Significance
Entry of enveloped viruses into the cell requires the activation of viral glycoproteins, often mediated by cellular receptors. Herpesviruses infect cells via a multipartite system, which includes species-specific glycoproteins plus conserved apparatus gH/gL and gB. HSV makes use of αvβ6- or αvβ8-integrins as gH/gL receptors. The interaction of HSV gH/gL with integrins resulted in the dissociation of gL. The dissociation took place if all the actors of the entry apparatus were present, i.e., under conditions that lead to glycoprotein activation and virus entry. We propose that (i) gL is a regulator of gH and prevents its activation until integrins promote gL dissociation from gH/gL. (ii) Dissociation from an inhibitory regulator represents a previously unidentified mechanism of activation of viral fusion glycoproteins.
Keywords: herpes simplex virus, glycoprotein, gH, gL, virus entry
Abstract
Herpes simplex virus (HSV) is an important human pathogen. It enters cells through an orchestrated process that requires four essential glycoproteins, gD, gH/gL, and gB, activated in cascade fashion by receptor-binding and signaling. gH/gL heterodimer is conserved across the Herpesviridae family. HSV entry is enabled by gH/gL interaction with αvβ6- or αvβ8-integrin receptors. We report that the interaction of virion gH/gL with integrins resulted in gL dissociation and its release in the medium. gL dissociation occurred if all components of the entry apparatus—receptor-bound gD and gB—were present and was prevented if entry was blocked by a neutralizing monoclonal antibody to gH or by a mutation in gH. We propose that (i) gL dissociation from gH/gL is part of the activation of HSV glycoproteins, critical for HSV entry; and (ii) gL is a functional inhibitor of gH and maintains gH in an inhibited form until receptor-bound gD and integrins signal to gH/gL.
Entry of herpesviruses into the cell is an orchestrated process that necessitates a multipartite glycoprotein system, rather than one-two glycoproteins, as is the case for the vast majority of viruses (1–4). For herpes simplex virus (HSV), the entry-fusion apparatus consists of four essential glycoproteins —gD, the heterodimer gH/gL, and gB—plus cognate cellular receptors. gD is the major determinant of HSV tropism. Structurally, it exhibits an Ig-folded core with N- and C-terminal extensions (5, 6). The structure of the gH/gL heterodimer does not resemble that of any known protein (7–9). The binding site of gL in gH maps to the N-terminal domain I. Whether gH/gL has a profusion activity in itself or is only an intermediate between gD and gB in the chain of activation of the glycoproteins is an open question (10–13). gB is considered the fusogenic glycoprotein, based on structural features, including a trimeric fold and a bipartite fusion loop (14, 15). gH, gL, and gB constitute the conserved-entry glycoproteins across the Herpesviridae family. The ability of gH to heterodimerize with gL is also conserved across the family, highlighting that the heterodimeric structure is critical to the function of two glycoproteins.
The prevailing model of HSV entry envisions that following a first virion attachment to cells mediated by heparan sulfate glycosaminoglycans, the interaction of gD with one of its receptors, nectin1 and HVEM (herpesvirus entry mediator), results in conformational changes to gD, in particular to the ectodomain C terminus, which harbors the profusion domain (5, 6, 16, 17). The activated gD recruits gH/gL, which, in turn, recruits gB. gB executes the virus–cell fusion (2–4, 18, 19). We observed that the glycoproteins are already in complex in resting virions (17, 18). In contrast with the view that glycoproteins are stepwise-recruited to a complex, we favor the view that the process of activation of the viral glycoproteins results from the interaction of preassembled glycoproteins’ complexes with cellular receptors and from a signaling cascade, which is likely triggered by receptor-induced conformational changes (18).
The more speculative part of the HSV-entry model concerns the roles of gH and of gL and why gH has evolved to be a heterodimer with gL. Recently, we discovered that αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL. They play two distinct roles in infection (20). They enable virus entry, as inferred by inhibition of infection following integrin depletion by siRNAs or exposure of cells to anti-integrin antibodies. Second, they promote HSV endocytosis into acidic endosomes (20); the latter function is nonessential because the virus may enter some cells also by fusion with plasma membranes or with neutral endosomes (21–23). Remarkably, the use of integrins as receptors is a common feature among herpesviruses. Integrins serve as receptors also for gH/gL of EBV (Epstein Barr virus), of human cytomegalovirus and equine herpesvirus, and for gB of Kaposi’s sarcoma-associated herpesvirus (24–28). Most likely, they play a common role.
Here, we asked whether αvβ6- or αvβ8-integrin induce conformational changes to HSV gH/gL, as part of the process of glycoprotein activation in virus entry. We report that αvβ6- and αvβ8-integrin promote the dissociation of gL from gH/gL. Conditions for the dissociation were the presence of gD, its receptor nectin1, and gB, i.e., conditions that lead to activation of the entry machinery, including the virion glycoproteins.
Results
Ectodomain and the C-Tail of αvβ6-Integrin Play Distinct Roles in HSV Entry.
αvβ6- and αvβ8-integrin play two distinct roles in HSV infection of human cells: they enable HSV-1 entry by an unknown mechanism; and they promote virus endocytosis into acidic endosomes (20). To differentiate between these two functions and define the integrin domains where they map, we generated the β6N1 chimera, in which the transmembrane and C-tail portions of β6-integrin were replaced with those of nectin1. The β subunit was selected because this is the signaling portion.
J cells are negative for HSV gD receptors and resistant to infection. They become infectable upon transfection with a gD receptor, nectin1 or HVEM (29). J cells express endogenous integrins in limited amounts; overexpression of human αvβ6- or αvβ8-integrin in nectin1-positive J cells increases the extent of infection (20). Expression of αvβ6- or αvβ8-integrin in the absence of nectin1 or HVEM does not enable infection.
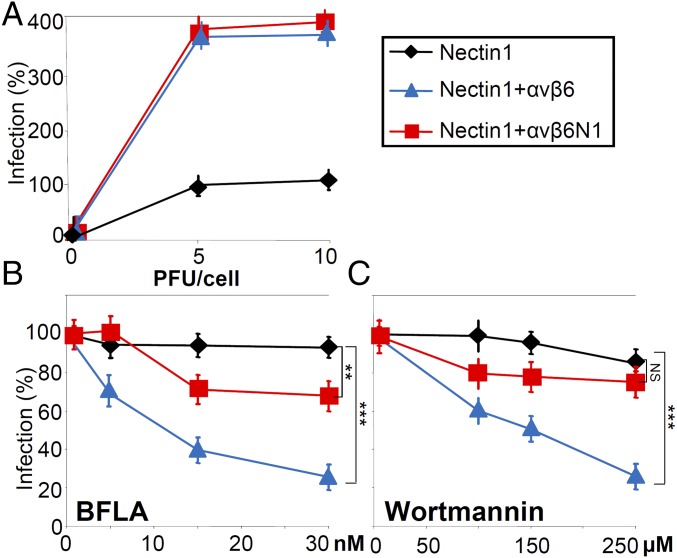
Here, J cells were transfected with nectin1 alone, nectin1 plus αvβ6-integrin, or nectin1 plus αvβ6N1 and infected with the HSV recombinant R8102, which carries the reporter Lac-Z under the α27 promoter (29). The extent of R8102 infection can be quantified as β-gal activity. Fig. 1A shows that both WT–αvβ6-integrin and αvβ6N1 chimera increased HSV entry, suggesting that the integrin-mediated increase in infection is independent of type of C-tail and most likely mediated by αvβ6-integrin ectodomain. This integrin portion binds gH/gL, as seen also by surface plasmon resonance (20).
Fig. 1.
The ectodomain and the C-tail of αvβ6-integrin play distinct roles in HSV infection. (A–C) Entry of HSV-1 strain F [HSV-1 (F)] into J cells expressing nectin1 (♦), nectin1 plus WT–αvβ6-integrin ( ), or nectin1 plus the chimera αvβ6N1 (
), or nectin1 plus the chimera αvβ6N1 ( ). J cells were transfected with the indicated receptors and infected with the HSV-1 recombinant R8102. Infection was quantified 16–18 h later by means of β-gal. (A) Comparison of extent of infection in J cells expressing the indicated receptors. Cells were infected at 5 or 10 PFU per cell. Infection is expressed as percentage of infection in J cells expressing nectin1 alone. (B and C) Effect of bafilomycin A (BFLA) (B) or wortmannin (C) on R8102 infection of J cells expressing the indicated receptors. Cells were exposed to the indicated concentrations of the inhibitors from 1 h before infection until harvesting. Infection is expressed as percentage of infection in the absence of inhibitor. Each point represents the mean of triplicate samples ± SD. **P < 0.01, ***P < 0.001. NS, not significant.
). J cells were transfected with the indicated receptors and infected with the HSV-1 recombinant R8102. Infection was quantified 16–18 h later by means of β-gal. (A) Comparison of extent of infection in J cells expressing the indicated receptors. Cells were infected at 5 or 10 PFU per cell. Infection is expressed as percentage of infection in J cells expressing nectin1 alone. (B and C) Effect of bafilomycin A (BFLA) (B) or wortmannin (C) on R8102 infection of J cells expressing the indicated receptors. Cells were exposed to the indicated concentrations of the inhibitors from 1 h before infection until harvesting. Infection is expressed as percentage of infection in the absence of inhibitor. Each point represents the mean of triplicate samples ± SD. **P < 0.01, ***P < 0.001. NS, not significant.
To differentiate among the entry pathways taken by HSV, infected cells were exposed to bafilomycin A (BFLA), an inhibitor of vacuolar H+ ATPase, hence of endocytosis into acidic endosomes, or to wortmannin, an inhibitor of phosphoinositide3-kinases. Fig. 1 B and C shows that infection mediated by WT–αvβ6-integrin was completely inhibited by BFLA and wortmannin, whereas infection mediated by the αvβ6N1 chimera was only slightly inhibited. The results indicate that the region of αvβ6-integrin critical for HSV entry is the ectodomain and that the signaling C-tail is a determinant of the entry pathway into acidic endosomes.
