Significance
According to the World Health Organization, anxiety and depressive disorders are a leading source of disability, affecting hundreds of millions of people. Children can inherit an extremely anxious temperament, which is a prominent risk factor for the later development of anxiety, depression, and comorbid substance abuse. This study uses high-resolution functional and structural imaging in our well-established developmental nonhuman primate model to identify the heritable neural substrate that underlies extreme childhood anxious temperament. Using a large multigenerational family pedigree, genetic correlation analyses revealed a tripartite neural circuit where metabolism likely shares a genetic substrate with early-life dispositional anxiety. Interestingly, we found that brain function—not structure—is the critical intermediary between genetics and the childhood risk to develop stress-related psychopathology.
Keywords: anxiety, primate, heritability, positron emission tomography, brain volume
Abstract
Understanding the heritability of neural systems linked to psychopathology is not sufficient to implicate them as intergenerational neural mediators. By closely examining how individual differences in neural phenotypes and psychopathology cosegregate as they fall through the family tree, we can identify the brain systems that underlie the parent-to-child transmission of psychopathology. Although research has identified genes and neural circuits that contribute to the risk of developing anxiety and depression, the specific neural systems that mediate the inborn risk for these debilitating disorders remain unknown. In a sample of 592 young rhesus monkeys that are part of an extended multigenerational pedigree, we demonstrate that metabolism within a tripartite prefrontal-limbic-midbrain circuit mediates some of the inborn risk for developing anxiety and depression. Importantly, although brain volume is highly heritable early in life, it is brain metabolism—not brain structure—that is the critical intermediary between genetics and the childhood risk to develop stress-related psychopathology.
Parents with anxiety and depressive disorders are considerably more likely to have children with an extremely anxious temperament (AT) (1–3). Extreme-AT children have heightened behavioral and physiological reactivity to potential threat and have a markedly increased risk to develop anxiety and depressive disorders (4, 5). These disorders emerge as inborn tendencies and environmental factors converge to disrupt the neural systems that mediate adaptive anxiety; as many as 50% of children with extreme-AT develop a psychiatric disorder (6). In addition to environmental influences that facilitate the cross-generational transfer of psychopathology (e.g., parent–child interactions), genetic variance accounts for ∼35% of the likelihood that a child will develop an anxiety disorder (7, 8) The neural substrates of AT are distributed throughout the brain and range from primitive brainstem structures to primate-specific cortical subfields. Multiple brain regions causally contribute to AT, and damage to any one of these regions is sufficient to decrease, although not abolish, anxiety (9–14). Thus, the inherited risk to develop stress-related psychopathology likely manifests via its effects on multiple components of the neural circuit underlying AT. Here we use a genetic correlation approach to identify brain regions where function and structure contribute to the intergenerational transmission of AT. Genetic correlation analyses are crucial for identifying regions that are likely to mediate the genetic contributions to AT, and to distinguish them from regions that, although heritable, rely on an independent set of genetic variations.
The recent evolutionary divergence of humans and rhesus monkeys is reflected in their shared capacity for higher-order cognition, complex social behavior, and homologous neural circuits, which make the young rhesus monkey an ideal model for understanding the neural substrates of childhood AT. Our group developed and validated a paradigm for studying the neural bases of primate AT that combines [18-F] deoxyglucose positron emission tomography (FDG-PET) imaging with behavioral and neuroendocrine responses to a potentially threatening human intruder making no eye contact (NEC) with the monkey. The NEC context is designed to elicit naturalistic adaptive defensive behaviors and captures the evolutionarily conserved tendency of high-AT individuals to inhibit behaviors that otherwise could attract the attention of potential predators. Because it measures brain metabolism over ∼30 min, FDG-PET imaging is ideally suited to examine the sustained neural responses that underlie trait-like measures, such as AT. Following FDG injection, the monkey is placed in the NEC context. As FDG is taken-up into metabolically active cells, the monkey behaves freely in the NEC context, revealing its anxious disposition. The post-NEC PET scan measures the integrated brain metabolism that occurred during exposure to the ethologically relevant NEC context.
To identify the brain regions that underlie the parent-to-child transmission of psychopathology, it is critical to understand the pattern of cosegregation between the AT phenotype and its neural circuit alterations within the family tree. This approach first requires demonstrating that the phenotype is heritable, identifying brain regions associated with the phenotype, and determining which of these brain regions are heritable. Once this is accomplished, a genetic correlation analysis between brain function/structure and the phenotype is critical to identify the neural mediators of the heritable parent-to-child transfer of risk.
