Significance
The stereotyped cellular organization found within the mammalian auditory epithelium is key to its proper function. Differentiation of this structure occurs under strict spatial and temporal regulation to ensure that proper patterning is achieved. Unlike other neuronal structures (e.g. the retina and cortex), where terminal mitosis and differentiation are linked, these processes remain distinctly separated within the developing auditory epithelium. How coordination is achieved remains largely unknown. Here we show that the RNA-binding protein LIN28B times auditory prosensory cell cycle withdrawal and differentiation through both let-7–dependent and let-7–independent mechanisms. Additionally, we show that manipulation of the LIN28B/let-7 axis alters the capacity for postnatal production of sensory hair cells (HC) in the absence of Notch signaling, revealing this axis as a potential candidate for future HC regeneration therapies.
Keywords: Lin28b, Let-7, hair cell, cochlea, regeneration
Abstract
Proper tissue development requires strict coordination of proliferation, growth, and differentiation. Strict coordination is particularly important for the auditory sensory epithelium, where deviations from the normal spatial and temporal pattern of auditory progenitor cell (prosensory cell) proliferation and differentiation result in abnormal cellular organization and, thus, auditory dysfunction. The molecular mechanisms involved in the timing and coordination of auditory prosensory proliferation and differentiation are poorly understood. Here we identify the RNA-binding protein LIN28B as a critical regulator of developmental timing in the murine cochlea. We show that Lin28b and its opposing let-7 miRNAs are differentially expressed in the auditory sensory lineage, with Lin28b being highly expressed in undifferentiated prosensory cells and let-7 miRNAs being highly expressed in their progeny—hair cells (HCs) and supporting cells (SCs). Using recently developed transgenic mouse models for LIN28B and let-7g, we demonstrate that prolonged LIN28B expression delays prosensory cell cycle withdrawal and differentiation, resulting in HC and SC patterning and maturation defects. Surprisingly, let-7g overexpression, although capable of inducing premature prosensory cell cycle exit, failed to induce premature HC differentiation, suggesting that LIN28B’s functional role in the timing of differentiation uses let-7 independent mechanisms. Finally, we demonstrate that overexpression of LIN28B or let-7g can significantly alter the postnatal production of HCs in response to Notch inhibition; LIN28B has a positive effect on HC production, whereas let-7 antagonizes this process. Together, these results implicate a key role for the LIN28B/let-7 axis in regulating postnatal SC plasticity.
The auditory sensory epithelium, housed in the inner ear cochlea, is critical for our ability to perceive sound. This bilayered structure is composed of mechano-sensory hair cells (HCs), which lie atop a layer of glial-like supporting cells (SCs). Stereotyped organization of these cells is essential for proper functioning of the mature cochlea. HCs and SCs arise from a common pool of progenitor cells (prosensory cells), which in mammals withdraw from the cell cycle in a highly synchronized apical-to-basal wave (1) that is closely followed by an inverse basal-to-apical wave of differentiation (2). This unique spatial and temporal pattern of cell cycle withdrawal and differentiation holds postmitotic prosensory cells in an undifferentiated state for varying lengths of time, depending on their basal-to-apical location, and is thought to ensure the proper patterning of HCs and SCs. Over the past several years, key regulators of prosensory cell proliferation and differentiation have been identified (3, 4). P27/Kip1 (CDKN1B), a cyclin-dependent kinase inhibitor, controls prosensory cell cycle withdrawal (5), whereas ATOH1, a basic helix-loop-helix transcriptional activator, controls HC and SC differentiation (6, 7). Atoh1 and p27/Kip1 loss-of-function studies indicate that prosensory cell cycle exit and differentiation occur independently from each other (5, 8); however, the molecular mechanisms coordinating the timing of these processes remain unknown.
Using microarray-based transcriptional profiling, we recently identified Lin28b to be highly expressed in prosensory cells. Lin28 genes encode for evolutionarily highly conserved RNA binding proteins (9) known to regulate larval developmental timing (heterochrony) in Caenorhabditis elegans (10). In humans and mice, Lin28a and its homolog Lin28b are critical regulators of stemness, organismal growth, metabolism, tumorigenesis, and tissue repair (11). LIN28A and LIN28B proteins promote a stem cell/progenitor-like state through two distinct mechanisms. First, LIN28 proteins bind to and stabilize mRNAs encoding for cell cycle regulators and growth stimulating genes, leading to increases in their protein abundance (12–15). Second, LIN28 proteins block let-7 microRNA (miRNA) biogenesis (16–19). Mature miRNAs are small noncoding RNAs that interact with their targets by partial base pairing with complementary sequences commonly found within the 3′ untranslated region (3′ UTR) of the target mRNA. In the majority of cases, miRNA binding inhibits translation and/or destabilizes the target mRNA (20). Similar to lin-28, let-7 was initially identified in C. elegans as a heterochronic gene (10, 21). Let-7 miRNAs inhibit stem cell/progenitor cell proliferation and promote differentiation by targeting cell cycle and growth-associated genes (22–24). The Lin28 genes possess multiple let-7 binding sites in their 3′ UTR and are subject to negative regulation by let-7 miRNAs, establishing a double negative feedback loop (19). There is emerging evidence for a critical role of the Lin28/let-7 axis in controlling self-renewal, lineage commitment, and differentiation during neurogenesis (25). For instance, experiments in retinal explants provide compelling evidence that Lin28b and its opposing miRNAs, let-7, mir-9, and mir-125, regulate the developmental timing of retinal neurogenesis (26). Here, using recently developed iLIN28B and ilet-7g transgenic mouse lines (27), we show that Lin28b functions as a developmental timer in the murine cochlea through both let-7–dependent and let-7–independent mechanisms. Furthermore, we demonstrate that reexpression of LIN28B enhances the ability of early postnatal SCs to switch cell fate and transdifferentiate into HCs in response to Notch inhibition and that let-7 overexpression antagonizes this process. Together, these results suggest an important role for the LIN28B/let-7 axis in regulating SC plasticity.
Results
Lin28b and let-7 miRNAs Are Differentially Expressed in the Auditory Sensory Lineage.
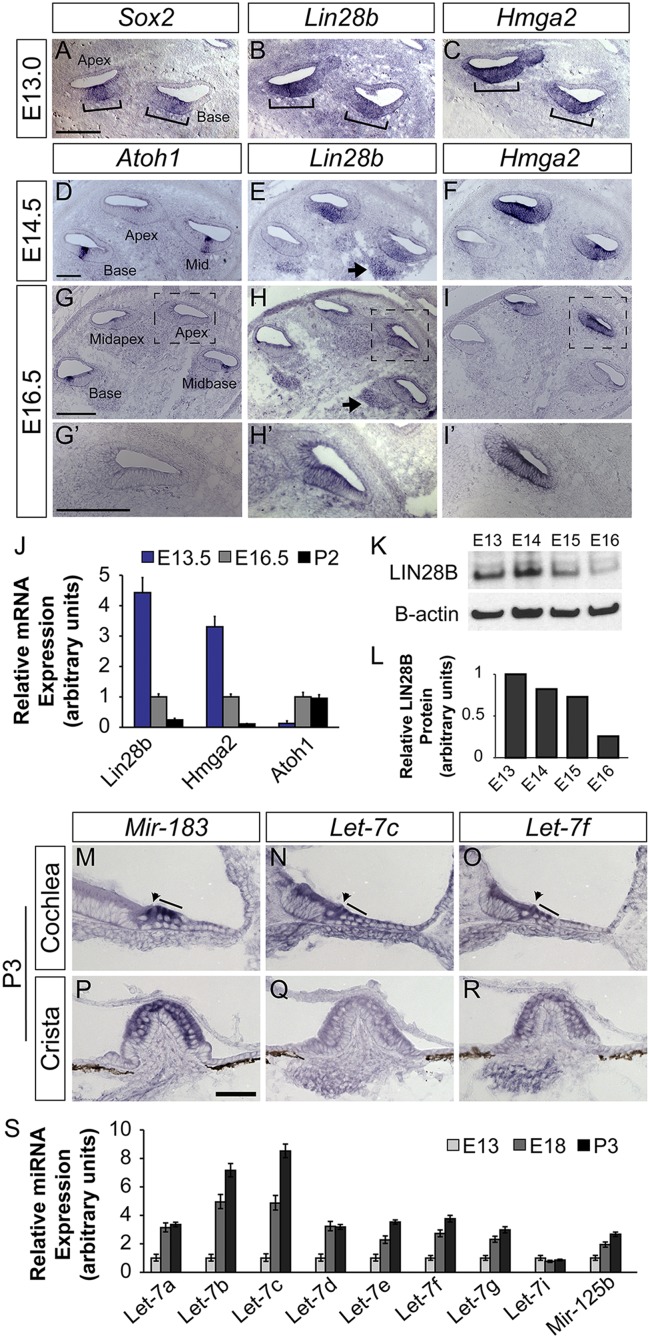
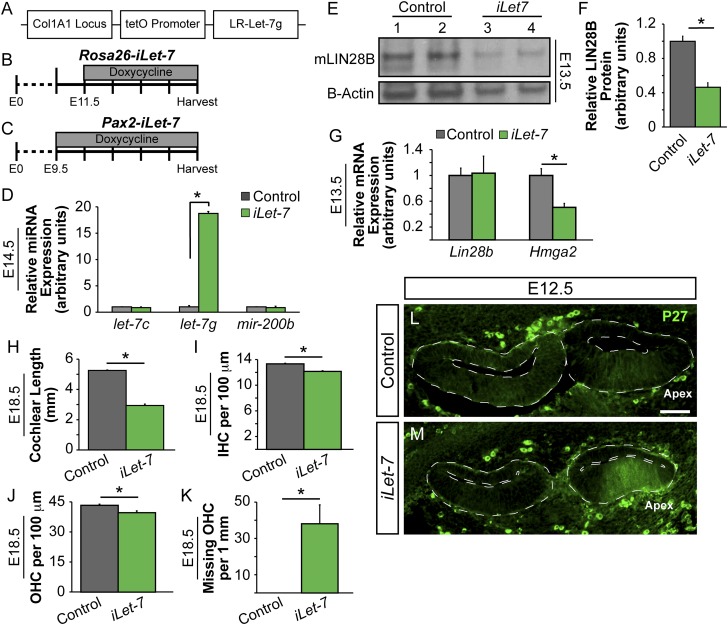
To characterize the spatial and temporal expression pattern of the Lin28b/let-7 axis during cochlear differentiation, a series of RNA in situ hybridization (ISH) and quantitative PCR (qPCR) experiments were performed. In addition to Lin28b, we characterized the expression of the high mobility group transcription factor Hmga2, which has been shown to promote organismal growth and stemness in other systems (28, 29). Similar to Lin28b, both human and murine Hmga2 harbor let-7 binding sites in their 3′ UTRs and are negatively regulated by the let-7 miRNAs (23, 24, 30, 31). In the mammalian cochlea, prosensory cell differentiation follows a steep basal-to-apical gradient, whereby midbasally located HCs differentiate before more apically located HCs. Our analysis revealed that both Lin28b and Hmga2 transcripts are expressed in prosensory cells but are rapidly down-regulated in a basal-to-apical fashion upon the onset of HC differentiation (Fig. 1 A–I′). At embryonic day (E)13.0, Lin28b and Hmga2 were coexpressed with Sox2 in prosensory cells (Fig. 1 A–C) (32). At E14.5, following the onset of Atoh1 expression in nascent HCs, Lin28b and Hmga2 transcript expression was reduced in the differentiating cochlear base and mid turn (Fig. 1 D–F). At the peak of HC differentiation (E16.5), Lin28b and Hmga2 expression was only maintained in the most apical segment of the cochlear duct, which had not yet differentiated (Fig. 1 G–I′). Quantification by qPCR and Western blot revealed that the more than fourfold reduction in Lin28b transcript levels between E13.5 and E16.5 (Fig. 1J) was accompanied by a nearly fivefold decline in LIN28B protein levels (Fig. 1 K and L).
