Significance
As a central hormone in metabolism, leptin functions to suppress food intake and to dissipate energy. Mutations of leptin or its receptor result in profound obesity. Data from classic parabiosis experiments (a procedure to surgically connect the vascular systems of two mice together) by Douglas Coleman over 40 years ago and recent studies of leptin treatment, have suggested that leptin might require a cofactor to exert its full metabolic strength. However, we found that a leptin-binding protein, clusterin, is dispensable for the normal function of leptin. Rather, the parabiosis procedure itself appears to potentiate the metabolic action of leptin, leading to chronic starvation and lethality of the mice. Our results have resolved a long-standing puzzle in leptin biology.
Keywords: leptin, parabiosis, db mutant, clusterin, obesity
Abstract
In this study we set out to explain the differing effects of parabiosis with genetically diabetic (db) mice versus administration of recombinant leptin. Parabiosis of db mutant, which overexpress leptin, to wildtype (WT) or genetically obese (ob) mice has been reported to cause death by starvation, whereas leptin infusions do not produce lethality at any dose or mode of delivery tested. Leptin is not posttranslationally modified other than a single disulphide bond, raising the possibility that it might require additional factor(s) to exert the maximal appetite-suppressing effect. We reconfirmed the lethal effect of parabiosis of db mutant on WT mice and further showed that this lethality could not be rescued by administration of ghrelin or growth hormone. We then initiated a biochemical fractionation of a high-molecular-weight leptin complex from human plasma and identified clusterin as a major component of this leptin-containing complex. However, in contrast to previous reports, we failed to observe a leptin-potentiating effect of either exogenous or endogenous clusterin, and parabiosis of db clusterin−/− double-mutant to WT mice still caused lethality. Intriguingly, in parabiotic pairs of two WT mice, leptin infusion into one of the mice led to an enhanced starvation response during calorie restriction as evidenced by increased plasma ghrelin and growth-hormone levels. Moreover, leptin treatment resulted in death of the parabiotic pairs. These data suggest that the appetite suppression in WT mice after parabiosis to db mutants is the result of induced hyperleptinemia combined with the stress or other aspect(s) of the parabiosis procedure.
Leptin functions as an afferent signal in a negative feedback loop that maintains constancy of adipose tissue mass. Leptin acts on its receptors located in the hypothalamus and elsewhere to regulate the neuroendocrine axis, food intake, metabolism, and body weight. The existence of an appetite-suppressing hormone was first suggested by the results from a set of parabiosis experiments between genetically obese (ob) and diabetic (db) mice as well as in studies of the rats with electrolytic lesions of the ventromedial hypothalamus (VMH) that also causes obesity (1–3). The ob and db loci are fully penetrant autosomal-recessive mutations that cause extreme obesity and encode leptin and the leptin receptor, respectively (4, 5).
A key finding in these classic parabiosis experiments has been the observation that parabiosis of db mice or VMH-lesioned rats completely suppressed the appetite of wildtype (WT) or ob animals, leading to death by apparent starvation. The parabiotic partners of db mice were reported to be lean and hypoglycemic with little or no food in the stomach (1, 2). These studies suggested that db mice overproduce an endogenous appetite suppressant, and based on these studies, Coleman correctly predicted that the mouse ob gene encoded a novel hormone regulating body weight. The fact that db mice overproduce an appetite suppressant but were obese suggested that this gene encoded the (leptin) receptor (1, 2). The similar effect of VMH-lesioned animals on body weight of parabiotic partners further suggested that this receptor was localized in the hypothalamus (3). However, whereas parabiosis to db mice (or VMH-lesioned rats) leads to anorexia and death in the partners, this effect has not been recapitulated by administration of recombinant leptin at any dose or via any means of delivery yet tested (6). Thus, leptin produced in parabiosis appears to have a qualitatively different effect than recombinant leptin, although an explanation for this discrepancy has not been forthcoming in the 20 years since the ob gene was cloned.
One possibility to explain this difference was that in vivo, leptin is posttranslationally modified in a manner that is not reproduced in recombinant proteins. However, mass spectrometry confirmed that the molecular weight of recombinant leptin is identical to native leptin (other than an N-terminal methionine in the recombinant protein) with both forms having a single intramolecular disulphide bond (7). This finding pointed to an alternative possibility that leptin action could be potentiated by an additional factor(s) that is necessary for the hormone to exert the maximal catabolic effect in vivo. Consistent with this possibility, previous studies have shown that a fraction of circulating leptin forms distinct high-molecular-weight complexes and that the proportion of leptin in this complex(es) can differ between healthy and obese individuals (8, 9). The nature of these complexes has been studied in several reports, and the data have further suggested that the interaction between leptin and other plasma proteins might modulate the effective concentration of the hormone and thus influence its ability to activate downstream-signaling pathways (8–12). In addition, it was recently reported that clusterin/ApoJ, another circulating protein, can suppress food intake and body weight by itself and can also bind to leptin to potentiate leptin's catabolic effects (13, 14).
In this study, we sought to understand the basis for the differing effects of parabiosis of db mice versus treatment with recombinant leptin. We reconfirmed the lethal effect of parabiosis of db mice to WT mice and observed that this lethality was unaffected by administration of ghrelin or growth hormone, two hormones central for survival during calorie restriction (15, 16). We then set out to biochemically purify leptin-interacting protein(s) from human plasma and showed that ∼25% of leptin circulates in a high-molecular-weight complex. Although significant enrichment of the complex was achieved, our data indicated that the complex was too unstable to enable purification to homogeneity. We did find, however, that clusterin/ApoJ, which was previously shown to bind leptin (10), cofractionated with leptin in the high-molecular-weight complex through each of several chromatographic steps, prompting us to further evaluate clusterin on leptin's action. In contrast to recent reports (13, 14), we failed to find any physiological effect of central or peripheral clusterin on food intake or body weight when it was administered by itself or in combination with leptin. In addition, clusterin was not required for the lethal effect of parabiosis of db mice. However, we observed that treatment of two WT mice parabiosed to one another with recombinant leptin resulted in an enhanced starvation response and death, suggesting that the lethal effect of parabiosis in this setting is likely a result of the hyperleptinemia combined with the stress or other aspect(s) of the procedure itself. The results provide functional data on the presence and role of leptin-binding proteins in vivo and resolve a long-standing conundrum in leptin biology.
Results
Parabiosis Between db and WT Mice Leads to Lethality.