Glycoproteins’ Complex Pulled Down by αvβ6- or αvβ8-Integrin Plus Nectin1, but Not by Nectin1 Alone, Lacks gL.
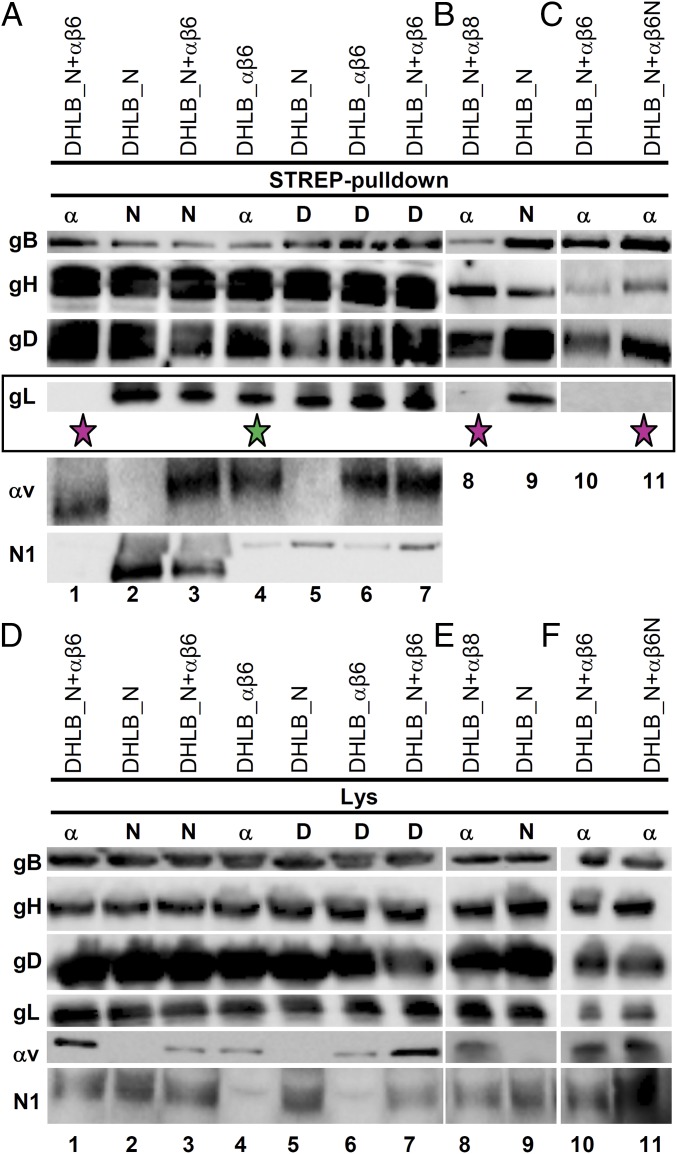
We hypothesized that the interaction of αvβ6- or αvβ8-integrin ectodomain with gH/gL might contribute to HSV entry by inducing conformational changes to gH/gL and that such changes might result in gL dissociation from gH/gL. We verified this hypothesis first in transfected cells. We compared the composition of the glycoproteins’ complexes pulled down by αvβ6-integrin alone, αvβ6-integrin in the presence of nectin1, or nectin1 alone. J cells were transfected with the glycoprotein quartet, gD, gH, gL, gB, αv- and β6-integrin, and nectin1, in appropriate combinations. Either αv-integrin or nectin1 were Strep-tagged to enable pulldown experiments (30). The complex harvested directly by means of gDSTREP was also examined. Analysis of the complex composition was performed by Western blot (WB) of the pulled-down glycoproteins. Fig. 2A shows that the complex pulled down by αvSTREPβ6-integrin (Strep-pulldown α) in the presence of nectin1 lacked gL (Fig. 2A, lane 1, fuchsia star). By contrast, the complex pulled down by αvβ6-integrin in the absence of nectin1 did contain gL (Fig. 2A, lane 4, green star), as did the complex pulled down by nectin1 alone (Fig. 2A, lane 2) or by nectin1 in combination with αvβ6-integrin (Fig. 2A, lane 3). gDSTREP (Strep-pulldown D) pulled down all four glycoproteins in any of the above conditions (Fig. 2A, lanes 5–7). The results show that the glycoproteins’ complex aggregated with αvβ6-integrin and containing gD, gB, and nectin1 does not contain gL.
Fig. 2.
Composition of HSV glycoprotein complexes assembled on αvβ6-integrin, αvβ8-integrin, αvβ6N1-integrin chimera, nectin1, or gD. J cells were transfected with gD, gH, gL, gB (DHLB) plus nectin1 (N), αv-integrin (α), β6-integrin (β6), β8-integrin (β8), or β6N1-integrin chimera (β6N), as indicated. To carry out pulldown experiments, one of the following—αv-integrin (α), nectin1 (N), or gD (D)—was Strep-tagged (Strep-pulldown) (30). Twenty-four hours after transfection, complexes were harvested by means of Strep-Tactin Sepharose (17) and separated by SDS/PAGE. The glycoproteins and receptors indicated to the Left were identified by WB. For each sample, three replicate gels were developed as follows: one for gB and gD, one for gH and gL, and a third one for αv-integrin and nectin1. (A) The complex pulled down by αvSTREPβ6-integrin in cells transfected with the four glycoproteins, gD, gH, gL, and gB, plus nectin1, αv-integrin, and β6-integrin lacks gL (lane 1, fuchsia star). In contrast, the complex assembled onto αvβ6-integrin alone (lane 4, green star) or nectin1 alone (lane 2) contains gL. (B) The complex assembled on αvβ8-integrin in cells transfected with the four glycoproteins, gD, gH, gL, and gB, plus nectin1, αv-integrin, and β8-integrin lacks gL (lane 8, fuchsia star). (C) The complex assembled on αvβ6N1-chimera in cells transfected with the four glycoproteins, gD, gH, gL, and gB, plus nectin1, αv-integrin, and β6N-chimera lacks gL (lane 11, fuchsia star). (D–F) WB analysis of lysates from samples A–C. For each sample, a lysate was made in 100 μL of EA1 buffer plus (17). The insoluble material was pelleted. Ten microliters of the supernatant was subjected to SDS/PAGE and WB with antibodies to the indicated proteins. The remaining 90 μL were used for the pulldown experiments shown in A–C.
Next, we analyzed the complex aggregated with αvβ8-integrin (Fig. 2B, lanes 8 and 9). Even the complex pulled down by αvSTREPβ8-integrin in the presence of nectin1 lacked gL (Fig. 2B, lane 8, fuchsia star). Thus, the absence of gL from the complex was seen irrespectively with αvβ6- or αvβ8-integrin. Most subsequent experiments were carried out with αvβ6-integrin.
We investigated whether the ectodomain or the C-tail of β6-integrin were involved in the assembly of the gL-less complex (Fig. 2C, lanes 10 and 11). J cells were transfected as in Fig. 2A, except that the β6-integrin subunit was either WT or the chimeric β6N1. Either form of β6-integrin failed to pull down gL (Fig. 2, lanes 10 and 11, fuchsia star). Thus, the ectodomain of β6-integrin is responsible for assembly of a glycoprotein complex from which gL is absent.
Together these results show that the compositions of the complexes aggregated with αvβ6- or αvβ8-integrin differ from those aggregated with nectin1 or gD, in that the complexes containing αvβ6- or αvβ8-integrin (in the presence of nectin1) do not contain gL. The complexes containing αvβ6-integrin alone or nectin1 alone do contain gL.
αvβ6-Integrin Does Not Induce gH Degradation.
We considered the possibility that the absence of gL from the glycoprotein complex assembled on αvβ6-integrin was the result not of the dissociation of gL from gH/gL, but of integrin-induced degradation of gH N terminus or of generalized gH degradation. If this were the case, the apparent mass of gH should be decreased (degradation of gH N terminus), or the amount of gH pulled down by αvβ6-integrin should be lower than the amount of gH pulled down by nectin1 alone. An aliquot of the samples shown in lanes 1, 2, and 8 was analyzed by WB with monoclonal antibody (MAb) H12 to gH. There was no evidence of gH degradation because the electrophoretic mobility and the amount of gH pulled down by nectin1 could not be differentiated from those of gH pulled down by αvβ6-integrin or αvβ8-integrin (Fig. S1). Furthermore, analysis of total cell extracts ruled out that the absence of gL from the complex harvested by means of αvβ6- or αvβ8-integrin reflected a lower expression of gL (Fig. 2 D–F).
Fig. S1.

Integrins do not induce degradation of gH. Aliquots of pulled-down gH from cells transfected with gD, gH, gL, gB, αvβ6-integrin plus nectin1 (lane DHLB_N+αβ6), or αvβ8-integrin plus nectin1 (lane DHLB_N+αβ8), or nectin1 alone in the absence of integrins (lane DHLB_N) (cells shown in Fig. 2) were subjected to SDS/PAGE and WB with MAb H12 to gH. It can be seen that the electrophoretic mobility and the amount of gH pulled down by nectin1 could not be differentiated from those of gH pulled down by αvβ6-integrin or αvβ8-integrin.
Cell Surface αvβ6-Integrin Promotes the Assembly of a Complex Carrying gH and Lacking gL on Adjacent Cells.
