Significance
Myosins are cellular motors that promote muscle contraction by converting chemical energy into mechanical force. The myosin molecule self-assembles through its coiled-coil rod domain into the highly ordered thick filaments of the sarcomeres, which represent the basic contractile unit of the muscle. Because there is limited information about the mechanisms of filament formation, and mutations in the rod domain cause muscle disease, we investigated the molecular properties and function of four regions of the rod containing an extra amino acid (skip residue) predicted to alter the regular organization of the coiled-coil. To our knowledge, this is the first study reporting that these regions fold into specialized structures engaged in promoting proper myosin assembly into the thick filaments.
Keywords: myosin, cardiac/skeletal myopathies, molecular dynamics, coiled-coils, protein structure
Abstract
The rod of sarcomeric myosins directs thick filament assembly and is characterized by the insertion of four skip residues that introduce discontinuities in the coiled-coil heptad repeats. We report here that the regions surrounding the first three skip residues share high structural similarity despite their low sequence homology. Near each of these skip residues, the coiled-coil transitions to a nonclose-packed structure inducing local relaxation of the superhelical pitch. Moreover, molecular dynamics suggest that these distorted regions can assume different conformationally stable states. In contrast, the last skip residue region constitutes a true molecular hinge, providing C-terminal rod flexibility. Assembly of myosin with mutated skip residues in cardiomyocytes shows that the functional importance of each skip residue is associated with rod position and reveals the unique role of the molecular hinge in promoting myosin antiparallel packing. By defining the biophysical properties of the rod, the structures and molecular dynamic calculations presented here provide insight into thick filament formation, and highlight the structural differences occurring between the coiled-coils of myosin and the stereotypical tropomyosin. In addition to extending our knowledge into the conformational and biological properties of coiled-coil discontinuities, the molecular characterization of the four myosin skip residues also provides a guide to modeling the effects of rod mutations causing cardiac and skeletal myopathies.
Muscle contraction is primarily driven by the interactions between actin and myosin and the associated ATP hydrolysis, but the long-range transmission of force is based on the intrinsic ability of both proteins to self-assemble into organized filaments. The myosin thick filament is a well-characterized bipolar structure. The central area, or bare zone, is ∼160-nm wide and is structurally defined by the packing of antiparallel myosin molecules cross-linked at the sarcomeric M-band by scaffold proteins (1, 2). On either side of the bare zone, parallel arrays of staggered myosin molecules assemble into the characteristic A-band that is ∼1.6 μm in length and is centered between two Z-lines, where actin filaments are cross-linked at the Z-disk (3, 4).
The motor activity of myosin resides in the globular N-terminal region or subfragment 1, whereas the remainder of the molecule forms an extended dimeric α-helical coiled-coil. This rod-like section can be divided into two parts: subfragment-2, which allows the motors to extend away from the thick filament, and light meromyosin (LMM), which promotes both parallel and antiparallel myosin filament formation (5–7).
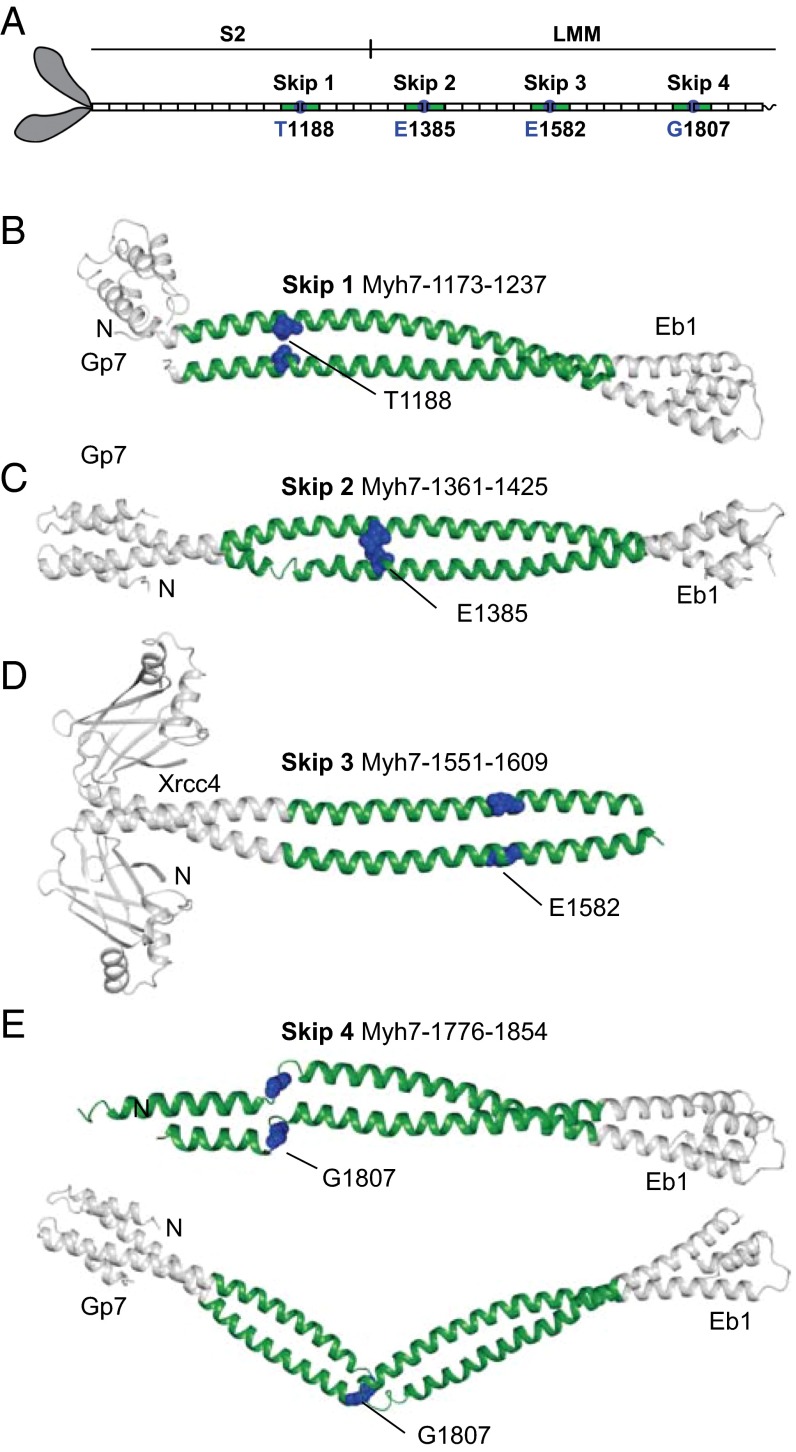
The sequence of the myosin rod shows the classic seven residue heptad repeat that is considered the hallmark of coiled-coil structures. However, it is also characterized by a remarkable dipolar charge profile, repeated every 28 amino acid residues, that is predicted to assist the staggered assembly of adjacent rods in the thick filament (5). In sarcomeric myosins, the cyclic pattern of 38 dipolar charge repeats is interrupted by four widely spaced extra amino acids, called skip residues (Fig. 1A). These residues are by convention located at the end of different 28-amino acid repeats following position c of the heptad motif (5). Insertion of a single residue (or deletion of six residues) introduces a discontinuity in the phasing of the heptad repeats that results in deformation of the α-helical coiled-coil. Such skip residues and stutters (deletions of three residues) are predicted to introduce regions of flexibility in the coiled-coil by causing local unwinding of the two α-helices; in contrast, stammers (deletions of four residues) are predicted to cause local overwinding of the supercoil (8). Although the number and spacing of the skip residues are conserved across all sarcomeric myosins, both smooth muscle and nonmuscle isoforms, which assemble differently, lack the second skip residue (9).
Fig. 1.
