zeiss Observer.Z1 inverted microscope equipped with a 40×/1.3 oil objective and AxioVision software. Alternatively, confocal time-lapse images were acquired with brightfield, GFP, and RFP (where applicable) illumination at 5-s intervals for 2.5 min using Slidebook or MetaMorph software and a 40×/0.3 oil objective on a Leica DMI6000 inverted microscope equipped with a Yokogawa CSU10 spinning disk. For all stimulation assays, 1 µM cAMP was added to the cells after two frames were acquired; on average, six independent wells were stimulated per cell line per experiment. Cells shown in Fig. 2C and Fig. S2C were pretreated with 1 µM Latrunculin A for 5–10 min before cAMP addition, although similar results were obtained with 5 µM and 10 µM Latrunculin A.
Micropipette Assays.
Chemotaxis assays using a micropipette were performed as described previously (37). Cells were differentiated with cAMP pulses in DB as above, diluted in DB to ∼2 × 105 cells/mL, plated in small drops on chambered coverglasses (Lab-Tek, Thermo Scientific, Nunc), allowed to adhere for a minimum of 5 min, and covered with DB. A micropipette filled with 10 µM cAMP was placed in the middle of the field of cells, and time-lapse images were acquired at 30-s intervals for 20–30 min using a Zeiss Observer.Z1 inverted microscope and AxioVision software. Cells shown in Fig. 5A and Fig. S3B were imaged with phase and GFP (Fig. S3B) illumination using a 20× objective; cells shown in Figs. 1, 3, 5 B and C, 7, and Fig. S3F were imaged with DIC, GFP, and RFP (where applicable) illumination using a 40×/1.3 oil objective. Chemotactic and motility speeds, chemotactic index, and directional persistence were measured using the Chemotaxis Quantification software as described previously, and statistical analysis was performed using an unpaired two-tailed Student’s t test (P ≤ 0.05 were considered significant) (35). Cells shown in Fig. S3B were treated with 50 µM LY294002 or a corresponding concentration of DMSO for 50 min before imaging. Alternatively, images in Fig. S5C were acquired at 10-s intervals for 10 min by brightfield and GFP illumination using a Zeiss Axiovert 100 inverted microscope equipped with a 40×/1.3 oil objective and IPLab software. The micropipette was relocated between frame acquisitions as indicated in the figure.
FRAP.
Cells were differentiated with cAMP pulses in DB as above for a total starvation time of 4.5 h, seeded into chambered coverglasses (Lab-Tek, Thermo Scientific, Nunc), and allowed to migrate randomly. For Fig. 2F, cells were pretreated for 5 min with 1 µM Latrunculin A or a corresponding concentration of DMSO. Images were acquired by laser scanning confocal microscopy using a Zeiss Axiovert 200 inverted microscope equipped with an LSM510-Meta confocal module and a 63×/1.4 oil objective using excitation with a 488-nm argon laser and Zen software. A total of 150 images were acquired with 2.5% laser power, and the region of interest was photobleached by 20 iterations of 100% laser power after the fourth scan. The time interval between scans varied from 25 to 500 ms; cells shown in Fig. 2 E and F were imaged at 500-ms and 200-ms time intervals, respectively. The cell shown in Fig. S3D was imaged at 250-ms intervals. Growing, undifferentiated cells were also assessed by FRAP; these cells were cultured on chambered coverglasses for several hours, then washed into low-fluorescence media for imaging (86).
Differentiation on Nonnutrient Agar.
For differentiation on nonnutrient agar, cells growing in HL5 media were washed with DB and plated at 5.25 × 105 cells/cm2 on freshly solidified DB agar in six-well plates (37). Several replicate plates were made for each cell line. Each hour after starvation was induced, the cells on one set of the replicate plates were imaged using a dissection scope before being washed off of the agar with DB and collected for further analysis. For microscopy, 1.5 × 105 cells in DB were seeded into eight-well chambered coverglasses (Lab-Tek, Thermo Scientific, Nunc) and allowed to adhere for 10 min before imaging with phase illumination on a Zeiss Axiovert 200 inverted microscope equipped with an automated stage and a 10× air objective. Multipoint time-lapse images were acquired simultaneously for all cell lines at 30-s intervals for 30 min for three different fields of cells per cell line per experiment using Volocity software. The remaining cells collected from nonnutrient agar were analyzed by immunoblotting with an anti-cAR1 antibody, as described below.