Following this strategy in a large familial sample of young rhesus monkeys, we (i) demonstrate the heritability of AT, (ii) identify neuroimaging measures that predict AT, (iii) assess the heritability of the relevant brain structural and functional measures, and finally, (iv) perform the relevant genetic correlation analyses between the neuroimaging measures and AT. To this end, NEC FDG-PET and structural brain imaging were performed in our large sample of 592 young rhesus monkeys from a large multigenerational pedigree (age: mean = 1.88 y; 327 males/265 females). Paralleling work in children, the monkey AT phenotype encompasses behavioral (freezing), communicative (decreased cooing), and physiological (increased cortisol) responses to potential threat. Specifically, our composite measure of AT was operationalized as the mean of the monkey’s relative freezing levels, inhibition of coo vocalizations, and plasma cortisol concentration (15–18). In humans, the features of AT, extreme behavioral inhibition, and heightened cortisol levels are early risk factors for the later development of anxiety and depressive disorders (6, 19–21). Children who respond to strangers and novel situations with excessive apprehension or physiological arousal are likely to modify their behavior in ways that are maladaptive and over time are indicative of stress-related psychopathology. Similar to humans, monkeys with extreme AT appear to be functionally impaired across laboratory and naturalistic social settings, making the rhesus monkey model of AT ideal for understanding the pathophysiology that underlies the risk to develop anxiety and depression (21).
Heritability of AT was estimated as the proportion of variation in the phenotype explained by the coefficient of relatedness in the extended multigenerational pedigree (see ref. 22 and Methods for details). This extended pedigree approach is powerful because it accounts for the phenotypic similarity of both close and distant relationships (see SI Appendix, SI Methods for details). In this preadolescent sample, there was no significant effect of sex on AT (t = 0.830, P = 0.407), but there was a significant decrease in AT with age (t = −10.013, P < 0.001). Accordingly, all analyses controlled for nuisance variance in age, age2, sex, and the age × sex interaction. Results demonstrated that the AT phenotype is heritable (AT: h2 = 0.29, P < 0.0001), consistent with prior findings in young monkeys as well as the heritability estimates for human anxiety and anxiety disorders (7, 23, 24).
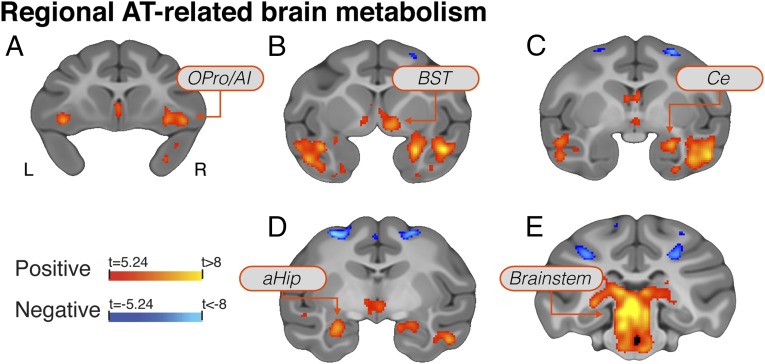
To identify brain regions where metabolism contributes to the cross-generational transmission of AT, it is first necessary to identify the regions where these measures predict variation in the anxious phenotype. Voxelwise robust regressions were performed between AT and FDG-PET. Results revealed significant relations between metabolism and AT (P < 0.05 Šidák-corrected; also see SI Appendix, Results and Discussion) in regions of the orbital frontal (OFC) and anterior insular (AI) cortices [including orbital proisocortex (OPro)/AI, as well as cytoarchitectonic areas 11/13/47], amygdala [including the central nucleus of the amygdala (Ce)], anterior hippocampus (aHip), bed nucleus of stria terminalis (BST), as well as midbrain regions that encompass the periaqueductal gray (PAG) (Fig. 1 and SI Appendix, Table S1; full voxelwise maps are available in Dataset S1). Importantly, these AT-related regions include areas that causally contribute to AT in mechanistic studies, including: orbital-prefrontal cortical areas involved in emotional valuation (25); the extended amygdala, an interface between emotions and their behavioral and physiological expression (26); and brainstem regions, including PAG, which are the downstream effectors required for the expression of defensive responses (27).