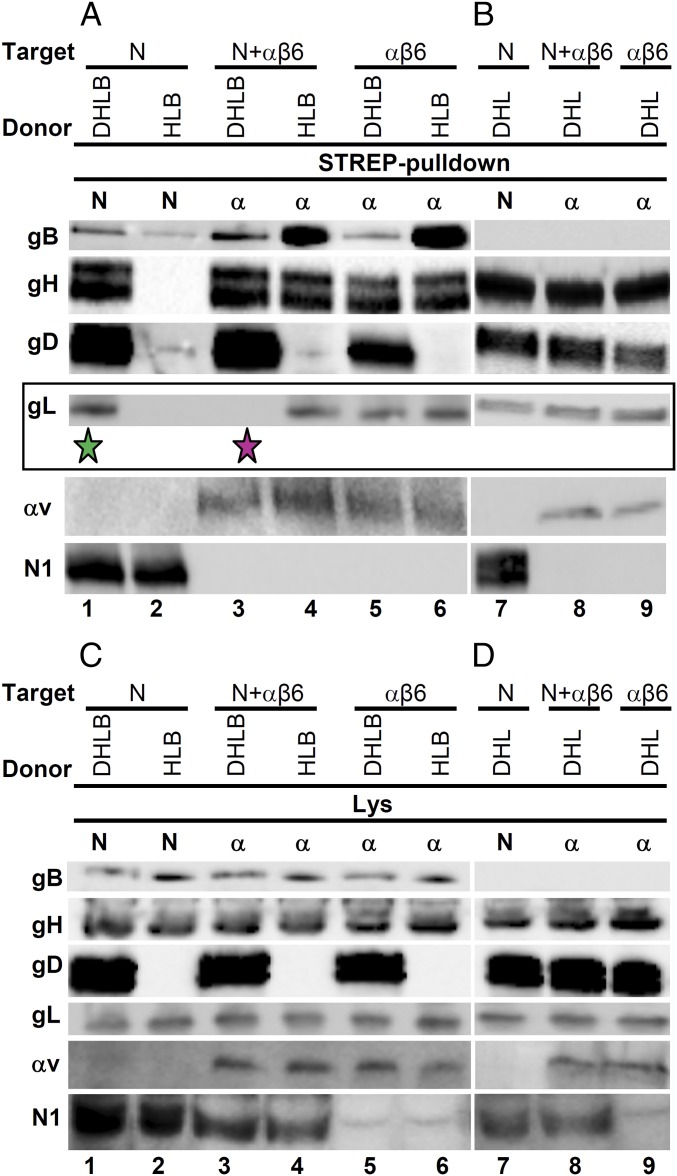
When HSV interacts with a cell, the envelope glycoproteins are located in trans relative to the cell surface receptors. This relative topology is mimicked when receptor-expressing target cells are cocultured with donor cells expressing the viral glycoproteins. This was our second experimental system. Target J cells transfected with a mixture of αvSTREP-integrin and β6-integrin with or without nectin1 (target) were cocultured with donor J cells expressing gD, gH, gL, and gB (donor) (Fig. 3A, lanes 1, 3, and 5). The control target cells expressed nectin1STREP and no αvβ6-integrin (Fig. 3A, lane 1). αvSTREPβ6-integrin pulled down gH, gD, gB, and no gL (Strep-pulldown α, Fig. 3A, lane 3, fuchsia star), whereas nectin1STREP pulled down all four glycoproteins (Strep-pulldown N, Fig. 3A, lane 1, green star). Thus, the αvβ6-integrin acts to dissociate gL both in cis, i.e., in the glycoprotein complexes on the same membrane, and in trans, i.e., in the glycoprotein complexes on the membranes of adjacent cells.
Fig. 3.
αvβ6-integrin, expressed on target cells together with nectin1, assembles a gL-less complex with the viral glycoproteins expressed on adjacent donor cells. Target J cells transfected with combinations of αv-integrin (α), β6-integrin (β6), and nectin1 (N) as indicated (Target) were cocultured with donor cells expressing gD, gH, gL, and gB (DHLB) or subsets thereof (HLB, DHL) (Donor). To carry out pulldown experiments, αv-integrin (α) or nectin1 (N) were Strep-tagged (Strep-pulldown). Twenty-four hours after coculture, complexes were harvested from cell lysates by means of Strep-Tactin resin, as described in ref. 17, and separated by SDS/PAGE. Glycoproteins and receptors were identified by WB. It can be seen that the complex assembled onto αvβ6-integrin in the presence of nectin1 lacks gL (lane 3, fuchsia star). When gD (lane 4, HLB), gB (lane 8, DHL), or nectin1 (lanes 5, 6, 9, and αβ6) were missing, the complexes contained gL. (C and D) WB analysis of lysates from samples A and B. For each sample, a lysate was made in 100 μL of EA1 buffer plus (17). The insoluble material was pelleted. Ten microliters of the supernatant was subjected to SDS/PAGE and WB with antibodies to the indicated proteins. The remaining 90 μL were used for the pulldown experiments shown in A and B.
Dissociation of gL Occurs if Nectin1, gD, and gB Are Present and Is Prevented by a Mutation in the RGD Motif of gH.
We investigated the requirements for the integrin-mediated dissociation of gL from gH/gL in the coculture system and focused on nectin1, gD, and gB. When nectin1 was omitted from the transfection mixture of target cells (Fig. 3A, lane 5), αvSTREPβ6-integrin pulled down gL, gH, gD, and gB. Hence, gL dissociation only took place if nectin1 was present (compare lanes 5 and 3 in Fig. 3A). Of note, αvβ6-integrin interacted with gH/gL even in the absence of nectin1. Parenthetically, we observe that the amount of gD pulled down by αvSTREPβ6-integrin alone (Fig. 3A, lane 5) was lower than that pulled down by nectin1 (Fig. 3A, lane 1) and likely reflects the amount of gD in complex with gH/gL.
When gD was omitted (Fig. 3A, lanes HLB, 2, 4, and 6) or gB was omitted (Fig. 3B, lanes DHL and 7–9), αvSTREPβ6-integrin expressed in the target cells alone (Fig. 3 A and B, lane αβ6) or in combination with nectin1 (N+αβ6) pulled down gL, gH, and gB (Fig. 3A, lanes 4 and 6) or gL, gH, and gD (Fig. 3B, lanes 8 and 9). In all cells, analysis of total cell extracts ruled out that the absence of gL from the complex harvested by means of αvβ6-integrin reflected a lower expression of gL (Fig. 3 C and D). We conclude that nectin1, gD, and gB were all required in order for αvβ6-integrin to assemble a complex in which gL was dissociated from gH/gL.
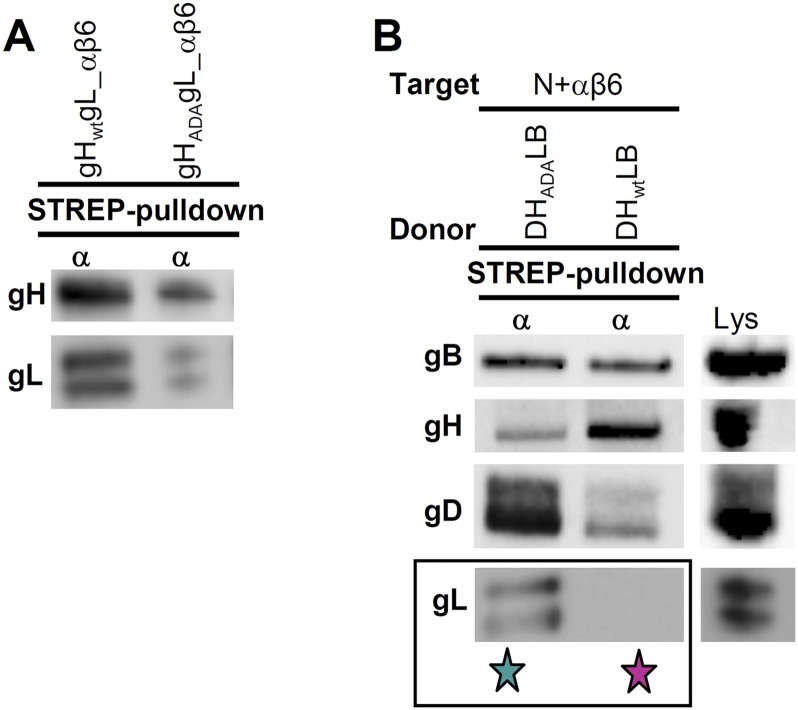
gH carries an RGD176–178 motif located in domain II at the boundaries between domains I and II. In an earlier work, we mutated this motif to ADA and found that virions carrying gHADA exhibited impaired, but not null, entry activity mediated by αvβ6-integrin. This implied that the RGD motif is important for HSV entry, even though the contact site between integrin and gH extends beyond it (20). We extended this conclusion in pulldown experiments (Fig. S2A) and verified that gHADA was impaired in the αvβ6-integrin–mediated dissociation of gL (Fig. S2B). Thus, a mutation in gH (RGD176–178 to ADA), which hampers virus entry, prevents αvβ6-integrin–mediated dissociation of gL.
Fig. S2.
Role of gH RGD motif in integrin-mediated dissociation of gL from gH/gL. (A) J cells were simultaneously cotransfected with WT-gH or gHADA, gL, plus αvSTREP-integrin (α), and β6-integrin (β6). Twenty-four hours after transfection, the complexes assembled on αvSTREP-integrin were harvested. It can be seen that the complex pulled down by αvSTREPβ6-integrin and containing gHADA carried less gH and gL than the complex containing WT-gH. (B) The substitution of WT-gH with gHADA prevents gL dissociation mediated by αvβ6-integrin. Donor cells were transfected with gD, gH, gL, and gB (DHLB); gH was WT or gHADA. Target cells were transfected with αvSTREP-integrin (α), β6-integrin (β6), and nectin1 (N) (N+αβ6). Following coculture for 24 h, the complex assembled onto αvSTREPβ6-integrin was harvested (Strep-pulldown). The substitution of WT-gH with gHADA prevented the dissociation of gL (green star). The absence of gL with WT-gH is highlighted with a fuchsia star. Lane Lys shows the immunoreactivity of transfected cell lysate.
Interaction of Virions with Cells Expressing αvβ6-Integrin Plus Nectin1, but Not Nectin1 Alone, Induced gL Dissociation.
The aim of subsequent experiments was to verify whether gL dissociation from gH/gL occurs when virions absorb to and enter cells.
