Significance
Developing effective antiangiogenesis strategies remains clinically challenging. Unlike physiological angiogenesis, pathological angiogenesis comprises of many microvessels that do not fully mature or develop functionally, because the cell fate decision about which endothelial cells become the tip and lead the following stalk cells is dysregulated. We devised a specific theoretical framework to decipher the cross-talk between two crucial players of the decision-making process of tip and stalk cell fate: VEGF and Notch-Delta-Jagged signaling. We find that high expression of Jagged, but not Delta, can destabilize the terminal differentiation into tip or stalk cells and give rise to a hybrid tip/stalk phenotype, a phenotype that can transform physiological into pathological angiogenesis. Our results offer insights into why tumor-stroma communication often implicates Jagged.
Keywords: angiogenesis, Notch signaling, Jagged, VEGF signaling, tumor angiogenesis
Abstract
Angiogenesis is critical during development, wound repair, and cancer progression. During angiogenesis, some endothelial cells adopt a tip phenotype to lead the formation of new branching vessels; the trailing stalk cells proliferate to develop the vessel. Notch and VEGF signaling mediate the selection of these tip endothelial cells. However, how Jagged, a Notch ligand that is overexpressed in cancer, affects angiogenesis remains elusive. Here, by developing a theoretical framework for Notch-Delta-Jagged-VEGF signaling, we found that higher production levels of Jagged destabilizes the tip and stalk cell fates and can give rise to a hybrid tip/stalk phenotype that leads to poorly perfused and chaotic angiogenesis, which is a hallmark of cancer. Consistently, the signaling interactions that restrict Notch-Jagged signaling, such as Fringe, cis-inhibition, and increased production of Delta, stabilize tip and stalk fates and limit the existence of hybrid tip/stalk phenotype. Our results underline how overexpression of Jagged can transform physiological angiogenesis into pathological one.
Angiogenesis, the formation of new blood vessels from existing ones, is a vital process during embryonic development, homeostasis, and tumor progression (1). This process starts when cells release angiogenic growth factors such as VEGF in response to hypoxia (lack of oxygen). These growth factors induce the formation of a new sprout, and the endothelial cell at the very front of this angiogenic sprout is called a “tip” cell. The tip cell extends numerous filopodia toward the source of these growth factors and migrates toward the direction of the upward gradient of the growth factor concentration, thereby leading a new angiogenic branch. The cells that follow the tip cell do not adopt a tip phenotype, but rather form the stalk of the branch and proliferate to form the vessel lumen (2). A well-regulated balance between the migration of tip cells and proliferation of stalk cells is essential for adequately shaped nascent sprouts (3).
The selection of the tip and the stalk cell fate is critical for developing a functional vessel. This decision is mediated by Notch signaling pathway (2), an evolutionarily conserved cell–cell communication pathway involved in cell fate decisions in multiple contexts. This pathway is activated when Notch (transmembrane receptor) belonging to a particular cell interacts with Delta or Jagged (transmembrane ligands) belonging to its neighboring cell (trans-activation), thereby releasing the Notch intracellular domain (NICD). NICD then enters the nucleus and modulates the expression of many target genes of the Notch pathway, including both the ligands Delta and Jagged. However, when Notch of a cell interacts with Delta or Jagged belonging to the same cell, no NICD is produced; rather, both the receptor (Notch) and ligand (Delta or Jagged) are degraded (cis-inhibition) and therefore the signaling is not activated (4).
Despite generating the same signal (NICD), Notch signaling activated via Delta and that via Jagged, or in other words, Notch-Delta (N-D) signaling and Notch-Jagged (N-J) signaling, have different dynamics, because NICD asymmetrically modulates the expression of the two ligands: it represses Delta but activates Jagged (5, 6) (Fig. 1A). Therefore, Notch-Delta signaling between two interacting cells forms an intercellular double negative feedback loop, and the two cells tend to adopt different fates: one cell behaves as a sender [high ligand (Delta), low receptor (Notch)] and the other one behaves as a receiver [low ligand (Delta), high receptor (Notch)]. This process of lateral inhibition has a crucial role in generating a checkerboard-like or “salt-and-pepper” pattern, as observed during bristle patterning in flies and inner ear patterning in vertebrates (7). Conversely, Notch-Jagged signaling generates an intercellular double positive feedback loop, enabling the two interacting cells to adopt similar fates: a hybrid sender/receiver [high ligand (Jagged), high receptor (Notch)] fate. This process of lateral induction is crucial during sensing development and the formation of a smooth muscle wall around a nascent artery (6, 8).
Fig. 1.
Overview of the intracellular and intercellular interplay between Notch and VEGF signaling pathways. (A) Notch signaling is activated when the transmembrane receptor of one cell (Notch) binds to the transmembrane ligand (Delta or Jagged) of the neighboring cell (trans-activation). This trans-activation cleaves Notch to produce Notch Intracellular Domain (NICD) that is released in the cytoplasm and then enters the nucleus to modulate the transcription of many target genes. NICD can activate Notch and Jagged and inhibit Delta and VEGF receptor 2 (VEGFR2). Glycosylation of Notch by Fringe modifies Notch to have a higher affinity for binding to Delta and a lower affinity for binding to Jagged. Interaction between Notch receptor and ligands (Delta or Jagged) of the same cell (cis-inhibition) leads to the degradation of both the receptor and the ligand; thus, no NICD is generated. VEGF-A binds to VEGFR2, thus activating VEGF signaling in the cell that activates Delta (DLL4). (B) Cells with high levels of Delta, VEGFR2, and active VEGF signaling develop filopodia and migrate toward the VEGF-A gradient, leading the formation of the new branch and are called tip cells. DLL4 from tip cells inhibits the neighboring cells to also adopt a tip phenotype, thereby forcing them to adopt the stalk fate (low Dll4, high Jagged1, and NICD). Stalk cells, by virtue of the lateral induction characteristics of Notch-Jagged signaling, can induce neighboring cells to adopt a stalk cell, therefore elongating the lumen.
Besides asymmetric modulation by NICD, N-D and N-J signaling can also be differentially regulated by glycosyltransferase Fringe. Fringe modifies Notch such that the modified (or glycosylated) Notch has a higher chance to bind to Delta, but a lower chance to bind to Jagged (9). Importantly, Fringe, can also be activated by NICD in some biological contexts (10).
