Abstract
Flowering plants possess an unrivaled diversity of mechanisms for achieving sexual and asexual reproduction, often simultaneously. The commonest type of asexual reproduction is clonal growth (vegetative propagation) in which parental genotypes (genets) produce vegetative modules (ramets) that are capable of independent growth, reproduction, and often dispersal. Clonal growth leads to an expansion in the size of genets and increased fitness because large floral displays increase fertility and opportunities for outcrossing. Moreover, the clonal dispersal of vegetative propagules can assist “mate finding,” particularly in aquatic plants. However, there are ecological circumstances in which functional antagonism between sexual and asexual reproductive modes can negatively affect the fitness of clonal plants. Populations of heterostylous and dioecious species have a small number of mating groups (two or three), which should occur at equal frequency in equilibrium populations. Extensive clonal growth and vegetative dispersal can disrupt the functioning of these sexual polymorphisms, resulting in biased morph ratios and populations with a single mating group, with consequences for fertility and mating. In populations in which clonal propagation predominates, mutations reducing fertility may lead to sexual dysfunction and even the loss of sex. Recent evidence suggests that somatic mutations can play a significant role in influencing fitness in clonal plants and may also help explain the occurrence of genetic diversity in sterile clonal populations. Highly polymorphic genetic markers offer outstanding opportunities for gaining novel insights into functional interactions between sexual and clonal reproduction in flowering plants.
Keywords: clonal growth, dioecy, geitonogamy, heterostyly, somatic mutations
Biological reproduction is the production of offspring by sexual or asexual processes. Among most groups of organisms are species that produce sexual and asexual offspring (e.g., bacteria, fungi, invertebrates), but flowering plants (angiosperms) display the widest range of strategies for achieving both reproductive modes. Angiosperms possess two distinct forms of asexual reproduction—vegetative reproduction and apomixis (asexually produced seed)—as well as diverse pollination and mating systems (1). Vegetative reproduction is more commonly represented than apomixis among angiosperm lineages, and it has been estimated that ∼80% possess some means of reproducing in this manner (2). Indeed, many terrestrial habitats are dominated by species that reproduce by vegetative reproduction, including saltmarshes, tundra, grasslands, sand dunes, and the herbaceous understory of woodlands. Vegetative reproduction is commonly associated with the perennial life form, longevity, and occurrence in habitats in which sexual recruitment is often restricted, as in many aquatic environments (3). The widespread distribution of vegetative reproduction among angiosperm lineages results from several ecological and evolutionary advantages that this form of reproduction provides although the diversity of clonal strategies indicates that particular ecological conditions favor some mechanisms over others.
Clonal propagation provides several ecological benefits compared with an exclusive reliance on sexual reproduction. These advantages include the ability of clones to “forage” for light, nutrients, and water in patchy environments; opportunities for physiological integration and spatial division of labor among shoots of a clone; reduced likelihood of the death of clonal genotypes because mortality risk is spread among numerous shoots; and the ability of clonal propagules to disperse to new environments where, being larger than seeds and lacking dormancy, they can multiply rapidly and compete effectively with other species (2). Clonal reproduction also provides several evolutionary benefits, including avoidance of the costs associated with sexual reproduction and a means by which adaptive genotypes can be replicated rapidly after colonization of new environments (4). Clonal populations often persist in habitats in which sexual reproduction is prevented, either because of the absence of mating partners, or where ecological conditions are unfavorable for seed set, seed germination, or seedling establishment.
Despite the many advantages of vegetative reproduction, there is a growing recognition that clonal growth can also affect sexual reproduction in ways that may be detrimental to fitness. Three particular influences have been identified (5). First, if there are strong trade-offs between investment in sexual and vegetative reproduction, rapid clonal expansion may limit allocation to flowering and seed production (6). Second, vegetative reproduction has the potential to interfere with pollination and mating, resulting in reductions in the quantity and quality of offspring. This situation can occur when clonal dispersal disrupts the functioning of sexual systems, leading to the absence of mating groups required for outcrossing, or where very large clones experience fitness costs (inbreeding depression and pollen discounting) associated with geitonogamous (between flower) self-pollination because of large floral displays (5, 7, 8). Finally, in highly clonal populations in which sexual reproduction is very limited or absent, there is growing evidence that the accumulation of somatic mutations may result in reductions in fertility, potentially leading to the loss of sex.
In this article, I consider the consequences of clonality for sexual reproduction in populations of flowering plants. I begin by briefly summarizing reproductive diversity in angiosperms and clarify several terminological issues related to clonal propagation. I then review evidence from species with sexual polymorphisms that shows the disruptive influence of clonal reproduction on various aspects of mating and fertility. I next consider the loss of sex in predominantly clonal populations and the role that somatic mutations may play in this process. Finally, I outline unresolved questions that future studies could address in clonal plants. Many of the examples that I have chosen to illustrate particular topics involve aquatic species. This focus is because clonal propagation is especially well-developed in aquatic plants, probably because life in and around water presents particular challenges for successful sexual recruitment.
Plant Reproductive Diversity
Flowers display greater variety than the reproductive structures of any other group of organisms (9). Associated with floral diversity is an equally impressive array of sexual systems and mating patterns (10). Studies using genetic markers indicate that mating patterns range from predominant self-fertilization to obligate outcrossing, with a significant proportion of species exhibiting a mixture of selfing and outcrossing (11). Because most plants produce multiple reproductive structures (flowers and inflorescences), sex in flowering plants can be highly promiscuous, with individuals mating with numerous sexual partners, and in some species, with themselves.
Vegetative reproduction involves the production of genetically identical individuals capable of independent existence and reproduction as a result of plant growth. Indeed, following Harper (12), many botanists prefer the term “clonal growth” and deliberately avoid using the terms “vegetative or clonal reproduction”. To quote Harper (ref. 12, p. 27), “clones are formed by growth—not reproduction.” This perspective has merit because it identifies the appropriate unit of selection—the clonal genotype—and has led to a focus on the developmental mechanisms and patterns of growth that produce clonal offspring. Nevertheless, the terms vegetative and clonal reproduction (or alternatively vegetative and clonal propagation) are widely used, and, in this article, I will use these terms because I prefer the broader definition of reproduction they imply.
Two other terms introduced by Harper (12) are now widely used in the plant literature: “Ramet” and “genet” refer to the unit or module of clonal growth (shoot) and the entire clone or genotype, respectively. A genet is therefore a collection of ramets descended from a single seedling arising from sexual reproduction. The ramets of a clone may or may not be physically separated and physiologically autonomous, depending on the type of clonal strategy. Because clonal growth does not involve sexual reproduction, botanists make a clear distinction between predominant self-fertilization and clonal reproduction, a separation that is not as evident in the literature involving some other groups: e.g., fungi and parasitic protozoa (13). Both forms of uniparental reproduction limit recombination and can result in similar signatures of population genetic structure. However, in plants, clonal growth and selfing result from entirely different structures and processes, and the selective forces responsible for their respective origins and maintenance are quite distinct.