Fig. 1.
Regions where brain metabolism was significantly associated with individual differences in AT (P < 0.05, Šidák-corrected for multiple comparisons across the whole-brain). Regions include the OPro/AI (A), subgenual anterior cingulate, temporal cortex, BST (B), Ce (C), aHip (D), and brainstem regions including the PAG (E).
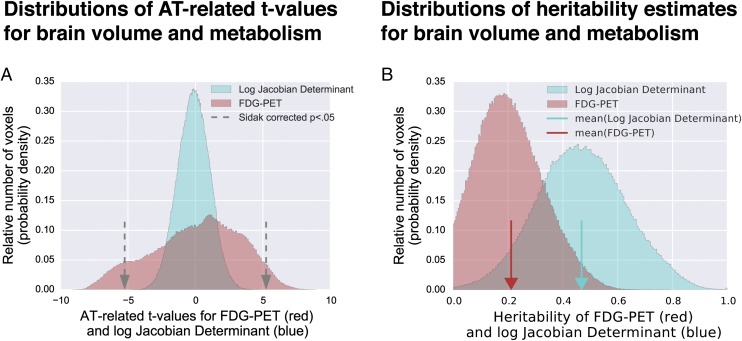
In parallel, to identify regions where brain volume was associated with AT, voxelwise analyses were performed to identify regions where brain volume predicted AT. Regional brain volume was measured using the log-Jacobian determinant of the nonlinear transformations to template space. Remarkably, there were no voxels in which brain volume significantly predicted AT [Šidák correction, P > 0.05, or false-discovery rate (FDR) q > 0.05 corrected] (Fig. 2A; full voxelwise maps are available in Dataset S1). Cross-validation analyses examining the predictive utility of brain volume to predict AT or its components using elastic-net regression and supervised learning yielded the same conclusion (see SI Appendix, Results and Discussion and Figs. S2 and S3 for details). Thus, at least early in life, variation in the expression of AT does not involve altered regional brain volume.
Fig. 2.
Histograms of voxelwise AT-related brain metabolism (red) and brain volume (blue) demonstrate significant relationships for brain metabolism but not brain volume (A). Gray arrows represent the threshold for reaching significance at a Šidák-corrected P < 0.05. Histograms of heritability estimates for brain metabolism (red) and brain volume (blue) demonstrated significant heritability for many voxels of both measures, and on average greater heritability of brain volume (see main text for details) (B). Red and blue arrows indicate the mean heritability estimates for FDG-PET and the log-Jacobian determinant, respectively.
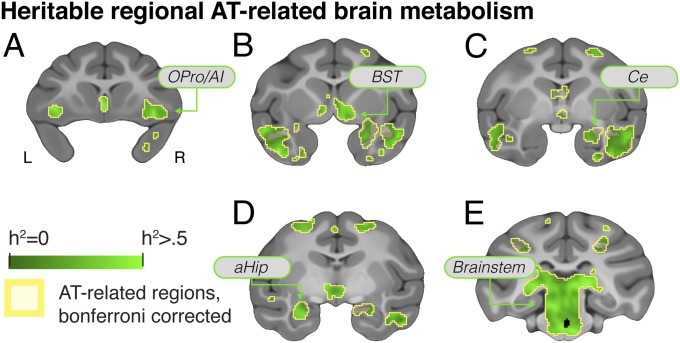
The heritability of brain metabolism was identified using voxelwise heritability analyses of FDG-PET that were performed by harnessing the computational resources of the Open Science Grid’s distributed high-throughput computing system (Methods). Results demonstrated significant heritability of glucose metabolism across the brain (Fig. 2B). We observed significantly heritable metabolic activity in nearly every AT-related region (FDR q < 0.05, corrected within regions where metabolism significantly predicted AT) (Fig. 3 and SI Appendix, SI Results and Discussion and Table S1). Within these AT-related heritable regions there was substantial variability in the magnitude of heritability estimates, with peak heritability estimates of 26% in the amygdala, 53% in the hippocampus, and 57% in the BST region. Estimates were as low as 9% in the superior temporal sulcus (full voxelwise maps are available in Dataset S1).
Fig. 3.