These different dynamics of Notch-Delta and Notch-Jagged signaling allow them to play complementary roles during angiogenesis. Notch-Delta signaling plays a crucial role in selecting the tip cell in response to VEGF (11). The binding of VEGF-A (the key ligand of VEGF family that responds to hypoxia) to VEGF receptor 2 (VEGFR2) (the main mediator of VEGF-A signaling during angiogenesis) up-regulates the production of Delta (DLL4) (12). DLL4 binds to Notch receptor on the neighboring cell and activates Notch signaling (NICD) in it. NICD inhibits VEGFR2, therefore making the adjacent cell less sensitive to the VEGF-A signal (12). The cell with high Delta (and low NICD) becomes the tip, and the adjacent ones with low levels of Delta (and high NICD) become the stalk (12). This interplay between Notch and VEGF pathways is quite tight and dose dependent, i.e., many neighboring cells dynamically compete to adopt the tip position but only one of them wins (13). However, unlike other contexts where Notch-Delta (N-D) signaling leads to salt-and-pepper patterns, i.e., pattern of alternate fates with a wavelength of one cell, in angiogenesis, the two tip cells are usually separated by a few stalk cells, all of which have low Delta but high Jagged (Jag1) levels (14). Thus, Notch-Jagged (N-J) signaling that regulates lateral induction (6, 8, 15), i.e., propagation of the same cell fate in adjacent cells, might decide the distance between two tip cells (Fig. 1B).
Based on these roles of N-D and N-J signaling, it is expected that increased production of Jagged would increase the distance between two tip cells by reinforcing the lateral induction mechanism between the stalk cells. However, the available experimental results are the exact opposite: i.e., higher production rates of Jagged leads to more tip cells (14). Further, one would also expect that increased production of Delta would lead to more tip cells, but as experimentally noted, Dll4 acts as a “brake” on sprouting angiogenesis (16). These conflicting observations call for an investigation of the underlying mechanisms of tip and stalk cell-fate selection mediated by Notch-Delta-Jagged (N-D-J) signaling.
Here, we propose a specific theoretical framework to study the interplay between N-D-J and VEGF signaling in the tip-stalk cell fate decision during sprouting angiogenesis. We show that cells can attain the stalk position by both lateral inhibition (through high levels of Delta in the neighboring tip cells) and lateral induction (through high levels of Jagged in the neighboring stalk cells). However, Delta and Jagged have opposite roles in stabilizing the tip position: whereas a higher production rate of Jagged makes it easier for a tip cell to lose its position to a neighboring stalk cell, a higher production rate of Delta decreases the dynamic competition between the two cells to adopt the tip position and consequently stabilizes the tip and stalk cell fates. Our results also suggest the existence of a hybrid or intermediate tip/stalk phenotype when Jagged is overexpressed compared with Delta. Cells in this hybrid tip/stalk fate have compromised migration traits compared with tip cells; therefore, the vessels led by these cells are expected to be smaller and poorly perfused compared with those led by the tip cells. These traits of the hybrid tip/stalk fate enhance dynamic lateral inhibition and can be critical for the emergence of a chaotic blood vessel network as seen during tumor angiogenesis. Finally, we evaluate the role of both Fringe and cis-inhibition in the tip-stalk cell fate decision.
Results
The Theoretical Framework.
To explore the effects of Jagged in cell fate determination during angiogenesis, we generalized our earlier theoretical framework of Notch-Delta-Jagged signaling (15) to incorporate VEGF signaling. The equations that describe the dynamics of Notch (N), Delta (D), Jagged (J), NICD (I), VEGFR2 (), and active VEGF signaling in a cell (V) are
| [1] |
| [2] |
| [3] |
| [4] |
| [5] |
| [6] |
where γ represents the degradation rate of N, D, J, , and is the degradation rate of I and V. , , , and represent innate production rates of the Notch, Delta, Jagged, and VEGF receptors, respectively. , , and represent the amounts of external proteins, i.e., receptor Notch and ligands Delta and Jagged available from neighboring cells. Similarly, represent the amount of external VEGF. represents the cis-inhibition rate, and represent the trans-activation rates of Notch with its ligands (Delta and Jagged) and the activation rate of VEGF signaling. and represent the transcriptional activation of Notch (N) and Jagged (J) by the signal NICD (I), and denotes the repression of Delta (D) by I. , , , and are shifted Hill functions. Shifted Hill functions are defined as , where is inhibitory Hill function and is excitatory Hill function, and denotes the fold change in production of Y due to X (17, 18). For activation, shifted Hill functions are depicted by and ; for inhibition, they are depicted by and . denotes no effect. The effect of Fringe is considered to increase with the increase of the Notch signal (I) and is represented by the shifted Hill functions and (15, 19). We considered and to represent Fringe-mediated increase of Notch-Delta (N-D) binding affinity both for trans- and cis-interactions, and the decrease of the same for Notch-Jagged (N-J) interactions (20, 21).
The values of the parameters are detailed in SI Appendix, section S1 and Table S1. The details of model construction are discussed in SI Appendix, section S2. The models for two interacting cells and many interacting cells are presented in SI Appendix, sections S3 and S4, respectively. A discussion about the robustness of the model with respect to changes in parameter values is presented in SI Appendix, section S5 and Figs. S1 and S2. The computational analysis was performed in Python using IPython (22) and PyDSTool (23).
We analyze two cases of the model: (i) single cell driven by fixed values of external signals: Delta (), Notch (), and Jagged () representing the amount of proteins in the neighboring cells, and representing the external signal VEGF-A; and (ii) a multicell system, where cells are coupled with each other and communicate via the N-D-J signaling in the presence of an external concentration of VEGF-A signal ().
Notch Mediates Tip-Stalk Fate Decision.