Image Analysis.
All images were processed using ImageJ (National Institutes of Health).
To quantify the translocation of proteins upon cAMP stimulation as shown in Fig. 2D and Fig. S2D, the mean membrane and cytosolic fluorescence intensities were measured using ImageJ for each frame of the acquired time-lapse movies. To correct for the cytosolic background component of the membrane intensity values, half of the mean cytosolic intensity value was subtracted from the mean membrane intensity value; this was normalized to the mean cytosolic intensity for each cell. The plots shown represent the mean ± SD of several cells as indicated by the figure legends.
For the plots shown in Fig. 2 G and H, FRAP images were first aligned using the TurboReg plugin for ImageJ (87). Next, ImageJ was used to measure the mean fluroscence intensity of both the photobleached region and a nonphotobleached region of equal size for each frame and, after background subtraction, the ratio of intensities (the bleached region to the nonbleached region) was calculated and normalized such that the mean of the prebleach time points was set to one.
To quantify the polarity defects of cynA- cells for Fig. 4C and Fig. S4B, ImageJ was used to measure the morphology of cells from the last frame of acquired time-lapse images. First, cells were segmented by defining the cell edges (Find Edges), converting the image to binary (Make Binary), and smoothing the cell boundaries (Close-, Fill Holes). Next, the shape parameters of circularity, aspect ratio, and roundness were measured [Set Measurements: Shape descriptors; Analyze Particles: Size (pixel2) = 200-infinity, Show outlines, Include holes, Exclude on edges]. Circularity is defined as 4π × area/perimeter2, such that a perfect circle has a value of one and values closer to zero have a more elongated shape. Aspect ratio is defined as the length of the major axis divided by the length of the minor axis; axis lengths are determined by the best-fitting ellipse. Roundness is defined as 4 × area/(π × major_axis2), where the length of the major axis is determined by the best-fitting ellipse. The shape parameters of the most highly polarized cells were not measured because the cells failed to segment properly due to aggregation. For Fig. 4C, the resulting data were plotted as mean ± SEM as described in the figure legend, and statistical analysis was performed using an unpaired two-tailed Student’s t test (P ≤ 0.01 was considered significant). For the histograms shown in Fig. S4B, the data were binned using intervals of 0.05 for circularity and roundness or 0.1 for aspect ratio, and the number of cells falling within each binned range was plotted as a function of the total number of cells within the sample population. Fig. S4B also depicts the data as the cumulative percentage of cells falling within each binned range using the same intervals described for the histograms.
The LimEΔcoil-RFP tracks shown in Fig. 5C were generated from the RFP fluorescence intensities of the cells shown in Fig. 5B using ImageJ (Stacks: Z Project: Projection type = Max Intensity), such that each pixel shown in the image of the tracks corresponds to the maximum pixel intensity for that location over the course of the movie. The first 5 min of each movie, during which the cells were turning to orient toward the cAMP gradient, were excluded from analysis for simplification.
Quantification of Cell Tracks.
Cells were imaged during migration for 30 min at 30-s intervals. Cell centroids, denoted by zi = (xi,yi), i = 1,…,n, (n is the number of frames) were manually identified from these images and recorded over time using a custom-designed Matlab (Mathworks) script file. From these positions, we computed the displacement between frames: Δzi = zi+1 − zi = (Δxi, Δyi), and the associated angles: θi = arctan(Δyi/Δxi), using Matlab’s atan2d function (which makes the appropriate sign correction to account for the possibility of this movement being in any of the four quadrants). For the quantitation of randomly migrating cells shown in Fig. 4F, we used these values to calculate the total distance traveled: D = (Σ|Δzi|2)1/2, and the persistence: P = Σ|Δzi|cos(θi)/ Σ|Δzi|. The cell speed is obtained by dividing distance traveled by the duration of the cell track. For Fig. 4F, the resulting data were plotted as mean ± SEM as described in the figure legend, and statistical analyses of CynA cell lines vs. wild-type were performed using an unpaired two-tailed Student’s t test (P ≤ 0.05 with a Bonferroni correction was considered significant). The tracks plotted for these cells in Fig. 4E show the Δzi for each cell as a function of time, assuming that all cells start at the same point.
Image Processing for Kymographs.