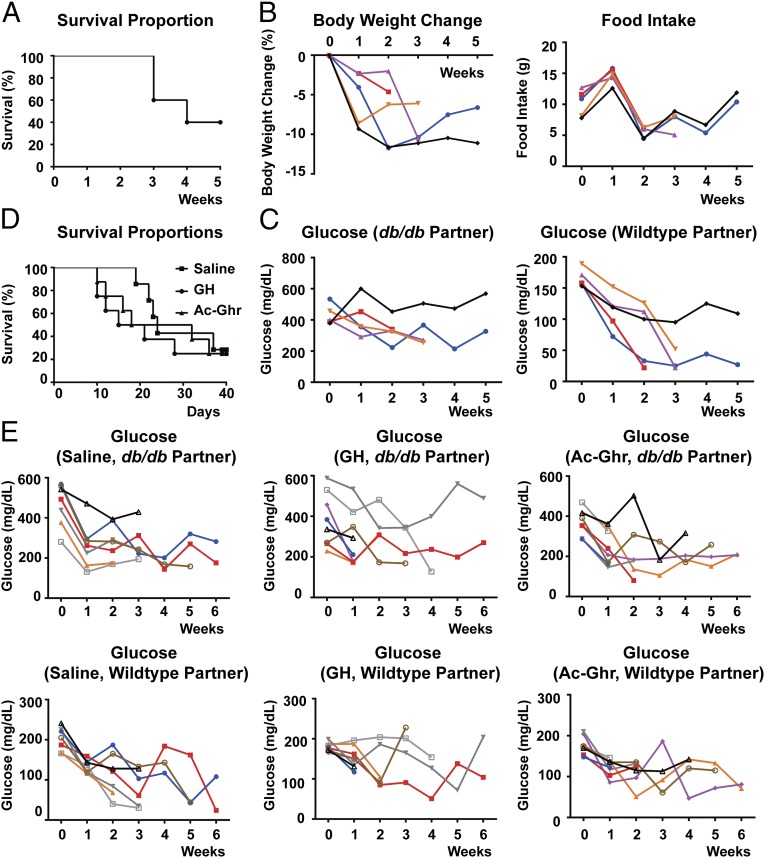
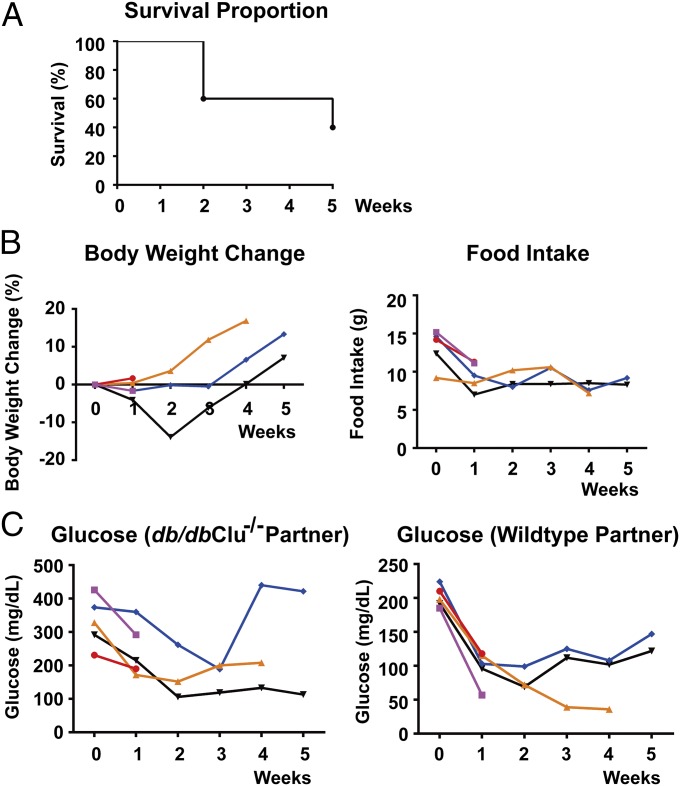
We first set out to replicate the results of classic parabiosis experiments between db and WT mice. Previously, Coleman showed that in parabiosis with db mice, WT and ob mice consumed less food and died of apparent starvation (1, 2). After parabiosis to db mutant animals, there was a 60% mortality of WT mice by 5 wk after the surgery, as shown in the Kaplan–Meier plot (Fig. 1A). This is comparable to the previous study, which showed that 12 out of 18 parabiotic pairs died by 7 wk (2). Due to the adjoining of the paired mice, only the combined body weight and food intake of each pair, but not individual animals, could be measured. Although the combined body weight and food intake of the pairs decreased after the first week (Fig. 1B), the significance of these results is less clear. In contrast, it has previously been shown that plasma glucose does not equilibrate across the parabiotic anastamosis owing to its short half-life, and therefore reliable individual measures of this parameter can be made. Again consistent with the previous data, the WT partners exhibited a steady decrease of plasma glucose levels before death occurred (Fig. 1C). Of note, the death of one partner in a parabiotic pair, which in this case is the hypoglycemic WT mouse, would quickly kill the remaining partner, even though the db mutant maintained significantly higher glucose levels.
Fig. 1.
Parabiosis of the WT mice with db mice leads to lethality. Parabiotic pairs between the WT and db mice were established as described in Materials and Methods. Survival of the pairs was monitored for 5 wk (A). Body weight and food intake of each pair (B) and plasma glucose levels of each partner in a pair (C) were measured every week. Alternatively, parabiotic pairs between the WT and db mice were treated with 625 ng/h GH or 1,250 ng/h acyl–ghrelin (Ac-Ghr) via s.c. osmotic pumps 2 d after parabiosis procedure. Survival of the pairs was monitored for 6 wk (D), and plasma glucose levels of each partner in a pair were measured every week (E). Measures of each pair or each partner in a pair are plotted separately in different colors.
Ghrelin is a peptide hormone secreted by the gastrointestinal tract and functions to stimulate the release of growth hormone (GH) from the pituitary. Recent studies have demonstrated that the ghrelin–GH axis is central for survival during chronic calorie restriction and that food-restricted mice with genetic deletion of ghrelin or ghrelin O-acyltransferase succumbed due to hypoglycemia (15, 16). We therefore tested whether administration of exogenous ghrelin or GH would be able to rescue the lethality of parabiosis of db mice. The WT mice were treated with 625 ng/h GH or 1,250 ng/h acyl–ghrelin (Ac-Ghr) via s.c. osmotic pumps 2 d after the parabiosis procedure. GH or ghrelin treatment of WT mice parabiosed to db mice did not alter the mortality rate (see Kaplan–Meier plots, Fig. 1D), and the WT mice still showed similar levels of hypoglycemia (Fig. 1E). These data suggest that the lethal effect of db parabiosis is not secondary to an alteration of the ghrelin–GH prosurvival axis.
In our parabiosis experiments, the average plasma leptin level was 56 ng/mL in the db mice versus 20 ng/mL in the parabiosed WT mice, consistent with the previous report that plasma leptin does not fully equilibrate across the parabiotic anastamosis (17). Previously, we showed that administering recombinant leptin at 500 ng/h by s.c. osmotic pumps increased the plasma leptin levels to ∼25 ng/mL, comparable to those of the WT mice parabiosed to db mutant (6). However, in this prior report, the hyperleptinemia only led to the loss of adipose tissue mass, after which body weight stabilized for the duration of the infusion without lethality (6). In the same report, high doses of leptin administered intracerebroventricularly (ICV) also failed to recapitulate the lethality (6). Our reconfirmation of the lethal effect of parabiosis of db mice here thus raised the possibility that additional factor(s) may potentiate the catabolic effects of leptin in this context.
A Fraction of Leptin Circulates in the High-Molecular-Weight Complex.