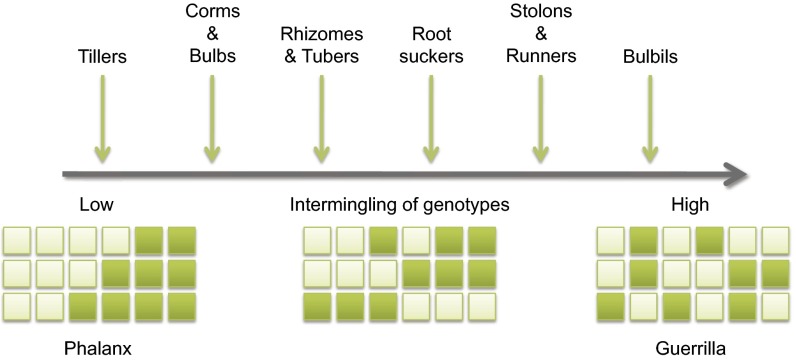
Plant clonality is manifested by a range of growth strategies involving variation in the spatial arrangement of ramets within a clone (clonal architecture) and diverse organs of propagation. The organs vary in the extent to which they are capable of establishing an autonomous existence and function in regeneration, multiplication, and dispersal. Tillers, corms, bulbs, rhizomes, and tubers are usually produced relatively close to the parent plant. In contrast, stolons, runners, and bulbils usually result in ramets located at further distances from the parent plant. This variation gives rise to different levels of clonal aggregation within populations and has led to the identification of two contrasting clonal strategies that differ in the degree of intermingling of clones (14). The “phalanx strategy” is characterized by the close aggregation of ramets that are often tightly packed around the parental shoot, limiting the mixing of ramets of different clones. In contrast, the “guerrilla strategy” involves extensive intermingling of ramets from different clones, as occurs when ramets are produced on long stolons or runners, or when clonal propagules are dispersed by water, animals, or human activities. In reality, a continuum exists in the degree to which the various organs of clonal growth promote intermingling of clonal genotypes and thus opportunities for outcrossing (Fig. 1). Clone sizes can vary enormously among species. Depending on the intermingling of clones and proximity of mating partners, this variation can have important implications for mating and fertility.
Fig. 1.
The influence of organs of clonal growth on the intermingling of genets and opportunities for outcrossing in clonal plants. The order from left to right reflects the distance that daughter ramets are usually produced from the parent plant; phalanx and guerrilla strategies represent two contrasting clonal strategies distinguished by this distance. Reprinted with permission from ref. 5.
Clonality in Plants with Sexual Polymorphisms
Many flowering plants possess sexual polymorphisms in which populations are composed of different mating types. The most common is homomorphic self-incompatibility, in which the mating types are morphologically indistinguishable and numerous. A second class of polymorphism involves species in which mating types are morphologically distinct and field observations can reveal their number and frequency in populations. Heterostyly and dioecy are the best-known sexual polymorphism of this type, with heterostylous populations usually composed of two (distyly) or three (tristyly) style morphs (15), and dioecious populations composed of female and male plants (16). Disassortative mating in sexually polymorphic populations results in negative frequency-dependent selection on style morph or sex ratios and at equilibrium should give rise to equal ratios of the sexual morphs. However, in clonal species with sexual polymorphisms, this expectation is frequently not met.
Clonality, Morph Ratios, and Fertility.
Heterostylous species with extensive clonal propagation may be particularly sensitive to the restricted dispersal of compatible pollen as a result of local pollinator foraging. Unlike species with homomorphic incompatibility, heterostylous populations contain only two or three mating groups, and large clones may result in the spatial isolation of flowers from compatible pollen sources, with consequences for fertility. An investigation of style morph ratios and female fertility in populations of the distylous, clonal aquatic Nymphoides peltata (Menyanthaceae) provides evidence of how clonality can disrupt cross-pollination and reduce seed production (17). A survey of 30 populations from China revealed that biased morph ratios predominated, with 30% of populations having only one morph. Monomorphic populations arise through the dispersal of vegetative fragments, followed by rapid clonal propagation. Because N. peltata is strongly self-incompatible, at least in China, monomorphic populations produce very little seed and thus are vulnerable to desiccation if habitats dry out. The manipulation of style-morph ratios in an experimental population of N. peltata demonstrated frequency-dependent reproductive success. Over a wide range of morph ratios, fruit set averaged between 75% and 90%, but, once the minority morph dropped below ∼20%, as is common in natural populations (see figure 1 in ref. 17), fruit set declined steeply because of insufficient pollen for cross-fertilization, and reduced fruit set occurred regardless of which morph was in the minority. The spatial patterns of fruit set in a large clone of N. peltata provided further evidence of the influence of clonality on pollen dispersal and fertility. Flowers at the periphery of the clone closest to the opposite style morph set abundant fruit, but fertility dropped significantly 2–3 m from the clone boundary due to dilution of compatible pollen. This study (17) demonstrates how extensive clonal propagation and biased morph ratios disrupt the maintenance of heterostyly, leading to reduced fertility and sterile populations. Other examples of reduced fertility owing to insufficient pollen delivery are also reported from clonal species with other sexual polymorphisms [e.g., gynodioecy in Glechoma hederacea (18) and homomorphic self-incompatibility in Filipendula rubra (19)].
Clonality, Morph Ratios, and Mating.
The occurrence of different mechanisms of clonal reproduction among related species suggests that clonal strategies are relatively labile and that their evolution is not strongly constrained. The freshwater aquatic family Pontederiaceae is instructive in this regard. Despite being relatively small (35-40 spp.), it contains four different mechanisms of clonal propagation (stem fragmentation, stolons, rhizomes, and tubers), each of which is adapted to different wetland and riverine habitats and associated with contrasting life forms (20, 21). Comparative studies of the closely related heterostylous species Eichhornia azurea and Eichhornia crassipes illustrate how differences between species in clonal strategy can have dramatic effects on morph-ratio variation, with consequences for mating and sexual reproduction.
E. azurea and E. crassipes are highly clonal neotropical species that are widespread in distribution and superficially similar in morphology. They often grow together in aquatic habitats of lowland South America, particularly along the Amazon and Paraná rivers and in the Pantanal wetlands. Both possess showy tristylous flowers pollinated by specialized long-tongued bees. However, they differ in two important ways that affect style-morph ratios and the functioning of heterostyly. First, E. azurea possesses a trimorphic incompatibility system that limits opportunities for selfing (22). In contrast, E. crassipes is self-compatible, allowing selfed seed to be produced in abundance in populations monomorphic for style morph (23). Theoretical studies of morph-ratio evolution in tristylous populations indicate that opportunities for selfing weaken the strength of frequency-dependent selection and can lead to biased morph ratios and morph loss (24). Second, the two species differ in their mode of clonal propagation and commitment to an aquatic “lifestyle.” Although floating clonal propagules are an effective means of dispersal and mate finding in both species, they differ in the extent to which this form of dispersal occurs. E. azurea is rhizomatous, and large floating mats commonly occupy river shorelines where the species is tethered to the substrate by roots. The species is classified as a floating-leaved aquatic (3) because it requires terrestrial conditions for establishment and colony growth. Propagation and dispersal result from stem fragments of varying size that break away from established colonies in strong water currents. In contrast, E. crassipes is a free-floating aquatic (3) that requires only a brief period “on land” before becoming entirely aquatic. Once seedlings establish in wet soil and become submerged, they abscise their roots, float to the surface, and begin to clone rapidly through daughter rosettes produced on delicate stolons that are easily severed by water currents. E. crassipes has prolific powers of clonal propagation and dispersal and is considered one of the world’s most invasive aquatic weeds (25). Large floating mats are common in areas where it has been introduced in tropical and subtropical regions of the New and Old World. Although E. azurea is widely distributed in the neotropics, and has also been introduced to the Old World as a pond ornamental, it shows no signs of becoming invasive.