Cells were segmented by a multistep process using commands from the Image Processing Toolbox in Matlab, v.2013a (MathWorks). When multiple fluorescent channels were available, the fluorescence intensities from the separate channels were summed to create a total intensity. This was particularly useful when the two markers were found preferentially at different regions (front vs. rear) of the cell. At each frame, the resultant intensities were first converted to a binary image using Otsu’s method (Matlab’s graythresh command). Following morphological closing (command: imclose), an active contour based on the Chan–Vese algorithm was used to refine the segmentation (command: activecontour). This was followed by morphological erosion (command: imerode). This process resulted in a set of pixels Bj = {zi}, i = 1,…,nj, on frame j. In practice, these pixels were not always aligned with the regions of high fluorescence. To ensure that we captured the fluorescence around the edge, at each pixel zi we computed the least-squares tangent line through pixels zi−3,… zi+3. We then computed a straight-line normal to the edge of length seven, centered at zi. We associated the highest fluorescence intensity among those of these seven pixels with boundary pixels zi. This process was carried out for each frame. This fluorescence value was also adjusted to account for photobleaching by computing the average fluorescence intensity of the corresponding channel in a region inside the cell. This region was selected by further erosion (seven pixels) of the boundary.
To create the kymographs required that we align two different boundary points Bj and Bj+1 that, in general, were of different lengths (nj and nj+1, respectively). This was done by finding the alignment that minimized the Euclidean distance between the two sets of boundary pixels: that is, finding . The boundaries were then rearranged so that a manually selected point, corresponding to the center of the rear fraction of the cell boundary, in the first frame appeared in the center of the kymograph. Subsequent frames were rearranged so that they were centered with the initial frame. Finally, the intensity of each channel was adjusted so that the lowest and highest intensities over the complete kymograph were 0 and 255, respectively.
For the quantification shown in Fig. 6E, raw fluorescence intensity values of the cell boundary were taken from the frame during which the maximal chemoattractant response occurred (∼7 s after stimulus was added), adjusted such that the mean of the five brightest pixels for each cell was 255, aligned such that the center of each cell rear was designated as 0°, and averaged across cells. Statistical analyses were performed using unpaired two-tailed Student’s t tests to compare the RFP intensity values at the rear vs. nonrear regions of the cell boundaries, as well as intensity values at the cell rear of null vs. overexpressing cells (P < 0.01 was considered significant). For the quantification shown in Fig. S2B, fluorescence intensity values of the cell boundary were normalized to the mean cytosolic fluorescence intensity for each time point; the resulting values for the first two time points, before the addition of cAMP, were averaged and set to one.
Immunoblotting.
Cells were collected from nonnutrient agar as described above, pelleted by centrifugation, and resuspended in SDS sample buffer to a final concentration of 1 × 108 cells/mL. Electrophoresis was performed using 4–15% Tris⋅HCl polyacrylamide gels (Criterion, Bio-Rad), and proteins were then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% (wt/vol) skim milk in TBST [Tris-buffered saline/0.1% (vol/vol) Tween 20] and incubated with an anti-cAR1 1° antibody in 5% (wt/vol) BSA in TBST. Membranes were then washed with TBST, incubated with an HRP-conjugated anti-rabbit 2° antibody (GE Healthcare) at a 1:10,000 dilution in 5% (wt/vol) skim milk in TBST, and washed in TBST before the signal was detected by chemiluminescence using ECL Western blotting detection reagent (GE Healthcare).
Lipid Strips.
Cells expressing CynA-GFP, PHCRAC-GFP, Crac-GFP, cytosolic GFP, or an empty vector control were washed and resuspended in DB at 2 × 107 cells/mL, starved without cAMP pulses for 3 h, washed twice in ice cold 10 mM sodium phosphate buffer (pH 7.2), resuspended in 10 mM sodium phosphate buffer (pH 7.2) with added protease inhibitor mixture (cOmplete tablets, Roche) at 5 × 107 cells/mL, and placed on ice. Meanwhile, PIP strips (Echelon) were blocked with 3% (wt/vol) fatty-acid–free BSA in PBST [PBS/0.1% (vol/vol) Tween 20]. Cells were then filter-lysed using a syringe fitted with Whatman filter holders (#420200) and double 5-µm pore filters (Whatman, #110613); samples were collected for Western blotting, and the remaining lysate was centrifuged for 10 min at 10,000 × g and 4 °C. Samples of the resulting supernatant and pellet fractions were collected for Western blotting, and the remaining supernatant was added to an equal volume of 2× Binding Buffer [300 mM NaCl, 10 mM sodium phosphate buffer (pH 7.2), 0.5% (vol/vol) Nonidet P-40] and incubated with the preblocked PIP strips at 4 °C for 3 h. PIP strips were also incubated with Binding Buffer alone as a control. After incubation with cell supernatants, a sample of the supernatant was collected for Western blotting, and the PIP strips were washed with PBST before incubation with an anti-GFP 1° antibody (mouse, monoclonal; Roche) at a 1:1,000 dilution in 3% (wt/vol) fatty-acid-free BSA in PBST. The PIP strips were then washed with PBST, incubated with an HRP-conjugated anti-mouse 2° antibody (GE Healthcare) at a 1:10,000 dilution in 3% (wt/vol) BSA in PBST, and washed in PBST before the signal was detected by chemiluminescence using ECL Western blotting detection reagent (GE Healthcare).