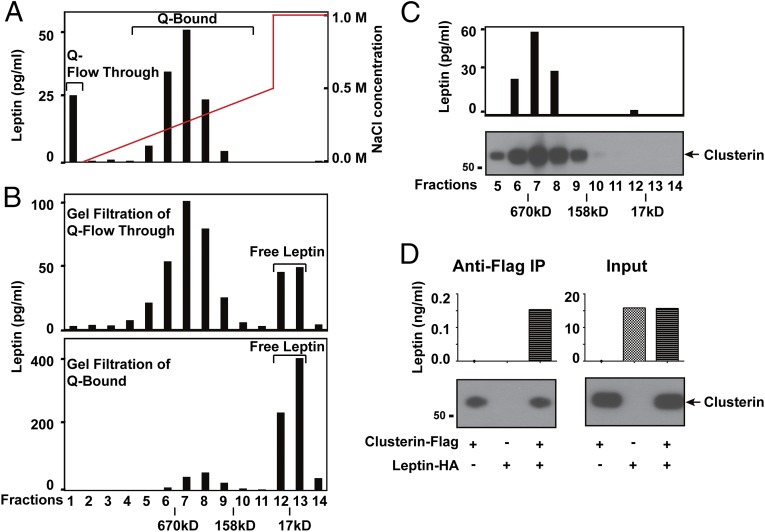
Previous studies have shown that a fraction of plasma leptin circulates in high-molecular-weight complexes, raising the possibility that additional factor(s) could potentiate leptin's action, although these putative factor(s) have not been identified. To begin, we fractionated pooled plasma from healthy human donors through a series of chromatographic steps and followed leptin distribution by ELISA. Plasma leptin was first separated into two distinct populations on the anion-exchange Q-Sepharose column (Fig. 2A). When the flowthrough of Q-Sepharose was loaded on a Superose 6 gel filtration column, ∼75% of the leptin was present in a high-molecular-weight form peaking around 450 kDa (with a range of 158–670 kDa), as shown on the chromatogram in Fig. 2B, Top (with the chromatogram of standard-molecular-weight markers labeled at the bottom). In contrast, the Q-bound fractions entered the gel filtration column and yielded a predominant peak of leptin around a molecular weight of 16 kDa, the size of the monomeric form of the hormone, together with a minor amount of a complex (Fig. 2B, Bottom). We estimate that 25% of total leptin in plasma is in the high-molecular-weight form. We then fractionated the plasma through a total of five chromatographic steps in tandem, including Q-Sepharose, Heparin-Sepharose, SP-Sepharose, Blue-Sepharose, and finally, Superose 6. In each of the first four steps, the complex was found in the flowthrough fraction, with each step yielding an ∼5–10-fold enrichment of the 450-kDa leptin complex. We were unsuccessful in all attempts to identify a chromatographic matrix to which the complex would specifically bind, which limited our ability to purify the complex to homogeneity. Similar to a previous report (9), we observed that exogenous recombinant mouse leptin could displace endogenous human leptin from the complex, indicating that leptin was not tightly bound to other component(s) in the complex. Thus, although we were able to enrich the complex over 2,500-fold compared with unfractionated plasma, we were unable to purify it for direct identification of protein component(s) using mass spectrometry or equivalent methods. We thus tested whether proteins shown to interact with leptin might be part of this complex.
Fig. 2.
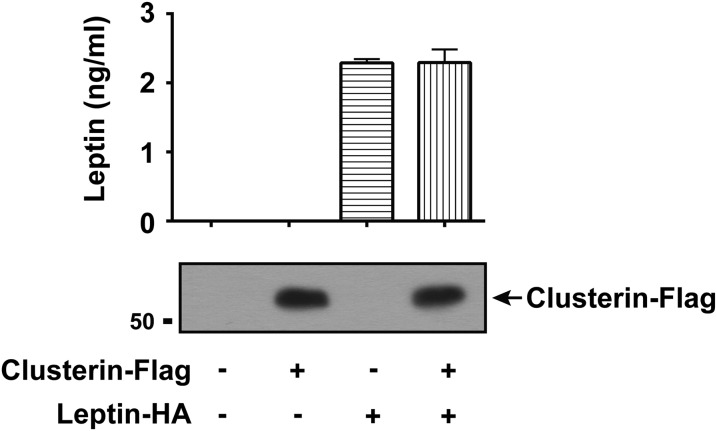
Clusterin is a major component of the leptin-containing complex in human plasma. (A to C) Chromagraphic separation of distinct populations of leptin in human plasma. (A) A portion of leptin is present in complexed form in human plasma. Plasma from healthy human donors was loaded onto an anion-exchange Q-Sepharose column and eluted with a gradient of NaCl, as illustrated by the red line. Leptin was separated into Q-flowthrough (fraction #1) and Q-bound (fraction #6–8). (B) Q-flowthrough and Q-bound fractions were further separated on a Superose 6 gel filtration column. The chromatogram of standard molecular weight markers was indicated at the bottom. (C) Clusterin is a major component of the leptin-containing complex in human plasma. Leptin-containing complex in Q-flowthrough was separated by four additional steps of chromatography (flowthrough of Heparin-Sepharose, flowthrough of SP-Sepharose, flowthrough of Blue-Sepharose, and Superose 6 gel filtration). Fractions of the final gel filtration column were shown, and the chromatogram of standard molecular weight markers was indicated at the bottom. Leptin and clusterin in all of the chromatographic fractions were detected by ELISA and immunoblot, respectively. (D) Clusterin and leptin form the complex. HEK293T cells were cotransfected with plasmids expressing leptin-HA and clusterin-Flag. Anti-Flag immunoprecipitation of clusterin-Flag from the culture medium was performed 48 h after transfection. Enrichment of leptin-HA and clusterin-Flag after immunoprecipitation (Left) and secretion of the proteins in the culture medium (Right) were determined by ELISA and immunoblot, respectively.
Previous reports have used affinity chromatography of GST-tagged recombinant leptin bound to Sepharose beads to identify clusterin (also known as Apoliprotein J) as a leptin-binding protein (10). Although clusterin is an 80-kDa protein, it is known to circulate in multimeric forms. We thus considered the possibility that the 450-kDa complex included both leptin and clusterin. To test this, we performed immunoblot to the fractions after each of the five chromatographic steps used for purifying the high-molecular-weight leptin complex. Clusterin was cofractionated with the leptin complex through all of the chromatographic steps that were performed (Fig. 2C). These data support the possibility that a fraction of circulating leptin binds to clusterin in human plasma.
To confirm clusterin binding to leptin, we cotransfected HEK293T cells with plasmids expressing leptin-HA and clusterin-Flag proteins and assayed for presence of the leptin/clusterin complex in the culture medium. Immunoprecipitation of clusterin-Flag with the anti-Flag M2 antibody coprecipitated leptin-HA from the medium (Fig. 2D). These biochemical results are consistent with the finding that clusterin is a component of the leptin-containing complex in human plasma, although we cannot exclude the possibility that the complex might contain additional protein(s).
Two recent reports presented data showing that clusterin can reduce body weight and food intake in mice by itself and also can bind to leptin and potentiate the engagement of the leptin receptor (13, 14). Our confirmation that clusterin as a major component of the leptin-containing complex thus prompted us to analyze its physiological function in vivo.
Exogenous Clusterin Does Not Potentiate the Catabolic Function of Leptin.