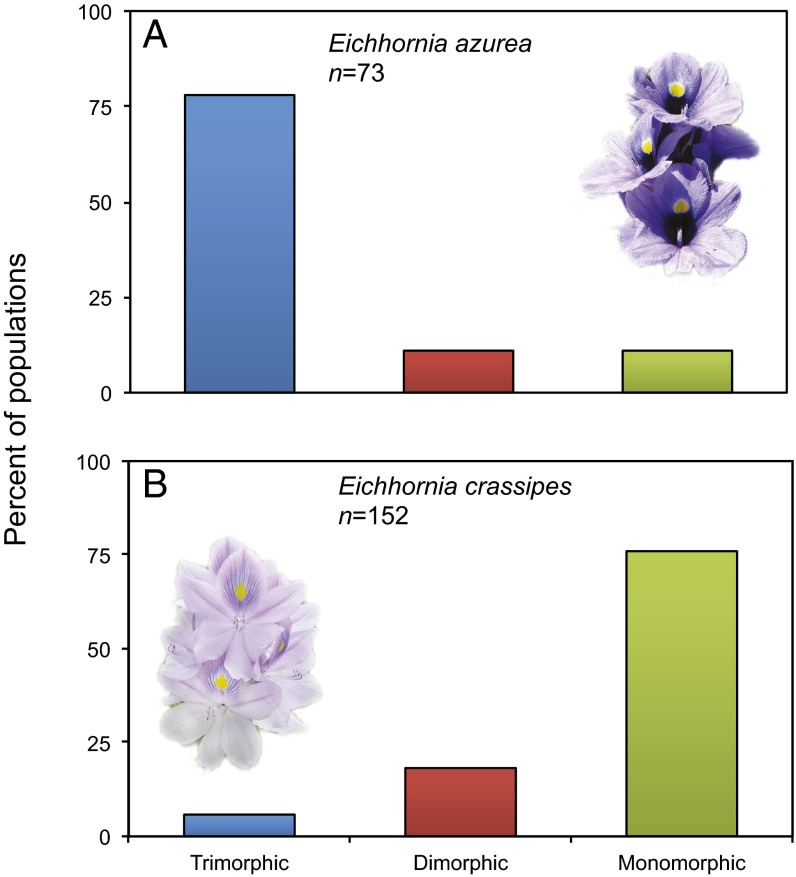
Extensive surveys of style morph frequencies reveal a striking difference in population morph structure between the Eichhornia species as a result of their contrasting clonal strategies (Fig. 2). The difference in morph structure has important consequences for the functioning of tristyly. Sampling of morph ratios in 73 populations of E. azurea in the Pantanal wetlands of Brazil revealed that 78% were trimorphic, including 18 with equal frequencies of the style morphs (26). The remaining 22% of populations were equally divided between dimorphic and monomorphic populations. Although the majority of populations were trimorphic, the pervasive occurrence of biased morph ratios strongly suggests that most populations are in a nonequilibrium state. Local founder events associated with clonal dispersal followed by vegetative propagation probably account for the observed patterns. In contrast, populations of E. crassipes are rarely trimorphic in the native range, thus limiting opportunities for disassortative mating. A survey of morph representation in 154 South American populations revealed that 76.0% were monomorphic and only 5.8% were trimorphic (27). Although sampling in E. crassipes was more geographically extensive than in E. azurea, monomorphic populations dominated in all regions, and no population contained equal frequencies of the style morphs.
Fig. 2.
Contrast in population style-morph structure between (A) E. azurea and (B) E. crassipes, two closely related, tristylous aquatic plants with different methods of clonal propagation and dispersal. Population surveys of E. azurea (26) and E. crassipes (27) were conducted in the Pantanal wetlands of Brazil and various regions of lowland tropical South America, respectively.
In E. crassipes, the high mobility of the free-floating life form and explosive clonal growth through stolon formation often lead to one or a few clones dominating in populations. A molecular study of clonal diversity in both the native and introduced range of E. crassipes supports this suggestion (28). For example, in the introduced range, 80% of the populations sampled contained a single clone, with one particular clone dominating in 74.5% of the populations sampled. Thus, a strong genetic bottleneck is associated with invasion, and the mid-styled morph dominates in most populations and the short-styled morph is entirely absent from the introduced range (23, 28). The function of tristyly in promoting cross-pollination clearly differs in the two Eichhornia species as a result of their contrasting clonal strategies. Most populations of E. azurea sampled in the Pantanal were trimorphic, and, despite biased morph ratios, abundant outcrossed seed was produced in populations. In contrast, E. crassipes populations are largely monomorphic, even in the native range, and large clone size and self-compatibility guarantee high levels of geitonogamous self-pollination.
There is evidence in both Eichhornia species that the disruptive influence of clonal dispersal is associated with the breakdown of tristyly and the evolution of self-fertilization. Selfing variants occur in geographically isolated populations in E. azurea (29, 30) and in the introduced range of E. crassipes (31, 32). For example, in Costa Rica, selfing forms of E. crassipes are reported from a seasonal marsh in which clones desiccate during the dry season and sexual reproduction is important for recruitment (31). Insufficient pollinator service limits seed set in such geographically isolated populations and favors variants that have the capacity for autonomous self-pollination, as a mechanism of reproductive assurance. The tempo and extent of transitions to selfing is much slower and less common in E. azurea and E. crassipes in comparison with the annual tristylous Eichhornia paniculata, in which selfing predominates at range margins and there have been multiple transitions to selfing (33). The high degree of clonality and limited sexual recruitment in most E. azurea and E. crassipes populations probably retard opportunities for the spread of mating system modifier genes promoting selfing.
Clonality and Sex Ratios in Dioecious Species.
The widespread occurrence in dioecious species of both sexual and clonal reproduction provides an opportunity to investigate how these two reproductive modes interact to influence sex ratios and fitness in plant populations. The classical theoretical arguments of Düsing (34), and Fisher (35) predict 1:1 sex ratios in dioecious populations, but recent comparative studies of plant populations indicate considerable variation in sex ratios both within and among species (36, 37). Determining the ecological, demographic, and genetic factors responsible for this variation is an important problem in evolutionary biology. Several caveats are, however, necessary when interpreting sex ratio bias in plants, especially those that are clonal. Data on sex ratios largely come from sampling flowering individuals because the sex of individuals can usually be determined only at reproductive maturity (but see ref. 38). Also, clones are difficult to identify without genetic markers, and sex ratio estimates are usually based on the sampling of ramets rather than genets. Thus, unlike many animal studies, flowering or “operational” sex ratios are reported in most studies, and there are very few estimates of primary sex ratios in plant populations.