Fig. 1.
Members of the Lin28b/let-7 axis are differentially expressed in the developing cochlea. (A–I′) ISH-based analysis of Lin28b and Hmga2 expression before (A–C) and during cochlear differentiation (D–I′). Sox2 (A) marks prosensory cells, and Atoh1 (D, G, and G′) marks HCs. Brackets (A–C) indicate the prosensory domain. Arrows (E and H) indicate Lin28b expression within the developing spiral ganglion. High power images of the apical turn of G–I are shown in G′–I′. (J) RT-qPCR analysis of relative Lin28b, Hmga2, and Atoh1 mRNA expression within the cochlear epithelium before (E13.5), during (E16.5), and following (P2) differentiation. Rpl19 was used as an endogenous reference gene. Data are mean ± SEM. (K and L) LIN28B protein quantification within the cochlea epithelium at stages acutely surrounding the onset of HC differentiation. (M–R) ISH-based analysis of let-7c and let-7f expression within the early postnatal (P3) cochlea (M–O) and vestibular crista (P–R). Mir-183 (M and P) marks cochlear and vestibular HCs. Arrowheads and lines (M–O) indicate cochlear IHCs and OHCs. (Scale bars: A–I′, 100 μm; M–R, 50 μm.) (S) RT-qPCR analysis of mature let-7 miRNA expression within the cochlear epithelium before (E13), during (E18), and following (P2) differentiation. The snoRNA U6 was used as an endogenous control. Data are mean ± SEM.
This dramatic decline in LIN28B levels in the differentiating cochlea strongly correlated with a rise in let-7 miRNA expression (Fig. 1 M–S and Fig. S1). The murine let-7 family is comprised of nine mature let-7 miRNA species (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i, and mir-98) (33). Our qPCR analysis revealed that eight of nine mature let-7 miRNA species were expressed at detectable levels in the developing cochlea and with the exception of let-7i, their expression steadily increased with the advancement of cochlear differentiation and maturation (Fig. 1S). We also analyzed the expression of mir-125b, a vertebrate homolog of the C. elegans heterochronic gene lin-4 (34), known to negatively regulate Lin28a/b expression (35). Our qPCR analysis revealed that similar to mature let-7 miRNAs, expression of mature mir-125b increased with the advancement of cochlear differentiation (Fig. 1S). ISH-based analysis using locked nucleic acid (LNA) probes revealed that in the terminally differentiated cochlea postnatal day (P)3 mature let-7f and let-7c miRNAs were expressed in cochlear and vestibular HCs and cells of the cochlear and vestibular ganglion. In contrast to mir-183, whose expression was confined to HCs and cells of the cochlear and vestibular ganglion (36, 37), let-7f and let-7c were also expressed in SCs and nonsensory epithelial cells of the greater epithelial ridge (Fig. 1 M–R). In the differentiating cochlea (E16.0), let-7c and let-7f were expressed in nascent HCs, and in the case of let-7c, in surrounding SCs, closely following the basal-to-apical wave of HC differentiation (Fig. S1). In summary, our expression analysis revealed reciprocal expression of Lin28b and mature let-7 miRNAs in the developing cochlea, with Lin28b being highly expressed in undifferentiated prosensory cells, and mature let-7 miRNA species being highly expressed in terminally differentiated HCs and SCs.
Fig. S1.
Let-7 miRNAs are expressed in the differentiating cochlear epithelium. ISH-based analysis of mir-183, let-7c, and let-7f expression within the differentiating (E16.0) cochlear epithelium. Red arrows in A′–C′ indicate differentiated HCs in the basal cochlear turn. (Scale bars: 50 μm.)
LIN28B Overexpression Delays Prosensory Cell Differentiation.
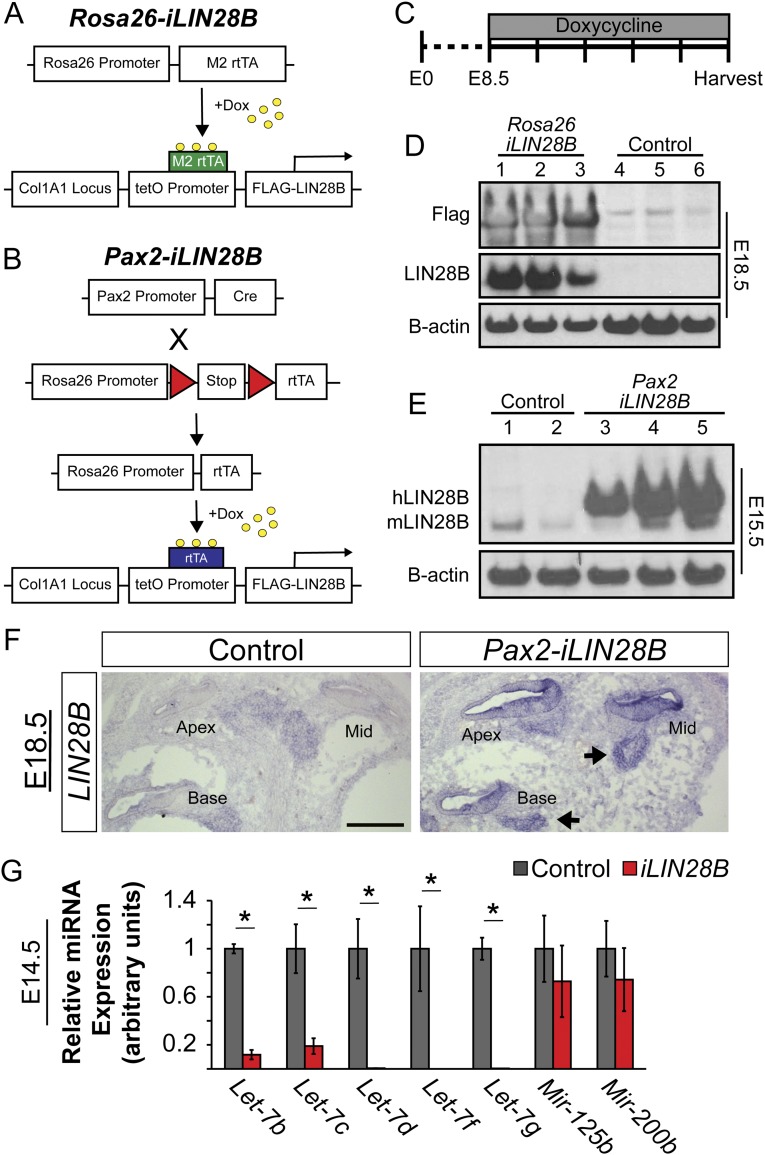
Based on the opposing expression pattern of Lin28b and let-7 miRNAs in the differentiating murine cochlea, and the known heterochronic function of this axis in C. elegans larval development (10), we hypothesized that Lin28b might be functioning as an intrinsic regulator of developmental timing in the mammalian cochlea. To address the function of the Lin28b/let-7 axis in the murine cochlea, we made use of the iLIN28B transgenic mouse line (27). In this mouse line, a flag-tagged human LIN28B transgene is under the control of a tetracycline-responsive promoter element (TRE). When combined with a ubiquitous (Rosa26) or inner ear-specific (Pax2) reverse tetracycline transactivator (rtTA) transgene, doxycycline (dox) administration (beginning at E8.5) resulted in robust LIN28B overexpression within the developing cochlear duct (Fig. S2 A–F). In addition, because of LIN28B’s inhibitory function on let-7 miRNA biogenesis, LIN28B overexpression resulted in more than an 80% reduction in mature let-7 miRNA expression within the developing cochleae of iLIN28B transgenic embryos (Fig. S2G).
Fig. S2.
LIN28B overexpression in the embryonic cochlea. (A) The iLIN28B transgene, containing flag-tagged human LIN28B ORF driven by the tetracycline inducible promoter, and the M2 reverse tetracycline transactivator (rtTA) transgene under control of the ubiquitously expressed Rosa26 promoter, were combined to drive global LIN28B expression (Rosa26-iLIN28B). (B) Inner ear-specific overexpression of LIN28B was achieved by using a trigenic approach. This rtTA line contained a floxed stop cassette between the Rosa26 promoter and the rtTA. Crossing in Pax2-driven CRE recombinase confined LIN28B overexpression to the Pax2+ cells of the cochlear epithelium and spiral ganglion (Pax2-iLIN28B). (C) To induce iLIN28B overexpression, time-mated females were given ad libitum access to feed containing 2 g of dox per kg of feed beginning at E8.5. Dox treatment was continued until embryos were harvested. Unless otherwise stated, iLIN28B was always induced at E8.5. (D) Dox administration resulted in robust flag-tagged LIN28B protein overexpression within differentiated (E18) Rosa26-iLIN28B (iLIN28B tg/+; Rosa26 M2-rtTA tg/+) inner ears but not nontransgenic littermate controls (Rosa26 M2-rtTA tg/+). (E) Dox administration resulted in human LIN28B overexpression within differentiating (E15.5) Pax2-iLIN28B (Pax2-Cre tg/+; rtTA tg/+; iLIN28B tg/+) cochlear epithelia but not nontransgenic littermate controls (Pax2-Cre tg/+; rtTA tg/+). Endogenous mouse Lin28b protein expression was seen in both transgenic and nontransgenic epithelia. (F) ISH based analysis of LIN28B expression in E18.5 control and Pax2-iLIN28B cochleae. Arrow points to LIN28B expression within the spiral ganglion. (Scale bar: 100 μm.) (G) RT-qPCR analysis of mature let-7 miRNA expression in E14.5 control (gray) and Pax2-iLIN28B (red) cochlear epithelia. Data are mean ± SEM (n = 3–5, *P < 0.05).