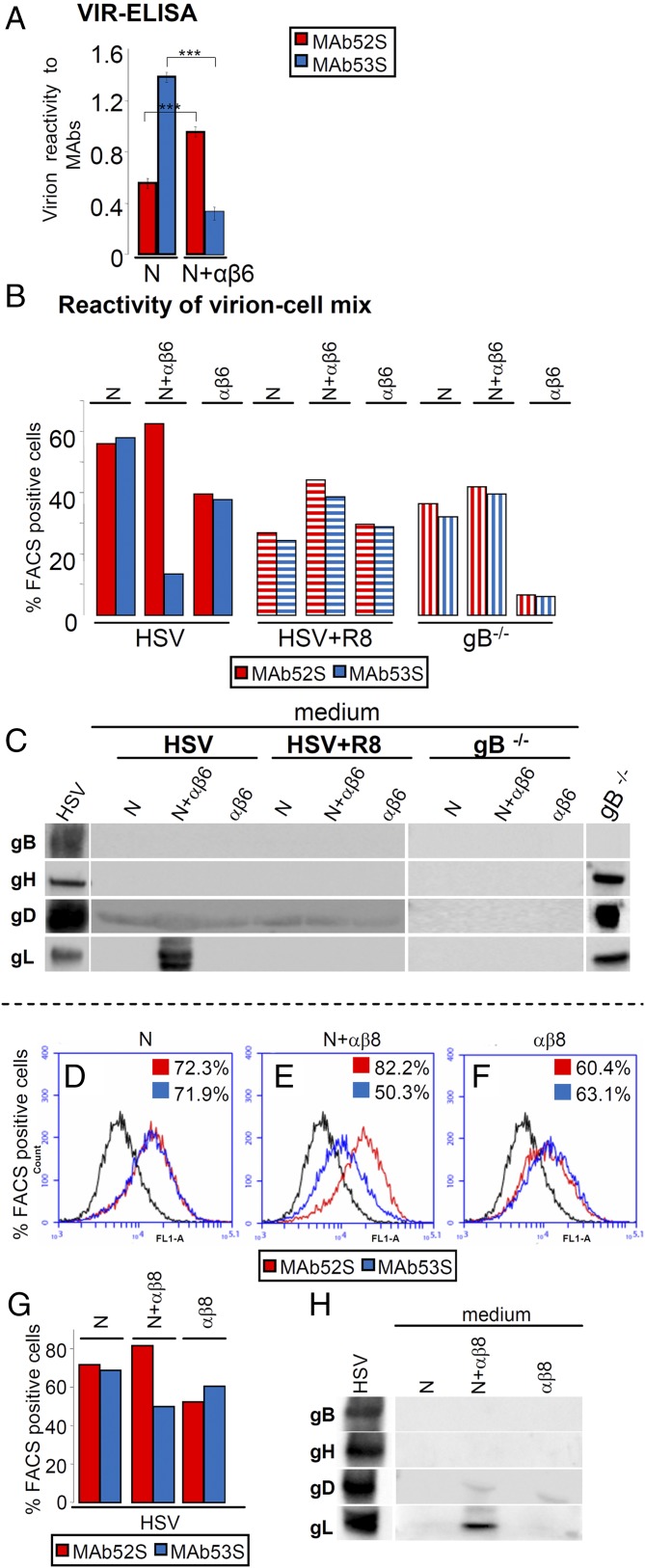
In the first two series of experiments, we took advantage of the different reactivity of two anti-gH MAbs. MAb 52S recognizes a gL-independent epitope. MAb 53S recognizes an epitope in gH, which is formed only when gH heterodimerizes with gL (gL-dependent gH epitope) (31). We reasoned that, should the interaction of virion gH/gL with αvβ6-integrin result in gL dissociation, the reactivity to MAb 53S should decrease relative to that to MAb 52S and relative to that seen when virions interact with cells expressing nectin1 alone. We probed this idea first in a VIR-ELISA test (Fig. 4A). Virions immobilized onto a 96-well plate were incubated with a suspension of J cells expressing αvβ6-integrin plus nectin1 (N+αβ6) or nectin1 alone (N). After cell removal, the virions were reacted with MAbs 52S or 53S. The 53S reactivity was much lower (76% reduction) in virions incubated with cells expressing αvβ6-integrin plus nectin1 than in virions incubated with cells expressing nectin1 alone (Fig. 4A). This decrease did not reflect loss of virions from the plate or their degradation because the 52S reactivity of virions exposed to cells expressing αvβ6-integrin plus nectin1 was even higher than that of virions exposed to cells expressing nectin1 alone (Fig. 4A). The results demonstrate that the exposure of virions to cells expressing αvβ6-integrin plus nectin1 caused a dramatic decrease in the gL-dependent gH epitope (53S) and imply that αvβ6-integrin promotes the dissociation of gL at virus absorption/entry.
Fig. 4.
Dissociation of gL from virion gH/gL and release of gL in the culture medium upon HSV infection of J cells expressing αvβ6- or αvβ8-integrin plus nectin1. (A) VIR-ELISA. Virions (1 × 108 PFU per well) were immobilized onto 96 wells and then reacted with a suspension of J cells expressing αvβ6-integrin plus nectin1 (N+αβ6) or nectin1 alone (N). After cell removal, virion immunoreactivity to MAbs 52S (red) or 53S (blue) was measured. (B–H) Immunoreactivity of cells carrying absorbed virions to MAbs 52S (red) and 53S (blue) and release of gL in medium. Virions were absorbed for 30 min at 37 °C to J cells expressing αvβ6-integrin plus nectin1 (N+αβ6), nectin1 alone (N), or αvβ6-integrin alone (αβ6) (B and C) or to J cells expressing αvβ8-integrin plus nectin1 (N+αβ8), nectin1 alone (N), or αvβ8-integrin alone (αβ8) (D–H). Virions were WT–HSV-1(F) (HSV), HSV pretreated with R8 PAb to gD (HSV+R8), or gB−/−. Cells carrying absorbed virions were then reacted with MAbs 52S (red) or 53S (blue) and analyzed by flow cytometry (see graphs in Fig. S3 and D–F). The percentage of FACS (fluorescent activated cell sorter) positive cells to each MAb was determined (B and D–G). (C and H) Release of gL in medium. WB reactivity of the media harvested after the 30-min virus absorption to cells (same samples as in B or in D–F) for PAb gH/gL, MAb gB, and MAb gD. The lanes to the Left marked as HSV show the electrophoretic mobility of HSV glycoproteins. In C, the lane to the Right marked as gB−/− shows the electrophoretic mobility of the gB−/− virion glycoproteins. It can be seen that gL was released in the medium when WT-HSV absorbed to J cells expressing αvβ6-integrin or αvβ8-integrin plus nectin1. No other glycoprotein was released in the medium. Virion preincubation with anti-gD PAb R8 or gB−/− virions resulted in no release of gL. In A, ***P < 0.001.
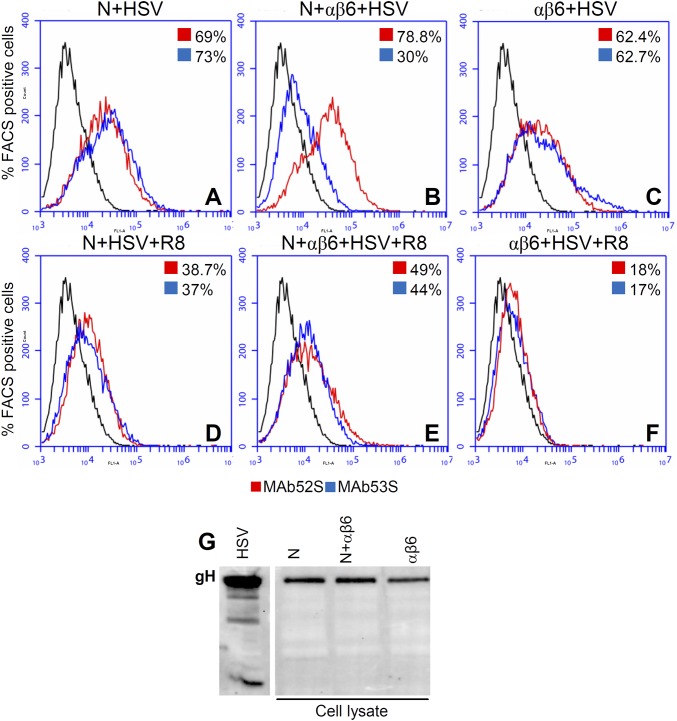
In the second set of experiments, we compared the 52S and 53S reactivity of virions absorbed to cells by flow cytometry (Fig. 4B). Virions were allowed to absorb to cells expressing αvβ6-integrin plus (N+αβ6) or minus nectin1 (αβ6) or nectin1 alone (N). The amount of absorbed virions was quantified as 52S reactivity; the dissociation of gL was measured as a decrease in 53S reactivity. The results shown in Fig. S3 and summarized in Fig. 4B show that the 52S reactivity (binding of virions to cells) was similar when cells expressed αvβ6-integrin plus nectin1 (62.5%) and nectin1 alone (56%) and somewhat lower when cells expressed αvβ6-integrin alone (40%). By contrast, the 53S reactivity was high when cells expressed nectin1 (58%) and decreased sharply (13%) when cells expressed αvβ6-integrin plus nectin1. Thus, even by flow cytometry we demonstrate that virions lost the gL-dependent gH epitope following absorption to cells expressing αvβ6-integrin plus nectin1. Of note, the change in 53S reactivity documents a conformational change to gH.
Fig. S3.
Dissociation of gL from virion gH/gL upon HSV infection of J cells expressing αvβ6-integrin plus nectin1, αvβ6-integrin alone, or nectin1 alone, detected by flow cytometry. (A–F) Virions were absorbed to J cells expressing αvβ6-integrin plus nectin1 (N+αβ6), nectin1 alone (N), or αvβ6-integrin alone (αβ6) for 30 min at 37 °C. Virions were WT–HSV-1(F) (HSV), HSV pretreated with R8 PAb to gD (HSV+R8). Immunoreactivity of cells carrying absorbed virions to MAbs 52S (red) and 53S (blue) was expressed as the percentage of FACS (fluorescent activated cell sorter) positive cells. (G) Virion gH does not undergo degradation upon virus absorption to J cells expressing αvβ6-integrin plus nectin1. Aliquots of the virion–cell mixtures described in Fig. 4B were subjected to PAGE and WB with MAb H12 to gH.