A comparative analysis of sex ratios in 243 angiosperm species representing 61 families revealed that male-biased sex ratios were twice as common as female-biased sex ratios, with male bias evident in 61% of the families surveyed (37). The common occurrence of male-biased sex ratios may be, in part, associated with differences in the costs of reproduction between the sexes. If reproductive costs are greater in females than males owing to fruit production, females may delay flowering, flower less often, or experience greater mortality compared with males. These effects could be especially important in clonal species in which cumulative episodes of sexual reproduction may result in sexual dimorphism in rates of clonal reproduction and the number of flowering ramets per clone (39, 40). Interestingly, in the comparative analysis conducted by Field et al. (37), the opposite pattern was found in both the species level and phylogenetically controlled analyses. Clonality was associated with female-biased rather than male-biased sex ratios, particularly in herbaceous species. The cause of this pattern is unclear but may be because of the correlation of clonality with other life-history variables (e.g., abiotic pollination was correlated with female-biased sex ratios) and patterns of sexual size dimorphism (e.g., females are often larger than males in herbs) (39, 41).
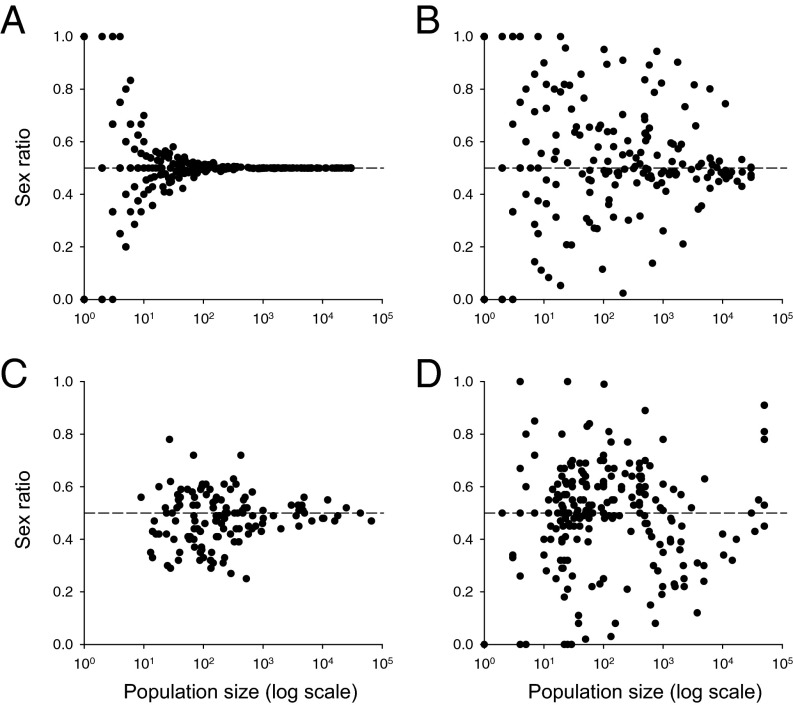
In common with clonal heterostylous species with biased style-morph ratios, stochastic variation is likely to play an important role in causing variation in sex ratios among dioecious populations. Restrictions on sexual recruitment and high levels of clonal propagation can preserve nonequilibrium sex ratios for surprisingly long periods after the initiation of populations by founding genotypes (42). Historical contingency may therefore play an important role in contributing to the striking variation in sex ratio commonly observed among populations of clonal dioecious species. For example, in Sagittaria latifolia (Alismataceae), a widespread emergent aquatic that inhabits a variety of wetland habitats in North America, sex ratios range from female- to male-biased, including some populations with only one sex (43). A recent study examined the influence of nonequilibrium conditions, founder effects, and mode of reproduction on sex ratios by modeling hypothetical dioecious metapopulations that were either clonal or nonclonal and comparing the results with observed data on sex ratios from dioecious species (44). The model assumed that population size was positively correlated with time since colonization; although this assumption may be violated in some cases, it seems likely to be true for many dioecious populations, especially those that are clonal. As expected for populations reproducing exclusively by sexual reproduction, there was a rapid decline in the variance in sex ratio with population size whereas the decline was much less steep in clonal populations, and many populations, regardless of size, exhibited biased sex ratios (Fig. 3 A and B). Empirical data from clonal and nonclonal species (Fig. 3 C and D) showed similar patterns to those obtained from the model, indicating that vegetative propagation is likely a major determinant of variation in plant sex ratios in clonal species.
Fig. 3.
Contrasting patterns of variation in sex ratio in populations of different size for nonclonal (A and C) and clonal (B and D) dioecious species. A and B are the predicted patterns based on a metapopulation model with two founding individuals per population, and C and D are the observed patterns from populations of 9 and 14 nonclonal and clonal species, respectively (total populations n = 348; mean per species, nonclonal = 14.6; clonal = 15.5). Modified with permission from ref. 44.
Clonality in Populations with Combined Versus Separate Sexes.
Until recently, it has been difficult to investigate key features of clonality in plant populations because of the inability to identify genets accurately and determine their abundance and size. However, with the advent of highly polymorphic molecular markers, it is now possible to obtain a clearer picture of the clonal structure of plant populations and to investigate a variety of problems that were previously intractable. S. latifolia is a useful study system for investigating the interactions between clonality and sexual reproduction because of the wide range of sex ratios in this species. A particularly attractive feature of S. latifolia is that it largely comprises either monoecious or dioecious populations (45), allowing explicit intraspecific comparisons of clonality in populations with combined versus separate sexes. Below, I outline several recent findings from S. latifolia obtained by spatially explicit sampling of vegetative and reproductive ramets in monoecious and dioecious populations and multilocus genotyping of clones using variation at microsatellite loci.
As discussed earlier, it has been suggested that clone size may differ between females and males in some species due to differences in their costs of reproduction. If, as might be expected, larger clones produce more ramets, then sexual dimorphism in clone size could result in weak correspondence between ramet and genet sex ratios. The relation between ramet and genet sex ratios could be further weakened if, for example, clones of one sex were not only larger but also exhibited a greater fraction of total ramets that flowered each year. These predictions were investigated in a study of clone size in 11 dioecious populations of S. latifolia (46). Although average clone size differed significantly among populations, there was no overall difference in the size of female and male clones or differences between them in the proportion of ramets within a clone that were in flower. Thus, at least in S. latifolia, estimates of flowering ramet sex ratios can be used as a reliable surrogate for genet sex ratios in large-scale surveys (e.g., ref. 43) in which molecular analyses of clone size would be impractical.
In common with most cases of the origin of dioecy in angiosperms, separate sexes in S. latifolia have been derived from hermaphroditism: in this case, monoecy. Marker-based estimates of selfing rates and inbreeding depression indicate that some monoecious populations exhibit values for these parameters that satisfy the conditions of theoretical models for evolution of dioecy (45), at least those involving simple nuclear gene control of sex phenotype, as occurs in S. latifolia (47). Clonality has been implicated in the evolution of dioecy, based on the assumption that large clone size increases the probability of geitonogamous selfing, which results in inbreeding depression and favors the spread of unisexual variants. According to this scenario, we might predict that constraints on clonal expansion in monoecious populations, due to the costs of geitonogamy, would be relaxed in dioecious populations, resulting in larger clones than in monoecious populations. However, this prediction was rejected in S. latifolia because genetic estimates of clone size indicated the opposite pattern, with larger clones in monoecious than dioecious populations (46). Monoecious populations are adapted to temporary aquatic habitats (e.g., ditches, farm ponds), which probably explains why they have higher vegetative growth rates and corm production than dioecious populations, which are more commonly found in permanent wetlands (48). This difference in life history may explain the observed difference in clone size between the sexual systems.