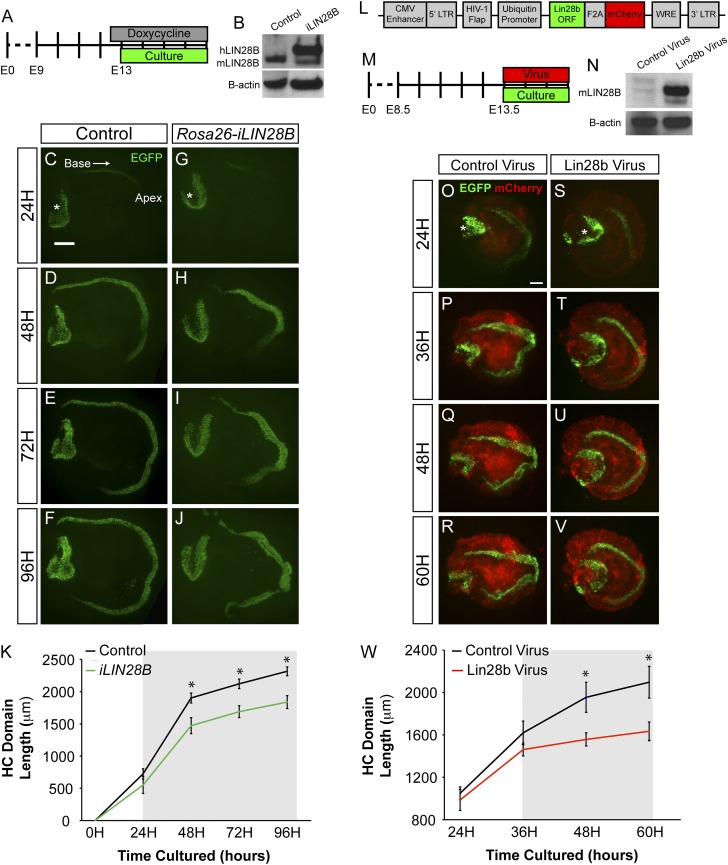
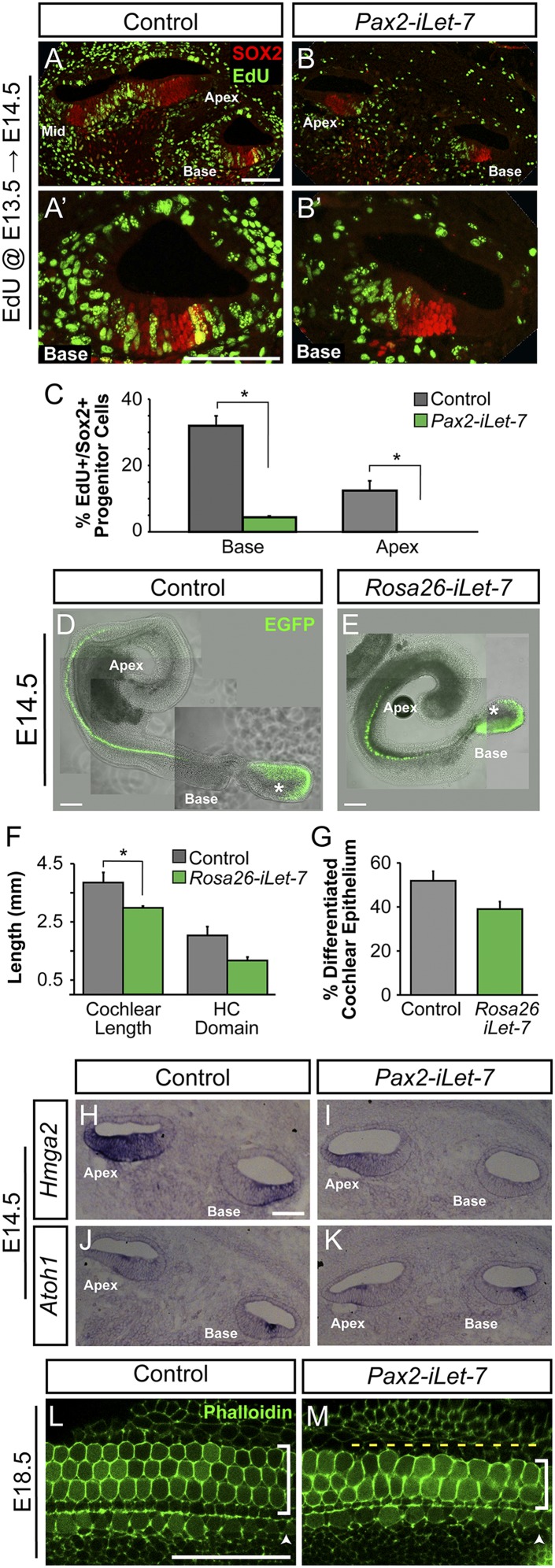
To determine whether LIN28B overexpression might inhibit or delay prosensory cell differentiation, dox was continuously administered starting at E8.5, and HC-specific Atoh1/nEGFP reporter expression was used to monitor HC differentiation in E13.0 Rosa26-iLIN28B and control cochlear explants over 3 d (Fig. 2 A–J). HC differentiation follows a steep basal-to-apical gradient and a less steep medial-to-lateral gradient, causing basally located HCs to differentiate before more apically located HCs and medial IHCs to differentiate before more lateral OHCs (2, 8). After 16 h in vitro (HIV), a stripe of GFP+ IHCs was visible in the cochlear base of control explants (Fig. 2 B and J). In contrast, LIN28B overexpressing cochlear explants remained undifferentiated after 16 HIV (Fig. 2 C and J). Sixteen hours later (32 HIV), IHCs in LIN28B overexpressing cochlear cultures became apparent (Fig. 2 E and J) and over the next 32 h, HC differentiation progressed at a similar rate in control and LIN28B overexpressing cultures (Fig. 2J). Consistent with the observed delay in HC differentiation in vitro, both IHC and OHC differentiation was less advanced in acutely isolated E15.5 Rosa26-iLIN28B cochlear tissue compared with control (Fig. 2S).
Fig. 2.
LIN28B overexpression delays auditory HC differentiation. (A) Experimental design: iLIN28B overexpression was induced beginning at E8.5 and Rosa26-iLIN28B (iLIN28B tg/+; Rosa26 M2-rtTA tg/+) and nontransgenic littermate control (Rosa26 M2-rtTA tg/+) cochlear epithelia were cultured beginning at E13.0. In vitro HC differentiation was monitored over the following 3 d by using the Atoh1/nEGFP reporter. (B–I) Atoh1/nEGFP reporter expression (EGFP, green) marks nascent HCs. Asterisks indicate EGFP expression within HCs of the vestibular sacculus. (J) Length of the EGFP+ HC stripe was used to quantify the extent of HC differentiation in control versus Rosa26-iLIN28B cochlear cultures. Data expressed as mean ± SEM (n = 5 animals per group, *P < 0.05). (K and L) Overlays of low-power fluorescent images of acutely isolated E15.5 control (Pax2-Cre tg/+; rtTA tg/+) and Pax2-iLIN28B (Pax2-Cre tg/+; rtTA tg/+; iLIN28B tg/+) cochlear ducts stained for the hair cell specific protein MYO6 (white). Red arrowheads indicate the IHC domain, and yellow arrowheads indicate the OHC domain. (M–R) Cross-sections through the basal, mid, and apical turns of control and Pax2-iLIN28B cochleae. MYO6 staining (red) marks HCs, and p27/Kip1 (green) staining marks the postmitotic sensory domain. (S and T) Analysis of acutely isolated E15.5 cochlear ducts (as demonstrated in J and K) from both the global Rosa26-iLIN28B line and inner ear-specific Pax2-iLIN28B line compared with their nontransgenic littermates (control). Data expressed as mean ± SEM (n = 3–7 animals per group, *P < 0.05, n.s., not significant). (Scale bars: B–I, K, and L, 200 μm; M–R, 50 μm.)
To rule out the possibility that the observed delay in cochlear HC differentiation was due to a more general developmental delay, an inner ear-specific LIN28B overexpression approach was used (Fig. S2 B, C, E, and F). Dox was continuously administered starting at E8.5, and the basal-to-apical extent of HC differentiation was analyzed in acutely isolated E15.5 Pax2-iLIN28B and control cochlear tissue. Immunostaining for HC-specific protein myosin VI (MYO6) (38) revealed that similar to global overexpression (Rosa26-iLIN28B), selective, inner ear-specific LIN28B overexpression (Pax2-iLIN28B) delayed auditory HC differentiation. In E15.5 nontransgenic control animals, MYO6+ IHCs were present throughout nearly the entire length of the cochlear duct (Fig. 2 K, M, O, and T) and MYO6+ OHCs were already evident in the cochlear base (Fig. 2 K, M, and T). In contrast, cochlear tissue from Pax2-iLIN28B transgenic littermates contained no MYO6+ OHCs yet (Fig. 2 L, N, and T) and MYO6+ IHCs (Fig. 2 L, P, and T) were not found as far apically as in control cochlear tissue.
Next we wanted to determine whether more acute LIN28B overexpression was sufficient to delay the onset of HC differentiation. Dox-mediated induction of LIN28B protein takes ∼24 h (Fig. S3B). To account for this 24-h delay in induction, LIN28B expression was induced at E12.5 and cochlear explant cultures were obtained 12 h later, at E13.0 (Fig. S3A). Analysis of HC-specific Atoh1/nEGFP expression in these cultures revealed that HC differentiation in E13.0 LIN28B overexpressing cochlear explants was significantly delayed compared with controls (Fig. S3 C–K), suggesting that within hours of being expressed, LIN28B exerts its negative effect on HC differentiation. A qualitatively similar delay in the HC differentiation was also observed when E13.5 cochlear explants were acutely infected with murine Lin28b-expressing lentivirus (Fig. S3 L–W). Taken together, these data suggest that LIN28B overexpression just hours before HC differentiation is sufficient to delay its onset.
Fig. S3.
LIN28B overexpression delays auditory HC differentiation. (A–K) Acute overexpression of iLIN28B slows the progression of HC differentiation. (A) Experimental design: Rosa26-iLIN28B overexpression was induced at E12.5, and control and iLIN28B cochlear explants were cultured 12 h later at E13.0. HC differentiation was monitored over the following 4 d by using HC-specific Atoh1/nEGFP reporter expression. (B) Human LIN28B protein is overexpressed within the developing cochlear epithelium (E13.5) of Rosa26-iLIN28B mice 24 h after the start dox treatment. (C–J) Acute overexpression of iLIN28B slows the progression of HC differentiation. Atoh1/nEGFP reporter expression (EGFP, green) marks nascent HCs. Asterisks indicate EGFP expression within HCs of the vestibular sacculus. (K) Length of the EGFP+ HC stripe was used to quantify the extent of HC differentiation in control versus Rosa26-iLIN28B cochlear cultures. The gray box indicates the estimated time-point at which LIN28B overexpression reached a biologically relevant level. Data expressed as mean ± SEM (n = 7–13, *P < 0.01). (L–W) Lentiviral-driven overexpression of murine Lin28b delays HC differentiation. (L) Schematic of the experimental lentiviral construct, which contained ubiquitin promoter-driven murine Lin28b ORF linked to a mCherry reporter via an F2A sequence. The control lentiviral vector contained only mCherry. (M) Experimental design: E13.5 Atoh1/nEGFP cochlear explants were infected at plating with either Lin28b or mCherry (control) lentivirus. HC differentiation (EGFP) and lentiviral driven protein expression (mCherry) were monitored over the following 3 d. (N) Forty-eight hours after plating, Lin28b protein was robustly expressed in cochlear explants infected with Lin28b-containing lentivirus. Infection with control lentivirus did not impede the down-regulation of endogenous Lin28b. (O–V) Murine Lin28b overexpression delays HC differentiation. Atoh1/nEGFP reporter expression (EGFP, green) marks nascent HCs. Lentiviral-driven mCherry expression (red) marks infected cells. Asterisks indicate EGFP expression within HCs of the vestibular sacculus. (W) Length of the EGFP+ HC stripe was used to quantify the extent of HC differentiation in control virus versus Lin28b virus-treated cultures. The gray box indicates the estimated time-point at which the lentiviral-driven Lin28b overexpression reached a biologically relevant level. Data expressed as mean ± SEM (n = 4, *P < 0.05). (Scale bars: 200 μm.)
LIN28B Overexpression Results in a Delay in Prosensory Cell Cycle Exit.