Cartoon of their location in the myosin rod and structures of the four human β-cardiac myosin (MYH7) skip residues. (A) The relative location of the skip residues in the myosin rod which depicts the 38 dipolar charge repeats, each of which is formed by 28 amino acid residues (5). Each fusion protein consists of an N-terminal globular element, either Gp7 or Xrcc4 (white), before a section of MYH7 (green). A C-terminal fusion, Eb1 (white), is also present in all constructs except for Skip 3: Xrcc4-L1551-N1609. Each skip residue is colored in blue and depicted in sphere representation. The N terminus of each construct is indicated. (B) Gp7-K1173-I1238-Eb1 (Skip 1). (C) Gp7-L1361-I1425-Eb1 (Skip 2). (D) Xrcc4-L1551-N1609 (Skip 3). (E) Two crystallographically independent dimers within the asymmetric unit are shown for Gp7-A1777-T1854-Eb1 (Skip 4). Gp7 is disordered in the crystal lattice for the first of the two dimers shown in E.
The role of myosin skip residues has not yet been defined. Early studies have associated their positions with the four rod bends observed by EM on purified molecules (9), and linear modeling of the charge distribution of the rod has suggested that skip residues could play a role in properly staggering adjacent rod molecules (10). Nevertheless, individual deletion of two of the skip residues contained in the LMM does not alter myosin solubility or paracrystal formation in vitro (11).
To our knowledge, we report herein the first structural data, molecular dynamical properties, and role in myosin assembly of the regions encompassing the four skip residues of a sarcomeric cardiac myosin. Our data reveal that the first three skip residues are structurally comparable and induce a unique local relaxation of the coiled-coil superhelical pitch. However, we find that the functional importance of each of the first three skip residues in promoting myosin assembly in vivo is different. Surprisingly, we discovered that the fourth skip residue lies within a highly flexible molecular hinge that is necessary for myosin incorporation in the bare zone of sarcomeres.
Results
Three distinct lines of investigation were followed to gain insight into the role of the four skip residues in myosin filament formation. The 3D structures of the regions surrounding the four myosin skip residues were determined by X-ray crystallography. Molecular dynamics simulations were carried out to reveal the effect of skip residues on the structure and the dynamics of the region. Finally, myosin assembly into sarcomeres in cardiac cells examined the function of the skip residues in a biological context.
The Coiled-Coil Surrounding the First Three Skip Residues Is Locally Unwound.
Sections of the human β-cardiac myosin gene (MYH7; GenBank: M58018.1), ranging from 50 to 70 amino acids roughly centered on each of the four skip residues, were fused at their N termini to globular folding domains, Xrcc4 and Gp7, that have been shown to enhance crystallization propensity and solubility of isolated sections of extended coiled-coils (12, 13). Crystallization and structural determination of three of the structures was obtained only after the addition of the helical bundle domain from microtubule binding protein Eb1 at the C terminus of the myosin fragments to create double-sided fusions (SI Appendix, Fig. S1 and Tables S1 and S2) (13). In general, the stabilization domains might influence the folding, structure, or functional properties of coiled-coil fragments. However, Xrcc4 and Gp7 have been successfully used to determine the structures of overlapping segments of tropomyosin and a component of the yeast spindle pole body (12–14), and GCN4, the stereotypical leucine zipper, has been used extensively for structural and functional studies of segments of coiled-coil proteins (15).
Comparison of the crystal structures reveals that the regions surrounding the first three skip residues share a remarkable degree of structural similarity (Fig. 1). The Cα RMSD between Skip 1 and 2 and 3 are 0.92 Å and 1.1 Å, respectively. The close similarity of the first three structures and the presence of multiple copies in the asymmetric unit for Skip 1, which show an RMSD difference of 0.51 Å, suggest that the structural features described below are a fundamental property of the myosin rod and not an artifact of crystal packing.
The polypeptide chain surrounding T1188 (Skip 1), E1385 (Skip 2), and E1582 (Skip 3) adopts a noncanonical coiled-coil characterized by local unwinding. These regions, which extend ∼17 and 11 residues on the N- and C-terminal sides of the skip residues, respectively, show a substantial increase in the superhelical pitch such that the helices run approximately parallel to each other (16, 17). This effectively changes the regular a – g heptad designation of the residues surrounding each skip residue, as previously proposed (5). As a result, side chains that would be predicted to be solvent exposed by assignment to a regular heptad repeat are now buried along the interface. Furthermore, because the distortion extends over four heptads, it is difficult to attribute the changes in the coiled-coil to the insertion of one particular residue in this region. This unwinding was predicted from the original sequence analysis, although the structural similarity between the first three skip regions was not (5).
The Coiled-Coil Surrounding Skips 1, 2, and 3 Accommodates the Break in the Heptad Repeat Similarly.
Because the structures of Skips 1, 2, and 3 accommodate the insertion of the skip residue in a similar manner, the description of the structures will focus on Skip 3, which was determined to the highest resolution (SI Appendix, Table S2).
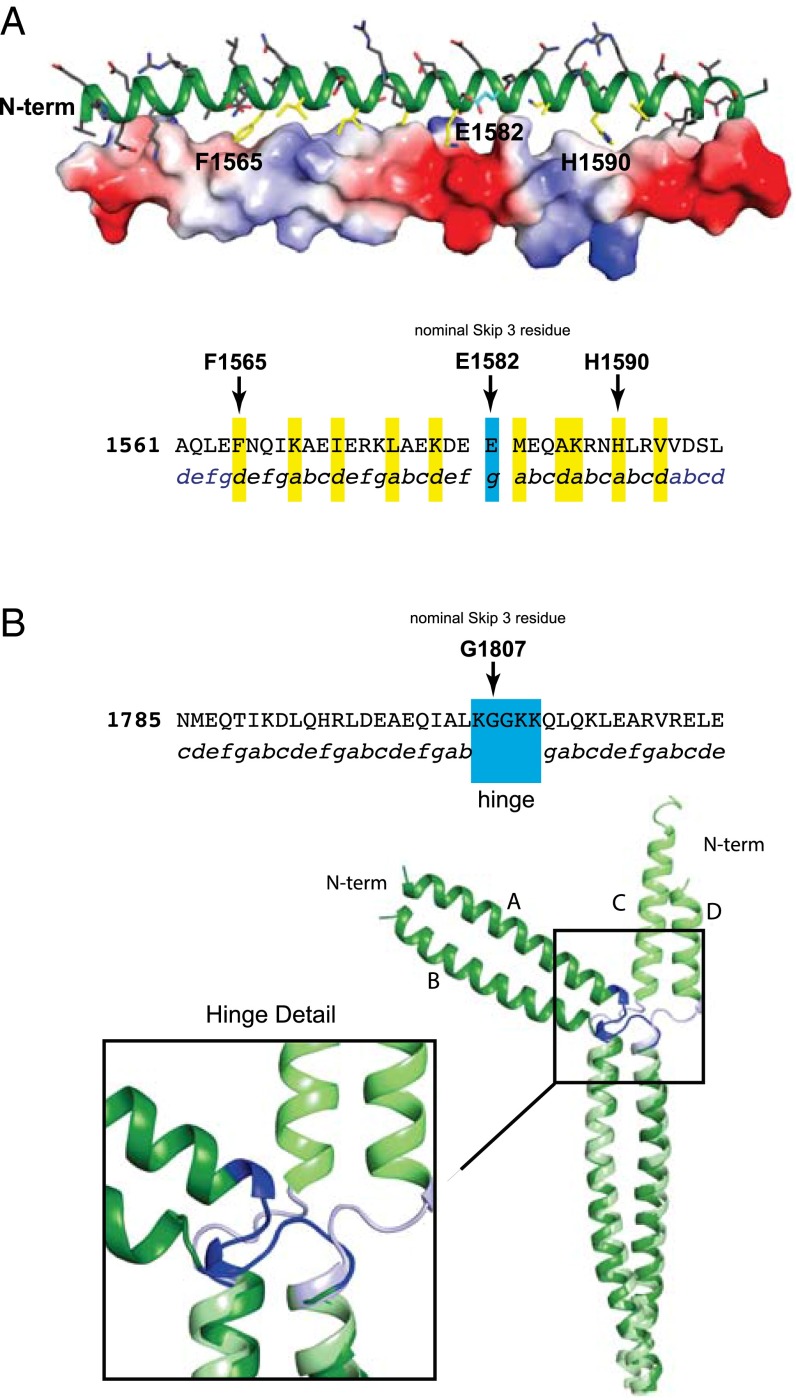
Based on the position of residues around the conventional coiled-coil helical wheel, Skip 3 residue, E1582, is solvent exposed and lies in the g position, which differs from the earlier prediction that it would lie between c and d (Fig. 2A) (5, 18). The approximate heptad designation of the 28 residue repeats either side of Skip 3 based on their helical disposition is shown in Fig. 2A, where the residues that contribute to the hydrophobic core of the helical assembly are designated either a or d according to their observed position in the helical interface.