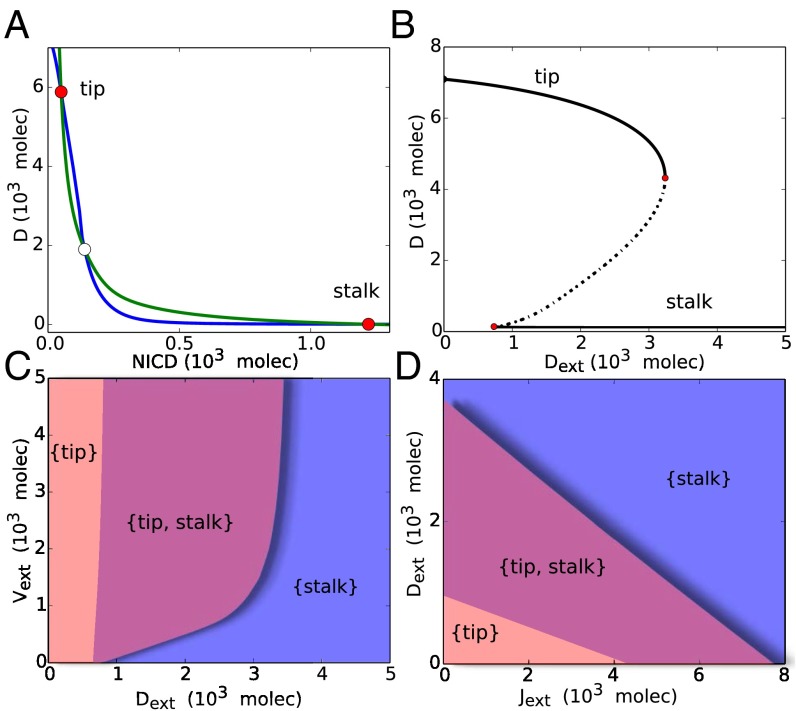
To evaluate the basic operating principles of cell fate decision between tip and stalk, we first analyze the dynamics of Notch-VEGF signaling by considering one cell in contact with the external signals: , , and —parameters that represent the concentration of Notch, Delta, and Jagged in the neighboring cells—and —the parameter that represents the amount of external VEGF released by cells under hypoxia. We find that the circuit is bistable with two stable states as (i) [high Delta (D), active VEGF signaling (V) and VEGF receptor (), and low NICD (I) and Jagged (J)] and (ii) [low Delta (D), active VEGF signaling (V) and VEGF receptor (), and high NICD (I) and Jagged (J)]. The former stable state corresponds to a tip phenotype, and the latter one corresponds to a stalk (Fig. 2A), i.e., the cell can adopt either of the two phenotypes: tip or stalk.
Fig. 2.
Nullcline, bifurcation curve, and phase diagrams for the case of a single cell driven by external proteins Notch, Delta, Jagged, and VEGF. (A) Nullclines for the case of one cell interacting with fixed levels of external proteins ( molecules). Blue nullcline is for the condition of all ODEs being set to zero except for and green nullcline is for the condition of all ODEs being set to zero except for (Eqs. 1–6). Unfilled circles represent unstable steady states, whereas red filled circles represent the two stable states: tip (high Delta, low NICD) and stalk (low Delta, high NICD). (B) Bifurcation curve of the levels of Delta (D) on the membrane as a function of the number of external Delta (). At low , the cell adopts the tip fate, whereas at high , the cell adopts the stalk fate. At intermediate , the cell can adopt either fate: tip or stalk. (C) Phase diagram (two-parameter bifurcation diagram) as a function of external Delta () and VEGF (). The monostable phase {tip} corresponds to the state [high Delta (D), VEGF receptor (), active VEGF signaling (V) and low NICD (I) and Jagged (J)], and monostable phase {stalk} corresponds to the state (low D, , and V; and high I and J). The bistable phase {tip, stalk} corresponds to a region of coexistence of both states: tip and stalk. (D) Phase diagram as a function of external Delta () and external Jagged (). Bifurcation curves of the levels of VEGF receptor (), active VEGF signaling (V), NICD (I), and Jagged (J) are included in SI Appendix, Fig. S3.
Further, we evaluate the different states or phenotypes a cell can adopt by varying levels of Delta in the neighboring cells (). For low values of , which mimics the case of neighboring cells being stalk cells, the cell adopts a tip phenotype (marked by the {tip} phase). Conversely, for high levels of , which mimics the case of neighboring cell(s) as tip(s), the cell adopts a stalk phenotype (marked by the {stalk} phase) (Fig. 2B and SI Appendix, Fig. S3). Interestingly, at intermediate levels of , we observe a range of bistability, i.e., the cell can be either tip or stalk (marked by the {tip, stalk} phase) (Fig. 2B and SI Appendix, Fig. S3), thereby reflecting phenotypic plasticity and leading to a dynamic lateral inhibition or “cell shuffling” as experimentally observed in angiogenesis (13, 24). This region of bistability—{tip, stalk} phase—exists for a large range of values of external VEGF signal, as long as is at intermediate levels, thereby indicating the tight coupling of Notch and VEGF signaling in tip-stalk fate decision (Fig. 2C). Similar behavior is found when varying instead of (SI Appendix, Fig. S4).
Next, we present the phase diagram driven by two control parameters— and —denoting the varying conditions for the different fates of the neighboring cells. We observe that the cells can attain the stalk fate for both high levels of and , i.e., stalk cell fate can be obtained by lateral inhibition mediated largely by Notch-Delta (when neighboring cell is a tip), as well as by lateral induction mediated largely by Notch-Jagged (when neighboring cell is a stalk) (Fig. 2D). Therefore, Notch-Jagged signaling can propagate the stalk cell fate, or in other words, a stalk cell can use Notch-Jagged signaling to induce its neighboring cells to adopt a stalk phenotype also. These stalk cells can contribute to lumen elongation and maintain the required ratio between tip and stalk cells for developing a functional blood vessel.
Overexpression of Jagged Leads to a Hybrid Tip/Stalk Phenotype.
Next, we investigate the role of Jagged alongside Delta in the tip-stalk decision making for the one-cell system. We evaluate a phase diagram as a function of parameters: both the levels of external Delta () and the different production levels of the ligands— (production rate of Jagged) and (production rate of Delta). Our results suggest that overexpression of Jagged leads to the emergence of a previously unidentified phenotype: a hybrid tip/stalk fate (marked by the {tip/stalk} phase), where the cell expresses intermediate levels of the proteins N, D, J, I, , and V (Fig. 3 A–C and SI Appendix, Fig. S5A). A similar hybrid state is obtained at low production rate of Delta (Fig. 3 D–F and SI Appendix, Fig. S5B), therefore suggesting that the relative production rate between Delta and Jagged in a cell determines the existence of this hybrid tip/stalk phenotype (Fig. 3 C and D and SI Appendix, Fig. S5). Consistently, at a higher production rate of Delta compared with that of Jagged, the circuit is bistable only, and therefore the cell can adopt either a tip or a stalk phenotype (marked by the {tip, stalk} phase), but not the hybrid tip/stalk one (Fig. 3 A and F).