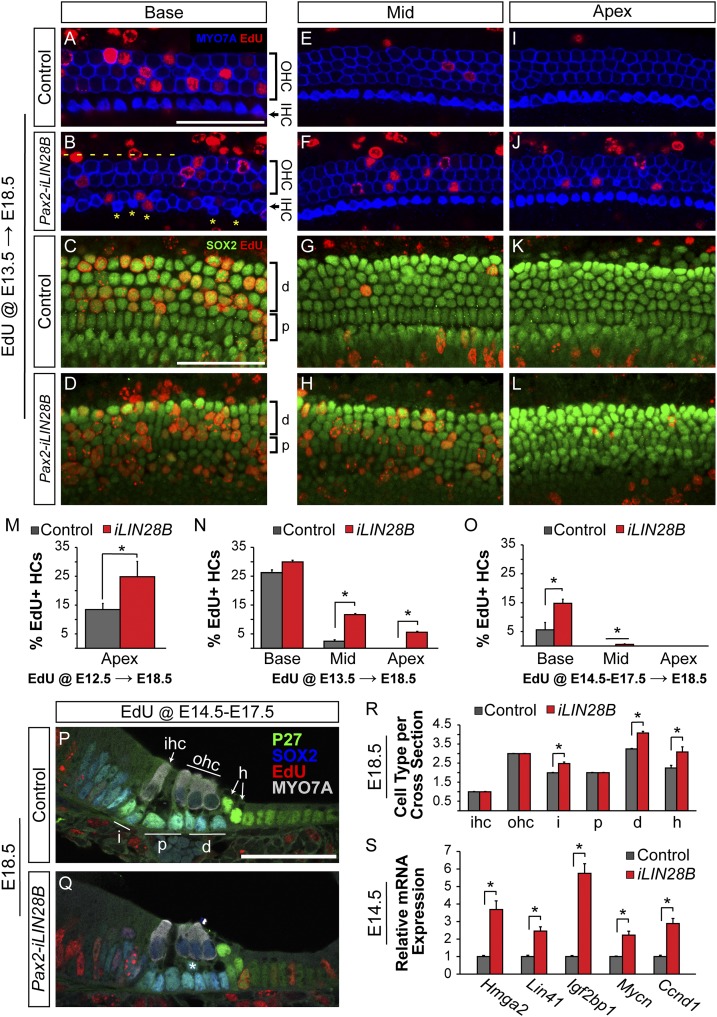
We next assayed the effects of LIN28B overexpression on prosensory cell proliferation. Lin28b functions as an oncogene in many tumors (39–41); however, little is known about Lin28b’s role in controlling cell proliferation during normal development. In the mammalian cochlea, prosensory cells withdraw from the cell cycle in a striking apical-to-basal gradient. Murine prosensory cells exit the cell cycle within a 48-h time window, with apical prosensory cells exiting the cell cycle as early as E12.5 and the most basal progenitors exiting as late as E14.5 (1, 42). To determine whether higher-than-normal LIN28B protein levels might delay prosensory cell cycle exit, a series of EdU pulse–chase experiments were performed by using the Pax2-iLIN28B transgenic line. Dox was induced starting at E8.5, and EdU incorporation in differentiated HCs and SCs of Pax2-iLIN28B transgenic and nontransgenic cochleae was analyzed at E18.5. Cytoplasmic myosin VII a (MYO7A) staining was used to identify HCs, and nuclear p27/Kip1 and SOX2 staining were used to identify SCs (5, 43). In the first set of experiments timed mated dams received a single injection of EdU at E13.5, the peak of cell cycle withdrawal. These experiments revealed that at stage E13.5, basally located HC and SC precursors in both control and LIN28B overexpressing cochlea were actively dividing and incorporated EdU at a similar rate (Fig. 3 A–D and N). However, whereas in control cochlear tissue HC and SC precursors located further apically had largely withdrawn from the cell cycle, in the LIN28B, overexpressing cochleae HC and SC precursors continued to proliferate and incorporate EdU at a significantly higher rate than controls (Fig. 3 E–L and N). Moreover, experiments conducted using the Rosa26-LIN28B transgenic line revealed that at E13.5 LIN28B overexpressing HC and SC precursors expressed P27/Kip1 protein at much lower levels than in control cochlea (Fig. S4 C–D′). To determine whether the altered pattern of HC and SC precursor proliferation was due to a delay in the initial onset of cell cycle withdrawal, a single injection of EdU was administered at E12.5 and EdU incorporation in apically located HCs was analyzed at E18.5. Our analysis revealed that at E12.5 the majority of apical prosensory cells was actively cycling in LIN28B overexpressing cochleae, whereas in control cochleae, prosensory cells had started to withdraw from the cell cycle (Fig. 3M). Consistent with a shift in timing rather than a permanent derailment of prosensory cell cycle withdrawal, EdU injections for three consecutive days beginning at E14.5, only labeled basally located HCs in LIN28B overexpressing cochleae. No EdU-labeled cells were observed within the auditory sensory epithelium further apically, indicating that once postmitotic, HC and SC precursors permanently withdrew from the cell cycle in the LIN28B overexpressing cochlea (Fig. 3 O–Q). Furthermore, no proliferation was detected in either control or iLIN28B sensory epithelia at E15.5 (Fig. S4 A and B).
Fig. 3.
LIN28B overexpression delays progenitor cell cycle withdrawal and causes mispatterning of the auditory sensory epithelium. (A–L) EdU incorporation (red) in HCs (MYO7a, blue) and SCs (SOX2, green) was analyzed at E18.5 following a single EdU pulse at E13.5. Shown are whole-mount preparations of basal, mid, and apical cochlear segments from control and Pax2-iLIN28B inner ears. Yellow asterisks mark ectopic IHCs, and dashes indicate missing OHCs. (M–O) Quantification of HC-specific EdU incorporation in E18.5 control (gray) and Pax2-iLIN28B (red) cochlear tissue following a single EdU pulse at E12.5 (M), a single EdU pulse at E13.5 (N), or once daily EdU pulses from E14.5 through E17.5 (O). Data expressed as mean ± SEM (n = 2, *P < 0.05). (P and Q) Cross-sections of E18.5 control and Pax2-iLIN28B cochleae. EdU (red) was given once daily from E14.5 to E17.5. HCs are marked by MYO7A (white) and SC subtypes are marked by P27 (green) and SOX2 (blue) immunostaining. Asterisk (Q) marks an ectopic SC disrupting the bilayered patterning of the cochlear epithelia in an iLIN28B cochlea. (R) Quantification of HC and SC subtypes per cochlear cross-section in control (gray) and Pax2-iLIN28B (red) cochleae. Data expressed as mean ± SEM (n = 3 animals per group, 5 cross-sections per animal; *P < 0.05). (S) RT-qPCR expression analysis of let-7 target genes associated with growth and proliferation in E14.5 control (gray) and Pax2-iLIN28B (red) cochlear epithelia. Data expressed as mean ± SEM (n = 3, *P < 0.05). d, Deiter’s cell; h, Hensen’s cell; i, phalangeal cell; p, pillar cell. (Scale bars: 50 μm.)
Fig. S4.
LIN28B overexpression delays progenitor cell cycle withdrawal and causes mispatterning of the cochlear epithelia. (A and B) Cross-sections of E15.5 control and Pax2-iLIN28B cochleae. A single EdU pulse was given 3 h before collecting. No EdU incorporation (red) was observed within the sensory domain (p27/Kip1, green) of control and iLIN28B cochleae. (C–D′) Whole-mount preparations of E13.5 control and Rosa26-iLIN28B cochlear ducts labeled with SOX2 (green) and P27 (red, C and D; white, C′ and D′) immunostaining. Asterisks (C and D) mark damaged portions of the cochlear epithelia. (E) Length measurements of E18.5 control (gray) and Pax2-iLIN28B (red) cochlear ducts. Data expressed as mean ± SEM (n = 14; n.s., not significant). (F and G) Quantification of IHC (F) and OHC (G) density within E18.5 control (gray) and Pax2-iLIN28B cochlear epithelia. Data expressed as mean ± SEM (n = 5, *P < 0.04). (H) Quantification of ectopic IHCs within the base, mid, and apex of E18.5 control (gray) and Pax2-iLIN28B (red) cochlear epithelia. Data expressed as mean ± SEM (n = 5, *P < 0.05). (I) Quantification of missing OHCs within the base, mid, and apex of E18.5 control (gray) and Pax2-iLIN28B (red) cochlear epithelia. Data expressed as mean ± SEM (n = 5, *P < 0.03). (J and K) Cross-sections through the midbasal turn of E18.5 control and Pax2-iLIN28B cochlea. HCs are marked by Atoh1/nEGFP expression (green) and SCs are marked by S100 (red) and SOX2 (blue) immunostaining. (Scale bars: A, B, J, and K, 50 μm; C–D′, 200 μm.)
At E18.5, HC differentiation and patterning is largely complete in the murine cochlea. In nontransgenic littermate control cochleae, the typical stereotyped pattern of one row of IHCs and three rows of OHCs (Fig. 3 A, E, and I) resting on a single layer of SCs (Fig. 3 C, G, K, and P) was observed throughout the length of the cochlear duct. In contrast, the stereotyped organization of HCs and SCs was disrupted in the cochlear base of E18.5 Pax2-iLIN28B transgenic embryos. Ectopic IHCs were frequently observed in the basal segment of the LIN28B overexpressing cochleae (Fig. 3B and Fig. S4H). In addition, OHCs were frequently missing, resulting in patches of sensory epithelium containing only two rows of OHCs (Fig. 3B and Fig. S4I). These HC patterning defects were mostly confined to the cochlear base; cellular patterning of the auditory sensory epithelium in more apical segments of the LIN28B overexpressing cochleae was largely normal (Fig. 3 F and J and Fig. S4 H and I). Interestingly, despite prolonged proliferation of both HC and SC precursors, only the number of SCs was increased in the iLIN28B cochleae (Fig. 3R and Fig. S4 F and G). SCs were more densely packed, and in contrast to the single SC layer in control cochleae (Fig. 3 C, G, K, and P), SCs were frequently found stacked on top of each other within LIN28B overexpressing cochleae (Fig. 3 D, H, L, and Q). In addition to the observed cellular patterning defects, maturation of HCs and SCs was delayed in iLIN28B cochleae as evidenced by delayed down-regulation of the prosensory marker SOX2 in HCs (Fig. 3 P and Q) and delayed up-regulation of the SC-specific marker S100 (Fig. S4 J and K).
What is the molecular basis of the LIN28B-mediated delay in prosensory cell cycle withdrawal and differentiation? One possible mechanism is that the up-regulation of let-7 target genes due to lower than normal let-7 levels alters the timing of prosensory proliferation and differentiation. Recent studies have revealed a critical role for the let-7 targets Igf2bp1 (Imp1), Hmga2, and Lin41 (Trim71) in neuronal stem cell/progenitor cell maintenance (29, 31, 44). Our analysis of let-7 target gene expression revealed that LIN28B overexpression significantly increased transcript levels of Igf2bp1, Lin41, and Hmga2 (Fig. 3S). Furthermore, LIN28B overexpression significantly increased the expression of the let-7 targets N-Myc (Mycn) and Cyclin D1 (ccnd1) (45, 46), which have been shown to positively regulate prosensory cell proliferation in the murine cochleae and vestibular organs (47–49) (Fig. 3S).
Let-7 Overexpression Results in Premature Progenitor Cell Cycle Exit but Does Not Cause Precocious Differentiation.
To test whether LIN28B regulates prosensory proliferation and differentiation via a let-7–dependent mechanism, we made use of transgenic mice that express a Lin28a/b-resistant form of let-7g under the control of a tetracycline response element (Fig. S5A) (27). When combined with either a ubiquitous (Rosa26 M2-rtTA) or inner ear-specific (Pax2-rtTA) rtTA, dox administration induced a more than 15-fold increase in let-7g miRNA expression within the developing cochlear duct (Fig. S5 B–D). Let-7g overexpression significantly reduced Hmga2 mRNA (Fig. 4 H and I and Fig. S5G) and LIN28B protein levels (Fig. S5 E and F). To determine whether higher than normal let-7 levels might cause precocious prosensory cell cycle withdrawal, dox-fed timed-mated dams received a single injection of EdU at E13.5 and EdU incorporation within Pax2-iLet-7 transgenic and control embryos was analyzed 24 h later. Our EdU incorporation analysis revealed a dramatic decrease in the number of proliferating SOX2+ prosensory cells upon let-7g overexpression (Fig. 4 A–C). In let-7g overexpressing cochleae, less than 5% of basally located prosensory cells incorporated EdU compared with more than 30% in control (Fig. 4C). This reduced rate of prosensory cell proliferation is likely due to the premature up-regulation of p27/Kip1. At E12.5, broad P27/Kip1 protein expression was seen in the apical segment of Pax2-iLet-7 cochlear ducts, whereas control cochlear sensory epithelia did not yet express p27/Kip1 protein (Fig. S5 L and M). Unsurprisingly, global (Rosa26-iLet-7) as well as inner ear-specific (Pax2-iLet-7) let-7 overexpression caused defects in cochlear outgrowth (Fig. 4F and Fig. S5H), and at E18.5, Pax2-iLet-7 cochleae were 40% shorter than controls (Fig. S5H). Likely due to premature prosensory cell cycle withdrawal, both IHC and OHC density was significantly reduced (Fig. S5 I and J) and the third row of OHCs was frequently missing in the Pax2-iLet-7 cochleae (Fig. 4 L and M and Fig. S5K). Surprisingly, the timing of HC differentiation appeared to be unaffected by let-7 overexpression. At E14.5, both Rosa26-iLet-7 cochleae and nontransgenic littermate control cochleae had a similar extent of HC differentiation along the length of the cochlear duct. Atoh1/nEGFP-positive IHCs were observed only within the basal half of the cochlear duct; the apical half remained undifferentiated (Fig. 4 D–G). Similarly, no difference in HC-specific Atoh1 mRNA expression was observed in E14.5 Pax2-iLet-7 transgenic cochlear tissue compared with nontransgenic littermate controls (Fig. 4 J and K). In summary, our analysis suggests that the Lin28b/let-7 circuit controls the timing of prosensory cell cycle withdrawal in the developing cochlea. However, in contrast to LIN28B overexpression, let-7g overexpression had little effect on the timing of HC differentiation, suggesting that Lin28b uses a distinct, let-7–independent mechanism in regulating the timing of HC differentiation.