Fig. 2.
Structural analysis of Skip 3 and Skip 4. (A, Upper) A cartoon representation of the coiled-coil surrounding E1582 (Skip 3) and with a surface electrostatic representation shown on the lower-helix. (Lower) The protein sequence surrounding Skip 3. Packing residues in the nonconical coiled-coil region of the upper-helix are colored in yellow on the upper-helix. The skip residue is colored in cyan. The protein sequence surrounding the nominal skip residue, E1582, with the observed coiled-coil position registry shown below. Residues that are in a standard packing arrangement are in blue and the atypical region is colored black. Residues packing along the distorted interface are highlighted in yellow and the skip residue is in cyan. (B) The sequence and structure of Skip 4. The protein sequence surrounding the nominal Skip 4 residue, G1807, is shown with the coiled-coil position registry below. Residues involved in the hinge region are indicated. A C-terminal structural alignment of residues Q1811–T1854 in chains A and B superimposed on C and D for the two independent molecules in the asymmetric unit for Skip 4 is also shown. This reveals the conformational variability in the Skip 4 hinge. The stabilization and folding domains were omitted from all structural figures.
The transition from a canonical coiled-coil starts at ∼F1565, where the phenyl side chains on opposing polypeptide chains stack against each other in the hydrophobic interface, which necessitates a distortion of the coiled-coil that places the α-carbon in a nominal d position rather than a (Fig. 2A). Thereafter, the helices run approximately parallel to each other with the overall positions of the side chains following a distorted abcdefg repeat until the skip residue, E1582. The 11 residues on the C-terminal side of Skip 3 exhibit greater deviations from the canonical repeat showing an unconventional abcdabcabcd distribution; subsequently, the residues in the coiled-coil return to a conventional repeat. Given the overall structural similarity between Skip 1, 2, and 3, it is not surprising that the distribution of hydrophobic residues that constitute the interface is also conserved among the skip regions.
Interestingly, there are numerous solvent exposed hydrophobic residues surrounding the Skip 3 residue; these include L1559, L1563, I1568, and L1591. Their biological significance is unclear; however, this pattern of hydrophobic exposed residues is highly conserved across species (SI Appendix, Fig. S2). Similarly, as judged by sequence alignment, there is also remarkable similarity in the electrostatic surfaces of Skip 1, 2, and 3 (SI Appendix, Figs. S2 and S3). The existence of exposed hydrophobic side chains makes a priori assignment of residues to an accurate heptad pattern difficult.
The Fourth Skip Residue Forms a Highly Flexible Hinge.
Remarkably, the fourth skip residue, G1807, is contained within a highly flexible hinge that disrupts the heptad repeat in a completely different manner from that observed for the first three skip residues (Fig. 1E). There are two crystallographically independent dimers present within the unit cell where both dimers show ordered electron density for the hinge. Importantly, each dimer adopts a dramatically different conformation for its hinge (Fig. 1E). The key implication from these structures is that the hinge could adopt a set of conformations in vivo that are likely to be different from the ones seen in vitro, because the latter reflect the necessities of crystallographic packing. Although the role of glycine residues as sites of flexibility had not been previously recognized, the hinge encompassing Skip 4 includes two of them, which probably contributes to its function. Glycine residues are uncommon in canonical coiled-coils and are often viewed as helix breakers; however, the myosin rod contains 12 of them in the LMM portion (19).
The hinge, extending from residues 1806–1810 (Fig. 2B), introduces a discontinuity in the coiled-coil; however, the α-helices on either side of the break follow a uniform and normal superhelical pitch. The helical regions on either side of the discontinuity are highly similar in both crystallographically independent dimers, where they align with a RMSD of 0.57 Å and 1.09 Å for the N- and C-terminal regions, respectively (Fig. 2B). As the residues surrounding Skip 4 adopt a canonical coiled-coil, there are some noteworthy features. First, the d and a positions of the heptad repeat preceding Skip 4 are occupied by alanine residues that reduce the hydrophobicity of the dimer interface. Second, the hydrophobic moieties (I1803 and L1805) in the heptad preceding the hinge are solvent-exposed. Finally, two glutamine residues (Q1811 and Q1813) immediately C terminal to the hinge are located such that they may hydrogen bond to exposed carbonyl oxygen atoms resulting from the break in the helicity. The unique nature of this hinge and its importance in bipolar thick filaments is observed in both molecular dynamics simulations and in vivo experiments, as discussed below.
Dynamics of the Coiled-Coil Regions Flanking the Skip Residues.
Because the crystal structure provides only a static view of the myosin rod, the structural dynamics of the coiled-coil surrounding all four skip residues were assessed by molecular dynamics. To achieve simulations on the microsecond time scale, graphical processing units along with implicit solvent models were used (SI Appendix, Fig. S4). Comparative analysis demonstrated that the conformational dynamics are not altered by the addition of the globular folding domains or the use of explicit solvent (SI Appendix, Figs. S4 and S5). Consequently, all simulations were performed solely on sections of myosin coiled-coil containing the skip residues.
The Skip Residue Is an Integral Component of the Coiled-Coil.
The crystal structures show that the designated skip residues are only one residue in substantial regions of distorted coiled-coil. Thus, the question arises whether the skip residues lead to coiled-coil instability or if the surrounding regions have adopted a stable state that accommodates their insertion. To address this question, simulations of the four regions encompassed by the crystallographic studies were performed with and without (ΔS constructs) the presence of each skip residue to probe the role of the skip residue within each structural environment (SI Appendix, Table S3).
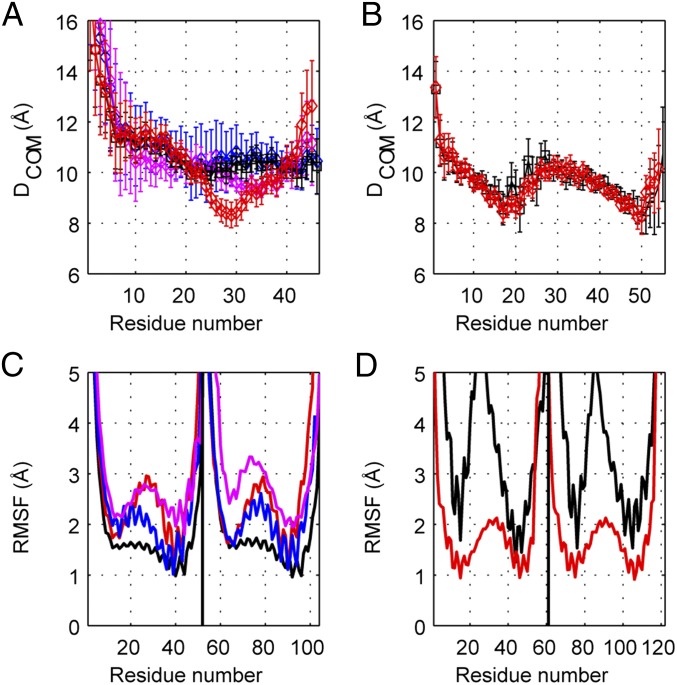
The simulations were analyzed in three ways. At a static level, the effect of removing the skip residues was assessed by examining the average distance between the center of mass of the two helices (DCOM) for the final 500 ns of each simulation, whereas the conformational variability was determined from both the root mean square fluctuations (RMSF) of individual residues and clustering analysis of the models. DCOM was determined as a moving window covering seven consecutive α-carbons to measure the degree of coiling: smaller values of DCOM imply tighter packing of the helices and a lowered super helical pitch, whereas a larger DCOM value indicates a nonclose-packed state and an increased super helical pitch. Analysis of the simulations for Skip 3 residue shows that its removal allows the region to move as a whole toward a canonical coiled-coil, with a decreased super helical pitch (Fig. 3A). This effect is especially evident for the C-terminal side of the skip residue. Skips 1 and 2 follow the same pattern, although to a lesser extent (SI Appendix, Fig. S6). Thus, the residues identified with Skips 1, 2, and 3 are associated with an increase in the helical pitch over a stretch of ∼29 residues. In contrast, deletion of Skip residue 4 has a different effect. The flanking sections of coiled-coil are not significantly disturbed by the absence of G1807, likely because of the fact that in the WT structure this residue resides in a highly flexible loop flanked by canonical coiled-coils (Fig. 3B). The differences between Skip 4 and the other skip residues are also seen in the conformational dynamics.