Fig. 3.
Dynamical characteristics of the one-cell system for different levels of production rates of the ligands. Bifurcation curves represent the levels of Delta in response to varying for different production rates of the ligands Delta and Jagged. (A) , ; (C) , ; (D) , ; and (F) , (all units in molecules/h). The phenotype diagrams (center) show the different possible phases when the circuit is driven by variable levels of external Delta (), production rate of Delta (), and that of Jagged (). (B) Phenotype diagram for variable levels of external Delta () and production rate of Jagged (). (E) Phenotype diagram for variable levels of external Delta () and production rate of Delta (). Bifurcation curve of the levels of VEGF receptor (), active VEGF signaling (V), NICD (I), and Jagged (J) for cases C and D are included in SI Appendix, Fig. S5.
We further investigate the dynamics of the circuit for two cells interacting via N-D-J signaling for different values of the production rate of ligands Delta () and Jagged () and fixed levels of . Our results indicate that at low production rates of both Delta and Jagged, both cells attain the stalk fate (both cells in monostable {stalk} phase). However, in the case of a higher production rate of Delta, one cell adopts the tip position, whereas the other become a stalk (bistable {tip, stalk} phase), but when the production rate of Jagged is high, both cells attain the hybrid tip/stalk phenotype (both cells in monostable {tip/stalk} phase), thereby being consistent with the canonical role of Notch-Delta in diversifying cell fates (tip and stalk in this context) and that of Notch-Jagged in unifying them (the hybrid tip/stalk here) (7, 15, 19) (SI Appendix, Fig. S6).
The hybrid tip/stalk phenotype, obtained under high levels of Jagged, is reminiscent of and might correspond to the tip-like thin cytoplasmic projections that extend across the vessel lumen of the tumor endothelium but not necessarily a nontumor endothelium (25).
Overexpression of Jagged Destabilizes the Tip and Stalk Cell Fates.
To elucidate how overexpression of Jagged affects the stability of the three different cell fates (tip, stalk, and hybrid tip/stalk), we represent the phase space of two interacting cells by an effective potential. The phase space is presented in terms of the levels of Delta of each cell (, ), such that (high , low ) corresponds to cell 1 as a tip cell and cell 2 as a stalk cell, and vice versa. The z axis represents the effective potential that is defined as , where is the probability density in the 2D phase space ( × ) (26–28). This probability is calculated by using the Euler–Maruyama method to approximate the ordinary differential equation to a stochastic differential equation that can evaluate the behavior of the cells in the presence of biological noise. In this representation, a deep basin of attraction represents that the corresponding cell fate or steady state is very stable, or in other words, the cell is not likely to switch its fate to a different one unless under a large amount of biological noise. Conversely, a shallow basin of attraction facilitates a more dynamic fate exchange (plasticity).
Using this representation, we found that a two-cell system communicating via N-D-J signaling and responding to external VEGF behaves differently for different values of the production rates of Jagged (). At low production rates of Jagged in both cells, the system has two stable steady states, both of which comprises of one cell in the tip (high levels of Delta) fate or phenotype, and the other in stalk (low levels of Delta) phenotype. In one of these two states, cell 1 is a tip cell, and cell 2 is a stalk cell (high , low ); and in the other state, its vice versa: cell 2 is a tip cell, and cell 1 is a stalk cell (low , high ). Both these states have a deep potential or basin of attraction, suggesting that these cell fates are very stable and that a large perturbation is required such that the tip cell (irrespective of whether cell 1 or cell 2 is the tip cell) loses its position, or in other words, changes its cell fate (Fig. 4A). These results are consistent with previous experimental and theoretical observations that VEGF-VEGFR-Dll4-Notch-VEGFR intercellular feedback loop can mediate a stable tip and stalk fate decision (11, 29), especially under conditions of nonpathological angiogenesis: low Jag1 and VEGF levels.
Fig. 4.
3D representation of the effective potential as a function of Delta in cell 1 () and in cell 2 (). The effective potential is defined as , where is the probability density calculated by solving the differential equations stochastically using the Euler–Maruyama method. A represents the case of low production rate of Jagged ( molecules/h). B–D represent increasingly high production rates of Jagged: molecules/h, molecules/h, and molecules/h, respectively. (E) Cell fate exchange rate (a measure of plasticity of the system) for increasing values of production rates of Jagged (). (F) Cell fate exchange rate for increasing values of production rates of Delta (). Red dot represents the standard value as presented in SI Appendix, Table S1.
However, as Jagged levels in the cells increase due to increased , the potential for these two states becomes increasingly shallow, and only a small amount of noise can be sufficient to induce a cell fate transition or exchange, i.e., a tip cell can become a stalk cell and vice versa (Fig. 4 B and C), hence indicating that high levels of Jagged in the cells destabilize the tip and stalk cell fates and facilitate a dynamic competition for the tip position. For very high levels of Jagged, both cells no longer maintain their distinct tip and stalk states or phenotypes, but rather adopt the intermediate tip/stalk state with intermediate levels of Delta (Fig. 4D).
Further, we calculate how the two ligands Delta and Jagged differently regulate the switching of cell fates between tip and stalk fates. Experimental observations on dynamic lateral inhibition shows that the cell at the tip position is replaced by another cell in ∼2 h (13), i.e., the cell fate exchange rate is around 0.5/h. We first determine the amount of noise in this two-cell system that can allow a fate exchange rate of 0.5/h (for and molecules/h; SI Appendix, Table S1) and then calculate this rate for different values of the production of Jagged () and Delta (), with both cases explored for the same level of noise as determined earlier. We observed that an increase in increases this tip position exchange rate (Fig. 4E). Oppositely, an increase in the production rate of Delta () significantly decreases the same (Fig. 4F).
JAG1 (Jagged) and DLL4 (Delta) have been reported to play opposite roles during angiogenesis (14). Thus, unlike high levels of Jagged, high levels of Delta lead to a lower tip position exchange rate and more stable tip and stalk cell fates, therefore suggesting mutually competing roles of the two ligands in stabilizing the tip and stalk cell fates (Fig. 4F and SI Appendix, Fig. S7).
Production Rate of the Two Ligands Regulate Angiogenesis Differently.