Sexual Dysfunction, Somatic Mutations, and Sterility in Clonal Populations
Very low seed production and even complete sterility are common features of clonal plant populations. Sterility can be caused by a variety of environmental and genetic factors influencing various stages in the process of sexual reproduction. Environmental factors such as a lack of pollinators and unsuitable ecological conditions for seed maturation, seed germination, and seedling establishment commonly limit fertility, especially in geographically marginal populations (49). Long-distance dispersal of only one mating type to a region, especially in invasive species, may disable sexual systems, resulting in sterility. This process has occurred in introduced populations of Canadian pondweed (Elodea canadensis), which in Europe are represented only by males but have nevertheless spread prolifically by clonal reproduction to many parts of the continent (3). Similarly, invasive populations of gynodioecious Japanese Knotweed (Fallopia japonica var. japonica) seem to be represented in the United Kingdom by a single female clone, with virtually all reproduction occurring by rhizome fragmentation (50). Sterility also commonly arises through hybridization between species of contrasting ploidy level, with the resulting hybrid populations maintained entirely by vegetative propagation (49). Fertility in clonal populations can occasionally be restored, as occurred with the allohexaploid hybrid species Mimulus peregrinus recently discovered in the United Kingdom (51), which originated from the sterile clonal triploid Mimulus × robertsii (2x Mimulus guttatus × 4x Mimulus luteus) from either unreduced gametes or somatic mutations. The occurrence of sterility in clonal plants raises many intriguing questions concerning its origins and consequences for the ecology of populations and their evolutionary prospects.
The Loss of Sex.
There are relatively few well-studied examples of the evolutionary loss of sex in clonal species as a result of the fixation of sterility mutations in populations. The best case involves Decodon verticillatus (Lythraceae), a tristylous clonal aquatic native to eastern North America. Populations at the northern range limit of the species are composed of single multilocus genotypes and are maintained entirely by clonal propagation through adventitious rooting at the tips of trailing branches. In contrast, populations in the southern portion of the range are tristylous, produce abundant seed, and maintain considerable variation at allozyme loci (52, 53). Glasshouse studies of D. verticillatus indicate that both environmental and genetic factors are responsible for the dramatic reduction in seed production in northern populations. Crossing studies have revealed that dysfunctional pollen tube growth is probably the major cause of sterility and that the genetic basis for infertility may be simple, involving a single recessive mutation, at least in some populations (54). Common garden studies of sterile and fertile clones from numerous northern populations indicate greater winter survival of sterile genotypes, which should provide an advantage at the periphery of the range where sex is limited by environmental conditions such as temperature (55). In D. verticillatus, the origin of sterility seems to result from selection of genes with pleiotropic effects on vegetative performance through relaxation of the trade-off between sexual fertility and survival. The suppression of sexual activities in geographically marginal populations may be important in governing the range limits of other clonal plants, but, in most cases, the roles of environmental and genetic factors have not been investigated.
Somatic Mutations.
The influence of somatic mutations on fitness is relevant in plants because of their indeterminate growth and lack of separation between somatic and germ-line tissues, thus violating Weismann’s doctrine. The frequency of somatic mutation should be correlated with the number of mitotic divisions between zygote formation and gamete production. Thus, the great size and longevity of many trees and clonal plants is predicted to be associated with the accumulation of numerous somatic mutations in cell lineages (56, 57). Some have argued for the adaptive benefits of this type of variation because of the formation of genetic mosaics and their role in plant defense (58). However, in most cases, the gradual accumulation of deleterious mutations during growth is more likely to negatively influence fitness because these mutations can be potentially transmitted from shoot meristems to gametes when flowering occurs. As long as somatic mutations do not limit clonal growth, they should accumulate over the life span of genets. Unfortunately, despite the pioneering efforts of Klekowski (56), there has been very little empirical study of the potential fitness effects of somatic mutations in plants. However, two recent studies (59, 60) point the way forward and provide novel insights on the potential evolutionary significance of somatic mutations in clonal plants.
Fitness Effects of Somatic Mutations.
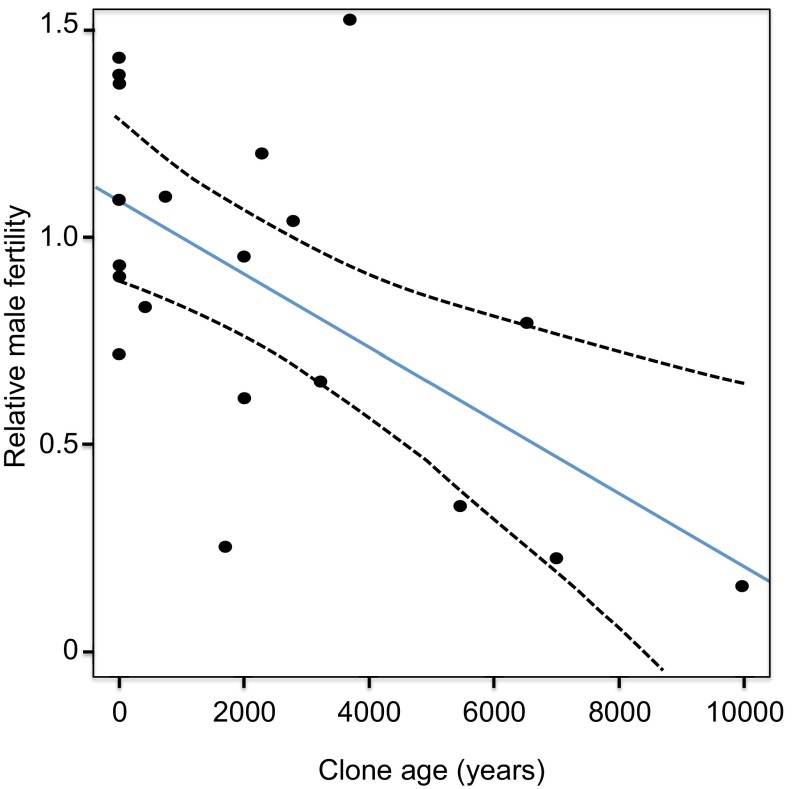
Quaking aspen (Populus tremuloides: Salicaceae) is a dioecious, deciduous tree that is native to North America and forms very large clones that can live for thousands of years. Indeed, in parts of Western North America, clones cover enormous areas where little if any sexual reproduction occurs owing to a long-term change in climate from historically moist to semiarid conditions, preventing seedlings from establishing (61). Exploiting the wide range of clone ages commonly found in P. tremuloides, Ally et al. (59) investigated clone age and pollen sterility. They found a striking relation, with older clones exhibiting higher levels of sterility than younger clones (Fig. 4). The average loss of male fertility in the sample of clones was 8%, with the oldest clone losing 75% of its pollen fertility compared with the estimated fertility of ancestral clones. Although there are various alternative hypotheses that could account for the observed association (e.g., resource allocation trade-offs due to negative pleiotropy, architectural effects, epigenetic variation), these hypotheses currently have no strong support, and deleterious somatic mutations seem to be the most likely mechanism.