Fig. 3.
Analysis of the molecular dynamics simulations for Skip 3 and Skip 4. DCOM and RMSF for the regions surrounding Skip 3 (A and C, respectively) and Skip 4 (B and D, respectively). The analyses of the WT simulations are shown in black, the skip residue deletion simulations (ΔS) are depicted in red. The simulation of the recoiled Skip 3 WT sequence (S3-R) in which the distribution of hydrophobic residues matches that expected for a canonical coiled coil, but still includes Skip 3 is shown in pink, whereas the recoiled Skip 3 deletion (ΔS3-R) is depicted in blue. DCOM is the distance between two α-helices calculated from the center of masses of Cα atoms for seven consecutive amino acids and is inversely correlated with the degree of coiling in the simulations of models. Cα-RMSF values are an indication of the degree of flexibility, as well as stability. Regions with higher RMSF values have larger degrees of flexibility. The measurements were averaged over the final 500 ns of each simulation to allow sufficient sampling for relaxation and ensure convergence of ensembles. Residue numbers correspond to L1551-D1602 and K1783-S1843 for Skip 3 and Skip 4, respectively. The recoiled WT sequence is: (LEHEEGKILRAQLEFNQIKAEIERLAAEVDEELEQAVRNHLRVVDSLQTSLD) where Skip 3 is underlined and the mutated residues are shown in bold.
Deletions of Skip 3 and Skip 4 Have Different Effects on the Conformational Dynamics and Structural Stability.
Analysis of the Cα-based RMSF in the simulations with WT and without the Skip residues (ΔS1, ΔS2, ΔS3, and ΔS4) shows that removal of Skip 1, 2, or 3 results in greater fluctuations than the WT (Fig. 3C and SI Appendix, Fig. S6). This finding implies that deletion of these residues would lead to enhanced flexibility in these skip regions. Conversely, removal of Skip 4 leads to less variation in the positions of individual residues (Fig. 3D).
The clustering analysis is consistent with the local fluctuations. In the presence of Skip 3 there is one structural cluster that persists throughout the simulation, whereas in its absence (ΔS3) there is a large increase in the number of well-populated structural clusters (Fig. 4 A and B). The same trend is seen for Skip 1 and 2 (SI Appendix, Fig. S6). In contrast, the WT structure for the Skip 4 region shows a very large distribution of structural states throughout the simulation, which are reduced dramatically on deletion of that residue (Fig. 4 C and D). Importantly, both WT and ΔS4 simulations maintain a structural ensemble dominated by canonical coiled-coils but removal of the Skip 4 residue reduced the conformational diversity within the ensemble.
Fig. 4.
Diversity of ensembles formed by the regions surround Skip 3 and 4 in the presence and absence of the skip residues. (A) Skip 3 WT, (B) Skip 3 deletion ΔS3, (C) Skip 4 WT, (D) Skip 4 deletion ΔS4, (E) recoiled Skip 3 WT sequence, and (F) recoiled Skip 3 deletion (ΔS3-R). In this figure, Cα-RMSD with respect to the representative member of the most populated cluster was plotted against simulation time. The color-coding represents the different clusters formed where blue depicts the dominating clusters. The Cα-alignments of representative members onto the crystal structure and model structure, for Skip 3 and Skip 4, respectively, are shown on the right where the representative and initial structures are shown in blue and gray, respectively. Skip residues in the WT crystal structures are depicted in green. The percentage of each cluster and the Cα-RMSD to initial structure is given under each structure.
Computational Recoiling of Skip 3.
Deletion of Skip 1, 2, or 3 is predicted to lead to greater conformational variability, which is consistent with the concept that these regions have evolved to accommodate the presence of an additional residue through local relaxation of the superhelical pitch. The simulations also predict that deletion (ΔS1, ΔS2, and ΔS3) moves the structure toward a more canonical coiled-coil, as indicated by the smaller values of DCOM, although the resulting pattern of hydrophobic amino acid residues is inconsistent with a normal heptad repeat pattern. Thus, any potential deleterious effects caused by deletion of the skip residues could be attributed to either the absence of the residue itself or destabilization of the surrounding regions. To resolve this issue, the sequence surrounding Skip 3 was reconfigured to retain the same charge distribution but exhibit the normal pattern of hydrophobic residues found in a canonical coiled-coil interface (Fig. 3). This was simulated with and without the nominal Skip 3 residue where the recoiled WT sequence and recoiled Skip 3 deletion are designated S3-R and ΔS3-R, respectively. These models show that introduction of a canonical coiled-coil pattern leads to greater conformational flexibility in the presence of Skip 3; but in the absence leads to less conformational variability in the clustering analysis and lower RMSFs (Figs. 3 A and C and 4 E and F).
The Role of the Skip Residues in the Assembly of Bipolar Thick Filaments.
To test the biological role of the skip residues, rat α-cardiac myosin rod constructs (WT and deleted for individual skip residues) tagged with GFP at their amino termini were transfected into neonatal rat ventricular cardiac myocytes (NRVMs) and examined for their ability to be incorporated into thick filaments by live-cell imaging (20). When single cardiomyocytes were surveyed by confocal microscopy, we found that no skip residue deletion fundamentally prevented myosin incorporation into the endogenous sarcomeres (Fig. 5A). However, deletion of Skip 3 (ΔS3) caused myosin cytoplasmic aggregates that show a sponge-like structure in about 40–50% of the transfected cells (Fig. 5A and SI Appendix, Fig. S7A). When the expression levels of the mutants were boosted, no negative effects on sarcomere structure were observed, but a higher number of large aggregates were detected in ΔS3 transfected cells (SI Appendix, Fig. S7B). Cardiomyocytes were cotransfected with both mutant GFP- and mCherry-tagged WT myosin rods to determine if the skip residue deletions alter the distribution of myosin within the thick filament. As previously reported, GFP- and mCherry-tagged labeled WT myosin rods are uniformly incorporated and fully colocalized along the ∼1.6-μm-long A-band (20) (Fig. 5B, WT). Furthermore, the fluorescence signals are absent from the I-band corresponding to the thin actin filaments, as well as from the H-band corresponding to the central part of the sarcomere where myosin antiparallel interactions take place in the bare zone. Lastly, the lack of fluorescence in the bare zone indicates that both the GFP and mCherry molecules are correctly positioned at the beginning of the parallel interactions; these occur on each side of the sarcomere at an estimated relative distance of about ∼1,600 Å, which also corresponds to the theoretical myosin rod length (21). Cotransfection of the mCherry-tagged WT and GFP-ΔS3 myosin rods (Fig. 5B and SI Appendix, Fig. S7C) confirmed the phenotype of the ΔS3 construct but also revealed its dominant-negative effects, as judged by the presence of WT mCherry-tagged myosin molecules in the cytoplasmic aggregates (Fig. 5B, ΔS3). However, this kind of analysis exposed an unexpected and critical role of the Skip 4 residue in promoting antiparallel assembly of myosin. Surprisingly, the emission signals of the ΔS4 GFP- and WT mCherry-tagged myosin molecules do not visibly overlap close to the center of the bipolar myosin filaments. Indeed, the red mCherry fluorescence is predominantly detectable in this region (Fig. 5B, ΔS4). In contrast, the WT and ΔS4-tagged molecules are homogenously distributed along the rest of the thick filaments where myosin rods pack in a parallel manner. To better characterize the ΔS4 phenotype, Linescan analysis, which measures the intensity value of each fluorophore over the length of the sarcomere, was then performed. The plot derived from an evaluation of 425 single sarcomeres imaged from three independent transfections revealed that the ΔS4 green fluorescence signal has both an offset of the peaks toward the Z-lines, as well as a substantially deeper valley in proximity of the bare zone (Fig. 5C). Thus, lack of GFP signal strongly suggests that the ΔS4 mutant is incapable of forming the antiparallel interactions occurring in the core of the bare zone, where full overlap of the rods appear to be demanded (21). To confirm the ΔS4 phenotype in different experimental conditions, cardiomyocytes were then stained with an antibody recognizing only the endogenous full-length myosin molecules or imaged in time-course experiments. The same peculiar distribution and spatial relationship with the WT myosin molecules was also observed in fixed cells (Fig. 5D, Ab-F59) as well as shortly after transfection, when the transcription rate is high (Fig. 5D, 36 h and 48 h).