Next, we evaluate the dynamics of the circuit at the tissue level, i.e., an array of cells interacting via the N-D-J signaling. We considered the case of a 2D layer of interacting identical cells exposed to a fixed level of external VEGF (). For low levels of (production rate of Jagged), there are, on average, more than one stalk cell between two tip cells, thereby allowing adequate development of the lumen (that is comprised of stalk cells) and hence a proper and robust development of the vessel branch: the case of physiological angiogenesis. However, as the production rate of Jagged () increases, some cells adopt the hybrid tip/stalk phenotype. These cells, with somewhat compromised tip characteristics, are expected to develop less filopodia and migrate less than the tip cells, however, yet initiate a sprout; therefore, the vessels led by these cells are expected to be relatively smaller and poorly perfused. Thus, one would expect proper development of vessels but with a higher vessel density: the case of suboptimal angiogenesis. Last, when Jagged is overexpressed, most cells can adopt the hybrid tip/stalk phenotype, leading to an excessive number of small blood vessels with quite poor perfusion: a case of nonproductive or pathological angiogenesis as typically observed in cancer (Fig. 5 A–C and SI Appendix, Fig. S8).
Fig. 5.
Patterning at the tissue level. (A) Cartoon representation of physiological, suboptimal, and pathological angiogenesis. In physiological angiogenesis, two tip cells are separated by a few stalk cells, allowing a proper and robust development of the blood vessel. In the suboptimal case, angiogenesis is increased by a decrease in the number of stalk cells and the emergence of some hybrid tip/stalk cells that lead to some small blood vessels and poor perfusion. For pathological angiogenesis, an excessive number of tip/stalk cells lead to a large number of small blood vessels, leading to excessive but nonproductive angiogenesis. (B) Average of the fraction of cells in (tip), (tip/stalk), or (tip) state as a function of the production of Jagged (). (C) Cartoon representation of 1D layer of interacting cells for increased values of . (D) Average of the fraction of cells in (tip), (tip/stalk), or (tip) state as a function of the production of Delta (). (E) Cartoon representation of 1D layer of interacting cells for increased values of . The averages were taken over 100 simulations of a 2D layer of 100 × 100 interacting cells with a periodic boundary condition. The states of the cells are defined according to the amount of VEGF signal (V): stalk, ; tip/stalk, ; and tip, molecules. Bidimensional patterning figures representing the levels of V, I, J, and D are presented in SI Appendix, Fig. S8.
The exact opposite results are observed when varying the production rate of Delta (). High and intermediate levels of ensure physiological angiogenesis; but for low levels of , the number of the hybrid tip/stalk cells increase, thus giving rise to many sprouts but a poorly perfused chaotic network, representing nonproductive or pathological angiogenesis (Fig. 5 A, D, and E). Our results are consistent with experimental evidence showing that deletion or inhibition of DLL4 promotes nonproductive angiogenesis with poorly perfused vessels (30, 31). It may be noted that here we do not consider the effect of proliferation of stalk cells and that of VEGF gradient: two key factors that can alter the number of tip cells and stalk cells, as well as their spatial distribution.
Interplay Between Notch Signaling and the VEGF Gradient Guides the Selection of Tip Cell.
Besides the production rates of the two ligands, VEGF gradient has been shown to influence the vascular patterning (the spatial distribution of the tip and stalk cells) (12). Therefore, we next incorporate a VEGF gradient in our two-cell system to evaluate how it alters the relative stability of the different cell fates the cells attain. Unlike previous cases, now, cell 2 is exposed to a higher external VEGF signal () compared with cell 1 (Fig. 6A). Similar to the earlier case of equal for both cells (Fig. 4B), we observed two stable steady states: (high , low ) or that cell 1 is a tip cell and cell 2 is a stalk cell and (low , high ) or that cell 1 is a stalk cell and cell 2 is a tip cell. However, in this case, both these stable states are not equally stable; rather, the (low , high ) state is more stable than the (low , high ) state, or in other words, the cell that receives higher levels of external VEGF signal, cell 2, is more likely to be the leading tip cell (Fig. 6B). Therefore, the Notch-VEGF interplay tends to ensure that the leading cell of a vascular sprout moves in the direction of the upward gradient of VEGF. We further show that the fate exchange rate decreases with the increase in steepness of the VEGF gradient, indicating that the cell that receives higher VEGF signal is more likely to be a tip cell and maintain its fate (Fig. 6C).
Fig. 6.
Effect of VEGF gradient on tip and stalk fate decision. (A) Cartoon representation. We simulate two cells interacting via Notch signaling in the presence of a VEGF gradient: cell 2 receives more VEGF-A signal than cell 1. (B) Effective potential representation for the case of molecules for cell 1 and molecules for cell 2. (C) Fate exchange rate for different values of for cell 2, whereas for cell 1 remains constant ( molecules).
Fringe Stabilizes the Tip and Stalk Cell Fates.
Fringe is a glycosyltransferase protein that is activated by NICD. It mediates the posttranslational modifications of Notch and consequently modulates the binding of Notch to Delta and to Jagged. The glycosylated (or Fringe-modified) Notch has a higher binding affinity to Delta but lower binding affinity to Jagged (20, 21). To evaluate the role of the glycosyltransferase Fringe in the tip-stalk fate decisions, we calculate the effective potential of a two-cell system interacting via N-D-J signaling and under the influence of fixed external VEGF levels. Including the effect of Fringe makes the basin of attraction of the two states—(high , low ) and (low , high )—deeper, thereby stabilizing the tip and stalk fates (Fig. 7 A and B). We further evaluate the effect of Fringe at the tissue level and show that loss of Fringe leads to an increase in the number of cells in the hybrid tip/stalk phenotype, thereby leading to small and poorly perfused blood vessels, typical of tumor angiogenesis (Fig. 7 C and D). Importantly, this stabilization effect of Fringe is observed even when Fringe is not included in the model as a downstream target of NICD, but rather as an independent variable (SI Appendix, Fig. S9).
Fig. 7.