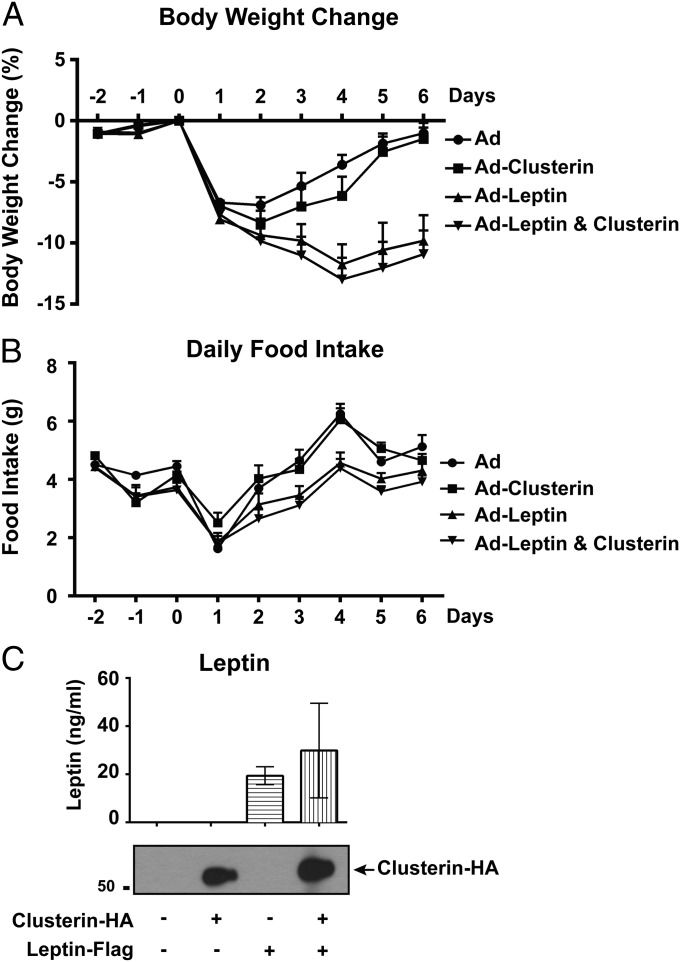
We first examined the metabolic effect of peripherally administrated clusterin alone or in combination with leptin using recombinant adenoviruses. Consistent with the previous report (18), an adenovirus-expressing leptin caused a significant decrease of body weight and food intake in mice compared with the GFP-expressing control virus (Fig. 3 A and B). However, in contrast to recent reports (13, 14), i.v. delivery of the clusterin-expressing adenovirus failed to elicit any significant effect on body weight or food intake. In addition, coinjection of the clusterin-expressing virus with leptin-expressing virus produced effects similar to those observed with the leptin-expressing virus alone (Fig. 3 A and B). Both adenoviral vectors led to high plasma levels of the corresponding protein as assessed by ELISA for leptin and by immunoblot for clusterin (Fig. 3C). Of note, similar to the previous report (18), the peripheral overexpression of leptin from adenovirus with or without clusterin did not result in lethality.
Fig. 3.
Peripheral expression of clusterin does not alter body weight and food intake. Recombinant adenoviruses expressing human leptin-Flag and clusterin-HA were administrated via i.v. injection. Body weight (A) and food intake (B) with each condition were measured daily before and after the viral delivery. Presence of human leptin and clusterin in plasma was confirmed by ELISA and immunoblot, respectively (C). All of the values are presented as mean ± SEM.
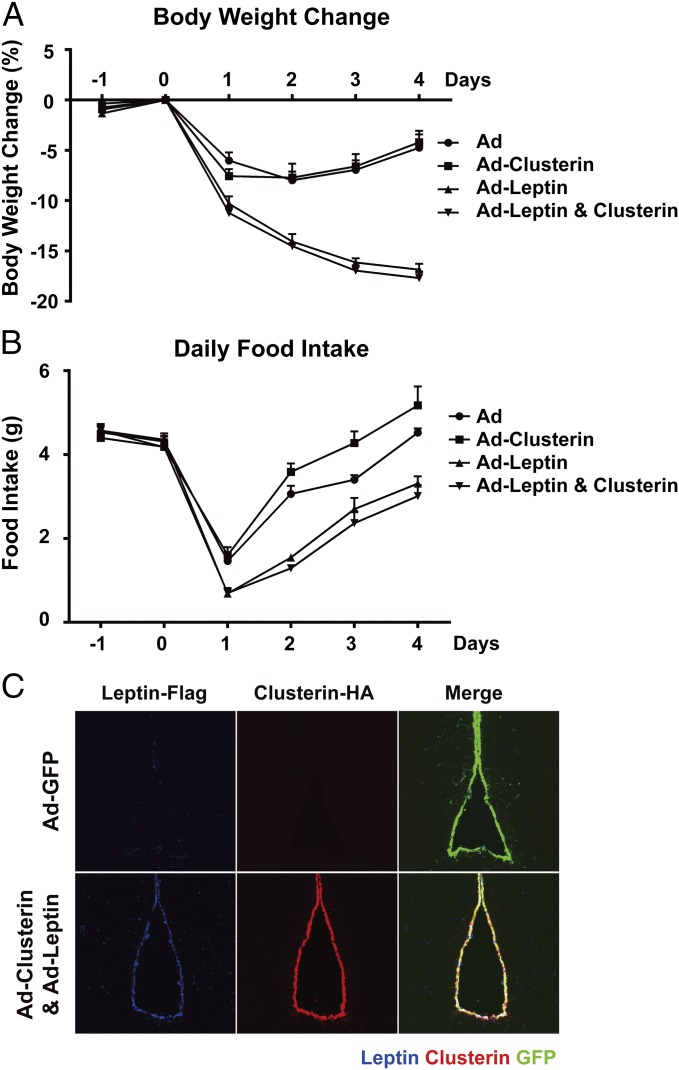
We next tested whether central administration of clusterin could affect energy balance. ICV delivery of the leptin-expressing adenovirus dramatically decreased body weight and food intake, even to a greater extent compared with the peripheral expression of the hormone (Fig. 4 A and B). In contrast, ICV delivery of the clusterin-expressing adenovirus did not elicit any significant effect on body weight or food intake compared with the control virus (Fig. 4 A and B), despite the fact that robust expression of clusterin protein was detected using immunohistochemistry (Fig. 4C). Of note, because the viruses were delivered via ICV, the strongest expression of the proteins were in the cells surrounding the ventricles. The effects of coinjection of the clusterin-expressing virus together with the leptin-expressing virus did not differ from those of the leptin-expressing virus alone (Fig. 4 A and B). Here again, the central overexpression of leptin with or without clusterin did not result in lethality.
Fig. 4.
Central expression of clusterin does not alter body weight and food intake. Recombinant adenoviruses expressing human leptin-Flag and clusterin-HA were administrated via ICV injection. Body weight (A) and food intake (B) with each condition were measured daily before and after the viral delivery. Expression of recombinant clusterin and leptin in the third ventricles was confirmed by immunohistochemistry (C). All of the values are presented as mean ± SEM.
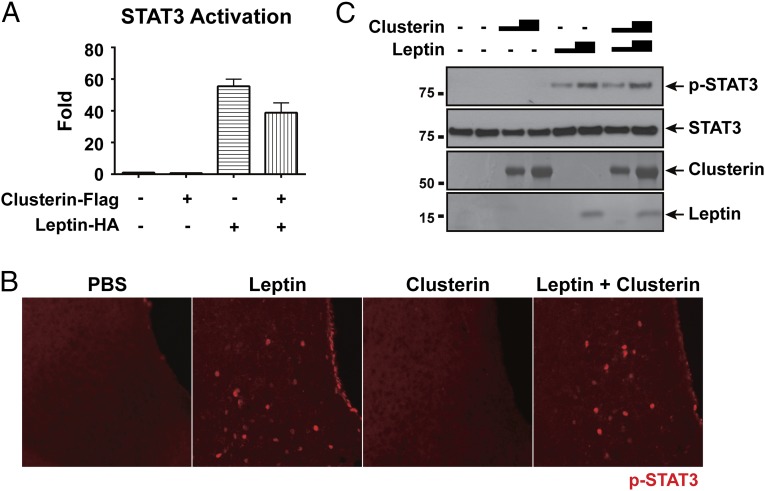
The two aforementioned reports also showed that clusterin potentiated leptin-stimulated activation of STAT3 in the hypothalamus by increasing engagement of the leptin receptor (13, 14). We first used a leptin-receptor/STAT3-luciferase reporter cell line to test for the potential synergy of leptin plus clusterin to activate STAT3 in vitro (19). Delivery of leptin alone to the reporter cells resulted in a significant STAT3 activation, as measured by an increase of luciferase activity (Fig. 5A). In contrast, clusterin alone showed no activation of STAT3-luciferase activity despite the protein being strongly expressed (Fig. S1). Moreover, we failed to observe any potentiating effect of clusterin on leptin-stimulated STAT3 activation (Fig. 5A). To determine the effect of clusterin on leptin signaling in vivo, the recombinant proteins were injected directly. Although ICV injection of recombinant leptin stimulated a robust increase of phospho-STAT3 in the hypothalamus as assessed by immunohistochemistry, recombinant clusterin alone did not cause any discernible increase in the amount of phospho-STAT3 (Fig. 5B). In addition, recombinant clusterin did not alter the level of leptin-stimulated STAT3 activation (Fig. 5B). The same result was obtained using immunoblot analysis of the protein samples prepared from the hypothalamus, i.e., clusterin protein failed to induce STAT3 phosphorylation by itself, nor did it enhance the leptin-stimulated STAT3 phosphorylation (Fig. 5C).