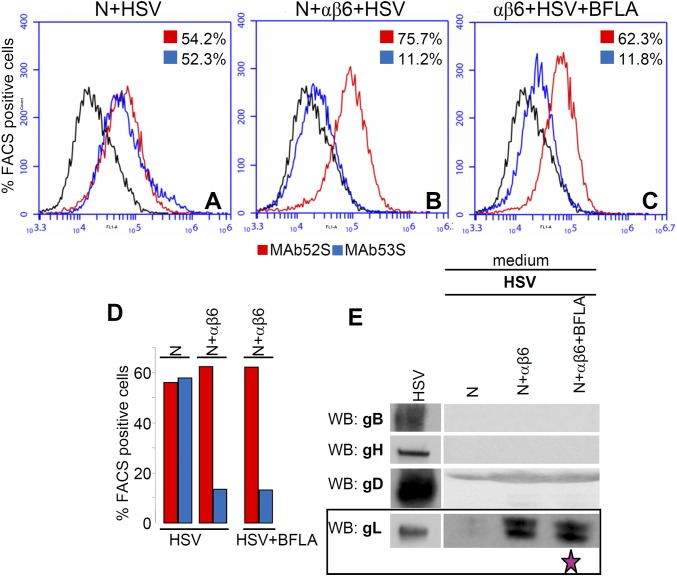
Next, we analyzed the requirements for gL dissociation. This enabled us to ask whether the change in gH conformation was prevented under conditions that block virus entry. The effect of inhibiting the gD interaction with nectin1 was assessed by preincubation of virions with a neutralizing serum to gD [polyclonal antibody (PAb R8)] (HSV+R8). The effect of the absence of gB was assessed by means of gB−/− virions (gB−/−). In both cases (Fig. 4B), the 53S reactivity did not decrease relative to the 52S reactivity, confirming that the αvβ6-integrin–mediated dissociation of gL occurred only when gD, gB, and nectin1 were also present. Finally, inhibition of virus endocytosis by BFLA left the 53S reactivity as low as that seen in the absence of the inhibitor (∼13%) (Fig. S4 A–D), indicating that the release of gL took place irrespective of whether virions were or not endocytosed, possibly before endocytosis.
Fig. S4.
Inhibition of endocytosis by BFLA does not alter the gL dissociation. (A–C) WT–HSV-1 virions were absorbed to J cells expressing αvβ6-integrin plus nectin1 (N+αβ6) or nectin1 alone (N) for 30 min at 37 °C. Cells were either untreated or exposed to BFLA (30 nM) from 1 h before infection until harvesting. Immunoreactivity of cells carrying absorbed virions to MAbs 52S (red) and 53S (blue) was expressed as the percentage of FACS (fluorescent activated cell sorter) positive cells. (D) gL dissociation was measured in A–C and expressed as the relative decrease in 53S reactivity. (E) Release of gL in the medium of virion–cell mixture. Inhibition of entry by BFLA left unaltered the release of gL in the medium (fuchsia star).
We looked for direct evidence of gL release by searching gL in the medium of the virion–cell mixture (Fig. 4C). Media were withdrawn at the end of virus absorption to cells (same experiment as in Fig. 4B) and analyzed by WB. It can be seen that gL was present in the medium when virions absorbed to cells positive for αvβ6-integrin and nectin1 (Fig. 4C). In all other conditions, except inhibition by BFLA, gL was absent (Fig. S4E). Together, gL was clearly and specifically released from virions absorbed to cells expressing αvβ6-integrin plus nectin1, but not nectin1 alone or αvβ6-integrin alone. gL was not released from virions preincubated with the anti-gD serum or from gB−/− virions (Fig. 4C).
gL was the only glycoprotein detected in the media. Under no condition did the media exhibit gH, gB, or gD reactivity (Fig. 4C). These results rule out shedding of virion glycoproteins at virus entry.
We ruled out that the presence of gL in the medium was the result of degradation of the gH N terminus or of generalized gH degradation (Fig. S3G). Aliquots of the virion–cell mixtures shown in Fig. 4B were analyzed by WB. The electrophoretic mobility and the overall quantity of gH were not modified when virions were absorbed to cells expressing nectin1 alone or αvβ6-integrin plus nectin1 (Fig. S3G). The small decrease in amount seen with virions absorbed to cells expressing only αvβ6-integrin likely reflects the lower efficiency of virus absorption in the absence of nectin1.
Together, these results indicate that (i) gL was dissociated from gH at virus absorption/entry; (ii) the dissociation was promoted by αvβ6-integrin and (iii) required all of the components of the entry apparatus; and (iv) gH underwent a conformational change at interaction with integrin. The change was prevented when virus entry was blocked (e.g., by antibodies to gD or absence of gB); most likely it reflects an active conformation of gH. The results argue for a role of gL dissociation in the activation of the entry machinery, in particular of gH, and raise the possibility that gL may exert an inhibitory activity on gH before virion encounter with integrin-positive cells.
αvβ8-Integrin Promotes the Dissociation of Virion gL.
We asked whether also αvβ8-integrin promoted the dissociation of virion gL. Virions were absorbed to cells expressing αvβ8-integrin plus (N+αβ8) or minus nectin1 (αβ8) or nectin1 alone (N) for 30 min. The reactivity to MAb 53S was significantly lower in the αvβ8-integrin plus nectin1 than in the nectin1-alone or αvβ8-integrin–alone cells (Fig. 4 D–F). A quantification is reported in Fig. 4G. We note that the extent of transgenic expression of αvβ8-integrin in J cells was usually lower than that of αvβ6-integrin. In this experiment the αvβ8-integrin–positive cells were 33%, whereas the αvβ6-integrin–positive cells were usually about 60%. This explains why the decrease in 53S reactivity was lower with αvβ8-integrin than with αvβ6-integrin (compare Fig. 4 B and G). Concomitantly with the decrease in 53S reactivity, gL was released in the medium of cells positive for αvβ8-integrin and nectin1 (Fig. 4H).
Dissociation of Virion gL Detected by Fluorescence Microscopy.
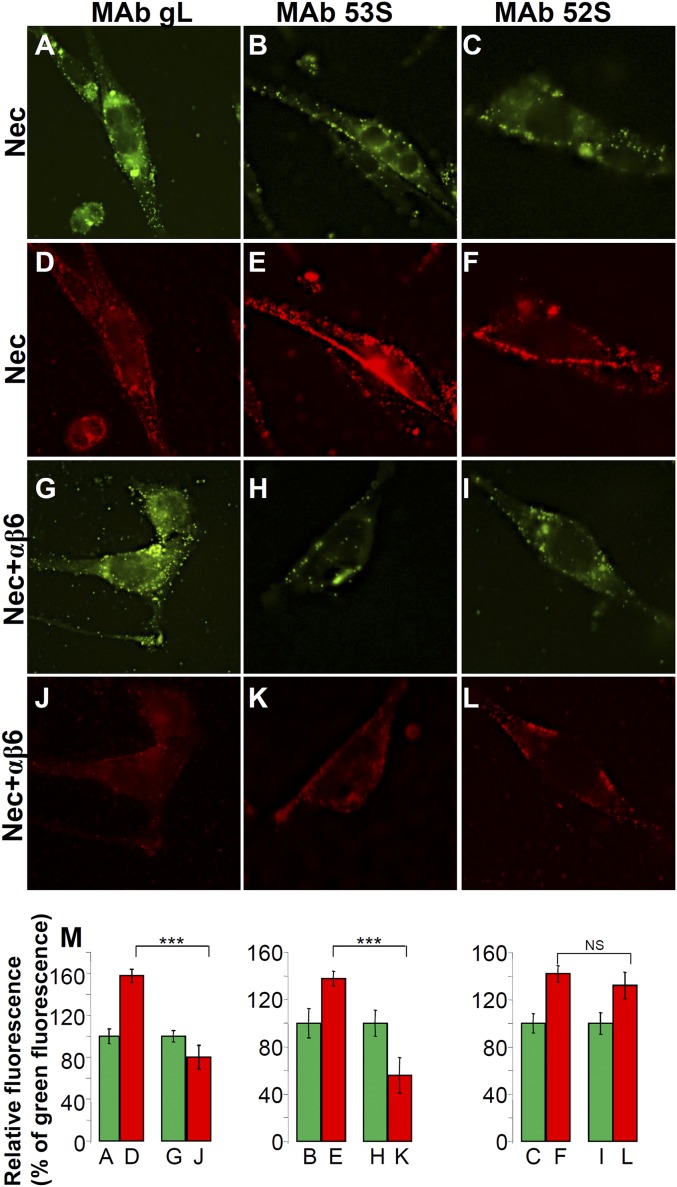
The dissociation of gL from virions was further documented by fluorescence microscopy (Fig. 5). J cells expressing αvβ6-integrin and nectin1 were infected with K26GFP virions, which carry the capsid protein ICP26 fused to GFP (32). After 30 min absorption, cells were fixed and reacted to MAb to gL, MAbs 53S and 52S to gH. The gL reactivity (Fig. 5 D and J) and the 53S reactivity (Fig. 5 E and K) were decreased when virions were absorbed to cells expressing αvβ6-integrin plus nectin1 relative to cells expressing nectin1 alone (compare Fig. 5 J vs. D and Fig. 5 K vs. E). The 52S reactivity (gL-independent epitope of gH) was essentially similar in the two types of cells (compare Fig. 5 L and F). A quantification of this experiment was carried out as follows. We measured the GFP (green) and MAb (red) fluorescence in 30–40 cells, calculated the mean fluorescence intensities, and plotted the red fluorescence as the percentage of green fluorescence (Fig. 5M). In cells expressing αvβ6-integrin and nectin1, the 53S and gL mean fluorescence intensities were about 50% of those obtained in cells expressing nectin1 alone (Fig. 5 J vs. D and Fig. 5 K vs. E). These data provide morphological evidence for gL dissociation when virions absorb to cells expressing αvβ6-integrin and nectin1.
Fig. 5.