Fig. 4.
The decline in male fertility with increasing clone age in the long-lived clonal tree P. tremuloides. For details of the estimation of relative male fertility and clone age, see ref. 59. Modified with permission from ref. 55.
An alternative approach for investigating the effects of somatic mutations on plant fitness was proposed by Schultz and Scofield (57) and involves measuring “autogamy depression,” the difference in fitness of inbred offspring produced from hand pollinations of flowers on the same inflorescence (“autogamous” pollinations) compared with pollinations between flowers from widely separated inflorescences on different segments of the plant (“geitonogamous” pollinations). Inbred offspring from autogamous pollinations should exhibit greater inbreeding depression than progeny from geitonogamous pollinations. This fitness reduction is because the somatic mutation hypothesis predicts that the number of different mutations carried by gametes will increase with the growth distance and number of mitotic cell divisions from the ancestral tissue from which flowers used in the two types of pollinations originated. A virtue of this procedure is that pollinations can be conducted reciprocally, and thus any differences observed in fruit and seed set between the two types of pollinations are unlikely to be the result of position-dependent effects associated with development or plant architecture.
Bobiwash et al. (60) recently implemented this novel approach by using estimates of early-acting inbreeding depression in the long-lived clonal shrub Vaccinium angustifolium (Ericaceae). The results obtained were consistent with the predictions of Schultz and Scofield (57), with significantly lower fruit set obtained from autogamous pollinations. The authors also analyzed data from the literature in which these types of controlled crosses were conducted and found additional evidence for autogamy depression, particularly in woody plants. In future, it would be worthwhile to extend this experimental approach to other clonal plant species. However, those that exhibit clonal fragmentation would be unsuitable because, in these species, the spatial distance between flowers used in pollinations is unlikely to reflect the number of mitotic divisions that separate the two from the ancestral cells from which they ultimately originated, especially in species with well-developed clonal dispersal. Clonal species with phalanx rather than guerrilla strategies would be better for this type of analysis.
Origins of Genetic Diversity in Sterile Populations.
Many clonal species are highly successful plant invaders, and, in some cases, their populations seem to contain little if any genetic diversity. In such cases, phenotypic plasticity is thought to play a major role in enabling populations to cope with environmental heterogeneity (62). A classic example is Oxalis pes-caprae (Oxalidaceae), a tristylous clonal geophyte that multiplies from bulbils and is native to South Africa, where populations are almost exclusively diploid or tetraploid. In contrast, invasive populations in Australia, California, Chile, and the Mediterranean are largely composed of a sterile, short-styled pentaploid (5x) cytotype, which remarkably is known only from a few localities in the native range near Cape Town (63, 64). The renowned botanist Herbert G. Baker used the sterile 5x form of O. pes-caprae to illustrate his concept of the “general-purpose genotype” involving a “jack-of-all-trades-master-of-none” strategy based on high phenotypic plasticity (65). How the 5x cytotype of O. pes-caprae originated and whether it represents a single clone has been of long-standing interest.
Investigations of variation at amplified fragment length polymorphism (AFLP) (66) and microsatellite loci (64) have revealed that 5x populations in the Mediterranean are not genetically uniform, as often assumed, and instead contain low levels of genetic diversity. Moreover, surveys in the Iberian Peninsula have recently discovered the occurrence of sexual tetraploids (4x) of all three style morphs (67). These discoveries raise the possibility that they may have originated by residual sexuality in the 5x cytotype (63) and could explain how genetic diversity may have arisen in supposedly asexual populations. Plants with odd ploidy levels often produce viable gametes, and, indeed, some viable seed has been produced from intramorph crosses of the 5x cytotype of O. pes-caprae (68) although it is unclear whether these seeds enable sexual recruitment in populations.
A recent study comparing genetic relationships among native and introduced populations of all ploidy levels of O. pes-caprae cast doubt on the de novo origin of 4x sexual morphs from the 5x cytotype (64). Rather, the observed patterns of differentiation at microsatellite loci indicated that 4x sexual morphs are more likely the result of independent introductions from the native range. If this inference is true, it raises the question of what mechanisms are responsible for the observed genetic diversity in asexual 5x populations. Sexual recruitment is certainly one possibility, but another is somatic mutations, which have been reported at microsatellite loci in other highly clonal species (69). It would be interesting to search for evidence for local adaptation in populations of the 5x cytotype of O. pes-caprae, given the broad range of climatic conditions that the species encounters across its extensive introduced range. Phenotypic plasticity may not be the only mechanism enabling clonal invaders to colonize widely, and the role of somatic mutations and epigenetic variation would be worth investigating in the future.
Future Directions
This article has focused on functional interactions between sexual and asexual reproductive modes that negatively affect the fitness of clonal plants. However, clonality is widely distributed among angiosperms, and large clone size has net benefits to fitness because of increased resource capture, greater competitive ability, and higher reproductive success through both female and male function. An important unresolved issue concerns the quality of mating that arises from different clonal strategies and the extent to which increases in clone size inevitably result in greater levels of geitonogamous selfing and reduced fitness (5, 7, 8). A recent study casts doubt on this frequent assumption by examining alternative strategies for the deployment of flowers and the influence that flower distribution has on pollinator behavior and pollen dispersal (70). Experiments with captive bumblebees and artificial flowers demonstrated that, rather than increasing geitonogamy, clonality reduced this component of selfing when the same number of flowers was distributed among several inflorescences rather than one. Interestingly, the assumption that clonality interferes with outcrossed mating success has also been challenged by Van Drunen et al. (71), based on a spatially explicit model involving fitness through male and female function and trade-offs between clonal expansion and sexual reproduction. They show that clonal expansion enhances overall genet fitness under conditions of spatially restricted pollen and seed dispersal because the outward expansion of clones alleviates dispersal limitation, especially where clones are intermingled. Future experimental studies using genetic markers could be usefully employed to investigate how clonal architecture and size, floral display, and genet density influence pollen dispersal and mating in clonal plants.
Acknowledgments
I thank Marcel Dorken and Lawrence Harder for providing unpublished manuscripts and for valuable discussion on the topic of clonality and Bill Cole for preparing the figures. This work was funded by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chair’s Program.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution IX: Clonal Reproduction: Alternatives to Sex,” held January 9–10, 2015, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/ILE_IX_Clonal_Reproduction.
This article is a PNAS Direct Submission.
References
- 1.Richards AJ. Plant Breeding Systems. Chapman and Hall; London, UK: 1997. [Google Scholar]
- 2.Klimes L, Klimesov J, Hendriks R, van Groenendael JM, de Kroon H. 1997. Clonal plant architecture: A comparative analysis of form and function. The Ecology and Evolution of Clonal Plants, eds de Kroon H, van Groenendael (Backhuys, Leiden, The Netherlands), pp 1–29.