Fig. 5.
Clusterin does not potentiate leptin-stimulated neuronal activation of STAT3. (A) Clusterin failed to potentiate STAT3 activation by leptin in the leptin-receptor/STAT3-luciferase reporter cells. The reporter cells were transfected with plasmids expressing leptin-HA and clusterin-Flag, and the luciferase activity with each condition was measured at 48 h after transfection. The values are presented as mean ± SEM. (B and C) Clusterin failed to potentiate STAT3 activation by leptin in the hypothalamus. Recombinant leptin and clusterin proteins were administrated into the lateral ventricles of mice via ICV injection. Sixty minutes after the injection, STAT3 phosphorylation in the hypothalamus was examined by immunohistochemistry (B) or by immunoblot (C, Top). The purity of recombinant leptin and clusterin proteins were examined by SDS/PAGE followed by coomassie blue staining (C, Bottom).
Fig. S1.
Expression of leptin and clusterin proteins in leptin-receptor/STAT3-luciferase reporter cells. The leptin-receptor/STAT3-luciferase reporter cells were transfected with plasmids expressing leptin-HA or clusterin-Flag. Secretion of the proteins in the culture media at 48 h after transfection was detected by ELISA and immunoblot, respectively.
Overall, our results did not support a physiological function of peripherally or centrally administered clusterin on body weight or food intake by itself or a potentiating effect on leptin's action. In addition, we failed to observe any effect of exogenous clusterin on the leptin-stimulated STAT3 activation either in the reporter cells or in the hypothalamus. We therefore decided to evaluate whether endogenous clusterin is necessary for the catabolic function of leptin.
Endogenous Clusterin Is Dispensable for the Catabolic Function of Leptin.
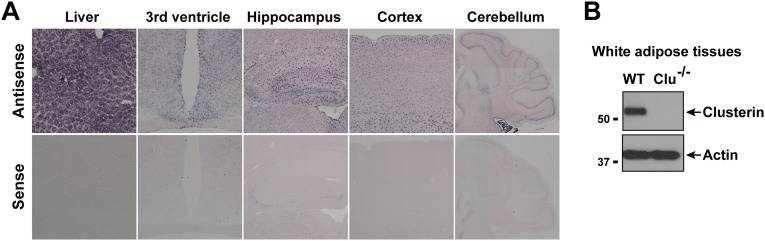
We determined the expression profile of clusterin in mice. In situ hybridization and immunoblot analysis showed that clusterin is expressed in white adipose tissue but also in many other peripheral tissues, including a high level of expression in liver (Fig. S2). Clusterin is also broadly expressed in the central nervous system, including in the hypothalamus and other brain regions (Fig. S2). Consistent with a previous report (20), clusterin expression appears highly enriched in nonneuronal glial cells.
Fig. S2.
Expression profile of endogenous clusterin in mice. (A) Expression of clusterin mRNA in the liver and the central nervous system (hypothalamus, hippocampus, cortex, and cerebellum) was determined by in situ hybridization. The sense probe was included to confirm the specificity of hybridization signals. Each of the cerebellum panels is a composite of multiple images. (B) Expression of clusterin protein in white adipose tissues was determined by immunoblot. The sample from clusterin-knockout mice was included to confirm the antibody specificity.
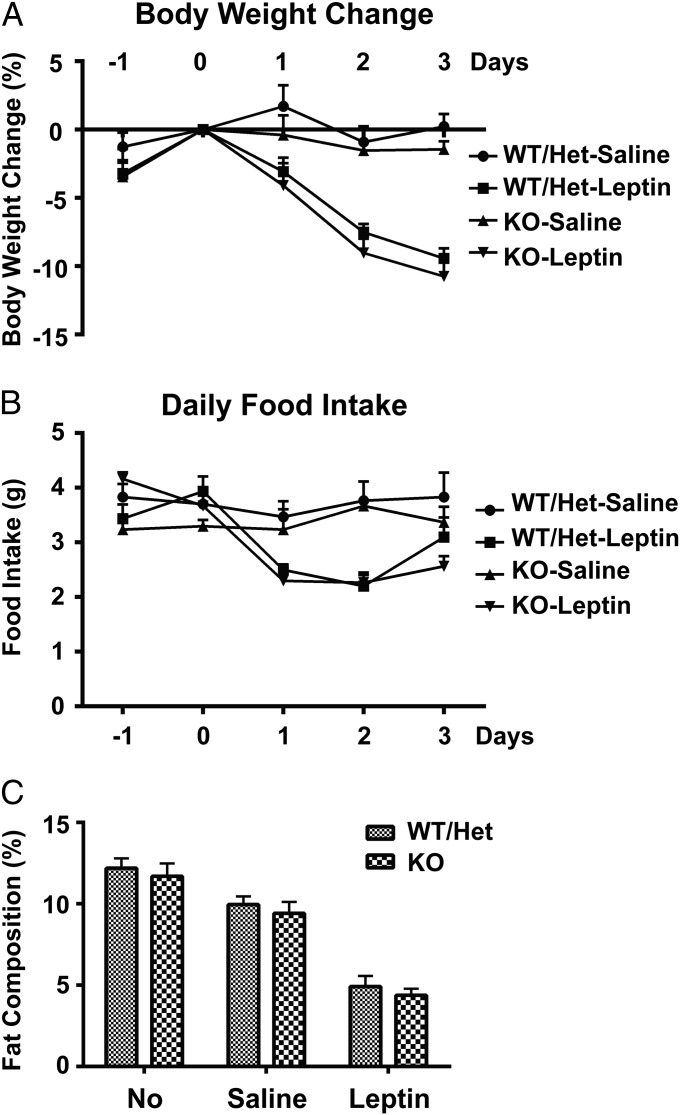
To evaluate the in vivo function of clusterin, we obtained clusterin-knockout mice (21). As reported, at 6 mo of age, clusterin-knockout mice do not show any gross difference in body weight, food intake, or activity compared with their WT littermates. To examine whether endogenous clusterin might modulate the catabolic function of leptin, recombinant leptin was peripherally administrated to clusterin-knockout mice via s.c. osmotic pumps. Leptin treatment reduced body weight and food intake to the same extent in clusterin-knockout mice as in the WT mice (Fig. 6 A and B). In addition, clusterin-knockout mice had normal fat composition at baseline condition, as measured by MRI, and peripheral administration of leptin caused a similar decrease of fat mass in the clusterin-knockout and WT mice (Fig. 6C). These data together suggest that endogenous clusterin is not essential for leptin's action.