Fig. 5.
Effects of skip residue deletions on myosin incorporation into sarcomeres. (A) Cardiomyocytes electroporated with WT and mutant GFP-tagged skip residue deletion constructs (ΔS) were imaged by confocal microscopy 96 h later. (Scale bar, 10 μm.) (B) Cardiomyocytes were cotransfected with mutant GFP- and WT mCherry-tagged constructs as indicated. Cells were imaged by confocal microscopy 96 h later. The two boxes in the WT and ΔS4 merge panels show the high magnification view of the sarcomeric I band and H zone (the latter corresponding to the bare zone) and the lack of colocalization between the mutant GFP- and the WT mCherry-tagged myosins. (Scale bar, 5 μm.) (C) Linescan analysis showing the relative intensity across the sarcomere of WT GFP and mCherry (Left) and ΔS4 GFP- and WT mCherry- tagged myosins (Right). Cells from three independent transfections were imaged and a total of 420 sarcomeres for each graph analyzed; data were obtained by averaging the two fluorescence signals. x axis: pixel distance (0.086 μm per pixel); y axis: fluorescence intensity. The location of the I-band and H-zone are reported. (D) Colocalization of ΔS4 GFP construct with the endogenous myosin, and time course incorporation into the sarcomeres. (Ab-F59): cardiomyocytes were transfected with WT or ΔS4 GFP-tagged myosin constructs; 96 h later cells were fixed and stained with F59 antimyosin primary antibody that recognizes only the myosin head domain, and the Alexa Fluor 568 secondary antibody with orange-red emission color. All panels are overlays of GFP and mCherry fluorescence signals. (36 h, 48 h): cardiomyocytes cotranfected with ΔS4 GFP- and WT mCherry-tagged myosin rod constructs were imaged by confocal microscopy 36 and 48 h later. (Scale bar, 5 μm.)
Skip Residues Are Coupled with Proximal Noncanonical Residues.
Mutations predicted to convert or recoil the skip regions into more stable structures were then introduced to determine whether unwinding of the regions surrounding Skip 1, 2, or 3 observed in the WT structures is functionally important. As seen with the skip residue deletions, the recoiled myosins were also incorporated into the endogenous sarcomeres (SI Appendix, Fig. S7D). The number of cells showing cytoplasmic aggregates did not increase when transfected with the Skip 3 deletion recoiling construct (ΔS3-R) (∼40–50%), but higher magnification inspection showed that many of the thick filaments containing the ΔS3-R protein have a less-defined structure (ΔS3-R HMV). Moreover, higher expression levels caused, as previously observed with the ΔS3 construct, a greater number of large mutant aggregates (SI Appendix, Fig. S7D, ΔS3-R). Myosin aggregates were also observed in a small percentage of cells (∼2–4%) transfected with the Skip 1 recoiled construct (SI Appendix, Fig. S7D, ΔS1-R). Interestingly, recoiling/mutagenesis converting the Skip 2 region into the corresponding sequence of nonmuscle/smooth muscle myosins, which lack Skip 2 residue (SI Appendix, Fig. S4), did not cause any noticeable myosin phenotype (SI Appendix, Fig. S7D, ΔS2-R). Cotransfection with mCherry-tagged WT and ΔS-R constructs validated the importance of Skip 3 for proper myosin assembly (SI Appendix, Fig. S8, ΔS3-R) but failed to detect any phenotype for either Skip 1 or 2 recoiling mutants (SI Appendix, Fig. S8, ΔS1-R and ΔS2-R). The lack of aberrant formation of parallel myosin arrangement and the more obvious exclusion from the bare zone for ΔS4-R, as observed for ΔS4, confirmed the specialized role of the Skip 4 residue/hinge region in bipolar myosin assembly (SI Appendix, Fig. S8, ΔS4-R).
Finally, to address whether the distortion or flexibility is important for the Skip 3 region, the skip residue was reintroduced into the recoiled mutant (S3-R) and its effect examined in vivo. In this case, the skip residue introduces instability into an otherwise stable coiled-coil as demonstrated by molecular dynamics simulation (Figs. 3 A and C and 4 E and F). Cells expressing this mutant show the same cytoplasmic aggregates observed with ΔS3-R mutant (SI Appendix, Fig. S7, S3-R). This finding implies that a structurally stable but unwound structure surrounding Skip 3 is functionally important.
Interchangeability of the Skip Regions.
The finding that deletion of Skip 3 and 4 causes myosin aggregates or exclusion from the bare zone raises the question whether their biological function is defined by the location of the distortion in the coiled coil or the specific sequence at that location. To address this question, 14 residues on either side of Skip 3 and Skip 4 were substituted with the corresponding region of Skip 2 that we have shown does not itself play an important role in myosin assembly, and shares only structural similarity—but limited sequence identity—with the Skip 3 region (Fig. 6A).
Fig. 6.
Functional activity of mutants carrying duplications of 28 amino acids encompassing the Skip 2 residue. (A, Upper) Topology of the duplications showing the number of amino acids separating the skip residues from each other or from the beginning/end of the myosin rod. (A, Lower) alignment of 28 amino acids surrounding the Skip 2 residue with the corresponding Skip 3 and 4 regions (replaced by the duplications). The observed coiled-coil position registry is shown above and below the sequences together with the conserved charge distribution for Skip 2 and 3. (B) Cardiomyocytes were transfected with GFP-tagged myosin constructs as indicated. (C) Cardiomyocytes were cotransfected with mutant GFP- and WT mCherry-tagged myosin constructs as indicated. The box in the S2-S4 Repl merge images shows the high magnification view of the of the sarcomeric I-band and H-zone. Cells in both B and C were imaged by confocal microscopy 96 h after transfection. (Scale bars, 5 μm.) (D) Linescan analysis showing the relative intensity across five sarcomeres of S2-S3 Repl GFP- and WT mCherry-tagged myosins (Left) and S2-S4 Repl GFP- and WT mCherry-tagged myosins (Right). x axis: pixel distance (0.086 μm per pixel); y axis: fluorescence intensity. The location of the I-band and H-zone are reported.
Examination of GFP replacement mutants, transfected into NRVMs alone, revealed no anomalous distribution of the tagged myosins in the cytoplasm or defects in the incorporation into the sarcomere (Fig. 6B, S2-S3 Repl and S2-S4 Repl). However, cotransfection with WT mCherry myosin shows an assembly defect associated only with the Skip 4 replacement, which is exactly the opposite of what was observed when Skip 4 was deleted: imaging and linescan analysis revealed accumulation but not exclusion of the S2-S4 construct in the bare zone that becomes completely filled by the GFP fluorescence signal (Fig. 6C, S2-S4 Repl, and Fig. 6D).
Discussion
Considerable effort has been devoted at the ultrastructural level to determine the organization of the myosin rods in the sarcomeric thick filament (4, 21). Similarly, there is a large database of sequences that indicate that the myosin rod is highly conserved (22). What is lacking is an understanding of the connection between sequence and macromolecular assembly. The structural and functional data presented in this study shed new light on the function of the four skip residues that interrupt the cyclic pattern of the myosin rod. It has been previously argued that by introducing conformational instability in the coiled-coil, skip residues allow myosin rods to wrap along the cylindrical thick filament (9, 23). Our structural studies show that the first three skip residues cause the coiled-coil to unwind for a region of ∼29 amino acid residues over which the α-helices run approximately parallel to each other. As such, the designation of a single residue as a “skip” residue is very difficult because the distortion extends over ∼four heptad repeats. Rather, the discussion of the role of the skip residues in the assembly of the myosin rod must focus on the structural properties of the entire region more than a single residue.