Voxelwise heritability of brain metabolism demonstrated that nearly every region that significantly predicted AT was also significantly heritable (q < 0.05, FDR-corrected within AT-related regions). Regions include the OPro/AI (A), subgenual anterior cingulate, temporal cortex, BST) (B), Ce (C), aHip (D), and brainstem regions including the PAG (E).
We assessed the heritability of regional brain volume using the same approach. Interestingly, brain volume was generally more heritable than brain metabolism (χ2 > 1,000, P < 0.001), with the average h2 for brain volume ∼0.5, whereas the average h2 for regional brain metabolism was ∼0.2. Within the specific subset of brain regions where metabolism predicted AT, variation in volume was significantly heritable (full voxelwise maps are available in Dataset S1). For example, volume in the AT-related amygdala and OFC regions was more than 60% heritable. Nevertheless, the surprising result that brain volume did not predict that AT provides compelling evidence that these highly heritable early-life structural differences do not mediate the intergenerational transmission of the risk for anxiety and depressive disorders.
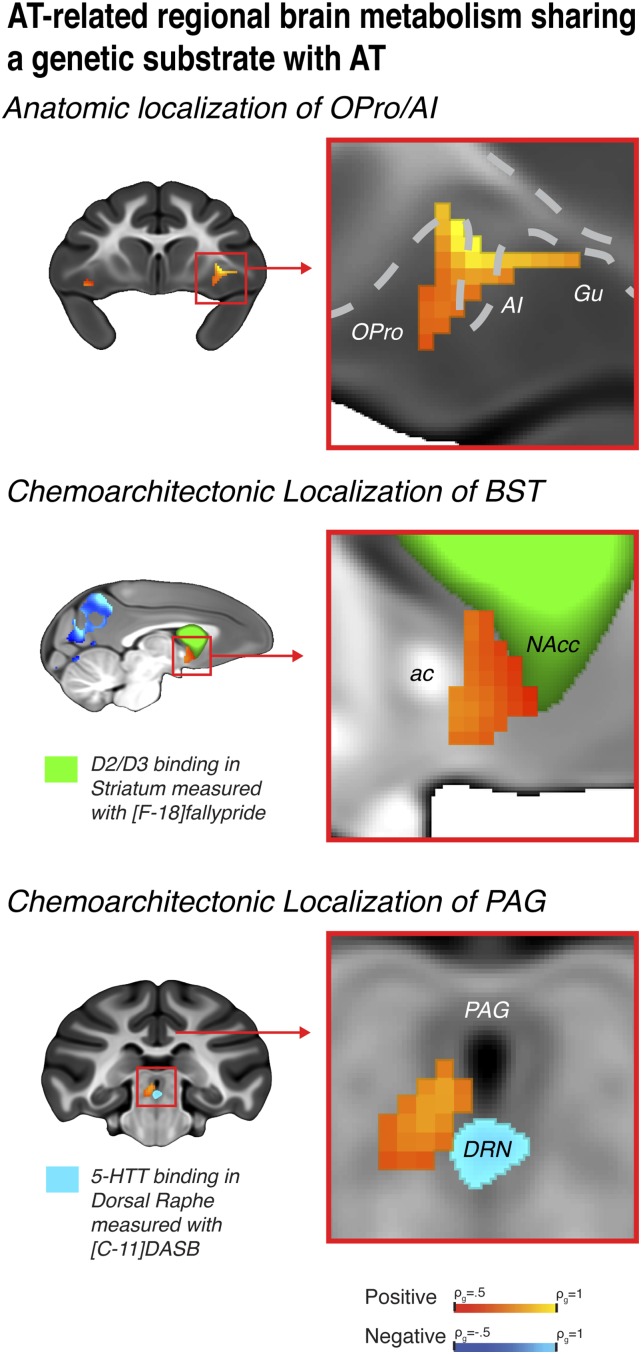
Demonstrating significant heritability of AT-related brain regions does not implicate these regions in the intergenerational transfer of AT. Because of the importance of identifying regions where brain metabolism and AT similarly fell through the family tree, we performed voxelwise genetic correlation analyses. Genetic correlation analyses are crucial for dissociating brain regions that share an overlapping genetic basis with AT from heritable regions where metabolism is driven by genes that are unrelated to the AT-phenotype. We computed bivariate genetic correlations (ρg) between AT and heritable AT-related brain metabolism. Results demonstrate significant genetic correlations with AT in regions that encompass portions of the OFC/AI, extended amygdala, and brainstem (Fig. 4 and SI Appendix, Fig. S4 and Table S2). To our knowledge, these data provide the first evidence for a coheritable substrate for AT and AT-related brain metabolism in a circuit, wherein the extended amygdala links prefrontal regulatory mechanisms to brainstem effector sites that initiate anxiety-related responses. Metabolism within this tripartite neural circuit is likely to share a genetic substrate with AT through which it mediates the inherited risk to develop stress-related psychopathology.