Fig. 6.
Clusterin is dispensable for the metabolic function of leptin. Clusterin-knockout and their control littermates (WT/heterozygote) were peripherally administrated with leptin via s.c. osmotic pumps. Body weight (A) and food intake (B) were measured daily before and after leptin treatment. Body fat composition was measured by MRI before and 3 d after the treatment (C). All of the values are presented as mean ± SEM.
Clusterin Is Dispensable for db-Mutant–Induced Lethality in Parabiosis.
We then considered the possibility that clusterin might be required for the lethality of the WT mice parabiosed to db mutant. To test this, we generated db/db; clusterin−/− double-mutant mice, which appeared phenotypically similar for body weight compared with db mutant. Parabiosis of WT mice to the double mutant resulted in similar lethality as that observed after parabiosis of the WT to db single-mutant mice (Fig. 7A). Body weight and food intake of the parabiotic pairs were measured once a week, but as noted above, these data corresponded to the combined measures (Fig. 7B). Similar to that observed in parabiosis with db mice, a continuous decrease of plasma glucose levels in the WT mice parabiosed to the double-mutant mice occurred (Fig. 7C). Thus, clusterin is dispensable for the long-term catabolic effects in parabiosis to db mice and therefore does not provide an explanation for the differing effect of parabiosis to db mice versus chronic administration of recombinant leptin.
Fig. 7.
Clusterin is dispensable for db-mutant–induced lethality in parabiosis. Parabiosis between the WT and db/db; clusterin−/− double-mutant mice was established. Survival of the pairs was monitored for 5 wk postsurgery (A). Body weight and food intake of each pair (B) and plasma glucose levels of each partner in a pair (C) were measured every week. Measures of each pair or each partner in a pair are plotted separately in different colors.
Leptin Treatment of WT Mice in Parabiosis Causes Lethality.
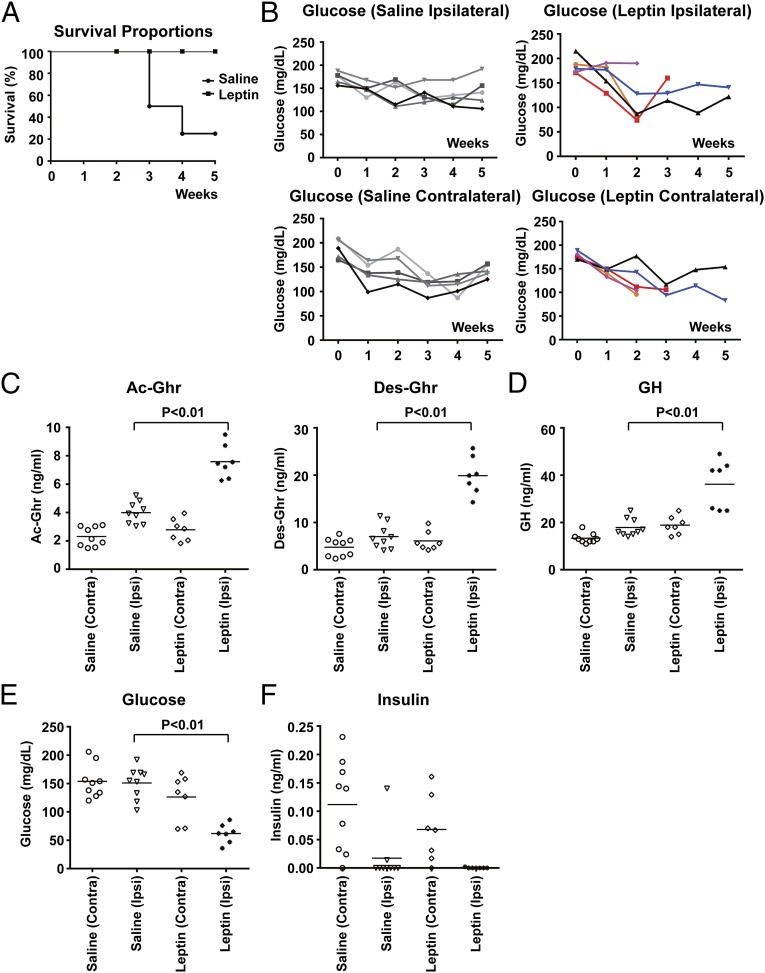
We finally considered the possibility that leptin treatment plus the parabiosis procedure could have a more profound effect than leptin alone. To test this, we parabiosed two WT mice to one another and then delivered recombinant leptin at 500 ng/h to one partner in each pair via s.c. osmotic pumps 2 d after the parabiosis procedure. The parabiosed mice directly receiving the leptin infusion had plasma leptin levels of ∼20–30 ng/mL, comparable to those in the WT mice parabiosed with db mice, as noted above. The plasma leptin levels in the paired mice not directly receiving the leptin infusion were ∼5 ng/mL, and those of saline-treated parabiotic pairs were ∼2 ng/mL Intriguingly, as shown in the Kaplan–Meier plot, the parabiosed WT mice treated with leptin died with a similar mortality rate as that of the WT mice parabiosed to db mutant (Fig. 8A). In addition, there was a significant decrease of plasma glucose levels in the leptin-infused mice (Fig. 8B). We examined whether this hypoglycemia of leptin-treated parabiosed WT mice could be due to an alteration of the ghrelin–GH axis. Two weeks after parabiosis procedure, the mice were subjected to a 23-h calorie restriction, which has previously been shown to dramatically stimulate the release of ghrelin and GH (15, 22). Compared with the saline-treated control mice, the leptin-treated parabiosed mice had significantly higher plasma levels of ghrelin (and its degradation product, desacyl–ghrelin) and GH (Fig. 8 C and D). In addition, the leptin-treated parabiosed mice showed extreme hypoglycemia with undetectable insulin levels (Fig. 8 E and F). These results suggest that the leptin-treated parabiosed mice had an appropriate response of the ghrelin–GH axis and that leptin plus parabiosis leads to hypoglycemia downstream of the production of these two glucoregulatory peptides. This finding is consistent with the failure of GH or ghrelin to improve survival of the WT mice parabiosed to db mutant. The data together suggest that the lethal effect of parabiosis to db mice is the result of hyperleptinemia combined with the stress or some other aspect(s) of the parabiosis surgery.
Fig. 8.
Chronic leptin treatment of the WT mice in parabiosis leads to lethality. Parabiosis between the two WT mice was established, and one partner of the pair was then administrated with saline or leptin via s.c. osmotic pumps. Survival of the pairs was monitored for 5 wk postsurgery (A). Plasma glucose levels of each partner in a pair were measured every week (B). Alternatively, 2 wk after the parabiosis procedure, the mice were subjected to a 23-h calorie restriction. Plasma levels of acyl–ghrelin (Ac-Ghr) and desacyl–ghrelin (Des-Ghr) (C), growth hormone (GH) (D), glucose (E), and insulin (F) in each partner of a pair were measured. Measures of each partner in a parabiotic pair are plotted separately.