Fig. S5.
Let-7g overexpression strategy. (A) Schematic of the iLet-7 construct, which expresses a LIN28A/B-resistant form of let-7g driven by a tetracycline-inducible promoter (tetO) within the Col1A1 locus (27). (B) To achieve global iLet-7 overexpression, the Rosa26 M2-rtTA transgene was used to drive let-7g expression (Rosa26-iLet-7). Dox administration to timed-mated females was begun at E11.5 to avoid embryonic lethality. Dox treatment was continued until tissue was harvested. (C) For inner ear-specific iLet-7 overexpression a trigenic approach was used. This rtTA line contained a floxed stop cassette between the Rosa26 promoter and the rtTA. Crossing in Pax2-driven CRE recombinase confined let-7g overexpression to the Pax2+ cells of the cochlear epithelium and spiral ganglion (Pax2-iLet-7). Dox administration to timed-mated females was begun at E9.5 and continued until tissue was harvested. (D) RT-qPCR analysis showing that mature let-7g is specifically overexpressed within the cochlear epithelium of E14.5 iLet-7 transgenic mice after dox treatment. Data are expressed as mean ± SEM (n = 3, *P < 0.001). Shown is Pax2-rtTA driven overexpression (dox E9.5); similar overexpression is achieved by using the Rosa26-iLet-7 strategy (dox E11.5). (E–G) Let-7 targets are down-regulated at the transcript or protein level following iLet-7 overexpression. (E and F) LIN28B protein levels in E13.5 control and Pax2-iLet-7 cochlear epithelia. Data expressed as mean ± SEM (n = 2, *P < 0.05). (G) Relative Lin28b and Hmga2 transcript expression within E13.5 control (gray) and Pax2-iLet-7 (green) cochlear epithelia. Data expressed as mean ± SEM (n = 3, *P < 0.05). (H–K) Quantification of cochlear duct length (H) HC density (I and J) and missing OHCs (K) in E18.5 Pax2-iLet-7 (green) and control (gray) cochlear epithelia. Data expressed as mean ± SEM (n = 3, *P < 0.05). (L and M) Cross-sections of E12.5 control and Pax2-iLet-7 cochlear ducts (dotted outline) immunostained for P27 (green). (Scale bar: 50 μm.)
Fig. 4.
Let-7 overexpression accelerates progenitor cell cycle withdrawal but not differentiation. (A–B′) Cross-sections of E14.5 control and Pax2 iLet-7 cochleae. A single EdU pulse was given at E13.5 and EdU incorporation (green) within SOX2+ (red) prosensory progenitor cells was analyzed after 24 h. High-power images of the basal turn are shown in A′ and B′. (C) Quantification of EdU incorporation shown in A–B′. Data expressed as mean ± SEM (n = 2, *P < 0.05). (D and E) Bright field and green fluorescence image overlays of acutely isolated E14.5 control and Rosa26 iLet-7 cochlear ducts. Atoh1/nEGFP reporter (EGFP, green) marks nascent HCs. Asterisks denote EGFP expression within HCs of the vestibular sacculus. (F and G) Analysis of the extent of HC differentiation in E14.5 control and Rosa26-iLet-7 cochlear ducts shown in D and E. Data expressed as mean ± SEM (n = 4, *P < 0.05). (H–K) ISH-based analysis of Hmga2 (H and I) and Atoh1 (J and K) expression in E14.5 Pax2-iLet-7 and control cochlear tissue. (L and M) HC phenotype in E18.5 control and Pax2-iLet-7 cochlear whole mounts. HCs are marked by phalloidin (green). Shown is the basal turn. Arrowheads indicate IHC domain, brackets indicate OHC domain, and yellow dashes indicate missing OHCs. (Scale bars: A–B′ and H–K, 50 μm; D and E, 200 μm.)
The LIN28B/let-7 Axis Modulates the Capacity for SC Conversion in the Absence of Notch Signaling.
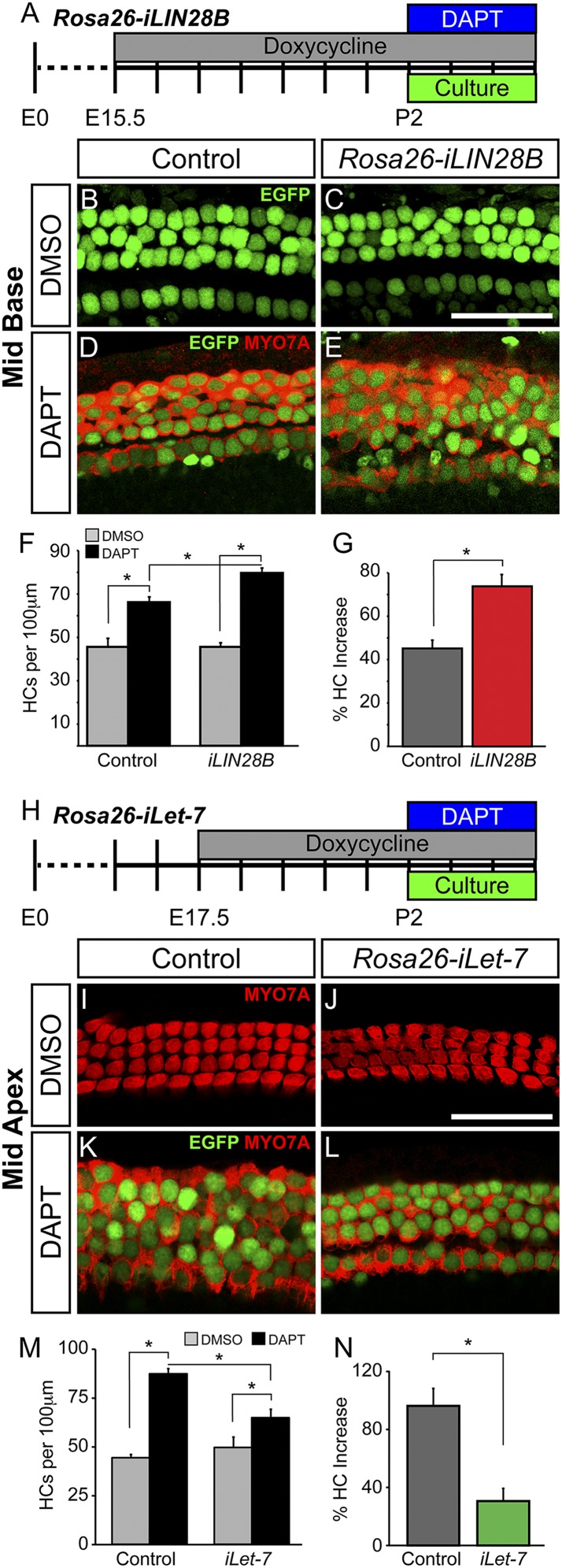
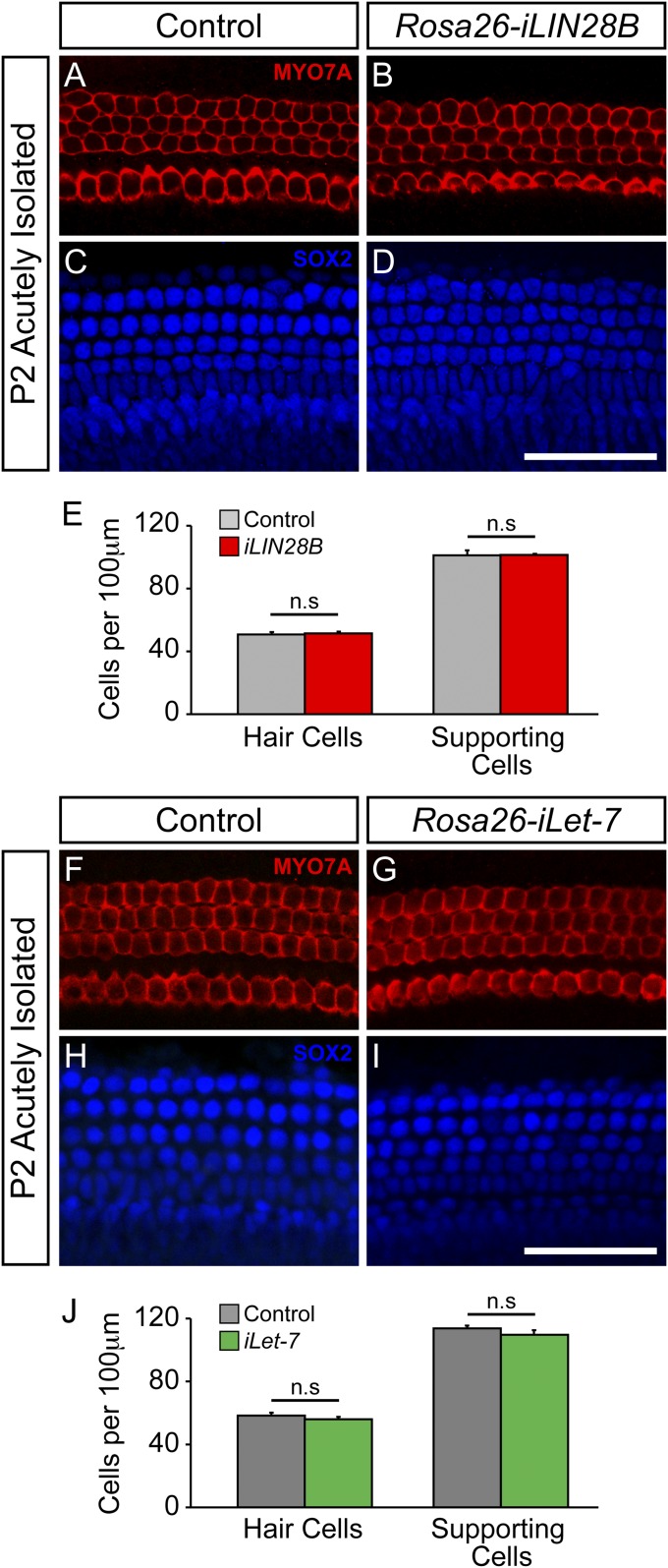
In the mammalian cochlea, HC production is restricted to embryonic development. Loss of HCs is permanent and is a leading cause of human deafness. However, recent studies suggest that in a permissive environment, murine SCs can function as HC progenitors and generate/regenerate HCs (50). One of the key pathways limiting SC-to-HC conversion is the Notch signaling pathway. During embryonic development, activation of Notch signaling within a subset of prosensory cells inhibits the activation of a HC-specific program, limiting these cells to a SC fate (51–53). Inhibition of Notch signaling using γ-secretase inhibitors results in the generation of new HCs within the embryonic and neonatal murine cochlea (54–56); although the ability of SCs to dedifferentiate and convert to a HC fate declines rapidly within the first postnatal week of life. In the adult animal, only SCs located in the cochlear apex respond to Notch inhibition and infrequently convert to a HC fate (57). Recently, two studies have suggested that Lin28a/b reexpression can counteract the developmental decline in cell regeneration and tissue repair. In both cases, let-7 repression was necessary to mediate this effect, although let-7 repression alone was not sufficient to enhance tissue repair (58, 59). To determine whether LIN28B reexpression might enhance SC plasticity, LIN28B was reexpressed in the late embryonic (E15.5) cochlea and the ability of SCs to generate new HCs in response to γ-secretase inhibitor treatment was analyzed in early postnatal (P2) cochlear explant cultures (Fig. 5A). We limited our analysis to the cochlear midbase where early postnatal SCs only infrequently convert to HCs in response to Notch inhibition (54). In contrast to early embryonic (E8.5) LIN28B induction, late embryonic LIN28B induction starting at E15.5 did not alter the number or organization of HCs and SCs (Fig. S6 A–E), and no difference in the number of HCs was observed in untreated P2 control and Rosa26-iLIN28B cochlear explants after 3 d in culture (Fig. 5 B, C, and F). Treatment with the γ-secretase inhibitor DAPT for 3 d significantly increased the number of MYO7A and Atoh1/nEGFP-positive HCs in both P2 control and Rosa26-iLIN28B cochlear explants (Fig. 5 D–G); however, LIN28B overexpressing cochlear explants generated significantly more new HCs than controls (Fig. 5 F and G). Interestingly, these newly generated HCs largely lacked EdU incorporation, suggesting that they originated from postmitotic cells. Taken together, our finding suggests that LIN28B reexpression increased the frequency of direct SC-to-HC conversion in response to Notch inhibition.