Effect of Fringe on tip and stalk fate decision. (A) 3D representation of the effective potential as a function of Delta in cell 1 () and in cell 2 () for the case of no Fringe effect (, i.e., ). B represents the effective potential after including Fringe effect (, i.e., , ). The state with high and low , i.e., the one with high levels of Delta in cell 1 but not in cell 2, corresponds to (cell 1 as tip and cell 2 as stalk); the state with high and low corresponds to (cell 1 as stalk and cell 2 as tip). (C) Average of the fraction of cells in (stalk), (tip/stalk), or (tip) state as a function of the Fringe effect. The averages were taken over 100 simulations of a 2D layer of 100 × 100 interacting cells in a square lattice with periodic boundary conditions. (D) Cartoon representation of a 1D layer of interacting cells for increased values of the effect of Fringe. The states of the cells are defined according to the amount of active VEGF signaling (V): stalk (), tip/stalk (), and tip ( molecules). The Fringe effect is represented by the variable f. The case represents the no Fringe effect, i.e., , i.e., binding affinity of Notch to Delta and to Jagged is the same. As f increases, the values of and linearly increase and decrease, respectively, such that at , and (SI Appendix, Table S1), i.e., Notch has higher binding affinity to Delta and lower to Jagged. Therefore, () and ().
These results offer an explanation into why aggressive tumor types such as basal-like breast cancer often show a loss of Fringe (32–34) and have increased microvessel density (MVD) and high microvessel proliferation (MVP) compared with the relatively less aggressive ER-positive and HER2-driven subtypes (35).
cis-Inhibition Stabilizes the Tip and Stalk Cell Fates.
cis-Inhibition, the intracellular binding and consequent degradation of the Notch receptor and ligands (both Delta and Jagged), has been considered to be critical for lateral inhibition and pattern formation in multiple developmental contexts (36, 37). However, its role in angiogenesis remains enigmatic. cis-Inhibition between Notch and Jagged in the stalk cells has been suggested to compromise the tip-to-stalk signaling (14). Thus, we decided to explore the role of cis-inhibition between Notch and Delta (N-D) and Notch and Jagged (N-J) both individually and together in the context of the tip selection process during angiogenesis.
To evaluate the role of cis-inhibition between Notch and both its ligands Dll4 and Jag1 in angiogenesis, we analyze its effect on the stability of the tip and stalk cell fates, by representing the phase space by an effective potential for the case of both lower and higher cis-inhibition rate (). In both cases, two stable states are present: one cell as tip and the other as stalk and vice versa [(high , low ), and (low , high )]. However, at higher values of , the basin of attraction for the stable states are deeper, therefore suggesting that cis-inhibition has an important role in stabilizing the tip position (Fig. 8). These results are consistent with previous experimental and theoretical observations that cis-inhibition facilitates pattern formation and usually confers a greater robustness to noise during adoption of alternate fates between neighboring cells (36, 38, 39).
Fig. 8.
Effect of cis-inhibition on tip and stalk fate decision. (A) 3D representation of the effective potential as a function of Delta in cell 1 () and in cell 2 () for the case of a decrease in 10% of the cis-inhibition strength compared with its standard value (). B represents the case of an increase in 10% of the cis-inhibition strength (). The state with high and low , i.e., the one with high levels of Delta in cell 1 but not in cell 2, corresponds to (cell 1 as tip and cell 2 as stalk); that with high and low corresponds to (cell 1 as stalk and cell 2 as tip).
We also evaluate the effect of cis-inhibition between Notch-Delta and Notch-Jagged individually, by changing only for N-D interactions (SI Appendix, Fig. S10 A and B), and then only for N-J interactions (SI Appendix, Fig. S10 C and D). Our results suggest that N-J cis-inhibition stabilizes the tip and stalk cell fates much strongly compared with N-D cis-inhibition, i.e., the increase in (cis-inhibition of N-J interactions only) leads to a much deeper potential well (or basin of attraction) for the two states—(high , low ) and (low , high )—whereas increasing (cis-inhibition of N-D interactions only) has little effect (SI Appendix, Fig. S10 A and B), again highlighting the fact that high Jagged levels can destabilize the tip and stalk cell fates and contribute to the rich cellular plasticity and chaotic behavior of tumor-mediated angiogenesis.
It has been speculated that cis-inhibition between Notch and Jagged in the stalk cells would reduce the signaling ability of Delta from the tip cell and hence compromise the tip-to-stalk signaling (14). Our results, however, suggest the opposite, i.e., that cis-inhibition has a fundamental role in stabilizing the tip position. More specifically, we suggest that Notch-Delta cis-inhibition has relatively little effect in the stability of tip cells, probably due to the low levels of Notch receptor in the tip cells. In contrast, Notch-Jagged cis-inhibition has an important role in stabilizing the tip position, because it decreases the probability of tip and stalk cells communicating via Notch and Jagged, hence reducing the levels of NICD in the tip cells. Reduced NICD implies increased VEGFR2 and consequently high Dll4 in tip cells, thereby stabilizing the tip cell fate. If N-J cis-inhibition was low, dynamic competition for tip position would be elevated.
Discussion
Notch and VEGF signaling pathways play a crucial role during tip-stalk cell fate decisions in both physiological and pathological angiogenesis (1, 12). However, the underlying principles of tip-stalk fate selection mediated by the interplay of Notch and VEGF pathways remains largely elusive. Here, we introduced a specific theoretical framework to study this interplay. We show that tip-stalk decision is not a binary one; rather, cells can adopt a hybrid tip/stalk phenotype, when Notch-Jagged signaling dominates over Notch-Delta signaling. This phenotype can lead to form a new sprout but has a compromised ability to migrate and develop filopodia, thereby leading to poorly perfused blood vessels with high MVD. Therefore, the hybrid tip/stalk phenotype offers a key advantage in pathological conditions: it can confer rich plasticity to the leading cell that can rapidly exchange its position with a neighbor stalk, therefore inducing a fast but irregular vessel branch that can quickly supply oxygen in fast growing tumors. When many cells adopt this hybrid phenotype, the vasculature is expected to be quite chaotic: excessive number of small but poorly perfused vessels, resulting in pathological angiogenesis as observed during tumor growth (40). Therefore, our results offer a good unifying explanation for many experimental observations: (i) loss of Jagged significantly decreases vascular branching (14), (ii) loss of Delta leads to excessive nonproductive or poorly perfused angiogenesis (16), and (iii) loss of Fringe is correlated with increased MVD in tumors (35).