Discussion
In a set of classic parabiosis experiments using genetically obese mutant mice, Coleman predicted that food intake and body weight are regulated by a circulating molecule(s) and its receptor(s) (1, 2). Studies of obese rats with hypothalamic lesions further suggested that this receptor was localized in the hypothalamus (3). This prophetic view on the hormonal control of energy balance was later proven to be correct with the subsequent cloning of the ob and db genes and the identification of leptin and the leptin receptor. However, 20 years after the discovery of leptin, one question originating from the parabiosis experiments by Coleman still lingers. It was originally observed (1, 2), as we also reconfirmed here, that parabiosis of db mice to WT or ob mice led to lethality due to chronic starvation of the WT or ob partners. However, this effect cannot be recapitulated by administration of recombinant leptin at any dose or mode of delivery (6).
The differing effects of parabiosis and recombinant leptin have led to several potential hypotheses. For instance, native leptin produced in db mice might carry a posttranslational modification that was absent in the recombinant protein. This possibility was excluded by the finding that native and recombinant leptin proteins showed the same molecular weight on mass spectrometry, other than an N-terminal methionine in the recombinant protein (7). An alternative hypothesis is that a cofactor(s) produced in db mice, but that cannot be complexed to the recombinant protein, can potentiate the catabolic function of leptin. The observation that a fraction of circulating leptin forms distinct high-molecular-weight complex(es) in plasma appeared consistent with this possibility. Moreover, distribution of leptin complex(es) has been reported to differ between healthy and obese patients, suggesting that the high-molecular-weight leptin complex(es) might control the availability and/or activity of leptin (8–12). However, whether such a leptin-binding factor is responsible for the lethality associated with parabiosis of db mice was unresolved.
In this study, we sought to settle this question by determining the component(s) of leptin complex in plasma. A key assumption underlying our attempts to identify a leptin-potentiating cofactor(s) biochemically was that this putative factor(s) would directly interact with leptin. One explanation for the lethal effect of parabiosis to db mice would thus be that leptin is complexed to another factor in adipocytes before secretion and that this interaction either cannot occur or only does so at very low efficiency in plasma after administration of the recombinant protein. This would explain why recombinant leptin, which could in theory encounter and bind to any factors in plasma, failed to recapitulate the lethality at any dose or mode of delivery tested. We showed that in human plasma from healthy blood donors there is one major leptin-containing complex that accounts for ∼25% of the circulating leptin, with the remainder circulating as a leptin monomer of ∼16 kDa. We used a series of chromatographic steps to enrich for this high-molecular-weight complex by over 2,500-fold. However, because of the low starting concentration (1–2 ng/mL, as estimated by leptin ELISA), the instability of the complex as demonstrated using displacement assay, and the inability to find a matrix to which the complex would specifically bind, we were unable to achieve the 1 × 106-fold enrichment estimated to be required for purification to homogeneity. Rather than de novo identifying the component(s) of this complex, we therefore used a candidate approach, and in line with previous studies (10), our data are consistent with the possibility that clusterin/ApoJ is a major component in this leptin complex. In particular, clusterin was cofractionated with the leptin complex through all of the chromatographic steps, suggesting that circulating leptin binds to clusterin in human plasma. This is further supported by the data showing that leptin can directly interact with clusterin in cultured cells expressing both proteins.
However, despite the presence of leptin binding to clusterin, we failed to find any effect of exogenous or endogenous clusterin on body weight or food intake alone or in combination with leptin. For example, clusterin did not potentiate leptin-stimulated STAT3 activation both in vitro and in vivo or the catabolic function of peripherally or centrally administrated leptin. In addition, db clusterin−/− double-mutant mice caused similar mortality of the WT mice in parabiosis as that observed with db single-mutant, ruling out clusterin as contributing to the lethal effect in this context. These results were in contrast to recent reports claiming that clusterin decreased body weight and food intake by itself and potentiated the catabolic function of leptin (13, 14). The basis for this discrepancy is not clear and might be due to the fact that the metabolic effect of clusterin is relatively subtle and can only be observed under certain physiological condition that are different from those of our experiments. Alternatively, to exert its biological function, clusterin protein might require specific posttranslational modification(s) or binding partner(s) that can only be obtained in specific cell types, which could not be recapitulated by the recombinant protein or when the protein was expressed by an adenovirus. Of note, we delivered the recombinant adenoviruses via i.v. or ICV injections, and for ICV injection the viruses were administrated into the ventricles. These methods were different from the viral delivery into the hypothalamus used in previous reports, which might lead to a higher local concentration of clusterin protein there. However, ICV injection of the leptin-expressing virus was sufficient to induce substantial weight loss and appetite suppression, and because coinjection of the clusterin-expressing virus did not potentiate these leptin effects, the data nevertheless suggest that a leptin/clusterin complex is not more potent than leptin alone.
Administration of ghrelin or GH did not alleviate the mortality of WT mice parabiosed to db mice, and we unexpectedly observed that the lethal effect of parabiosis to db mice might be attributable to the procedure itself. Thus, although long-term administration of high dosages of leptin reduces weight and transiently decreases food intake until adipose tissue mass is depleted (6), administration of a relatively low dosage of leptin to the parabiotic pairs of two WT mice resulted in equivalent mortality to that observed in parabiosis to db mutant. This finding suggests that one does not need to invoke the existence of a leptin-potentiating factor to explain the differing effects of parabiosis versus administration of recombinant leptin. The specific attribute of the parabiosis procedure that accounts for its “leptin-potentiating” effect is not immediately clear but could be a result of the operative and postoperative stress of the procedure, including limitation on motility, disruption of social behaviors (e.g., grooming), or chronic inflammation. In fact, parabiosis of two WT mice caused a moderate decrease (15–30%) of plasma glucose levels in the first 2 wk after the procedure (Fig. 8B). Because the parabiosis method has been exploited in many research fields, such as metabolism, immunity, and aging, the finding that there are procedure-associated complications should be considered when interpreting experimental results from any study using parabiosis. Whatever the particular effect of the procedure is, our findings suggest that the hypoglycemia and lethality in the WT mice parabiosed to db mutant is a combined result of moderate hyperleptinemia and the stress of the procedure. This resolves a long-standing mystery in leptin biology and vitiates the notion that an unidentified cofactor is required at least for the maximal catabolic effect of leptin in parabiosis, and in all likelihood, other functions.
Materials and Methods
Reagents.
Recombinant adenoviruses expressing clusterin and leptin were generated by ViraQuest. Recombinant leptin proteins were obtained from Amgen, recombinant mouse ghrelin protein was obtained from GenScript, and recombinant human growth-hormone protein was obtained from the National Hormone and Peptide Program of the National Institute of Diabetes and Digestive and Kidney Diseases. Recombinant clusterin protein and ELISA kits for human or mouse leptin were from R&D. ELISA kits for mouse acyl–ghrelin, desacyl–ghrelin, and growth hormone were from Cayman Chemical. ELISA kits for mouse insulin were from ALPCO. Osmotic pumps were from Alzet, and chromatographic columns were from GE Healthcare.
Rabbit anti-phospho-STAT3 and rabbit anti-STAT3 antibodies were from Cell Signaling, goat anti-clusterin was from Santa Cruz, chicken anti-GFP was from Abcam, rabbit anti-Flag was from Sigma, and rat anti-HA was from Roche. Anti-Flag M2 beads and other chemicals were from Sigma unless otherwise specified. Healthy donor plasma was obtained from the New York Blood Center. The study involving pooled human plasma was approved by and performed in compliance with the policy of the Institutional Review Board at The Rockefeller University.
Chromatography.