Supplementary Material
Acknowledgments
We thank Dr. M. N. Teruel for providing information in advance of publication; Dr. C. Wolberger for assistance with protein domain annotation; Dr. D. N. Robinson for providing myo II- cells and the mCherry-Myosin II construct, as well as for helpful discussions; B. Diplas and Y. Long for generating DNA constructs used in these studies; members of the Johns Hopkins School of Medicine Microscope Core Facility for assistance with microscopy; Drs. M. Iijima, G. Seydoux, and T. Inoue for advice and suggestions; and Drs. Y. Artemenko, H. Cai, Y. Kamimura, and other members of the P.N.D. and Robinson laboratories for helpful suggestions, sharing reagents, and technical advice. This work was supported by National Institutes of Health Grants GM28007 and GM34933 (to P.N.D.), and American Heart Association Fellowship 09PRE2300057 (to K.F.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509098112/-/DCSupplemental.
References
- 1.Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: A network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys. 2010;39:265–289. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151(6):1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114(2):215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 4.Servant G, et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287(5455):1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112(1):63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 6.Watton SJ, Downward J. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol. 1999;9(8):433–436. doi: 10.1016/s0960-9822(99)80192-4. [DOI] [PubMed] [Google Scholar]
- 7.Dormann D, Weijer G, Dowler S, Weijer CJ. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci. 2004;117(Pt 26):6497–6509. doi: 10.1242/jcs.01579. [DOI] [PubMed] [Google Scholar]
- 8.Gassama-Diagne A, et al. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8(9):963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- 9.Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev Cell. 2005;8(4):467–477. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Belmonte F, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128(2):383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moores SL, Sabry JH, Spudich JA. Myosin dynamics in live Dictyostelium cells. Proc Natl Acad Sci USA. 1996;93(1):443–446. doi: 10.1073/pnas.93.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinal N, et al. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16(2):140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 13.von Stein W, Ramrath A, Grimm A, Müller-Borg M, Wodarz A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development. 2005;132(7):1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Nørrelykke SF, Cox EC. Persistent cell motion in the absence of external signals: A search strategy for eukaryotic cells. PLoS ONE. 2008;3(5):e2093. doi: 10.1371/journal.pone.0002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoge M, et al. Cellular memory in eukaryotic chemotaxis. Proc Natl Acad Sci USA. 2014;111(40):14448–14453. doi: 10.1073/pnas.1412197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435(7038):43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemmon MA. Pleckstrin homology domains: Not just for phosphoinositides. Biochem Soc Trans. 2004;32(Pt 5):707–711. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 18.Park WS, et al. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol Cell. 2008;30(3):381–392. doi: 10.1016/j.molcel.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YE, et al. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol Biol Cell. 2003;14(5):1913–1922. doi: 10.1091/mbc.E02-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yumura S, Mori H, Fukui Y. Localization of actin and myosin for the study of ameboid movement in Dictyostelium using improved immunofluorescence. J Cell Biol. 1984;99(3):894–899. doi: 10.1083/jcb.99.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung CY, Firtel RA. PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J Cell Biol. 1999;147(3):559–576. doi: 10.1083/jcb.147.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faix J, et al. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 2001;20(14):3705–3715. doi: 10.1093/emboj/20.14.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller-Taubenberger A, et al. Differential localization of the Dictyostelium kinase DPAKa during cytokinesis and cell migration. J Muscle Res Cell Motil. 2002;23(7-8):751–763. doi: 10.1023/a:1024475628061. [DOI] [PubMed] [Google Scholar]
- 24.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109(5):599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 25.Bosgraaf L, et al. RasGEF-containing proteins GbpC and GbpD have differential effects on cell polarity and chemotaxis in Dictyostelium. J Cell Sci. 2005;118(Pt 9):1899–1910. doi: 10.1242/jcs.02317. [DOI] [PubMed] [Google Scholar]