Our results also attempt to resolve an apparent paradox between the canonical roles of Notch-Delta and Notch-Jagged signaling and the experimental observations about the overexpression of Delta and Jagged in angiogenesis. Neighboring cells interacting via Notch-Jagged signaling usually adopt a similar cell fate (lateral induction) (6, 8), whereas those interacting via Notch-Delta signaling adopt opposite fates (lateral inhibition) (7). Consequently, increased production of Jagged would be expected to reinforce the lateral induction mechanism between the stalk cells, hence elongating the lumen; increased production of Delta would lead to more tip cells. However, the experimental results are the exact opposite: i.e. higher Jagged levels increase vascular branching (14), and Dll4 acts as a brake on sprouting angiogenesis (16). These conflicting observations can be explained by the emergence of a hybrid tip/stalk phenotype on overexpression of Jagged. Cells in this hybrid phenotype can lead the formation of a vessel, albeit not so efficiently, thereby leading to more vascular branching. Overexpression of Delta can prevent cells from adopting this hybrid tip/stalk phenotype and can hence inhibit angiogenesis.
The emergence of a hybrid tip/stalk phenotype also lends support to the emerging notion “a black and white distinction between tip and stalk cells is an oversimplification” (1) and strengthens the increasingly accepted notion that a hybrid state that coexpresses markers of two lineages is a signature of enhanced plasticity (multipotency) of a system (41–43). We find that the tendency to adopt this hybrid phenotype is reduced at high levels of Fringe, a glycosyltransferase that promotes Notch-Delta signaling at the expense of Notch-Jagged signaling by modifying Notch to increase its affinity for Delta and decrease it for Jagged. Thus, Fringe stabilizes tip and stalk fates and can help promote physiological angiogenesis, hence acting as a critical molecular brake on deregulated/pathological angiogenesis. Loss of this brake, as seen in aggressive tumors such as basal-like breast cancer (32–34), can enable tumors to attain sustained angiogenesis (35), which is a hallmark of cancer (44). Overall, our results about Fringe are also consistent with experimental and theoretical observations that Fringe promotes lateral inhibition patterns (19, 45) and are reminiscent of how asymmetric modifications of transmembrane ligand-receptor pairs can govern tissue-level pattern formation (46).
The importance of Notch-Jagged signaling in delineating the difference between normal and tumor angiogenesis is further revealed by the role of cis-inhibition, specifically that between Notch and Jagged, in affecting tip selection. cis-Inhibition between Notch and Delta has been reported to offer greater robustness to noise during patterning (36), but ours is the first study, to the best of our knowledge, exploring the role of cis-inhibition between Notch and Jagged. Our results indicate that cis-inhibition between Notch-Jagged stabilizes tip-and-stalk fates more strongly than that between Notch-Delta, hence underlining the role of maintaining low levels of Jagged1 to ensure smooth and functional, i.e., physiological angiogenesis.
The critical role of overexpression of Jagged1 in mediating such abnormal angiogenesis might explain why tumor-stroma interplay often involves Notch-Jagged signaling (47). The increased Notch-Jagged signaling in tumor environment can be attributed to multiple specific traits of tumor endothelial cells (TECs): (i) they can secrete Jagged in the stroma (48) that can potentially activate Notch-Jagged signaling in neighboring endothelium; (ii) they have a proinflammatory gene expression and the inflammation response regulators such as NF-κB and TNF-α can increase Jagged in them (14, 49); and (iii) they often adhere to inflammatory cells such as macrophages (25) that can increase Jagged in them via paracrine or juxtacrine signaling. Such an amplified Notch-Jagged signaling can give rise to hybrid tip/stalk cells that closely resemble the observed tip-like projections of the tumor vessels that might overlap with each other and even form loose connections (25).
As discussed here, whereas some predictions of our model are consistent with reported experimental results, the model offers some previously untested hypotheses that can be tested experimentally. Specifically, we predict that the interactions that cause enhanced Notch-Jagged signaling, such as overexpression of Jag1, repression of Dll4, and inhibition of Fringe, should lead to a more dynamic switching between tip and stalk cell fates, because all these cases can cause a larger number of cells to adopt the hybrid tip/stalk phenotype, hence enriching cellular plasticity. It might be noted that among the three different homologs of Fringe in mammals, the role of Lfng (Lunatic fringe) and Manic fringe (Mfng) might be more pertinent than that of Rfng (Radical fringe), as they both can promote N-D signaling (50).
To conclude, our theoretical bottom-up modeling framework offers important insights into the molecular interplay between Notch and VEGF signaling in regulating cell fate decisions during both physiological and pathological angiogenesis. Albeit we do not consider any spatial effects into account, our framework is amenable to be integrated with agent-based models on angiogenesis (29) and can be used, in an iterative way with experiments, to decipher the organizing principles of multilayer process of angiogenesis (51). Specifically, as reported here, the crucial role of Notch-Jagged signaling in mediating differences between physiological and pathological angiogenesis can be used for novel therapeutic benefits such as developing decoys that can target JAG/NOTCH selectively as recently attempted (52). Such attempts are likely to be more specific in targeting tumor angiogenesis and hence provide a viable and safer alternative to disrupting Notch signaling altogether (both via Delta and Jagged), a hallmark of most antiangiogenesis efforts.
Materials and Methods
The equations for the mathematical model are presented in The Theoretical Framework. The values of the parameters used for the model are given in SI Appendix, section S1. Model construction is discussed in SI Appendix, section S2; and the sensitivity analysis for the model is presented in SI Appendix, section S5. The computational analysis was performed in Python.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Grants PHY-1427654 and NSF-MCB-1214457 and the Cancer Prevention and Research Institute of Texas. M.B. was also supported by FAPESP (Sao Paulo Research Foundation) Grant 2013/14438-8. E.B.-J. was also supported by the Tauber Family Funds and the Maguy-Glass Chair in Physics of Complex Systems.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511814112/-/DCSupplemental.