Fig. 4.
Regions where brain metabolism demonstrated significant genetic correlation with AT, thus sharing a genetic substrate, include: the OPro/AI (Top), BST (Middle), and the PAG (Bottom). Using high-resolution anatomical and chemoarchitectonic imaging in a separate group of monkeys, regions were precisely localized as: agranular OPro/AI (Top); the BST region lying between the anterior commissure (ac) and the [18-F] fallypride identified D2/D3-rich ventral striatum that includes the nucleus accumbens (green; NAcc) (Middle); and the vlPAG-region in the gray matter surrounding the ventricle, superior to the [11-C] DASB identified serotonin transporter-rich DRN (Bottom).
To precisely identify the locations of peak activations within the identified tripartite prefrontal-limbic-midbrain circuit, we used high-precision diffeomorphic registration and chemoarchitectonic imaging (Methods). High-precision diffeomorphic registration allowed us to localize significant regions on a superresolution template brain, which reflects the mean rhesus monkey brain anatomy with submillimeter precision in well-aligned areas. This integrative approach revealed that the OFC/AI cluster included regions of agranular orbital and insular cortices, with the peak ρg located in OPro/AI (Fig. 4A). To refine the localization of subcortical clusters, we leveraged in vivo chemoarchitectonic imaging of dopamine D2/D3 receptor binding ([F-18]fallypride; n = 33) and serotonin transporter binding ([C-11]DASB; n = 34) derived from an independent sample of young rhesus monkeys (28, 29). Using D2/D3 receptor binding to demarcate the ventral striatum, we localized the peak ρg within the extended amygdala to be specifically located in the BST region between the anterior commissure and the ventral striatum (Fig. 4B). In addition to the peak-region in the BST, the extended amygdala cluster encompassed portions of the sublenticular extended amygdala immediately posterior to the shell of the nucleus accumbens. Using serotonin transporter binding to pinpoint the dorsal raphé nucleus (DRN), we localized the peak ρg within the brainstem cluster to the ventrolateral PAG (vlPAG), slightly superior and lateral to the DRN in the gray matter that encircles the ventricle (Fig. 4C).
To understand whether metabolism in the brain regions that are genetically correlated with AT are themselves genetically correlated, we performed interregion bivariate genetic correlations. We observed significant genetic correlations in metabolism between the BST and both the PAG (ρg = 0.56, P = 0.01) and OPro/AI (ρg = 0.63 P = 0.006), but failed to find a significant correlation between the PAG and OPro/AI (ρg = 0.40, P = 0.12). These data, demonstrating significant genetic correlations in metabolism across the identified tripartite circuit, suggest that the molecular mechanisms that jointly influence the function of these regions may, in part, mediate the heritable components of AT. These results pave the path for future molecular investigations of AT. For example, publically available datasets can be leveraged to suggest potential molecular mediators of metabolism in these regions by identifying genes that are overexpressed in the BST-PAG or BST-OPro/AI compared with the rest of the brain (SI Appendix, SI Methods). These exploratory analyses can reveal candidate genes and molecular mechanisms that may partially mediate the cross-generational transfer AT by altering brain function within the tripartite prefrontal-limbic-midbrain circuit [e.g., somatostatin, neuropeptide Y, or the “neuropeptide hormone activity” (GO:0005184)] (Datasets S2 and S3). By identifying those brain regions that are genetically correlated with AT, informatics and molecular investigations can serve to help to prioritize future mechanistic studies aimed at altering the pathophysiology of anxiety.