- 26.Cai H, et al. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J Cell Biol. 2010;190(2):233–245. doi: 10.1083/jcb.201001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meili R, et al. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18(8):2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95(1):81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167(3):505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yumura S. Reorganization of actin and myosin II in Dictyostelium amoeba during stimulation by cAMP. Cell Struct Funct. 1993;18(6):379–388. doi: 10.1247/csf.18.379. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Jiang F, Zhuo Z, Wu XH, Wu YD. A method for WD40 repeat detection and secondary structure prediction. PLoS ONE. 2013;8(6):e65705. doi: 10.1371/journal.pone.0065705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Källberg M, et al. Template-based protein structure modeling using the RaptorX web server. Nat Protoc. 2012;7(8):1511–1522. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cullinane AR, Schäffer AA, Huizing M. The BEACH is hot: A LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013;14(7):749–766. doi: 10.1111/tra.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loovers HM, et al. Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: A quantitative in vivo analysis by inhibition of PI3-kinase. Mol Biol Cell. 2006;17(4):1503–1513. doi: 10.1091/mbc.E05-09-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamimura Y, et al. PIP3-independent activation of TorC2 and PKB at the cell’s leading edge mediates chemotaxis. Curr Biol. 2008;18(14):1034–1043. doi: 10.1016/j.cub.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parent CA, Devreotes PN. Molecular genetics of signal transduction in Dictyostelium. Annu Rev Biochem. 1996;65:411–440. doi: 10.1146/annurev.bi.65.070196.002211. [DOI] [PubMed] [Google Scholar]
- 37.Artemenko Y, Swaney KF, Devreotes PN. Assessment of development and chemotaxis in Dictyostelium discoideum mutants. Methods Mol Biol. 2011;769:287–309. doi: 10.1007/978-1-61779-207-6_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devreotes PN. G protein-linked signaling pathways control the developmental program of Dictyostelium. Neuron. 1994;12(2):235–241. doi: 10.1016/0896-6273(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 39.Heid PJ, Geiger J, Wessels D, Voss E, Soll DR. Computer-assisted analysis of filopod formation and the role of myosin II heavy chain phosphorylation in Dictyostelium. J Cell Sci. 2005;118(Pt 10):2225–2237. doi: 10.1242/jcs.02342. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Shen Z, Robinson DN, Briggs S, Firtel RA. Involvement of the cytoskeleton in controlling leading-edge function during chemotaxis. Mol Biol Cell. 2010;21(11):1810–1824. doi: 10.1091/mbc.E10-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lombardi ML, Knecht DA, Dembo M, Lee J. Traction force microscopy in Dictyostelium reveals distinct roles for myosin II motor and actin-crosslinking activity in polarized cell movement. J Cell Sci. 2007;120(Pt 9):1624–1634. doi: 10.1242/jcs.002527. [DOI] [PubMed] [Google Scholar]
- 42.Wessels D, et al. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988;128(1):164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119(Pt 18):3833–3844. doi: 10.1242/jcs.03152. [DOI] [PubMed] [Google Scholar]
- 44.Bretschneider T, et al. Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr Biol. 2004;14(1):1–10. doi: 10.1016/j.cub.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Z, Zhang N, Murphy DB, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139(2):365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem. 2004;279(16):16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- 47.Rahdar M, et al. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA. 2009;106(2):480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth AM, et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang Y, et al. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5(6):e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem. 2003;278(52):52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- 51.Shen B, Fang Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J Biol Chem. 2011;286(51):44162–44176. doi: 10.1074/jbc.M111.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumann T, Affentranger S, Niggli V. Evidence for chemokine-mediated coalescence of preformed flotillin hetero-oligomers in human T-cells. J Biol Chem. 2012;287(47):39664–39672. doi: 10.1074/jbc.M112.412742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludwig A, et al. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol. 2010;191(4):771–781. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann-Giesen C, et al. Membrane and raft association of reggie-1/flotillin-2: Role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem J. 2004;378(Pt 2):509–518. doi: 10.1042/BJ20031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossy J, Schlicht D, Engelhardt B, Niggli V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS ONE. 2009;4(4):e5403. doi: 10.1371/journal.pone.0005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solis GP, et al. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403(2):313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima A, Ishihara S, Imoto D, Sawai S. Rectified directional sensing in long-range cell migration. Nat Commun. 2014;5:5367. doi: 10.1038/ncomms6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang MJ, Artemenko Y, Cai WJ, Iglesias PA, Devreotes PN. The directional response of chemotactic cells depends on a balance between cytoskeletal architecture and the external gradient. Cell Reports. 2014;9(3):1110–1121. doi: 10.1016/j.celrep.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kriebel PW, Barr VA, Parent CA. Adenylyl cyclase localization regulates streaming during chemotaxis. Cell. 2003;112(4):549–560. doi: 10.1016/s0092-8674(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 61.Parikh A, et al. Conserved developmental transcriptomes in evolutionarily divergent species. Genome Biol. 2010;11(3):R35. doi: 10.1186/gb-2010-11-3-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rot G, et al. dictyExpress: A Dictyostelium discoideum gene expression database with an explorative data analysis web-based interface. BMC Bioinformatics. 2009;10:265. doi: 10.1186/1471-2105-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, et al. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell. 2003;14(12):5028–5037. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia GL, Parent CA. Signal relay during chemotaxis. J Microsc. 2008;231(3):529–534. doi: 10.1111/j.1365-2818.2008.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butty AC, et al. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 2002;21(7):1565–1576. doi: 10.1093/emboj/21.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16(4):339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Cheng PL, Poo MM. Early events in axon/dendrite polarization. Annu Rev Neurosci. 2012;35:181–201. doi: 10.1146/annurev-neuro-061010-113618. [DOI] [PubMed] [Google Scholar]
- 68.Inoue T, Meyer T. Synthetic activation of endogenous PI3K and Rac identifies an AND-gate switch for cell polarization and migration. PLoS ONE. 2008;3(8):e3068. doi: 10.1371/journal.pone.0003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kozubowski L, et al. Symmetry-breaking polarization driven by a Cdc42p GEF-PAK complex. Curr Biol. 2008;18(22):1719–1726. doi: 10.1016/j.cub.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meinhardt H. Orientation of chemotactic cells and growth cones: Models and mechanisms. J Cell Sci. 1999;112(Pt 17):2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 71.Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. BioEssays. 2000;22(8):753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 72.Srinivasan S, et al. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160(3):375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb Perspect Biol. 2009;1(4):a002980. doi: 10.1101/cshperspect.a002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299(5610):1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 75.Weiner OD, et al. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4(7):509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu CF, Lew DJ. Beyond symmetry-breaking: Competition and negative feedback in GTPase regulation. Trends Cell Biol. 2013;23(10):476–483. doi: 10.1016/j.tcb.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114(2):201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 78.Cramer LP. Forming the cell rear first: Breaking cell symmetry to trigger directed cell migration. Nat Cell Biol. 2010;12(7):628–632. doi: 10.1038/ncb0710-628. [DOI] [PubMed] [Google Scholar]
- 79.Mseka T, Cramer LP. Actin depolymerization-based force retracts the cell rear in polarizing and migrating cells. Curr Biol. 2011;21(24):2085–2091. doi: 10.1016/j.cub.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9(1):11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- 81.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183(3):543–554. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yam PT, et al. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J Cell Biol. 2007;178(7):1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruppel KM, Uyeda TQ, Spudich JA. Role of highly conserved lysine 130 of myosin motor domain. In vivo and in vitro characterization of site specifically mutated myosin. J Biol Chem. 1994;269(29):18773–18780. [PubMed] [Google Scholar]
- 84.Faix J, Kreppel L, Shaulsky G, Schleicher M, Kimmel AR. A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 2004;32(19):e143. doi: 10.1093/nar/gnh136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pitt GS, et al. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell. 1992;69(2):305–315. doi: 10.1016/0092-8674(92)90411-5. [DOI] [PubMed] [Google Scholar]
- 86.Nagasaki A, de Hostos EL, Uyeda TQ. Genetic and morphological evidence for two parallel pathways of cell-cycle-coupled cytokinesis in Dictyostelium. J Cell Sci. 2002;115(Pt 10):2241–2251. doi: 10.1242/jcs.115.10.2241. [DOI] [PubMed] [Google Scholar]
- 87.Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7(1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.