References
- 1.Benedito R, Hellström M. Notch as a hub for signaling in angiogenesis. Exp Cell Res. 2013;319(9):1281–1288. doi: 10.1016/j.yexcr.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Phng LK, Gerhardt H. Angiogenesis: A team effort coordinated by notch. Dev Cell. 2009;16(2):196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138(21):4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 4.Andersson ER, Sandberg R, Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011;138(17):3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 5.Shimojo H, Ohtsuka T, Kageyama R. Dynamic expression of notch signaling genes in neural stem/progenitor cells. Front Neurosci. 2011;5:78. doi: 10.3389/fnins.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manderfield LJ, et al. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2012;125(2):314–323. doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaya O, Sprinzak D. From Notch signaling to fine-grained patterning: Modeling meets experiments. Curr Opin Genet Dev. 2011;21(6):732–739. doi: 10.1016/j.gde.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopan R, Ilagan MXG. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales AV, Yasuda Y, Ish-Horowicz D. Periodic Lunatic fringe expression is controlled during segmentation by a cyclic transcriptional enhancer responsive to notch signaling. Dev Cell. 2002;3(1):63–74. doi: 10.1016/s1534-5807(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 11.Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 12.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3(1):a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakobsson L, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12(10):943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 14.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Boareto M, et al. Jagged-Delta asymmetry in Notch signaling can give rise to a Sender/Receiver hybrid phenotype. Proc Natl Acad Sci USA. 2015;112(5):E402–E409. doi: 10.1073/pnas.1416287112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchting S, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104(9):3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, Jolly MK, Levine H, Onuchic JN, Ben-Jacob E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc Natl Acad Sci USA. 2013;110(45):18144–18149. doi: 10.1073/pnas.1318192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu M, Jolly MK, Onuchic J, Ben-Jacob E. Toward decoding the principles of cancer metastasis circuits. Cancer Res. 2014;74(17):4574–4587. doi: 10.1158/0008-5472.CAN-13-3367. [DOI] [PubMed] [Google Scholar]
- 19.Jolly MK, et al. Operating principles of Notch-Delta-Jagged module of cell-cell communication. New J Phys. 2015;17(5):055021. [Google Scholar]
- 20.Shimizu K, et al. Manic fringe and lunatic fringe modify different sites of the Notch2 extracellular region, resulting in different signaling modulation. J Biol Chem. 2001;276(28):25753–25758. doi: 10.1074/jbc.M103473200. [DOI] [PubMed] [Google Scholar]
- 21.Hicks C, et al. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol. 2000;2(8):515–520. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- 22.Prez F, Granger BE. IPython: A system for interactive scientific computing. Comput Sci Eng. 2007;9(3):21–29. [Google Scholar]
- 23.Clewley R. Hybrid models and biological model reduction with PyDSTool. PLOS Comput Biol. 2012;8(8):e1002628. doi: 10.1371/journal.pcbi.1002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siekmann AF, Affolter M, Belting HG. The tip cell concept 10 years after: New players tune in for a common theme. Exp Cell Res. 2013;319(9):1255–1263. doi: 10.1016/j.yexcr.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2(3):a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Wang J. Quantifying Waddington landscapes and paths of non-adiabatic cell fate decisions for differentiation, reprogramming and transdifferentiation. J R Soc Interface. 2013;10(89):20130787. doi: 10.1098/rsif.2013.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Zhang K, Xu L, Wang E. Quantifying the Waddington landscape and biological paths for development and differentiation. Proc Natl Acad Sci USA. 2011;108(20):8257–8262. doi: 10.1073/pnas.1017017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KY, Wang J. 2007 Potential energy landscape and robustness of a gene regulatory network: Toggle switch. PLoS Comput Biol 3(3):e60. [Google Scholar]
- 29.Bentley K, Gerhardt H, Bates PA. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol. 2008;250(1):25–36. doi: 10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Noguera-Troise I, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 31.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7(5):327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 32.Xu K, et al. Lunatic fringe deficiency cooperates with the Met/Caveolin gene amplicon to induce basal-like breast cancer. Cancer Cell. 2012;21(5):626–641. doi: 10.1016/j.ccr.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi F, Amarasinghe B, Dang TP. Manic fringe inhibits tumor growth by suppressing Notch3 degradation in lung cancer. Am J Cancer Res. 2013;3(5):490–499. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Chung WC, Wu G, Egan SE, Xu K. Tumor-suppressive activity of Lunatic Fringe in prostate through differential modulation of Notch receptor activation. Neoplasia. 2014;16(2):158–167. doi: 10.1593/neo.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krüger K, et al. Microvessel proliferation by co-expression of endothelial nestin and Ki-67 is associated with a basal-like phenotype and aggressive features in breast cancer. Breast. 2013;22(3):282–288. doi: 10.1016/j.breast.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Barad O, Rosin D, Hornstein E, Barkai N. Error minimization in lateral inhibition circuits. Sci Signal. 2010;3(129):ra51. doi: 10.1126/scisignal.2000857. [DOI] [PubMed] [Google Scholar]
- 37.Miller AC, Lyons EL, Herman TG. cis-Inhibition of Notch by endogenous Delta biases the outcome of lateral inhibition. Curr Biol. 2009;19(16):1378–1383. doi: 10.1016/j.cub.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprinzak D, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465(7294):86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprinzak D, Lakhanpal A, LeBon L, Garcia-Ojalvo J, Elowitz MB. Mutual inactivation of Notch receptors and ligands facilitates developmental patterning. PLOS Comput Biol. 2011;7(6):e1002069. doi: 10.1371/journal.pcbi.1002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou JX, Huang S. Understanding gene circuits at cell-fate branch points for rational cell reprogramming. Trends Genet. 2011;27(2):55–62. doi: 10.1016/j.tig.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Grosse-Wilde A, et al. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS One. 2015;10(5):e0126522. doi: 10.1371/journal.pone.0126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolly MK, et al. Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. J R Soc Interface. 2014;11(101):20140962. doi: 10.1098/rsif.2014.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Nikolaou N, et al. Lunatic fringe promotes the lateral inhibition of neurogenesis. Development. 2009;136(15):2523–2533. doi: 10.1242/dev.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jolly MK, Rizvi MS, Kumar A, Sinha P. Mathematical modeling of sub-cellular asymmetry of fat-dachsous heterodimer for generation of planar cell polarity. PLoS One. 2014;9(5):e97641. doi: 10.1371/journal.pone.0097641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Front Oncol. 2014;4:254. doi: 10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23(2):171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston DA, Dong B, Hughes CCW. TNF induction of jagged-1 in endothelial cells is NFkappaB-dependent. Gene. 2009;435(1-2):36–44. doi: 10.1016/j.gene.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LeBon L, Lee TV, Sprinzak D, Jafar-Nejad H, Elowitz MB. Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. eLife. 2014;3:e02950. doi: 10.7554/eLife.02950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bentley K, Jones M, Cruys B. Predicting the future: Towards symbiotic computational and experimental angiogenesis research. Exp Cell Res. 2013;319(9):1240–1246. doi: 10.1016/j.yexcr.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Kangsamaksin T, et al. NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov. 2015;5(2):182–197. doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.