- 3.Sculthorpe CD. The Biology of Aquatic Vascular Plants. Edward Arnold; London: 1967. [Google Scholar]
- 4.Maynard Smith J. The Evolution of Sex. Cambridge Univ Press; Cambridge, UK: 1978. [Google Scholar]
- 5.Vallejo-Marín M, Dorken ME, Barrett SCH. The ecological and evolutionary consequences of clonality for plant mating. Annu Rev Ecol Evol Syst. 2010;41:193–213. [Google Scholar]
- 6.Van Drunen WE, Dorken ME. Trade-offs between clonal and sexual reproduction in Sagittaria latifolia (Alismataceae) scale up to affect the fitness of entire clones. New Phytol. 2012;196(2):606–616. doi: 10.1111/j.1469-8137.2012.04260.x. [DOI] [PubMed] [Google Scholar]
- 7.Handel SN. The intrusion of clonal growth patterns on plant breeding systems. Am Nat. 1985;125:367–383. [Google Scholar]
- 8.Charpentier A. Consequences of clonal growth for plant mating. Evol Ecol. 2002;15:521–530. [Google Scholar]
- 9.Harder LD, Barrett SCH. The Ecology and Evolution of Flowers. Oxford Univ Press; Oxford: 2006. [Google Scholar]
- 10.Barrett SCH. The evolution of plant sexual diversity. Nat Rev Genet. 2002;3(4):274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- 11.Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annu Rev Ecol Evol Syst. 2005;36:47–79. [Google Scholar]
- 12.Harper JL. Population Biology of Plants. Academic; London: 1977. [Google Scholar]
- 13.Tibayrenc M, Ayala FJ. Reproductive clonality of pathogens: A perspective on pathogenic viruses, bacteria, fungi, and parasitic protozoa. Proc Natl Acad Sci USA. 2012;109(48):E3305–E3313. doi: 10.1073/pnas.1212452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovett-Doust L. Population dynamics and local specialization in a clonal perennial (Ranunculus repens). I. The dynamics of ramets in contrasting habitats. J Ecol. 1981;69:743–755. [Google Scholar]
- 15.Barrett SCH. Evolution and Function of Heterostyly. Springer; Berlin: 1992. [Google Scholar]
- 16.Geber MA, Dawson TE, Delph LF. Gender and Sexual Dimorphism in Flowering Plants. Springer; Berlin: 1999. [Google Scholar]
- 17.Wang Y, Wang QF, Guo YH, Barrett SCH. Reproductive consequences of interactions between clonal growth and sexual reproduction in Nymphoides peltata: A distylous aquatic plant. New Phytol. 2005;165(1):329–335. doi: 10.1111/j.1469-8137.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 18.Widén B, Widén M. Pollen limitation and distance-dependent fecundity in females of the clonal gynodioecious herb Glechoma hederacea (Lamiaceae) Oecologia. 1990;83(2):191–196. doi: 10.1007/BF00317751. [DOI] [PubMed] [Google Scholar]
- 19.Aspinwall N, Christian T. Pollination biology, seed production, and population structure in Queen-of-the-Prairie, Filipendula rubra (Rosaceae) at Botkin Fen, Missouri. Am J Bot. 1992;79:488–494. [Google Scholar]
- 20.Barrett SCH, Graham SW. Adaptive radiation in the aquatic plant family Pontederiaceae: Insights from phylogenetic analysis. In: Givnish TJ, Sytsma KJ, editors. Molecular Evolution and Adaptive Radiation. Cambridge Univ Press; Cambridge, UK: 1997. pp. 225–258. [Google Scholar]
- 21.Puentes A, Cole WW, Barrett SCH. Trimorphic incompatibility in Pontederia subovata (Pontederiaceae): An aquatic macrophyte from lowland South America. Int J Plant Sci. 2013;174:47–56. [Google Scholar]
- 22.Bianchi MB, Vesprini J, Barrett SCH. Trimorphic incompatibility in Eichhornia azurea (Pontederiaceae) Sex Plant Reprod. 2000;12:203–208. [Google Scholar]
- 23.Barrett SCH. Tristyly in Eichhornia crassipes (Mart.) Solms (Water Hyacinth) Biotropica. 1977;9:230–238. doi: 10.1111/j.1558-5646.1979.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 24.Eckert CG, Barrett SCH. Stochastic loss of style morphs from populations of tristylous Lythrum salicaria and Decodon verticillatus (Lythraceae) Evolution. 1992;46:1014–1029. doi: 10.1111/j.1558-5646.1992.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 25.Barrett SCH. Waterweed invasions. Sci Am. 1989;260:92–97. [Google Scholar]
- 26.Leme da Cunha N, Fischer E, Lorenz-Lemke AP, Barrett SCH. Floral variation and environmental heterogeneity in a tristylous clonal aquatic of the Pantanal wetlands of Brazil. Ann Bot (Lond) 2014;114(8):1637–1649. doi: 10.1093/aob/mcu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett SCH, Forno IW. Style morph distribution in New World populations of Eichhornia crassipes (Mart.) Solms-Laubach (Water Hyacinth) Aquat Bot. 1982;13:299–306. [Google Scholar]
- 28.Zhang YY, Zhang D-Y, Barrett SCH. Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol Ecol. 2010;19(9):1774–1786. doi: 10.1111/j.1365-294X.2010.04609.x. [DOI] [PubMed] [Google Scholar]
- 29.Barrett SCH. Floral biology of Eichhornia azurea (Swartz) Kunth (Pontederiaceae) Aquat Bot. 1978;5:217–228. [Google Scholar]
- 30.Alvos dos Santos I. Flower visiting bees and the breakdown of the tristylous breeding system of Eichhornia azurea (Swartz) Kunth (Pontederiaceae) Biol J Linn Soc Lond. 2002;77:499–507. [Google Scholar]
- 31.Barrett SCH. The evolutionary breakdown of tristyly in Eichhornia crassipes (Mart.) Solms (Water Hyacinth) Evolution. 1979;33:499–510. doi: 10.1111/j.1558-5646.1979.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 32.Ren M-X, Zhang Q-G, Zhang D-Y. Geographical variation in the breeding system of an invasive plant. Eichhornia crassipes in China. Acta Phytoecol Sin. 2004;28:753–760. [Google Scholar]
- 33.Barrett SCH, Ness RW, Vallejo-Marín M. Evolutionary pathways to self-fertilization in a tristylous plant species. New Phytol. 2009;183(3):546–556. doi: 10.1111/j.1469-8137.2009.02937.x. [DOI] [PubMed] [Google Scholar]
- 34.Düsing C. Die Regulierung des Geschlechtsverhältnisses bei der Vermehrung der Menschen, Tiere und Pflanzen. Fischer; Jean, Germany: 1884. [Google Scholar]
- 35.Fisher RA. The Genetical Theory of Natural Selection. Clarendon; Oxford: 1930. [Google Scholar]
- 36.Sinclair JP, Emlen J, Freeman DC. Biased sex ratios in plants: Theory and trends. Bot Rev. 2012;78:63–86. [Google Scholar]
- 37.Field DL, Pickup M, Barrett SCH. Comparative analyses of sex-ratio variation in dioecious flowering plants. Evolution. 2013;67(3):661–672. doi: 10.1111/evo.12001. [DOI] [PubMed] [Google Scholar]
- 38.Stehlik I, Barrett SCH. Mechanisms governing sex-ratio variation in dioecious Rumex nivalis. Evolution. 2005;59(4):814–825. [PubMed] [Google Scholar]
- 39.Lloyd DG, Webb CJ. Secondary sex characters in plants. Bot Rev. 1977;43:177–216. [Google Scholar]
- 40.Barrett SCH, Hough J. Sexual dimorphism in flowering plants. J Exp Bot. 2013;64(1):67–82. doi: 10.1093/jxb/ers308. [DOI] [PubMed] [Google Scholar]