Fig. 5.
The LIN28B/let-7 axis modulates the capacity for SC conversion in the absence of Notch signaling. (A–G) LIN28B reexpression promotes SC-to-HC conversion in the absence of Notch signaling. (A) Schematic of HC regeneration assay. LIN28B overexpression was induced starting at E15.5, and cochlear explants from P2 Rosa26-iLIN28B pups and nontransgenic littermates (control) were cultured in the presence of DAPT or DMSO. SC-to-HC conversion was quantified after 3 DIV. (B–E) Shown are midbasal segments of P2 control and iLIN28B cochlear explants after 3 d of DMSO (B and C) or DAPT (D and E) treatment. HCs are marked by Atoh1/nEGFP (green) and MYO7A (red). (F and G) Quantification of B–E. Data expressed as mean ± SEM (n = 3, *P < 0.03). (H–N) Let-7 overexpression antagonizes SC conversion in the absence of Notch signaling. (H) Schematic of HC regeneration assay. Let-7 overexpression was induced starting at E17.5 and cochlear explants from P2 Rosa26-iLet-7 pups and nontransgenic littermates (control) were cultured in the presence of DAPT or DMSO. SC-to-HC conversion was quantified after 3 DIV. (I–L) Shown are midapical segments of P2 control and iLet-7 cochlear explants after 3 d of DMSO (I and J) or DAPT (K and L) treatment. HCs are marked by Atoh1/nEGFP (green) and MYO7A (red). (M and N) Quantification of I–L. Data expressed as mean ± SEM (n = 4–5 per group, *P ≤ 0.02). (Scale bars: 50 μm.)
Fig. S6.
Late embryonic induction of iLIN28B or iLet-7 does not impact cellular patterning or the number of HC and SC being produced. (A–E) iLIN28B expression was induced at E15.5 and Rosa26-iLIN28B (iLIN28B tg/+; Rosa26 M2-rtTA tg/+), and nontransgenic littermate control (Rosa26 M2-rtTA tg/+) cochlear ducts were harvested at P2. (A–D) Shown are midbasal segments from acutely isolated P2 control and iLIN28B cochlear epithelia. HCs are marked by MYO7A (red; A and B), and SCs are marked by SOX2 (blue; C and D). (E) Quantification of A–D. Data expressed as mean ± SEM (n = 2 animals per group; n.s., not significant). (F–J) iLet-7 expression was induced at E17.5 and Rosa26-iLet-7 (iLet-7 tg/+; Rosa26 M2-rtTA tg/+), and nontransgenic littermate control (Rosa26 M2-rtTA tg/+) cochlear ducts were harvested at P2. (F–I) Shown are midapical segments from acutely isolated P2 control and iLet-7 cochlear epithelia. HCs are marked by MYO7A (red; F and G), and SCs are marked by SOX2 (blue; H and I). (J) Quantification of F–I. Data expressed as mean ± SEM (n = 2 animals per group; n.s., not significant). (Scale bars: 50 μm.)
To determine whether let-7 overexpression would have the opposite effect and limit SC plasticity, let-7g overexpression was induced in the late embryonic cochlea (E17.5) and HC generation in the absence of γ-secretase activity was analyzed in P2 cochlear explant cultures (Fig. 5H). For these experiments, we limited our analysis to the cochlear midapex. In this more apical segment, early postnatal SCs readily convert to HCs when Notch signaling is inhibited (54). Let-7 overexpression in the late embryonic (E17.5) cochlea did not alter the number or organization of HCs and SCs (Fig. S6 F–J), and no difference in the number of HCs was observed in untreated P2 control and Rosa26-iLet-7 cochlear explants after 3 d in culture (Fig. 5 I, J, and M). Strikingly, we found that let-7 overexpression significantly decreased the number of MYO7A and Atoh1/nEGFP-positive HCs that were generated following DAPT treatment (Fig. 5 K–N). Whereas P2 control explant cultures had more than a 95% increase in HCs following DAPT treatment, P2 Rosa26-iLet-7 cultures had only a 30% increase in HCs (Fig. 5N). Thus, our findings suggest that let-7 down-regulation is necessary for SC-to-HC conversion in the absence of Notch signaling and that let-7 overexpression antagonizes this process.
Discussion
The differentiating inner ear cochlea expresses hundreds of miRNA species and RNA binding proteins, indicating that posttranscriptional regulation is likely to play an important role in controlling the dynamics of cochlear development. Here we have demonstrated that the RNA-binding protein LIN28B is a critical component of a timing mechanism that regulates both prosensory cell cycle withdrawal and differentiation in the murine cochlea. Furthermore, our data suggests a role for the LIN28B/let-7 axis in regulating HC regeneration and SC plasticity, identifying a novel candidate pathway for future gene-based HC regeneration therapies.
Regulation of Lin28b/let-7 Expression in the Developing Cochlea.
We have found that the evolutionary conserved Lin28b/let-7 circuit is active in the auditory sensory lineage. Lin28b is specifically expressed in prosensory progenitors and is rapidly down-regulated upon differentiation, whereas levels of mature let-7 miRNAs rise during prosensory differentiation and are highly expressed in early postnatal HCs and SCs. LIN28B inhibits mature let-7 miRNA processing and likely serves a critical role in let-7’s posttranscriptional regulation within the developing cochlea. Despite the mutually antagonistic relationship between Lin28b and the let-7s, however, it is unlikely that the initial down-regulation of Lin28b is mediated by let-7 miRNAs. Instead, it is more plausible that the heterochronic miRNA mir-125b mediates Lin28b’s down-regulation. Similar to the let-7 miRNAs, mir-125b targets the expression of Lin28b; however, unlike the let-7 miRNAs, Lin28b and mir-125b do not share a mutually antagonistic relationship, and LIN28B is unable to regulate mir-125b’s expression (19, 35, 60). Alternatively, it is possible that in the developing cochlea, Lin28b’s expression is mainly regulated on a transcriptional level. In stark contrast to the wealth of knowledge related to Lin28b’s regulation by miRNAs, relatively little is known about the transcription factors controlling Lin28b’s expression during mammalian development, and further investigations are warranted.
Lin28b Acts Through a let-7–Dependent Mechanism To Time Prosensory Cell Cycle Withdrawal but Not Differentiation.
The regulatory functions of the RNA binding protein LIN28B and its opposing let-7 miRNAs have been extensively studied in stem cells, tumor models, and adult tissues; however, little is known about their function during embryonic development. Here we provide evidence that the LIN28B/let-7 circuit is critical for the proper timing of prosensory cell cycle exit in the murine cochlea. We show that higher-than-normal LIN28B protein levels delayed the onset of prosensory cell cycle exit, whereas increased let-7 levels resulted in premature cell cycle exit. The opposing effects of LIN28B and let-7g overexpression suggest that relative LIN28B/let-7 levels set the timing of prosensory cell cycle withdrawal in the murine cochlea. Furthermore, our finding that let-7g is sufficient to induce premature cell cycle withdrawal suggests that LIN28B times prosensory cell cycle exit, at least in part, by repressing let-7 biogenesis. Interestingly, although both global and cochlear-specific LIN28B overexpression was sufficient to delay the onset of HC differentiation, similar let-7g overexpression strategies failed to induce premature HC differentiation. The failure to induce earlier HC differentiation is in stark contrast to let-7’s differentiation-promoting function in other tissues. For example, overexpression of let-7 in neuronal progenitors not only inhibits proliferation, but induces premature neuronal differentiation as well (29, 44, 45, 61, 62). How can this be explained? In many tissues, including the developing brain, timing of differentiation is tightly linked to timing of progenitor cell cycle exit. However, in the murine cochlea the developmental timing of these processes is largely uncoupled (5, 8). This difference is illustrated by the divergent behavior of the let-7 target Mycn within different tissue models. Similar to let-7 overexpression, loss of Mycn causes premature prosensory cell cycle withdrawal without altering the timing of HC differentiation within the developing cochlea (48, 63). In contrast, brain-specific deletion of Mycn results in both premature progenitor cell cycle exit and premature neuronal differentiation (64). Alternatively, it could be reasoned that the failure of let-7 to induce premature HC differentiation is due to a lack of inductive cues that would allow for an earlier onset of HC differentiation. The lack of inductive cues being the reason is rather unlikely, however, because loss of inhibitory Sonic Hedgehog (Shh) signaling is sufficient to trigger premature HC differentiation (65–67).
Let-7–Independent Functions of LIN28B.
It is becoming increasingly clear that LIN28 proteins can function in a let-7–independent manner (68, 69). Several recent genomewide studies have revealed that LIN28A and LIN28B proteins directly alter the protein abundance of hundreds of their mRNA targets through transcript stabilization and/or modulation of translation efficiency (14, 15, 70–72). Consistent with an important role in gene regulation, among the top LIN28B targets are RNA binding proteins, transcriptional regulators, and RNA splicing factors (15, 70). How might LIN28B alter the timing of HC differentiation? To date two pathways, the Insulin-like growth factor (Igfr1)-phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the Shh pathway, have been shown to influence timing of HC differentiation within the murine cochlea (65–67, 73). LIN28B has been shown to positively regulate Igf1r-PI3K/Akt pathway (27); however, loss of Igfr1 within the developing cochlea does not mirror LIN28B’s effects on HC differentiation. In the Igfr1 mutant cochlea, prosensory cell cycle exit occurs prematurely while HC differentiation is delayed (73). Functional connections between the Shh signaling pathway and LIN28B have not yet been established; however, the tools described here will allow future examination of this link.
The LIN28B/let-7 Axis Influences SC Plasticity.