The structures determined here suggest that the regions surrounding the first three skip residues exhibit a stable but distorted conformational state. Moreover, molecular dynamics simulations show that the skip residues maintain the relaxation of the superhelical pitch and their removal increases the flexibility of the surrounding regions. Although not assessed by our calculations, the structures are consistent with enhanced long-range flexibility introduced by unwinding of the coiled-coil.
The myosin distortions introduced by the first three skip residues are both structurally and functionally different from those observed in tropomyosin and reflect the different roles that these molecules play in the sarcomere. The structure of the first three skip regions has evolved to ensure regional and adequate flexibility of the rod required for assembly of the long myosin coiled-coil into the thick filament. In contrast, tropomyosin conformational flexibility has been achieved by the inclusion of alanine residues in the a and d positions at seven locations (24, 25). This molecular arrangement, which results in looser packing of the α-helices but maintains the overall coiled-coil structure, allows the molecule to wrap continuously around the actin filament, and to adopt different local positions, depending upon its regulatory state (26, 27). Distortions from a canonical coiled-coil are common and are often implicated in mediating protein–protein interactions as observed in intermediate filaments (28). Although several structural examples of stammer/stutters structures have been reported, skip residues have been observed mainly in antiparallel single-chain coiled-coils but there are very few structures for parallel dimeric coiled-coils that include skip residues (29). Thus, the comparison between the first three and the last myosin skip residues reveals how the insertion of a single residue can be differently accommodated within a long parallel coiled-coil to diversify structural function, and highlights the importance of experimental characterization of regions that do not show a canonical repeat.
It is accepted that the myosin rod arose from gene duplication of an ancestral rod unit (30). Our data show that during myosin evolution the same unwound structure has been conserved, even though the amino acid sequence has diverged considerably over the passage of time. The question arises whether the first three skip regions, which are by themselves highly conserved across the myosin II superfamily, play an equal role in thick filament assembly. The cellular data presented here show that the first three skip residues are not functionally equivalent in promoting myosin assembly and their hierarchical importance appears to be set by their position along the rod. The modification of the region surrounding Skip 3 has a greater effect on assembly than modification of either Skip 1 or 2. Only deletion of the Skip 3 residue causes both aggregation in the cytoplasm and reduced incorporation of the mutant protein into the sarcomere. Indeed, any modifications of Skip 3 that change the pitch or introduce flexibility lead to the same cell phenotype. This speaks in favor of the importance of a defined structure. Myosin assembly is not affected by the replacement of the Skip 3 region with the corresponding Skip 2 region that introduces 23 of 28 amino acid changes, but maintains the same structure, and the overall charge and distribution of surface exposed hydrophobic residues (SI Appendix, Fig. S3) (10).
Interestingly, recoiling of Skip 1, which is located within the hinge between subfragment-2 and LMM and presumably allows the myosin heads to commute between the thick and thin filaments, shows only a mild phenotype (SI Appendix, Fig. S7D). Although NRVMs contract spontaneously, our assay only measures myosin incorporation into the thick filaments and not the effects on sarcomere contractility. Thus, a role for the Skip 1 region in controlling specific steps that take place during actomyosin sliding cannot be ruled out. The lack of phenotype observed by converting the Skip 2 region into nonmuscle/smooth myosin isoforms, which lack the Skip 2 residue, is also puzzling. Clearly, the formation of side-polar versus bipolar filaments is driven by more than the absence or presence of the Skip 2 region and implies that this region could play a sarcomeric role not shared with nonmuscle myosins (9).
Our data clearly show structural and functional differences between the first three skip residues and the last one. The Skip 4 residue is located in a highly flexible loop encompassing three to four amino acids separating conventional coiled-coil regions that constitute a true hinge. Thus, defining of one of these residues as the Skip 4 residue is somewhat arbitrary. Removal of the skip residue from the hinge does not prevent incorporation in the parallel arms of the sarcomere but leads to exclusion of the mutant myosin from the bare zone. This finding implies that the C-terminal portion of the myosin rod requires a high degree of movement to interact with other components of the sarcomere or to assemble in a different fashion in the bare zone. To our knowledge, this is the first evidence that myosin requires conformational flexibility to form antiparallel assemblies that constitute the bare zone.
Interestingly, the myosin–titin interaction site has been mapped to a 17-residue sequence located eight amino acids downstream of the Skip 4 residue. Because the two proteins run approximately parallel to each other along the sarcomere, it has been proposed that the bending of the rod induced by the skip 4 residue could allow the two molecules to interact (31). Thus, one explanation for the phenotype observed with the Skip 4 mutant constructs might be that the reduction in flexibility introduced by the mutations in the rod could affect the interaction between the two proteins. However, mutagenesis of the predicted titin binding site, which contains a unique rod arrangement of negative residues (RELENELE), does not compromise myosin assembly nor alter its ability to be correctly incorporated into the sarcomere (SI Appendix, Fig. S9). However, reduced hinge flexibility (as probably occurs in the S2-S4 mutant) could affect accurate myosin antiparallel assembly by causing loss of interaction with other M-resident proteins (7).
In contrast to parallel assembly, which shows a characteristic polar repeat consistent with a 98-residue stagger that underlies the 143 Å distance between crowns in the thick filament, there is no obvious sequence signature that would indicate how the myosin rods align antiparallel to each other in the bare zone (5, 32, 33). This finding implies that the assembly cannot be described by a simple binary association of only two antiparallel coiled-coils. The bare zone exhibits D32 dihedral symmetry that demands a regular assembly of myosin molecules (34) and image reconstruction shows a thickening in the bare zone consistent with the overlap of myosin rods. Therefore, a complex arrangement is expected because both parallel and antiparallel interactions take place in the bare zone. Based on packing considerations, each myosin rod is expected to interact with at least five other molecules in this densely packed region (23). Indeed, modeling of the thick filament predicts that the C-terminal portion of the rod assembles differently in the bare zone than in the arms of the sarcomere (21, 35). The observation that the hinge at Skip 4 is necessary for incorporation into the bare zone is suggestive of a discontinuous interaction between myosin rods or interaction with multiple molecules. Taken together, these findings provide evidence that only the Skip 3 and 4 residue regions are essential for parallel and antiparallel myosin interactions. Although the similar structure of the first three skip residues has been functionally diversified by their location along the rod, the more complex structure of the bare zone has required the evolution of a specialized hinge structure.
More than 130 mutations causing hypertrophic and dilated cardiomyopathy, as well as different distal myopathies, have been mapped to the β-myosin rod; of interest, some of them are located near the four skip residues. In particular, three of them, E1573K R1588P, and L1591P, which lie in the solvent exposed c, b, and b positions of the heptad repeat (see Fig. 2A), respectively, are located within the skip 3 region resolved in this study. The first of these mutations has been implicated in Ebstein anomaly, which is a rare congenital heart malformation affecting the tricuspid valve, as well as the right ventricle, whereas the others result in Laing distal myopathy, characterized by slowly progressive skeletal muscle weakness with variable degrees of cardiac impairment (36–38). To determine the potential impact of these mutations on the structure of the skip 3 region, molecular dynamics were then carried out (SI Appendix, Fig. S10). Although the E1573K mutation has little effect, which implies that this charge reversal influences packing interactions between adjacent myosin rods, the proline substitutions have a much greater effect. In fact, these substitutions cause greater local conformational variability and separation of the α-helices; moreover, they introduce a bend into the coiled-coil and a change in pitch. Thus, the proline substitutions analyzed are predicted to have both a local and long-range effects on the conformation of myosin, which are expected to alter the rod interactions within the thick filament.
In addition to providing, to our knowledge, the first structural and functional characterization of four coiled-coil regions of a sarcomeric myosin rod, the multidisciplinary approach adopted here lays the groundwork for defining the functional impact of disease-causing mutations on the coiled-coil structure of the myosin rod.
Materials and Methods
For details, see SI Appendix.
DNA Constructs.