Dissociation of gL from virions detected by fluorescence microscopy. K26-GFP virions carrying the ICP26 protein fused to GFP (32) were absorbed to J cells expressing αvβ6-integrin plus nectin1 (Nec+αβ6) or nectin1 alone (Nec). Cells carrying absorbed virions were fixed, reacted with MAbs to gL, 53S, and 52S, followed by DyLight 549-conjugated II antibody, and analyzed by fluorescence microscopy. The green dots in A–C and G–I represent the GFP-tagged virions. D–F and J–L show the immunofluorescence reactivity (red) to indicated MAbs. For each column, the histograms report the quantification of the red-to-green fluorescence, determined as follows. Membrane areas were circumscribed in 30–40 cells for each sample. The pixels present in the green and in the red channels were counted and expressed as mean fluorescence intensities. (M) For each couple of samples (A and D, B and E, C and F, G and J, H and K, and I and L), the green fluorescence intensity was made equal to 100 and the red fluorescence was expressed as a percentage of the green fluorescence. Histograms represent the relative percentages ± SD. ***P < 0.001. NS. Not significant.
gL Dissociation from Virion gH/gL Occurs in Human Epithelial Cells.
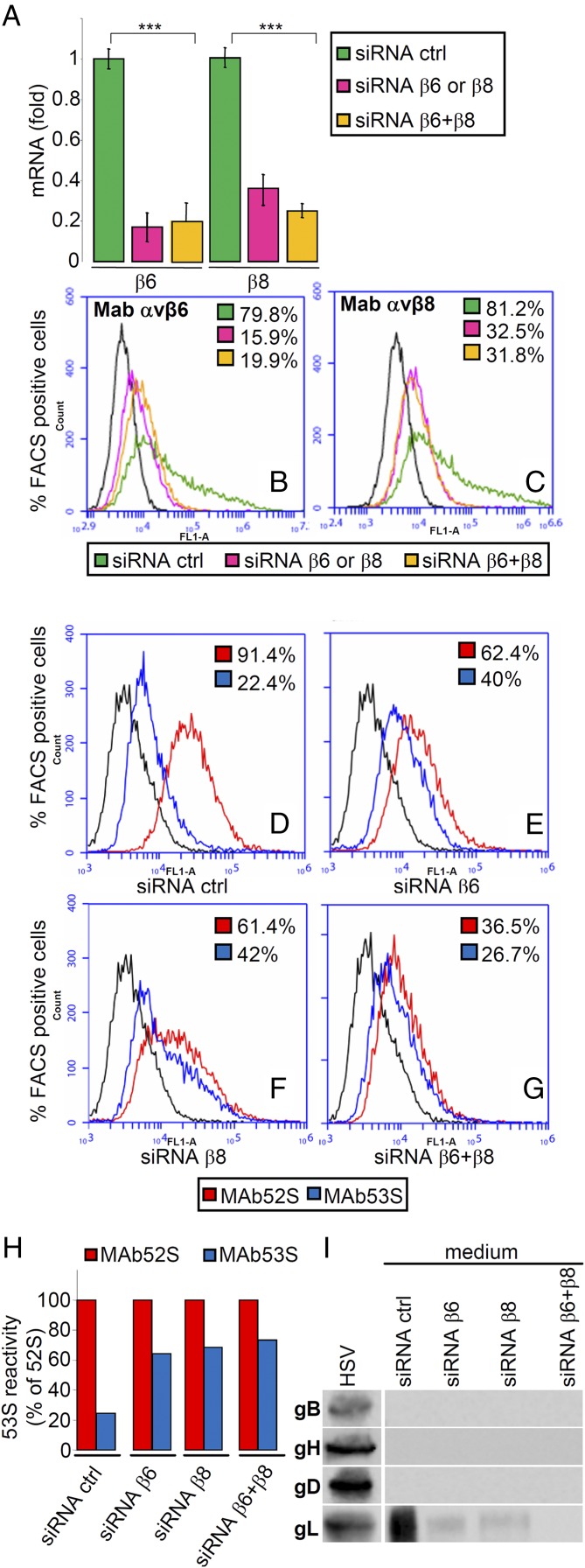
To verify whether the gL dissociation occurs during HSV attachment-entry in human cells, we chose the SW480 epithelial cell line, characterized by high expression of both integrins (20). We expected that RNAi-mediated depletion of integrins would result in decreased gL dissociation (measured as a relative increase in 53S reactivity) and, consequently, a reduction of gL present in the medium. Control cells received siRNA ctrl. The extent of integrin silencing ranged from 60 to 80%, as determined by qRT-PCR (Fig. 6A) and flow cytometry (Fig. 6 B and C). Fig. 6 D–G shows that after HSV absorption, the 52S reactivity of the virion–cell mixture, a measure of total virus binding, was 91% in control cells, 61% in β6- or β8-silenced cells, and 36% in cells silenced for both integrins. This decrease in 52S reactivity in integrin-depleted cells was expected, as β6- and β8-integrins contribute to HSV attachment-entry into cells, as shown in Fig. 1A and reported previously (20). In contrast, the 53S reactivity was 22% in control cells, ∼40% in β6- or β8-silenced cells, and 26% in double-silenced cells (Fig. 6 D–G). When expressed as a percentage of 52S reactivity (Fig. 6H), silencing resulted in a relative increase in 53S reactivity from 20 to 73%, strongly suggesting a decrease in gL dissociation. As expected, gL was present in the medium of control cells; it was highly reduced in the medium of β6- or β8-integrin depleted cells and undetectable in the medium of cells depleted of both integrins (Fig. 6I). These results show that the dissociation of gL from virion gH/gL occurs in human cells and that the depletion of αvβ6- or αvβ8-integrin results in inhibition of virus entry and inhibition of gL release and support the view that the dissociation of gL dependent on αvβ6- or αvβ8-integrin is critical for HSV entry.
Fig. 6.
Dissociation of gL from virion gH/gL at infection of human epithelial SW480 cell line. β6- or β8-integrins were silenced singly (siRNA β6 or siRNA β8), doubly (siRNA β6+β8), or mock-silenced (siRNA ctrl) in SW480 cells. HSV-1 was absorbed to the integrin-depleted cells for 90 min; virus absorption and dissociation of gL were quantified as flow cytometry reactivity to MAbs 52S (red) and 53S (blue), respectively. (A–C) Extent of silencing was measured in uninfected cells by qRT-PCR (A) and expressed as fold decrease relative to siRNA ctrl cells or by flow cytometry (B and C). In A, each column represents the average of triplicates from two independent experiments ± SD. ***P < 0.001. (D–G) Flow cytometry reactivity of cells carrying absorbed virions to MAbs 53S (blue) and 52S (red) was expressed as the percentage of positive cells (figures within each panel). (H) The 53S (blue) reactivity shown in D–G was expressed as a percentage of the 52S (red) reactivity. (I) At the end of virus absorption, the media from the SW480 cells shown in D–G were analyzed for the presence of released gL, as detailed in Fig. 4C. It can be seen that gL was released from virions absorbed to siRNA ctrl-treated SW480 cells, was released in a small amount from virions absorbed to singly silenced SW480 cells, and was undetectable in media of SW480 cells simultaneously silenced for both integrins. Lane HSV shows the WB immunoreactivity of the indicated virion glycoproteins.
Rescue of Infection with gH−/− Virions by Soluble gHt/gL and Release of gL Dependent on αvβ6- or αvβ8-Integrin.
The above results show that gL dissociation from virion gH/gL occurred under conditions that lead to virus entry and activation of the entry glycoproteins. They also document a conformational change in gH that likely reflects an active state. To gain further evidence that gL dissociation is part of gH activation critical for HSV entry, we tested whether a soluble form of gH/gL (gHt/gL) (33) rescued the infection of gH−/− virions and, concomitantly, whether gL was released in the medium. The rationale for this experiment was provided in earlier studies, as follows. Soluble gD (gDt) rescued the infection of gD−/− virions, providing indirect evidence that gD undergoes conformational changes upon interaction with receptor-positive cells (16). gHt/gL can promote cell–cell fusion in cells expressing gD (or exposed to gDt), gB, plus gD receptor (19). gHt/gL rescues the infection of gH−/− EBV virions (34).
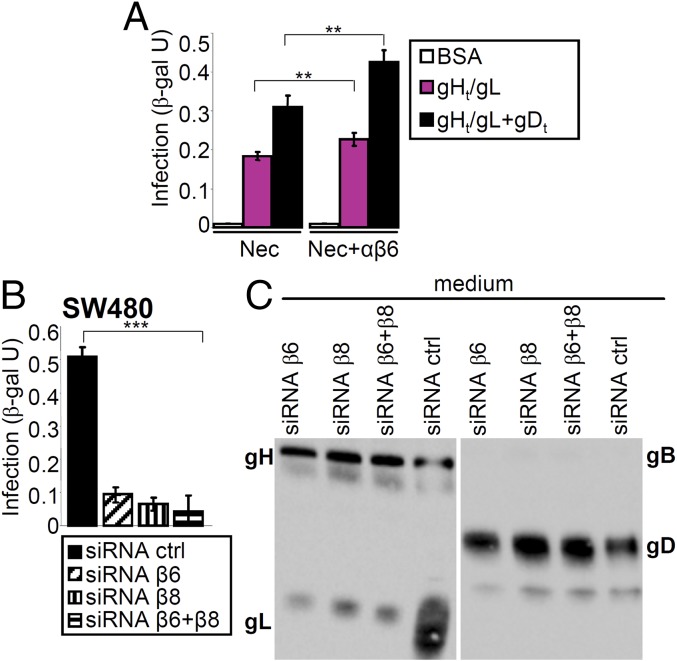
The noninfectious gH−/− virions are deleted in the gH gene and encode a β-gal reporter (35). In the first set of experiments, J-nectin1 or J-nectin1 plus αvβ6-integrin cells were exposed to gH−/− virions in the absence or presence of gHt/gL (33) or a mixture of gHt/gL plus gDt. Fig. 7A shows that gHt/gL, and to a higher extent the combination of gHt/gL plus gDt, rescued gH−/− virion infection. Although J cells express endogenous integrins, the rescue was higher in cells overexpressing human αvβ6-integrin.
Fig. 7.