Through a tripartite prefrontal-limbic-midbrain circuit, the OPro/AI, BST, and PAG regions work in concert to integrate and evaluate potentially threatening information to initiate and enact anxiety-related responses. Interestingly, this circuit, genetically correlated with individual differences in early-life anxiety, incorporates survival-related regions that span evolutionary history, where selective pressures shaped each stage of central nervous system development. The OPro/AI, BST, and PAG working together may also underlie the pathophysiology of anxiety and affective disorders (21, 30, 31). The primate OPro and AI are thought to be involved in predicting and maintaining affective representations of current and future events, which can be communicated to other AT-related brain regions to guide emotional responding (25, 32, 33). Increased OPro/AI activity may give rise to the excessive anticipatory anxiety associated with anxiety disorders (30, 34, 35). Furthermore, lesions to the monkey OFC that include the OPro/AI are sufficient to decrease anxiety-related behavior, as well as metabolism within the BST region (9, 10). The BST is required for the prolonged threat preparedness (16, 36–40) and is well-suited to integrate cortical affective inputs to coordinate emotional responses, via direct projections to the brain-stem regions required to mount defensive behaviors (31, 41). Although understudied in relation to human psychopathology, dysregulation of the BST likely underlies the hypervigilance characteristic of patients with extreme anxiety and anxiety disorders (31, 42). The evolutionarily old PAG is organized into functionally distinct columns that initiate specific behavioral and physiological responses, including those characteristic of anxiety, such as fleeing, inhibition of motoric activity, and increased passive coping (27, 43–45). It is likely that dysregulated PAG activity, in part, underlies the extreme behavioral inhibition, freezing behavior, and increased autonomic reactivity that is associated with panic symptoms that are common to anxiety disorders. Although temperamental anxiety is instantiated in distributed circuits throughout the brain, these new data specifically implicate the OPro/AI, BST, and PAG as key components that likely work together to mediate some of the inherited predisposition for extreme early-life temperamental anxiety.
Here, we identified previously unknown relationships between AT and metabolism in brain systems that regulate, initiate, and enact anxiety-related behavior that, when passed down from parent to child, likely result in early-life anxiety. We found that the genetic alterations underlying the inborn risk for anxiety and depressive disorders, which are only beginning to be identified, are likely instantiated within the molecular systems that alter metabolism within the OPro/AI, BST, and PAG. Furthermore, we demonstrated the utility of combining empirical data from this study with large-scale publicly available gene-expression databases to gain insight into the molecular systems underlying the heritable components of AT. Regions that do not share a genetic substrate with AT, including the amygdala and aHip, likely play a role in mediating environmental influences, such as caregiver-style, trauma, and other critical socio-emotional environmental factors on AT. Surprisingly, although early-life regional brain volume was highly heritable, we did not find any evidence linking it to early life anxiety. By identifying the neural systems that share a genetic substrate with AT and likely mediate a part of the genetic risk to develop stress-related psychopathology, these data provide a novel framework for understanding the relationship between genetic variation and early-life anxiety. Elucidating how inherited molecular alterations affect brain function in this tripartite anxiety circuit will help guide the development of novel interventions aimed at helping families with debilitating anxiety enhance the mental health of their at-risk children.
Methods
For this study, 592 young rhesus monkeys that were part of a large multigenerational pedigree were phenotyped with well-established behavioral, physiological, and brain-imaging methods. Each animal was injected with FDG and exposed to the potentially threatening NEC context in which a human intruder presents their profile to the monkey for 30 min before receiving a PET scan. This paradigm allows us to obtain simultaneous measurements of our composite AT measure and integrated regional brain metabolism during exposure to the NEC context. On a separate day, a structural T1-weighted MRI scan was acquired on each animal. Based on the T1-weighted MRI, the log-Jacobian determinant of the transformation to standard space was computed as to measure relative brain volume. Brain imaging measures were regressed against AT to identify AT-related brain regions. Heritability of local brain volume and brain metabolism was estimated at each voxel based on each pair of animals’ degree of relatedness. Bivariate heritability estimates were similarly computed to examine the degree to which AT and regional brain metabolism share a genetic substrate. All experiments were performed according to the federal guidelines of animal use and care and with the approval of the University of Wisconsin–Madison Institutional Animal Care and Use Committee. More information can be found in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
Authors thank E. Ahlers, B. Christian, L. Friedman, E. Larson, K. Mayer, T. Oakes, M. Riedel, P. Roseboom, J. Storey, B. Postle, W. Li, R. Jenison, D. Tromp, N. Vack, H. Van Valkenberg, and the staffs of the Harlow Center for Biological Psychology, HealthEmotions Research Institute, Waisman Center, Waisman Laboratory for Brain Imaging and Behavior, Unversity of Wisconsin Department of Psychiatry, and the Lane Imaging Laboratory for aid in data collection and conceptualization; Maria Jesson for her essential work in processing anatomical images; and Sneka Raveendran for valuable assistance in developing the pedigree information. This research was performed using the computer resources and assistance of the University of Wisconsin–Madison Center For High Throughput Computing in the Department of Computer Sciences, which is supported by the University of Wisconsin–Madison, the Advanced Computing Initiative, the Wisconsin Alumni Research Foundation, the Wisconsin Institutes for Discovery, and the National Science Foundation, and is an active member of the Open Science Grid, which is supported by the National Science Foundation and the US Department of Energy’s Office of Science. Research was supported in part by National Institutes of Health (NIH) Award P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin–Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program Grants RR15459-01 and RR020141-01. The authors of this work were supported by the NIH Grants R21MH91550, R01MH81884, R01MH46729, P50MH84051, P50MH100031, R21MH092581; HealthEmotions Research Institute; and the University of Maryland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The full voxelwise maps are available in Dataset S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508593112/-/DCSupplemental.