LIN28 proteins have been shown to promote cell reprogramming, tissue repair, and regeneration (58, 59, 74, 75). HC loss is among the leading causes of human deafness, because in contrast to nonmammalian vertebrates, mammals are unable to regenerate damaged HCs. Thus, uncovering mechanisms that promote HC regeneration/repair within the mammalian inner ear remains a major focus within the auditory research community. For nonmammalian vertebrates, SCs are the main source of postembryonic HC production and HC replenishment after trauma (76). Strikingly, mammalian SCs can generate HCs under certain permissive conditions, such as Notch inhibition or Wnt overstimulation, but this ability rapidly declines during the first week of life (57, 77–79). Here, we show that LIN28B reactivation in the early postnatal murine cochlea enhances the generation of new HCs in response to Notch inhibition. The vast majority of newly generated HCs in our experimental paradigm were generated by a nonmitotic process, suggesting that this increase in newly generated HCs was due to an increased number of SCs that converted into HCs in response to Notch inhibition. How does LIN28B reexpression alter the response of SCs? A recent study showed that LIN28A reactivation in adult mice boosts tissue repair by reprogramming cellular metabolism (58), and it is possible that enhancement of oxidative SC metabolism had a positive effect on the ability of SCs to switch cell fate and convert into HCs. Alternatively, it is possible that LIN28B reexpression altered the differentiation state of SCs by down-regulating let-7 miRNA expression. For instance, in zebrafish, Lin28 becomes reactivated in Müller glia after damage, and promotes Müller glia dedifferentiation and subsequent retina regeneration, at least in part by decreasing let-7 miRNA levels (80). Indeed, let-7 down-regulation was found to be necessary to promote cellular regeneration in multiple models of murine tissue repair (58). Similarly, we found that let-7 overexpression significantly antagonized SC-to-HC conversion in the absence of Notch signaling, suggesting that let-7 down-regulation is necessary for SC dedifferentiation in the murine cochlea. Could let-7 miRNAs contribute to the developmental decline in SC’s regenerative capacity? Let-7 miRNAs are highly expressed in early postnatal HCs and SCs, and there is tentative evidence that cochlear let-7 expression further increases during postnatal maturation (36). Future experiments are needed to link the rise in let-7 expression to an age-related decline in regenerative capacity.
In summary, we have demonstrated that the Lin28b/let-7 miRNA axis is active within the developing cochlea and plays a key role in coordinating the timing of progenitor cell cycle withdrawal and the onset of differentiation. Interestingly, LIN28B appears to act through both let-7–dependent and let-7independent mechanisms in coordinating these processes and further studies investigating these let-7–independent pathways are needed. Lastly, our findings indicate that the LIN28B/let-7 axis regulates the capacity for SC-to-HC conversion in the absence of Notch signaling, making it an interesting candidate for future HC regeneration therapies.
Materials and Methods
Mouse Breeding and Genotyping.
All experiments and procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committees protocol, and all experiments and procedures adhered to National Institutes of Health-approved standards. The Atoh1/nEGFP transgenic line was obtained from Jane Johnson (University of Texas Southwestern Medical Center, Dallas, TX) (81). The iLIN28B and ilet-7g transgenic lines were obtained from George Q. Daley (Children’s Hospital, Boston, MA) (27). The Pax2-Cre line was obtained from Andrew Groves (Baylor College, Houston, TX) (82). The M2-rtTA (stock no. 006965) and rtTA-EGFP (stock no. 005572) lines were purchased from Jackson Laboratories. Mice were genotyped by PCR and genotyping primers are listed in Table S1. Mice of both sexes were used in this study. All mouse lines were maintained on a mixed background of C57BL/6 and CD-1. Embryonic development was considered as E0.5 on the day a mating plug was observed.
Table S1.
Genotyping primers
| Mouse line | Primer name | Primer sequence |
| Col1A1 (iLIN28B, iLet-7) | ColfrtA | GCA CAG CAT TGC GGA CAT GC |
| ColfrtB | CCC TCC ATG TGT GAC CAA GG | |
| ColfrtC | GCA GAA GCG CGG CCG TCT GG | |
| M2-rtTA | rtTA A | GCG AAG AGT TTG TCC TCA ACC |
| rtTA B | AAA GTC GCT CTG AGT TGT TAT | |
| rtTA C | GGA GCG GGA GAA ATG GAT ATG | |
| rtTA-EGFP | rtTA mutant - F | GAG TTC TCT GCT GCC TCC TG |
| rtTA mutant - R | AAG ACC GCG AAG AGT TTG TC | |
| rtTA wild type - F | CGT GAT CTG CAA CTC CAG TC | |
| rtTA wild type - R | GGA GCG GGA GAA ATG GAT ATG | |
| Pax2-Cre | Cre - Forward | AAC ATG CTT CAT CGT CGG TCC GGG CTG C |
| Cre - Reverse | GAC GGA AAT CCA TCG CTC GAC CAG TTT A | |
| Atoh1/nEGFP | GFP - Forward | CGA AGG CTA CGT CCA GGA GCG CAC CAT |
| GFP - Reverse | GCA CGG GGC CGT CGC CGA TGG GGG TGT TCT GC |
To induce iLIN28B and iLet-7 expression, dox was delivered to time-mated females via ad libitum access to feed containing 2 g of doxycycline per kg of feed (Bioserv no. F3893), which was continued until the stage the tissue was harvested. Both Rosa26-iLIN28B and Pax2-iLIN28B overexpression were induced at E8.5, except for the Rosa26-iLIN28B litters used in the HC regeneration experiments, which were induced at E15.5. To induce Rosa26-iLet-7 expression, dox treatment was begun at E11.5. To induce Pax26-iLet-7 expression, dox treatment was begun at E9.5. For the iLet-7 hair cell regeneration experiments, Rosa26-iLet-7 was induced at E17.5. For the Rosa26-iLIN28B and iLet-7 lines, nontransgenic littermate controls expressed only the Rosa26 M2-rtTA transgene (M2-rtTA tg/+). For the Pax2-iLIN28B and iLet-7 lines nontransgenic littermate controls expressed only the rtTA-EGFP and Pax2-Cre transgenes (rtTA-EGFP tg/+; Pax2-Cre tg/+).
Tissue Isolation.
Embryos were removed from timed-mated females and staged by using the EMAP eMouse Atlas Project Theiler staging criteria (www.emouseatlas.org/emap/ema/home.html). Inner ears were collected and dissected in HBSS (Life Technologies). To free the cochlear epithelium (E13–E16), the vestibular portion of the inner ear was removed and the remaining cochlear portion was incubated in calcium-magnesium-free PBS (Life Technologies) containing dispase (1 mg/mL; Life Technologies) and collagenase (1 mg/mL; Worthington) for 8 min then placed in DMEM-F12 (Life Technologies) containing 10% (vol/vol) FBS for 30 min, the cochlear duct was then freed from surrounding mesenchyme by manual dissection with 30-gauge needles. To obtain cochlear sensory epithelia of stages E18 and older, the cochlear capsule and the spiral ganglion were removed before dispase/collagenase treatment.
RNA Extraction and qPCR.
Total RNA including mature miRNAs was extracted from cochlea epithelia samples by using the miRNeasy Micro Kit (QIAGEN). For mRNA expression analysis, mRNA was reverse transcribed into cDNA by using the iScript cDNA synthesis kit (Bio-Rad). SYBR Green-based qPCR was performed by using Fast SYBR Green Master Mix reagent (Applied Biosystems, Life Technologies no. 4385612) and gene-specific primers. For miRNA expression analysis, predesigned TaqMan Assays (Applied Biosystems, Life Technologies) were used according to manufacturer’s instructions for two-step RT-qPCR. qPCRs were carried out in triplicate by using a StepOne Plus Real-Time PCR System (Applied Biosystems, Life Technologies). Relative gene expression was analyzed by using the ΔΔCT method (83). The ribosomal gene Rpl19 was used as an endogenous reference gene for SYBR Green-based assays, and the snoRNA U6 was used as an endogenous reference gene for TaqMan-based miRNA measurements. qPCR primer sequences are listed in Table S2.
Table S2.
qPCR primers
| Gene | Forward primer | Reverse primer |
| Atoh1 | ATG CAC GGG CTG AAC CA | TCG TTG TTG AAG GAC GGG ATA |
| Ccnd1 | TCC GCA AGC ATG CAC AGA | GGT GGG TTG GAA ATG AAC TTC A |
| Hmga2 | CCC AAA GGC AGC AAA AAC AA | CCA ATG GTC TCT GCT TTC TTC TG |
| Igf2bp1 | GGA GCA GAC CAG GCA AGC TA | GGG CAT GGT TCT CCA GTT GA |
| Lin28a | TCC AAA GGA GAC AGG TGC TAC A | TTG CAT TCC TTG GCA TGA TG |
| Lin28b | CAG ACA GGT CAC CCC AAG AAG | TTT TGC TCT CCT ATT GCT GCA A |
| LIN28B | AAA GGG AAG ACA CTA CAG AAA AGA AAA | GAT GAT CAA GGC CAC CAC AGT |
| Lin41 | AGG TGG CCT CTT TCA CTG TCA | ATC AGG TCA CCT CCC GAA TG |
| Mycn | TCT AAC AAC AAG GCG GTA ACC A | GCC CAG AGC GGA GGT CTT |
| Rpl19 | GGT CTG GTT GGA TCC CAA TG | CCC GGG AAT GGA CAG TCA |
Immunoblotting.
Cochlear epithelia were placed in lysis buffer (50 mM Hepes, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 0.2% SDS, pH 7.9) supplemented with fresh Complete Protease Inhibitor (Roche). Equal amounts of cochlear lysate were resolved on NuPAGE 4–12% Bis-Tris Gels (Novex, Life Technologies) and transferred to PVDF membrane by electrophoresis. Membranes were blocked in 10% BSA in TBST and immunoblotted: rabbit anti-Lin28b 1:1,000 (Cell Signaling no. 5422), mouse anti-β-actin 1:3,000 (Ambion no. AM4302), rabbit anti-Flag 1:1,000 (Cell Signaling no. 2368). HRP-conjugated secondary antibodies from Jackson ImmunoResearch were used at a concentration of 1:7,500 (goat anti-rabbit IgG no. 111–035-003; sheep anti-mouse IgG no. 515–035-003). Signal was revealed by using a Western Lightening-ECL kit (Perkin-Elmer) according to manufacturer’s instructions.
ISH and Immunostaining.
Embryo heads (E11–E16) or inner ears (E18 and older) were fixed in 4% (vol/vol) paraformaldehyde in PBS overnight at 4 °C then cryoprotected through sucrose gradient: 10% (wt/vol) sucrose for 30 min at room temperature (RT), 15% sucrose for 1 h at room temperature, 50:50 30% sucrose:OCT overnight at 4 °C. Before cryosectioning, samples were submerged in OCT (Tissue-Tek, Sakura) and flash frozen. Fourteen micrometer sections were collected on SuperFrost Plus slides (Fisher). pBluescript II (Stratagene) and pGem-T easy (Promega) vectors containing full-length mouse Atoh1, Sox2, Lin28b, and Hmga2 cDNA were used as templates to synthesize digoxigenin-labeled antisense RNA probes according to the manufacturer’s specifications (Roche). Custom-made 5′ digoxygenin-labeled locked nucleic acid probes (miRCURY LNA) from Exiqon were used for let-7f and let-7c ISH. Probes were detected with the anti–DIG-AP (alkaline phosphatase) conjugated antibody (Roche), and the color reactions were developed by using BM Purple AP Substrate (Roche). Detailed protocol is available upon request.
Immunostaining was performed according to the manufacturer’s specifications. Primary antibodies: rabbit anti-myosin VI (1:1,000, Proteus no. 25–6791), rabbit anti-myosin VIIa (1:500, Proteus no. 25–6790), goat anti-SOX2 (1:500, Santa Cruz no. sc-17320), mouse anti-p27/Kip1 (1:200, NeoMarkers/Thermo no. MS-256-P1), rabbit anti-S100 (1:500, Abcam no. ab868), and rabbit anti-dsRed (1:500 Clontech no. 632496). Alex Fluor (488, 546, and 633) labeled secondary antibodies were used to visualize staining (1:1,000, Molecular Probes/Life Technologies). Stereocilia were visualized with fluorescently labeled phalloidin (1:500, Molecular Probes/ Life Technologies). For anti-p27/Kip1 immunostaining, sections underwent antigen retrieval by boiling in 1x Dako Ready-to-Use Target Retrieval Solution (Dako no. S1700) in a 95 °C water bath for 20 min, followed by a 45-min cooling incubation at RT.
Hair Cell Differentiation Assay.