- 41.Obeso JR. The costs of reproduction in plants. New Phytol. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 42.Barrett SCH, Yakimowski SB, Field DL, Pickup M. Ecological genetics of sex ratios in plant populations. Philos Trans R Soc Lond B Biol Sci. 2010;365(1552):2549–2557. doi: 10.1098/rstb.2010.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yakimowski SB, Barrett SCH. Variation and evolution of sex ratios at the northern range limit of a sexually polymorphic plant. J Evol Biol. 2014;27(7):1454–1466. doi: 10.1111/jeb.12322. [DOI] [PubMed] [Google Scholar]
- 44.Field DL, Pickup M, Barrett SCH. Ecological context and metapopulation dynamics affect sex-ratio variation among dioecious plant populations. Ann Bot (Lond) 2013;111(5):917–923. doi: 10.1093/aob/mct040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorken ME, Friedman J, Barrett SCH. The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae) Evolution. 2002;56(1):31–41. doi: 10.1111/j.0014-3820.2002.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 46.Yakimowski SB, Barrett SCH. Clonal genetic structure and diversity in populations of an aquatic plant with combined vs. separate sexes. Mol Ecol. 2014;23(12):2914–2928. doi: 10.1111/mec.12791. [DOI] [PubMed] [Google Scholar]
- 47.Dorken ME, Barrett SCH. Sex determination and evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae) Proc Biol Sci. 2004;271(1535):213–219. doi: 10.1098/rspb.2003.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorken ME, Barrett SCH. Life-history differentiation and the maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae) Evolution. 2003;57(9):1973–1988. doi: 10.1111/j.0014-3820.2003.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 49.Eckert CG. The loss of sex in clonal plants. Evol Ecol. 2002;15:501–520. [Google Scholar]
- 50.Hollingsworth ML, Bailey JP. Evidence for massive clonal growth in the invasive weed Fallopia japonica (Japanese Knotweed) Bot J Linn Soc. 2000;133:463–472. [Google Scholar]
- 51.Vallejo-Marín M. Mimulus peregrinus (Phrymaceae): A new British allopolyploid species. PhytoKeys. 2012;14(14):1–14. doi: 10.3897/phytokeys.14.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckert CG, Barrett SCH. Patterns of genotypic diversity and clonal reproduction in Decodon verticillatus (Lythaceae) Am J Bot. 1993;80:1175–1182. [Google Scholar]
- 53.Dorken ME, Eckert CG. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythaceae) J Ecol. 2001;89:339–350. [Google Scholar]
- 54.Eckert CG, Dorken ME, Mitchell SA. Loss of sex in clonal populations of a flowering plant, Decodon verticillatus (Lythraceae) Evolution. 1999;53:1079–1092. doi: 10.1111/j.1558-5646.1999.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 55.Dorken ME, Neville KJ, Eckert CG. Evolutionary vestigialization of sex in a clonal plant: selection versus neutral mutation in geographically peripheral populations. Proc Biol Sci. 2004;271(1555):2375–2380. doi: 10.1098/rspb.2004.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klekowski EJ. Mutation, Developmental Selection and Plant Evolution. Columbia Univ Press; New York: 1988. [Google Scholar]
- 57.Schultz ST, Scofield DG. Mutation accumulation in real branches: Fitness assays for genomic deleterious mutation rate and effect in large-statured plants. Am Nat. 2009;174(2):163–175. doi: 10.1086/600100. [DOI] [PubMed] [Google Scholar]
- 58.Whitham TG, Slobodchikoff CN. Evolution by individuals, plant herbivore interactions, and mosaics of genetic variability: The adaptive significance of somatic mutation in plants. Oecologia. 1981;49:287–292. doi: 10.1007/BF00347587. [DOI] [PubMed] [Google Scholar]
- 59.Ally D, Ritland K, Otto SP. Aging in a long-lived clonal tree. PLoS Biol. 2010;8(8):e1000454. doi: 10.1371/journal.pbio.1000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bobiwash K, Schultz ST, Schoen DJ. Somatic deleterious mutation rate in a woody plant: Estimation from phenotypic data. Heredity (Edinb) 2013;111(4):338–344. doi: 10.1038/hdy.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitton JB, Grant MC. Genetic variation and the natural history of quaking aspen. Bioscience. 1996;46:25–31. [Google Scholar]
- 62.Barrett SCH. Why reproductive systems matter for the invasion biology of plants. In: Richardson DM, editor. Fifty Years of Invasion Ecology: The Legacy of Charles Elton. Wiley-Blackwell; Oxford: 2011. pp. 195–210. [Google Scholar]
- 63.Ornduff R. Reproductive systems and chromosome races of Oxalis pes-caprae L. and their bearing on the genesis of a noxious weed. Ann Miss Bot Gard. 1987;74:79–84. [Google Scholar]
- 64.Ferrero V, et al. Invasion genetics of the Bermuda buttercup (Oxalis pes-caprae): Complex intercontinental patterns of genetic diversity, polyploidy and heterostyly characterize both native and introduced populations. Mol Ecol. 2014 doi: 10.1111/mec.13056. [DOI] [PubMed] [Google Scholar]
- 65.Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The Genetics of Colonizing Species. Academic; London: 1965. pp. 147–168. [Google Scholar]
- 66.Rottenberg A, Parker JS. Asexual populations of the invasive weed Oxalis pes-caprae are genetically variable. Proc Biol Sci. 2004;271(Suppl 4):S206–S208. doi: 10.1098/rsbl.2003.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castro S, et al. Reproductive strategy of the invasive Oxalis pes-caprae: Distribution patterns of floral morphs, ploidy levels and sexual reproduction. Biol Invasions. 2013;15:562–566. [Google Scholar]
- 68.Costa J, et al. Sexual reproduction of the pentaploid, short-styled Oxalis pes-caprae allows the production of viable offspring. Plant Biol (Stuttg) 2013;15:208–214. doi: 10.1111/plb.12010. [DOI] [PubMed] [Google Scholar]
- 69.Ally D, Ritland K, Otto SP. Can clone size serve as a proxy for clone age? An exploration using microsatellite divergence in Populus tremuloides. Mol Ecol. 2008;17(22):4897–4911. doi: 10.1111/j.1365-294X.2008.03962.x. [DOI] [PubMed] [Google Scholar]
- 70.Liao W-J, Harder LD. Consequences of multiple inflorescences and clonality for pollinator behavior and plant mating. Am Nat. 2014;184(5):580–592. doi: 10.1086/678117. [DOI] [PubMed] [Google Scholar]
- 71.Van Drunen WE, van Kleunen M, Dorken ME. The consequences of clonal expansion for sexual fitness: Clonal expansion enhances fitness under spatially restricted dispersal. Proc Natl Acad Sci USA. 2015;112:8929–8936. doi: 10.1073/pnas.1501720112. [DOI] [PMC free article] [PubMed] [Google Scholar]