Rescue of infection of J or SW480 cells with gH−/− virions by gHt/gL, plus or minus gDt, and release of gL in medium. (A) J cells expressing αvβ6-integrin plus nectin1 (Nec+αvβ6), or nectin1 alone (Nec) were exposed to gH−/− virions in the presence of gHt/gL (pink), gHt /gL plus gDt (black), or BSA (white). Infection was quantified as β-gal arbitrary units (β-gal U) from Lac-Z inserted in the viral genome. Results represent the mean of triplicate samples ± SD. **P < 0.01. (B and C) β6-integrin, β8-integrin, or β6 + β8-integrins were silenced in SW480 cells. Control cells received siRNA ctrl. (B) Cells were exposed to gH−/− virions in the presence of gHt/gL plus gDt. Infection was quantified as β-gal arbitrary units (β-gal U). Results represent the mean of triplicate samples ± SD. ***P < 0.001. (C) The media of the virion–cell mixtures described in B were harvested and analyzed by WB. The media contained the added soluble glycoproteins, gHt/gL and gDt. In siRNA-ctrl cells the medium contained a much higher quantity of gL, representing the displaced gL.
To better evaluate the integrin contribution to gHt/gL-mediated rescue of gH−/− virions, we silenced β6- and β8-integrin, singly or in combination, in SW480 cells (Fig. 7 B and C). Control cells received control siRNA (siRNA ctrl). Cells were exposed to gH−/− virions together with gHt/gL plus gDt. Fig. 7B shows that gHt/gL plus gDt rescued gH−/− virion infection in cells expressing β6- and β8-integrin (siRNA ctrl), but not in cells depleted of one or both integrins. Analysis of the culture media revealed that the medium of control cells (siRNA ctrl) contained gHt/gL and gDt, as expected, and a much higher amount of gL than the media of cells depleted of one or both integrins (Fig. 7C). These results provide a first line of evidence that infection of gH−/− virions can be rescued by gHt/gL and that the rescue occurs in an αvβ6-integrin–dependent fashion and results in the dissociation of gL and its release in the medium.
Neutralizing MAb LP11 Prevents the Integrin-Dependent Dissociation of gL.
We asked whether a block to gL dissociation from gH/gL prevents HSV infection. We reasoned that if gL dissociation is part of the process of gH activation and, ultimately, of HSV entry, some of the neutralizing MAbs to gH might inhibit HSV entry by preventing gL release. We tested two neutralizing MAbs to gH, 52S and LP11, which map to opposite faces of gH/gL (10, 31, 36). Virions were preincubated with MAbs 52S, LP11, the nonneutralizing 53S, or control IgGs and then absorbed to J cells expressing αvβ6-integrin plus nectin1 or nectin1 alone. After 30 min absorption, the media were analyzed by WB. Fig. 8 shows that gL was present in the medium of cells expressing αvβ6-integrin and nectin1, if virions were preincubated with MAbs 52S, 53S, or control IgGs. gL was absent if virions were preincubated with MAb LP11. As expected, there was no release of gL when virions were absorbed to cells expressing nectin1 alone. Thus, the potent neutralizing MAb LP11 prevents gL dissociation from virion gH/gL. These experiments demonstrate that gL dissociation and HSV entry are interdependent phenomena, suggest a mechanistic role for gL dissociation in virus entry, and raise the possibility that gL may exert an inhibitory activity on the entry glycoproteins, most likely on gH, before virion encounter with integrin-positive cells.
Fig. 8.
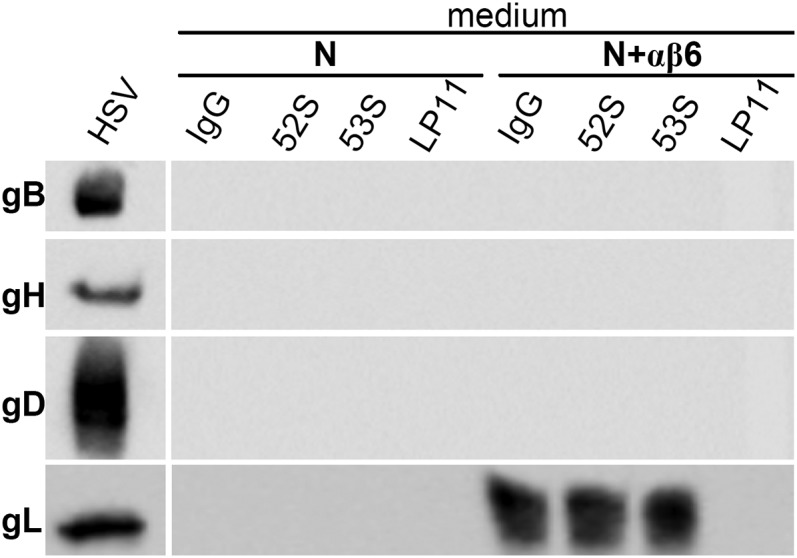
Block of HSV-1 infection by the neutralizing MAb LP11 to gH prevents the dissociation of gL from virion gH/gL. HSV-1(F) virions were preincubated with MAbs 52S, 53S, LP11, or IgGs. J cells expressing αvβ6-integrin plus nectin1 (N+αβ6), or nectin1 alone (N) were exposed to the preincubated virions. After 30 min, the media were harvested and subjected to SDS/PAGE and WB for gL. The medium of cells exposed to LP11-treated virions lacks gL. Lane HSV to the Left shows the WB reactivity of the indicated virion glycoproteins.
Discussion
We report that the interaction of HSV gH/gL with its receptor αvβ6- or αvβ8-integrin results in the dissociation of gL from the gH/gL heterodimer and the concomitant release of gL in medium of the virion–cell mixture. The gL dissociation occurs during virus absorption-entry into the cell, and requires the presence of gD, its receptor nectin1, and gB, i.e., conditions that lead to activation of the entry glycoproteins. The gL dissociation is prevented when virus entry is blocked with a neutralizing MAb to gH, and documents a conformational modification to gH, likely reflecting an active state of the glycoprotein. We propose that gL is a functional inhibitor of gH. This finding raises some key questions: how and why is gL dissociated from gH/gL; why did HSV and the entire Herpesviridae family evolve to have a hetererodimer as a conserved constituent of the entry machinery; and what is the impact on the model of HSV entry.
First, the experimental evidence in support of gL dissociation is severalfold. Thus, the dissociation of gL from gH/gL mediated by αvβ6- (or αvβ8)-integrin was consistently seen in three systems, i.e., in cells cotransfected with the four entry glycoproteins (gD, gH, gL, and gB) plus αvβ6-integrin (or αvβ8-integrin) and nectin1; in a coculture system where the donor cells expressed the glycoprotein quartet (and thus mimicked virions) and the target cells expressed the receptors αvβ6-integrin plus nectin1; and in virions at infection of cells positive for αvβ6-integrin and nectin1. In all three systems, the conditions for the gL dissociation were that gD, nectin1, and gB were also present. This is relevant because these conditions lead to gD activation by its receptor nectin1. In the absence of gD, nectin1, or gB, αvβ6-integrin maintained the ability to interact with gH/gL by a number of assays, including surface plasmon resonance (20). However, gL dissociation did not take place. The gL dissociation from gH/gL was observed in a human epithelial cell line and in the model receptor-negative J cell transgenically expressing αvβ6-integrin plus nectin1, and was not associated to acidic endosome entry pathway.
How Does Integrin Promote the Dissociation of gL from gH/gL?
Functional and structural data rule out that the binding site of gL in gH is one and the same as the binding site of integrin in gH, and consequently that gL and integrin compete for the same binding site to gH. Foremost, the binding of αvβ6- or αvβ8-integrin to gH/gL in the absence of nectin1, gD, or gB did not result in gL dissociation. Second, the crystal structures of HSV-2 gH/gL, of a fragment of pseudorabies virus gH (lacking domain I and gL), and of EBV gH/gL show that the integrin-binding RGD motif in gH, located at the boundaries between domain II and domain I, does not overlap with the binding site of gL in gH, located entirely within domain I (7–9). In agreement with the structural studies, current and previous studies with a mutant form of gH in RGD indicate that this motif is critical for the αvβ6-integrin–mediated virus entry and gL dissociation (20). Together, the data favor the view that gL dissociation results from conformational changes induced by integrins to gH/gL.
Why Is gL Released from gH/gL?
Several lines of evidence indicate that gL dissociation from gH/gL is part of the process of HSV entry. (i) Foremost, the infection-blocking MAb LP11 to gH prevented gL dissociation. The effect was specific because a nonneutralizing MAb and a neutralizing MAb directed to a different region of gH did not prevent gL dissociation. (ii) The gL dissociation occurred only when all of the actors of the entry process were simultaneously present and was prevented by a block in virus entry (e.g., by antibodies to gD or absence of gB) or by a mutation in gH (RGD to ADA), which hampers αvβ6-integrin–dependent entry. (iii) gH underwent a conformational change at virus absorption/entry that likely reflects an active state of the molecule. (iv) The infectivity of gH−/− virions was rescued by soluble gHt/gL (better so by the combination of soluble gHt/gL and gDt), with concomitant release of gL. Our interpretation of these findings is that gL dissociation enables virus entry and, consequently, that in resting virions, gL prevents the activation of the entry machinery, most likely of gH. We note that, although the preponderance of the results argue that release of gL promotes viral entry, causality has not been proven, and we cannot rule out that gL release follows entry. Further studies are needed to test the proposed model. The process of activation of a molecule upon conformational changes to the molecule and release of an inhibitor is well established. Just as an example, the activation of cytoplasmic NF-κB subunits (relA-relB) occurs through the release of the IκBα inhibitor (37). To our knowledge, such a mechanism has not been observed in the case of activation of the glycoproteins that form the entry machinery of viruses.
Why Did HSV Evolve to Have a Hetererodimer as a Conserved Constituent of the Entry Machinery?
gL enables gH to adopt a proper conformation at the time of biosynthesis in endoplasmic reticulum and enables gH transport along the exocytic pathway (38, 39). In essence, gH needs unknown molecular chaperones, of which gL may be part, for correct folding and transport. In contrast with the behavior of chaperon machines, inexplicably, gL remains in complex with gH after gH transport is completed. The proposed role of gL as an inhibitor of gH in virions before their encounter with receptor-positive cells adds a second function that would explain why gL remains associated to gH after its transport is completed.