E13.5 undifferentiated Atoh1/nEGFP cochlear epithelia, spiral ganglion, and surrounding mesenchyme were cultured on SPI black membranes (SPI Supplies, Structure Probe) in DMEM-F12 (Life Technologies) containing 1x N2 supplement (Life Technologies), 5 ng/mL EGF (Sigma), 2.5 ng/mL FGF (Sigma), 100 U/mL penicillin/streptomycin (Sigma), and 10 μg/mL dox (Sigma). Cultures were grown in a 5% CO2/20% O2 humidified incubator. To monitor HC differentiation, native nuclear GFP expression (Atoh1/nEGFP) was imaged every 8–12 h for 3 d by using fluorescent stereomicroscopy (Leica). Length of the GFP+ HC stripe was quantified by using ImageJ64 software (NIH).
Proliferation Assay.
EdU (5-ethynyl-2′-deoxyuridine, Life Technologies) was reconstituted in PBS and administered at 50 μg per gram of body weight to time-mated females by i.p. injection. Click-iT Alexa Fluor 546 Kit (Life Technologies) was used to detect incorporated EdU according to the manufacturer’s specifications. MYO7A and SOX2 immunostaining labeled HCs and SCs. Cochleae were divided into three equal portions (base, mid, apex) and high-power confocal (Zeiss) z-stack images (4 images per region, representing ∼700 microns) were taken through the HC and SC layers within each of these regions. The percentage of HCs and SCs that had incorporated EdU was manually quantified within Photoshop and ImageJ.
Cochlear Length and Cell Patterning Analysis.
Cochlear surface whole mounts were prepared from E18.5 iLIN28B and nontransgenic littermate embryos and immunostained for MYO7A and SOX2. Low-power fluorescent images of the HC layer were assembled in Photoshop CS5 (Adobe), and the length of the entire cochlear sensory epithelium was measured in ImageJ64 (NIH). For cell density and patterning analysis, cochleae were divided into three equal portions (base, mid, apex) and high-power confocal (Zeiss) z-stack images (4 images per region, representing ∼700 microns) were taken through the HC and SC layers within each of these regions. Images then were manually quantified within Photoshop and ImageJ.
Hair Cell Generation Assay.
P2 cochlear surface preparations were cultured on SPI black membranes (SPI Supplies, Structure Probe) in DMEM-F12 (Life Technologies) containing 1x N2 supplement (Life Technologies), 5 ng/mL EGF (Sigma), 2.5 ng/mL FGF (Sigma), 100 U/mL penicillin (Sigma), and 10 μg/mL dox (Sigma). Experimental cultures were treated with 10 μM DAPT (γ-secretase inhibitor IX, Calbiochem-EMD Biosciences) to block Notch signaling. Control cultures received DMSO (0.04%). Media was refreshed once at 24 h, and cultures were fixed at 72 h. To monitor proliferation, explants were grown in the presence of 3 μM EdU. For HC quantification, cochlear explant cultures were subdivided into four parts: apex (0–1,500 μM); mid apex (1,500–3,000 μM); mid base (3,000–4,500 μM); base (4,500–5,500). A sampling (four per region) of high-power confocal z-stack images were taken through the HC and SC layers within these regions, and HC density and proliferation were manually quantified by using Imaris software (Bitplane) and ImageJ. HCs were identified by MYO7A and Atoh1/nEGFP coexpression.
Lentiviral Expression System.
Control and experimental constructs (mCherry vs. Lin28bF2AmCherry) were maintained in FUW lentiviral-vector backbone. Multistep PCR was used to link full-length murine Lin28b ORF to a mCherry reporter via a F2A linker sequence (84). High titer lentiviral stocks were prepared as described (85). Undifferentiated Atoh1/nEGFP cochlear epithelial explants were incubated in a 1:1 mix of lentivirus and culture media at room temperature for 2-h before plating. Cultures were maintained as described above. Detailed protocols are available upon request.
Statistical Analysis.
Values are presented as mean ± SE (SEM), n = animals per group. All results were confirmed by at least two separate experiments. Two-tailed Student's t tests were used to determine confidence interval. P values ≤0.05 were considered significant. P values >0.05 were considered not significant.
Acknowledgments
We thank the members of the A.D. Laboratory and Center for Sensory Biology for the help and advice provided throughout the course of this study; Randy Reed, Mollie Meffert, Shan Sockanathan, and Seth Blackshaw for fruitful discussions regarding this project; Neil M. Neumann for training and patience with the Imaris software; Jane Johnson for the Atoh1/nEGFP mice; Andrew Groves for the Pax2-Cre mice; and George Q. Daley for iLIN28B and iLet-7 mice. This work was supported by March of Dimes Basil O’Connor Grant FY11-84 (to A.D.) and National Institutes of Health Grants DC012972 (to E.J.G.), DC013477 (to A.B.-G.), DC011571 (to A.D.), and DC005211 (Sensory Mechanisms Research Core Center).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.W.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501077112/-/DCSupplemental.
References
- 1.Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220:220–, 1–44. [PubMed] [Google Scholar]
- 2.Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- 3.Raft S, Groves AK. Segregating neural and mechanosensory fates in the developing ear: Patterning, signaling, and transcriptional control. Cell Tissue Res. 2015;359(1):315–332. doi: 10.1007/s00441-014-1917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schimmang T, Pirvola U. Coupling the cell cycle to development and regeneration of the inner ear. Semin Cell Dev Biol. 2013;24(5):507–513. doi: 10.1016/j.semcdb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 6.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 7.Bermingham NA, et al. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Johnson JE, Zoghbi HY, Segil N. 2002. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129(10):2495–2505. [DOI] [PubMed]
- 9.Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol. 2003;258(2):432–442. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 10.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226(4673):409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 11.Shyh-Chang N, Daley GQ. Lin28: Primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12(4):395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15(3):357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polesskaya A, et al. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21(9):1125–1138. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf R, et al. Identification of LIN28B-bound mRNAs reveals features of target recognition and regulation. RNA Biol. 2013;10(7):1146–1159. doi: 10.4161/rna.25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hafner M, et al. Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA. 2013;19(5):613–626. doi: 10.1261/rna.036491.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320(5872):97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo I, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32(2):276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14(8):1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybak A, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10(8):987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 20.Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014;15(9):565–576. doi: 10.1038/nrm3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SM, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YC, et al. Human TRIM71 and its nematode homologue are targets of let-7 microRNA and its zebrafish orthologue is essential for development. Mol Biol Evol. 2007;24(11):2525–2534. doi: 10.1093/molbev/msm195. [DOI] [PubMed] [Google Scholar]
- 25.Rehfeld F, Rohde AM, Nguyen DT, Wulczyn FG. Lin28 and let-7: Ancient milestones on the road from pluripotency to neurogenesis. Cell Tissue Res. 2014;359(1):145–170. doi: 10.1007/s00441-014-1872-2. [DOI] [PubMed] [Google Scholar]
- 26.La Torre A, Georgi S, Reh TA. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci USA. 2013;110(26):E2362–E2370. doi: 10.1073/pnas.1301837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, et al. DIAGRAM Consortium MAGIC Investigators The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Benson KF, Ashar HR, Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995;376(6543):771–774. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]
- 29.Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135(2):227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315(5818):1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maller Schulman BR, et al. The let-7 microRNA target gene, Mlin41/Trim71 is required for mouse embryonic survival and neural tube closure. Cell Cycle. 2008;7(24):3935–3942. doi: 10.4161/cc.7.24.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiernan AE, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434(7036):1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 33.Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012;22(9):474–482. doi: 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagos-Quintana M, et al. 2002. Identification of tissue-specific microRNAs from mouse. Curr Biol 12(9):735–739.
- 35.Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol Cell Biol. 2005;25(21):9198–9208. doi: 10.1128/MCB.25.21.9198-9208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111(1):95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Sacheli R, et al. Expression patterns of miR-96, miR-182 and miR-183 in the development inner ear. Gene Expr Patterns. 2009;9(5):364–370. doi: 10.1016/j.gep.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Avraham KB, et al. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11(4):369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan SR, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41(7):843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molenaar JJ, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44(11):1199–1206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 41.Urbach A, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes Dev. 2014;28(9):971–982. doi: 10.1101/gad.237149.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133(15):2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- 43.Hasson T, et al. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137(6):1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishino J, Kim S, Zhu Y, Zhu H, Morrison SJ. A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. eLife. 2013;2:e00924. doi: 10.7554/eLife.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao C, et al. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA. 2010;107(5):1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buechner J, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105(2):296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: Role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol. 2010;337(1):134–146. doi: 10.1016/j.ydbio.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez-Frutos E, et al. 2011. N-myc controls proliferation, morphogenesis, and patterning of the inner ear. J Neurosci 31(19):7178–7189.
- 49.Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn. 2011;240(6):1373–1390. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 51.Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132(19):4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 52.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133(7):1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 53.Lanford PJ, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21(3):289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 54.Doetzlhofer A, et al. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16(1):58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto N, et al. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med (Berl) 2006;84(1):37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- 56.Takebayashi S, et al. Multiple roles of Notch signaling in cochlear development. Dev Biol. 2007;307(1):165–178. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 57.Mizutari K, et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77(1):58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shyh-Chang N, et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155(4):778–792. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073):1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wulczyn FG, et al. Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB J. 2007;21(2):415–426. doi: 10.1096/fj.06-6130com. [DOI] [PubMed] [Google Scholar]
- 61.Zhao C, Sun G, Ye P, Li S, Shi Y. 2013. MicroRNA let-7d regulates the TLX/microRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep 3:1329.
- 62.Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136(5):913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. 2011. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn 240(6):1373–1390.
- 64.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16(20):2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benito-Gonzalez A, Doetzlhofer A. Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling. J Neurosci. 2014;34(38):12865–12876. doi: 10.1523/JNEUROSCI.1494-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tateya T, et al. Hedgehog signaling regulates prosensory cell properties during the basal-to-apical wave of hair cell differentiation in the mammalian cochlea. Development. 2013;140(18):3848–3857. doi: 10.1242/dev.095398. [DOI] [PubMed] [Google Scholar]
- 67.Bok J, Zenczak C, Hwang CH, Wu DK. Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc Natl Acad Sci USA. 2013;110(34):13869–13874. doi: 10.1073/pnas.1222341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balzer E, Heine C, Jiang Q, Lee VM, Moss EG. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137(6):891–900. doi: 10.1242/dev.042895. [DOI] [PubMed] [Google Scholar]
- 69.Nowak JS, Choudhury NR, de Lima Alves F, Rappsilber J, Michlewski G. Lin28a regulates neuronal differentiation and controls miR-9 production. Nat Commun. 2014;5:3687. doi: 10.1038/ncomms4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilbert ML, et al. LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Mol Cell. 2012;48(2):195–206. doi: 10.1016/j.molcel.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho J, et al. LIN28A is a suppressor of ER-associated translation in embryonic stem cells. Cell. 2012;151(4):765–777. doi: 10.1016/j.cell.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 72.Peng S, et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29(3):496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 73.Okano T, Xuan S, Kelley MW. 2011. Insulin-like growth factor signaling regulates the timing of sensory cell differentiation in the mouse cochlea. J Neurosci 31(49):18104–18118.
- 74.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462(7273):595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 76.Burns JC, Corwin JT. A historical to present-day account of efforts to answer the question: “What puts the brakes on mammalian hair cell regeneration?”. Hear Res. 2013;297:52–67. doi: 10.1016/j.heares.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi F, Hu L, Edge ASB. Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci USA. 2013;110(34):13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai R, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA. 2012;109(21):8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PLoS ONE. 2013;8(8):e73276. doi: 10.1371/journal.pone.0073276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12(11):1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lumpkin EA, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3(4):389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 82.Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38(4):195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 83.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 84.Klump H, et al. Retroviral vector-mediated expression of HoxB4 in hematopoietic cells using a novel coexpression strategy. Gene Ther. 2001;8(10):811–817. doi: 10.1038/sj.gt.3301447. [DOI] [PubMed] [Google Scholar]
- 85.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]