Vectors for crystallographic studies were prepared by QuikChange cloning. Sections of the MYH7 gene, purchased as an IMAGE clone from Open Biosystems, were inserted into modified pET vectors containing the desired fusion protein as previously described (12). QuikChange cloning allowed all constructs to be made without introducing cloning artifacts while maintaining the correct coiled-coil registration between the folding domains and the target gene fragment. The WT EGFP and mCherry myosin rod constructs were created by fusing each fluorescent reporter gene at amino acid 841 of the rat α-cardiac myosin gene, as previously described (20). All of the myosin mutants were generated by inverse PCR (39).
Protein Expression and Purification.
Myosin fusions were expressed in an Escherichia coli BL21-CodonPlus (DE3)-RIL cell line (Stratagene). Cells were grown in lysogeny broth (LB) at 16 °C, harvested by centrifugation, and then flash-frozen in liquid nitrogen before storage at −80 °C. Selenomethionine derivatives were prepared as previously described (40). The proteins were purified by nickel affinity chromatography followed by removal of the His-tag with tobacco etch virus protease. The proteins were flash-frozen in 30 μL droplets in liquid nitrogen and stored at −80 °C before crystallization.
Crystallization.
Crystals of Gp7-K1173-I1238-Eb1 (Skip 1), Gp7-L1361-I1425-Eb1 (Skip 2), and Xrcc4-L1551-N1609 (Skip 3) were grown by vapor diffusion at room temperature from a 1:1 mixture of protein at 10 mg/mL and a variety of organic precipitants as described in detail in SI Appendix. Gp7-A1776-T1854-Eb1 (Skip 4) was methylated to obtain suitably diffracting crystals with formaldehyde and dimethylamine borane complex (41) and subsequently crystallized at room temperature. All crystals were flash-frozen before X-ray data collection. Detailed crystallization conditions are given in SI Appendix.
Data Collection and Structure Determination.
Data were collected at 100 K at either SBC Beamline 19-ID or 19-BM (Advanced Photon Source, Argonne National Laboratory, Argonne, IL). Diffraction data were indexed, integrated, and scaled with HKL3000 (42). Initial solutions were obtained by molecular replacement using PHASER with the appropriate fusion protein as a search model (PDB ID codes 1NO4, 1IK9, or 1YIB) (43). Initial positions of the search models were optimized by rigid body refinement in Phenix Refine (44). Structure building was done both manually in COOT through iterative cycles and with Phenix Autobuild (45). All structures have a protein geometry MolProbity score of the 97th percentile or greater. Data collection and refinement statistics are shown in SI Appendix, Table S2.
Molecular Dynamics.
All simulations were carried out using the AMBER v12 Molecular Dynamics package with the f99SB force field improved with NMR observables (ff99SBnmr) (46–48). The Generalized Born implicit solvent approach was adopted using specifically the gb7 model as this is ∼100-fold faster compared with explicit solvent simulations when graphical processor units are used (49–51). Production gb7 simulations were carried out for 1,000 ns using 1 fs as the time step. Langevin dynamics was applied with a collision frequency of 20 ps−1 at 300 K. The SHAKE (52) algorithm was applied to bonds involving hydrogen with a tolerance of 10−5. Nonbonded cutoff was set up as 9,999 Å, and the maximum distance between atom pairs for Born radii calculations was set up to be 12 Å. Salt concentration was set to the physiological molarity of 0.15 M.
Explicit Solvent Control Simulations.
The validity of implicit solvent simulations was tested through explicit solvent simulations (SI Appendix, Fig. S4). In explicit solvent simulations, because of speed limitations, the simulations were carried out up to 160 ns. TIP3P water model was used along with ion parameters from Joung and Cheatham (53). SHAKE was applied to bonds involving hydrogen atoms. Andersen thermostat was selected to maintain the temperature at 300 K (54). Pressure was held constant at 1 bar with a relaxation time of 1 ps. The electrostatic interactions were treated with particle mesh Ewald with a grid size of about 1 Å, and Lennard–Jones interactions were treated with a cut-off of 12 Å.
NRVM Preparation, Culturing Transfection, and Immunostaining.
NRVMs were prepared as previously described (55). Cells were electroporated using the Rat Cardiomyocyte-Neonatal Nucleofector Kit (Lonza) according to the manufacturer’s protocol and plated onto 1% gelatin-coated glass-bottom Microwell Dishes (MatTeK). Following overnight recovery, cells were washed and then treated with 15 mM l-phenylephrine. Live-cell imaging was carried out at the time points specified in the figure legends. Detection of the endogenous myosin with the antisarcomeric myosin heavy-chain monoclonal F59 was carried out as follows: NRVMs were rinsed twice with PBS, fixed with 2% (vol/vol) paraformaldehyde for 5 min and permeabilized with 0.1% Triton X-100 for 5 min. After 3 PBS washes, cells were blocked with 1% BSA, 10% (vol/vol) goat serum, and 0.1% Triton X-100 at room temperature for 30 min and then incubated with the F59 antibody (1:10 dilution) at room temperature for 1 h. After three PBS washes, cells were incubated with the secondary antibody (Alexa Fluor 568 goat anti-mouse IgG) at room temperature for 1 h. After final washes with PBS, cells were rinsed in distilled water and mounted in Fluoromount.
Confocal Microscopy.
NRVMs were analyzed using the Nikon Eclipse TE 2000-U microscope coupled with an electron-multiplying charge-coupled device camera (Cascade II; Photometrics) and a Yokogawa spinning-disk confocal system (CSU-Xm2; Nikon). Images were taken with the 100× Nikon Plan Apo VC NA 1.4 oil objective. MetaMorph software was used for image acquisition; image analysis was performed with ImageJ (56).
Supplementary Material
Acknowledgments
We thank A. Robinson for neonatal rat ventricular myocyte preparations, and Joe Dragavon and the BioFrontiers Advanced Light Microscopy Core for microscopy support. This work was supported by National Institutes of Health Grants R21 HL111237 (to I.R. and Q.C.) and R01 GM29090 (to L.A.L.); the computational work was partially supported by NSF-CHE1300209 (to Q.C.). Use of the Structural Biology ID19 and BM19 beamlines, Argonne National Laboratory Advanced Photon Source was supported by the US Department of Energy, Office of Energy Research, under Contract W-31-109-ENG-38.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4XA1, 4XA3, 4XA4, and 4XA6).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505813112/-/DCSupplemental.