Plasma pooled from healthy human donors was diluted 10 times with Buffer Q-A (20 mM Tris-Cl at pH 7.5 and 10% (vol/vol) glycerol) and filtered through 0.22 μm filters. The plasma samples were then applied to Q-sepherose column and eluted with a linear gradient of Buffer Q-B [20 mM Tris-Cl at pH 7.5, 10% (vol/vol) glycerol, and 2 M NaCl]. The unbound Q-flowthrough fraction was adjusted with Hepes-Na to pH 7.0 and fractionated sequentially through Heparin-sepherose, SP-Sepharose, and Blue-Sepharose, with flowthrough of each column used for the next step. Flowthrough of the Blue-Sepharose column was concentrated and separated on Superose 6 gel filtration. Leptin and clusterin proteins in the fractions of each step of chromatography were detected by ELISA and immunoblot, respectively.
Animal Procedures.
The WT C57Bl6/J, db, and clusterin-knockout mice were from The Jackson Laboratory. All surgical procedures in mice were performed in compliance with the protocol approved by the Institutional Animal Care and Use Committee of The Rockefeller University. The mice were housed in colony cages with 12-h light/12-h dark cycles, with the dark cycle beginning at 7:00 PM. Unless otherwise specified, the mice were fed the chow diet (Teklad Mouse Diet 7002) ad libitum.
Parabiosis was established with 6–8-wk old mice, and each pair of animals were housed together for 1 wk before surgery. After anesthesia with isoflurane, a longitudinal incision was made along one side of each mouse, and skin was separated from underlying connective tissues. An incision of ∼8 mm was made on the peritoneum of each animal and sutured together to establish the connection of vascular systems. The scapulae and the dorsal skin of the mice were stitched, which helps hold the parabiotic pair together. To examine the effect of chronic leptin treatment, s.c. osmotic pumps with saline or leptin (delivery rate of 500 ng/h) were implanted in one partner of each pair 3 d after parabiosis surgery. To monitor plasma leptin levels by ELISA, blood samples were collected through orbital bleeding at 1 d before and 10 d after parabiosis surgery. Combined body weight and food intake of the pairs were measured weekly, and plasma glucose levels of individual mice were determined by Breeze2 glucometer (Bayer) from tail bleeding.
To examine the metabolic effect of clusterin, adenoviruses expressing clusterin or leptin were administrated into the WT mice via either bilateral ICV injection (2 × 109 viral particles per mouse) or i.v. injection (1 × 1011 viral particles per mouse). Body weight and food intake were measured daily before and after the viral delivery. To examine STAT3 activation in the hypothalamus, recombinant leptin (20 and 200 ng per mouse) or clusterin (100 and 1,000 ng per mouse) proteins were delivered into the lateral ventricles through ICV injection. Sixty minutes after the treatment, STAT3 phosphorylation in the neurons in the hypothalamus was determined by free-floating immunostaining, as previously described (23). Alternatively, the hypothalamus regions were freshly dissected, homogenized in SDS/PAGE loading buffer, and then analyzed by immunoblot.
To characterize the response of clusterin-knockout mice to leptin, s.c. osmotic pumps with saline or leptin (delivery rate of 500 ng/h) were set up. Body weight and food intake were measured daily before and after leptin treatment. Body fat composition of each mouse was determined by EchoMRI Body Composition Analyzer at 1 d before and 3 d after leptin treatment.
Peripheral administration of ghrelin and growth hormone as well as ELISA measurements of plasma hormones after 23-h calorie restriction were performed following the reported methods (15, 22).
Immunoprecipitation and STAT3-Luciferase Reporter Assay.
Cultured HEK293T cells were transfected with plasmids expressing leptin-HA and clusterin-Flag using Fugene 6 (Roche). Forty-eight hours after transfection, the culture media were collected and subjected to anti-Flag immunprecipitation using M2 beads. The bound proteins were eluted with 3xFlag peptide. Leptin and clusterin proteins in the eluates were detected by ELISA and immunoblot, respectively. For STAT3-luciferase reporter assay, the leptin-receptor/STAT3-luciferase reporter cells were transfected with plasmids expressing leptin-HA and clusterin-Flag. The luciferase activities under each condition were determined by Luciferase Assay System (Promega) at 48 h after transfection.
Acknowledgments
We thank the members of the J.M.F. laboratory for reagents, discussion, and suggestions. This work was supported by Howard Hughes Medical Institute at The Rockefeller University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510378112/-/DCSupplemental.
References
- 1.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 2.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217(5):1298–1304. doi: 10.1152/ajplegacy.1969.217.5.1298. [DOI] [PubMed] [Google Scholar]
- 3.Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959;145(2):336–352. doi: 10.1113/jphysiol.1959.sp006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 6.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94(16):8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SL, et al. Human leptin characterization. Nature. 1996;382(6592):589. doi: 10.1038/382589a0. [DOI] [PubMed] [Google Scholar]
- 8.Houseknecht KL, et al. Evidence for leptin binding to proteins in serum of rodents and humans: Modulation with obesity. Diabetes. 1996;45(11):1638–1643. doi: 10.2337/diab.45.11.1638. [DOI] [PubMed] [Google Scholar]
- 9.Sinha MK, et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest. 1996;98(6):1277–1282. doi: 10.1172/JCI118913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajari TM, Strasser V, Nimpf J, Schneider WJ. A model for modulation of leptin activity by association with clusterin. FASEB J. 2003;17(11):1505–1507. doi: 10.1096/fj.02-1106fje. [DOI] [PubMed] [Google Scholar]
- 11.Birkenmeier G, Kämpfer I, Kratzsch J, Schellenberger W. Human leptin forms complexes with alpha 2-macroglobulin which are recognized by the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Eur J Endocrinol. 1998;139(2):224–230. doi: 10.1530/eje.0.1390224. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Ioffe E, Fidahusein N, Connolly E, Friedman JM. Absence of soluble leptin receptor in plasma from dbPas/dbPas and other db/db mice. J Biol Chem. 1998;273(16):10078–10082. doi: 10.1074/jbc.273.16.10078. [DOI] [PubMed] [Google Scholar]
- 13.Byun K, et al. Clusterin/ApoJ enhances central leptin signaling through Lrp2-mediated endocytosis. EMBO Rep. 2014;15(7):801–808. doi: 10.15252/embr.201338317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil SY, et al. Clusterin and LRP2 are critical components of the hypothalamic feeding regulatory pathway. Nat Commun. 2013;4:1862–1870. doi: 10.1038/ncomms2896. [DOI] [PubMed] [Google Scholar]
- 15.Zhao TJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein JL, et al. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76:121–127. doi: 10.1101/sqb.2011.76.010447. [DOI] [PubMed] [Google Scholar]
- 17.Harris RB, Zhou J, Weigle DS, Kuijper JL. Recombinant leptin exchanges between parabiosed mice but does not reach equilibrium. Am J Physiol. 1997;272(6 Pt 2):R1800–R1808. doi: 10.1152/ajpregu.1997.272.6.R1800. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, et al. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc Natl Acad Sci USA. 1996;93(25):14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceccarini G, et al. PET imaging of leptin biodistribution and metabolism in rodents and primates. Cell Metab. 2009;10(2):148–159. doi: 10.1016/j.cmet.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel D, Moyse E, Trembleau A, Jourdan F, Brun G. Clusterin/ApoJ expression is associated with neuronal apoptosis in the olfactory mucosa of the adult mouse. J Cell Sci. 1997;110(Pt 14):1635–1645. doi: 10.1242/jcs.110.14.1635. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin L, et al. Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis. J Clin Invest. 2000;106(9):1105–1113. doi: 10.1172/JCI9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li RL, et al. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012;287(22):17942–17950. doi: 10.1074/jbc.M112.358051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE. 2010;5(6):e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]