References
- 1.Battaglia M, et al. Physiological and behavioral responses to minor stressors in offspring of patients with panic disorder. J Psychiatr Res. 1997;31(3):365–376. doi: 10.1016/s0022-3956(97)00003-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum JF, et al. Behavioral inhibition in children of parents with panic disorder and agoraphobia. A controlled study. Arch Gen Psychiatry. 1988;45(5):463–470. doi: 10.1001/archpsyc.1988.01800290083010. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum JF, et al. A controlled study of behavioral inhibition in children of parents with panic disorder and depression. Am J Psychiatry. 2000;157(12):2002–2010. doi: 10.1176/appi.ajp.157.12.2002. [DOI] [PubMed] [Google Scholar]
- 4.Hirshfeld DR, et al. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Kagan J. Temperament and the reactions to unfamiliarity. Child Dev. 1997;68(1):139–143. [PubMed] [Google Scholar]
- 6.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1066–1075.e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 8.Tambs K, et al. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry. 2009;195(4):301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox AS, et al. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30(20):7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62(10):1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci. 2009;30(12):2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- 14.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33(7):926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox AS, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci USA. 2012;109(44):18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3(7):e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oler JA, et al. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29(32):9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oler JA, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466(7308):864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagne JS, Vendlinski MK, Goldsmith HH. In: Handbook of Behavioral Genetics. Kim Y-K, editor. Springer; New York: 2009. pp. 251–267. [Google Scholar]
- 21.Fox AS, Kalin NH. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry. 2014;171(11):1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calboli FCF, et al. A genome-wide association study of neuroticism in a population-based sample. PLoS ONE. 2010;5(7):e11504. doi: 10.1371/journal.pone.0011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchard TJ, Jr, Loehlin JC. Genes, evolution, and personality. Behav Genet. 2001;31(3):243–273. doi: 10.1023/a:1012294324713. [DOI] [PubMed] [Google Scholar]
- 25.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15(1):13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 27.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci. 1994;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 28.Christian BT, et al. Serotonin transporter binding and genotype in the nonhuman primate brain using [C-11]DASB PET. Neuroimage. 2009;47(4):1230–1236. doi: 10.1016/j.neuroimage.2009.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian BT, et al. The distribution of D2/D3 receptor binding in the adolescent rhesus monkey using small animal PET imaging. Neuroimage. 2009;44(4):1334–1344. doi: 10.1016/j.neuroimage.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38(5):319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401(4):455–479. [PubMed] [Google Scholar]
- 33.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams LE, et al. Fear of the unknown: Uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology. 2015;40(6):1428–1435. doi: 10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 36.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58(10):796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mobbs D, et al. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA. 2010;107(47):20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68(5):416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1-3):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 41.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 42.Straube T, Mentzel H-J, Miltner WHR. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23(5):743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 44.Nashold BS, Jr, Wilson WP, Slaughter DG. Sensations evoked by stimulation in the midbrain of man. J Neurosurg. 1969;30(1):14–24. doi: 10.3171/jns.1969.30.1.0014. [DOI] [PubMed] [Google Scholar]
- 45.Satpute AB, et al. Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci USA. 2013;110(42):17101–17106. doi: 10.1073/pnas.1306095110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.