References
- 1.Craig R. Structure of A-segments from frog and rabbit skeletal muscle. J Mol Biol. 1977;109(1):69–81. doi: 10.1016/s0022-2836(77)80046-6. [DOI] [PubMed] [Google Scholar]
- 2.Gautel M. The sarcomeric cytoskeleton: Who picks up the strain? Curr Opin Cell Biol. 2011;23(1):39–46. doi: 10.1016/j.ceb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16(2):204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci USA. 2013;110(1):318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLachlan AD, Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- 6.Sohn RL, et al. A 29 residue region of the sarcomeric myosin rod is necessary for filament formation. J Mol Biol. 1997;266(2):317–330. doi: 10.1006/jmbi.1996.0790. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RC, Buvoli M, Buvoli A, Leinwand LA. Myosin filament assembly requires a cluster of four positive residues located in the rod domain. FEBS Lett. 2012;586(19):3008–3012. doi: 10.1016/j.febslet.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry DA, Fraser RD, Squire JM. Fifty years of coiled-coils and alpha-helical bundles: A close relationship between sequence and structure. J Struct Biol. 2008;163(3):258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Offer G. Skip residues correlate with bends in the myosin tail. J Mol Biol. 1990;216(2):213–218. doi: 10.1016/S0022-2836(05)80309-2. [DOI] [PubMed] [Google Scholar]
- 10.Straussman R, Squire JM, Ben-Ya’acov A, Ravid S. Skip residues and charge interactions in myosin II coiled-coils: Implications for molecular packing. J Mol Biol. 2005;353(3):613–628. doi: 10.1016/j.jmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson SJ, Stewart M. Expression in Escherichia coli of fragments of the coiled-coil rod domain of rabbit myosin: Influence of different regions of the molecule on aggregation and paracrystal formation. J Cell Sci. 1991;99(Pt 4):823–836. doi: 10.1242/jcs.99.4.823. [DOI] [PubMed] [Google Scholar]
- 12.Klenchin VA, Frye JJ, Jones MH, Winey M, Rayment I. Structure-function analysis of the C-terminal domain of CNM67, a core component of the Saccharomyces cerevisiae spindle pole body. J Biol Chem. 2011;286(20):18240–18250. doi: 10.1074/jbc.M111.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frye J, Klenchin VA, Rayment I. Structure of the tropomyosin overlap complex from chicken smooth muscle: Insight into the diversity of N-terminal recognition. Biochemistry. 2010;49(23):4908–4920. doi: 10.1021/bi100349a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitanai Y, Minakata S, Maeda K, Oda N, Maéda Y. Crystal structures of tropomyosin: Flexible coiled-coil. Adv Exp Med Biol. 2007;592:137–151. doi: 10.1007/978-4-431-38453-3_13. [DOI] [PubMed] [Google Scholar]
- 15.Deiss S, et al. Your personalized protein structure: Andrei N. Lupas fused to GCN4 adaptors. J Struct Biol. 2014;186(3):380–385. doi: 10.1016/j.jsb.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Crick FHC. The Fourier transform of a coiled-coil. Acta Crystallogr. 1953;6:685–689. [Google Scholar]
- 17.Grigoryan G, Degrado WF. Probing designability via a generalized model of helical bundle geometry. J Mol Biol. 2011;405(4):1079–1100. doi: 10.1016/j.jmb.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254(5031):539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 19.Gromiha MM, Parry DA. Characteristic features of amino acid residues in coiled-coil protein structures. Biophys Chem. 2004;111(2):95–103. doi: 10.1016/j.bpc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Buvoli M, Buvoli A, Leinwand LA. Effects of pathogenic proline mutations on myosin assembly. J Mol Biol. 2012;415(5):807–818. doi: 10.1016/j.jmb.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Khayat HA, Kensler RW, Morris EP, Squire JM. Three-dimensional structure of the M-region (bare zone) of vertebrate striated muscle myosin filaments by single-particle analysis. J Mol Biol. 2010;403(5):763–776. doi: 10.1016/j.jmb.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White GE, Erickson HP. Sequence divergence of coiled coils—Structural rods, myosin filament packing, and the extraordinary conservation of cohesins. J Struct Biol. 2006;154(2):111–121. doi: 10.1016/j.jsb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Chew MW, Squire JM. Packing of alpha-helical coiled-coil myosin rods in vertebrate muscle thick filaments. J Struct Biol. 1995;115(3):233–249. doi: 10.1006/jsbi.1995.1048. [DOI] [PubMed] [Google Scholar]
- 24.Brown JH, et al. Deciphering the design of the tropomyosin molecule. Proc Natl Acad Sci USA. 2001;98(15):8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JH. How sequence directs bending in tropomyosin and other two-stranded alpha-helical coiled coils. Protein Sci. 2010;19(7):1366–1375. doi: 10.1002/pro.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XE, Holmes KC, Lehman W, Jung H, Fischer S. The shape and flexibility of tropomyosin coiled coils: Implications for actin filament assembly and regulation. J Mol Biol. 2010;395(2):327–339. doi: 10.1016/j.jmb.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Barua B, Hitchcock-DeGregori SE. Probing the flexibility of tropomyosin and its binding to filamentous actin using molecular dynamics simulations. Biophys J. 2013;105(8):1882–1892. doi: 10.1016/j.bpj.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chernyatina AA, Guzenko D, Strelkov SV. Intermediate filament structure: The bottom-up approach. Curr Opin Cell Biol. 2015;32:65–72. doi: 10.1016/j.ceb.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Strelkov SV, Burkhard P. Analysis of alpha-helical coiled coils with the program TWISTER reveals a structural mechanism for stutter compensation. J Struct Biol. 2002;137(1-2):54–64. doi: 10.1006/jsbi.2002.4454. [DOI] [PubMed] [Google Scholar]
- 30.McLachlan AD. Analysis of gene duplication repeats in the myosin rod. J Mol Biol. 1983;169(1):15–30. doi: 10.1016/s0022-2836(83)80173-9. [DOI] [PubMed] [Google Scholar]
- 31.Houmeida A, Holt J, Tskhovrebova L, Trinick J. Studies of the interaction between titin and myosin. J Cell Biol. 1995;131(6 Pt 1):1471–1481. doi: 10.1083/jcb.131.6.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLachlan AD, Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- 33.Ricketson D, Johnston CA, Prehoda KE. Multiple tail domain interactions stabilize nonmuscle myosin II bipolar filaments. Proc Natl Acad Sci USA. 2010;107(49):20964–20969. doi: 10.1073/pnas.1007025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luther PK, Munro PM, Squire JM. Three-dimensional structure of the vertebrate muscle A-band. III. M-region structure and myosin filament symmetry. J Mol Biol. 1981;151(4):703–730. doi: 10.1016/0022-2836(81)90430-7. [DOI] [PubMed] [Google Scholar]
- 35.Squire JM. General model of myosin filament structure. 3. Molecular packing arrangements in myosin filaments. J Mol Biol. 1973;77(2):291–323. doi: 10.1016/0022-2836(73)90337-9. [DOI] [PubMed] [Google Scholar]
- 36.Postma AV, et al. Mutations in the sarcomere gene MYH7 in Ebstein anomaly. Circ Cardiovasc Genet. 2011;4(1):43–50. doi: 10.1161/CIRCGENETICS.110.957985. [DOI] [PubMed] [Google Scholar]
- 37.Cullup T, et al. Mutations in MYH7 cause Multi-minicore Disease (MmD) with variable cardiac involvement. Neuromuscul Disord. 2012;22(12):1096–1104. doi: 10.1016/j.nmd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Tasca G, et al. New phenotype and pathology features in MYH7-related distal myopathy. Neuromuscul Disord. 2012;22(7):640–647. doi: 10.1016/j.nmd.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Imai Y, Matsushima Y, Sugimura T, Terada M. A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 1991;19(10):2785. doi: 10.1093/nar/19.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol. 1993;229(1):105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 41.Rayment I. Reductive alkylation of lysine residues to alter crystallization properties of proteins. Methods Enzymol. 1997;276:171–179. [PubMed] [Google Scholar]
- 42.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276(Pt A):307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 43.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 46.Li DW, Brüschweiler R. NMR-based protein potentials. Angew Chem Int Ed Engl. 2010;49(38):6778–6780. doi: 10.1002/anie.201001898. [DOI] [PubMed] [Google Scholar]
- 47.Beauchamp KA, Lin YS, Das R, Pande VS. Are protein force fields getting better? A systematic benchmark on 524 diverse NMR measurements. J Chem Theory Comput. 2012;8(4):1409–1414. doi: 10.1021/ct2007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Case DA, et al. Univ of California; San Francisco, CA: 2012. AMBER 12. Available at ambermd.org/doc12/Amber12.pdf. Accessed June 26, 2015. [Google Scholar]
- 49.Götz AW, et al. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized Born. J Chem Theory Comput. 2012;8(5):1542–1555. doi: 10.1021/ct200909j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MS, Salsbury FR, Brooks CL. Novel generalized Born methods. J Chem Phys. 2002;116(24):10606–10614. [Google Scholar]
- 51.Mongan J, Simmerling C, McCammon JA, Case DA, Onufriev A. Generalized Born model with a simple, robust molecular volume correction. J Chem Theory Comput. 2007;3(1):156–169. doi: 10.1021/ct600085e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-integration of Cartesian equations of motion of a system with constraints: Molecular-dynamics of N-alkanes. J Comput Phys. 1977;23(3):327–341. [Google Scholar]
- 53.Joung IS, Cheatham TE., 3rd Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B. 2008;112(30):9020–9041. doi: 10.1021/jp8001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrea TA, Swope WC, Andersen HC. The role of long ranged forces in determining the structure and properties of liquid water. J Chem Phys. 1983;79(9):4576–4584. [Google Scholar]
- 55.Maass AH, Buvoli M. Cardiomyocyte preparation, culture, and gene transfer. Methods Mol Biol. 2007;366:321–330. doi: 10.1007/978-1-59745-030-0_18. [DOI] [PubMed] [Google Scholar]
- 56.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.