It has been proposed that HSV is a control freak (40). gL may provide yet another example of this lifestyle. We speculate that given the multipartite nature of the entry machinery of herpesviruses and their stepwise activation by cellular receptors, the presence of an Activator (gH), kept in an inactive form by an Inhibitor (gL), may ensure a tighter control on the process of activation of the glycoproteins. A premature activation might take place, for example, in the exocytic pathway of the cells during progeny virus maturation and assembly, and lead to the premature exhaustion of the fusion machinery. Different viruses deal with this problem with different strategies. The closest example is probably that of alpha and flaviviruses; cognate proteins enable the transport of the E1 (or E) fusion proteins; however, the cognate proteins are cleaved in the trans-Golgi network and, in contrast to gL, do not remain in heterodimeric association with their partners (41, 42).
Refinement of the Model of HSV Entry.
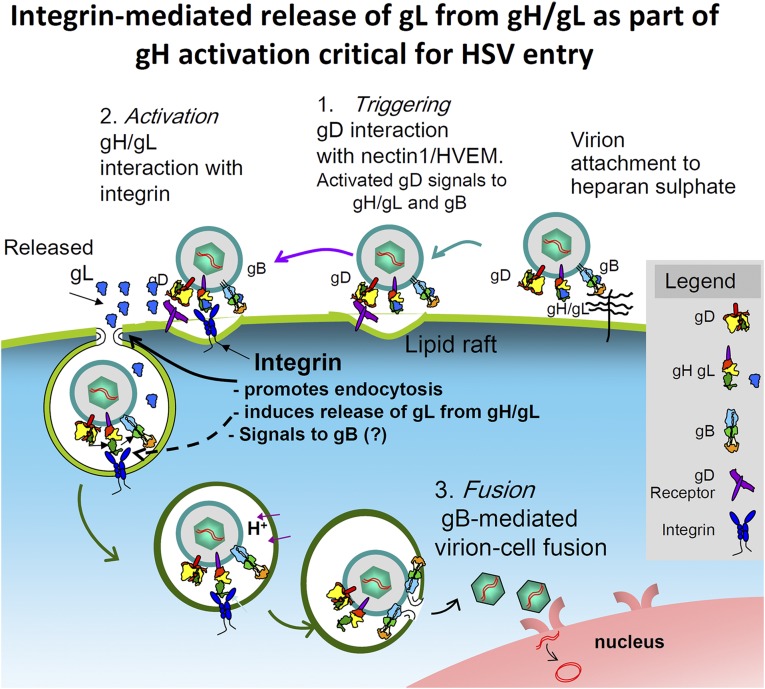
The current results and previous finding that αvβ6- and αvβ8-integrins serve as gH/gL receptors provide a better understanding of the process of HSV entry and more clearly define three steps, as follows (Fig. 9). (i) Triggering. This step occurs upon the interaction of virion gD with one of its alternative receptors, nectin1 or HVEM. (ii). Activation. The actor is gH (Activator). gH/gL receive two series of signals, one from receptor-bound gD and one from its integrin receptor. The end result is the dissociation of gL (Inhibitor) from gH/gL heterodimer. Activated gH then signals to gB. In a reciprocal process, the gH/gL-activated integrins promote endocytosis by way of the signaling C-tail of the β subunit. (iii) Fusion execution. The actor is gB. gB receives a signal from activated gH and, perhaps and possibly, from its own receptors.
Fig. 9.
Redefining the central role of the gH/gL heterodimer in the process of HSV entry. The “Triggering” event in the process is the binding of gD to one of its receptors. αvβ6 (or αvβ8)-integrin, together with receptor-bound gD, promote the dissociation of gL from gH/gH. This ensues in gH activation. In turn, the gH/gL-activated integrins promote virus endocytosis by way of the signaling C-tail of the β subunit. Activated gH signals to gB. gB executes the virion–cell fusion.
This schematic view provides a frame to interpret divergence in the entry mechanisms across the Herpesviridae family. Basically, steps 1 and 2 have undergone considerable species-specific diversity, finalized to diversify and expand the tropism of the various species. In some case, the “Activation” is carried out through the direct binding of integrins to gH/gL, without a prior triggering event. This is the case for EBV entry into epithelial cells and for equine herpesvirus entry. Alternatively, “Triggering” might be carried out by gH accessory proteins. This is the case for gp42, which binds MHC-II to enable EBV entry into lymphocytes. Members of the human cytomegalovirus pentamer or gH accessory proteins in human herpesvirus 6 may well fall into this category (43–46). In Kaposi’s sarcoma-associated herpesvirus, integrins directly activate gB (24).
Current findings raise the possibility that dissociation from an inhibitory regulator represents a previously unidentified mechanism of activation of viral fusion glycoproteins.
Materials and Methods
Detailed methods are available in SI Materials and Methods.
Viruses and Soluble Proteins.
The K26-GFP ΔgB-KΔT, ΔgH SCgHZ HSV mutants were previously described (32, 35, 47). Soluble gDΔ290t-299t (48) was a gift from G. H. Cohen and R. Eisenberg, University of Pennsylvania, Philadelphia. Soluble gHt/gL was previously described (33).
Strep-Tagged Forms of gD and Nectin1.
gD and nectin1 were tagged with the Strep epitope at the C terminus.
β6N1 Construct and Characterization.
The β6N1 chimera contains the ectodomain of β6-integrin fused to the transmembrane and C-tail of nectin1. Briefly, the ectodomain of nectin1 was removed from plasmid pCF18HNK (21), which carries the entire nectin1 ORF, and replaced with PCR-amplified β6-integrin.
Pulldown Experiments.
Pulldown experiments were carried out from lysates of transfected cells or of cocultured donor and target cells by means of Nectin1STREP, αvSTREP, and gDSTREP and Strep-Tactin Sepharose (IBA, Solutions for Life Sciences) (17).
Integrin Silencing and RT-PCR.
Integrins were silenced in SW480 cells by means of ON-TARGET plus, smart pools (Dharmacon), as previously described (49). Control cells were transfected with siRNA to E.coli-polA_0054 (20). Extent of silencing was determined by RT-PCR using TaqMan gene expression assay (Applied Biosystems) (20).
Flow Cytometry and Immunofluorescence Assay and Analysis of Media.
J cells transfected with the indicated receptors were exposed to HSV-1 or ΔgB-KΔT at 1 plaque forming unit (PFU) per cell and then fixed with 4% (wt/vol) paraformaldehyde and incubated with 53S or 52S MAbs and secondary antibody. At the end of virus absorption, the culture medium was concentrated, devoid of serum IgGs by Protein A-Sepharose, and subjected to SDS/PAGE.
Rescue of Infection with gH−/− Virions by Soluble Forms of gH.
gH−/− virions (5 μL corresponding to 30 PFU equivalents per cell) were mixed with soluble gHt/gL (33) or soluble gHt/gL plus soluble gDΔ290t-299t (48) (300 nM each) and added to the cells, grown in 24 wells. The extent of infection was expressed as β-gal arbitrary units.
VIRUS ELISA (VIR-ELISA).
HSV-1 virions (1 × 108 PFU per well) were immobilized onto 96 wells and then reacted with a suspension of J cells expressing αvβ6-integrin plus nectin1 or nectin1 alone for 1 h at 37 °C. Cells were removed. Virions were reacted with MAbs 52S or 53S, followed by peroxidase-conjugated secondary antibody. Immunoreactivity was measured by means of O-phenyilendiamine substrate and reading of the optical density at 490 nm.
Statistical Analyses.
When appropriate, data were expressed as the mean ± SD from at least three independent samples. For comparison of the mean of two groups, statistical significance was determined by Student’s t test. Statistical significance was defined as *P < 0.05, ** P < 0.01, ***P < 0.001.
SI Materials and Methods
Cells, Viruses, and Soluble Proteins.
J (a derivative of BHK-TK− cells lacking receptors for HSV gD) (29) cells were grown in DMEM containing 5% FBS. Colon carcinoma SW480 cells were grown in L15 medium. HSV-F, R8102, a HSV-1 recombinant carrying LacZ under the control of the α27 promoter, and K26-GFP, a HSV-1 recombinant expressing green fluorescent protein (GFP) in frame with VP26 were previously described (29, 32). The ΔgB-KΔT (47) and ΔgH SCgHZ (35) HSV mutants were grown and titrated in respective complementing cells. Soluble gDΔ290t-299t (48) was a generous gift from G. H. Cohen and R. Eisenberg, University of Pennsylvania, Philadelphia. Soluble gHt/gL carrying One- Strep-Tag epitope for affinity purification was previously described (33).
Antibodies.
R8 polyclonal antibody (PAb) to gD, H12 monoclonal antibody (MAb) to gH, and MAb CK6 to nectin1 were generously provided by G. H. Cohen and R. Eisenberg. MAb H170 reactive to gD and MAb H1817 reactive to gB were purchased from Goodwin Institute. PAb to gH and gL raised against the soluble form of gHt/gL was previously described (33). MAb VIII-62 reactive to gL was a generous gift from P. G. Spear, Department of Microbiology-Immunology, Feinberg School of Medicine, Northwestern University, Chicago. MAb 52S, 53S and LP11 were previously described (31, 36). MAb 52S reacts to a conformational dependent epitope in gH. MAb 53S and LP11 react to a conformational-dependent and gL-dependent epitope. MAb R1.302 to nectin1 was a gift from M. Lopez, INSERM, UMR1068/Centre de Recherche en Cancérologie de Marseille, Institut Paoli-Calmettes and University of Aix-Marseille, Marseille, France (29); MAb 2077Z to the αvβ6 integrin heterodimer and PAb 1930 to αv were from Chemicon. Strep-Tactin horseradish peroxidase conjugate antibody to Strep-Tag was provided by IBA, Solutions for Life Sciences.
Plasmids.
The mammalian expression plasmids encoding gH, gL, and gB in the MTS vector and gD in pcDNA3.1, all under the cytomegalovirus promoter, were previously described (50). Plasmids encoding nectin1, EGFR2NΔ (epithelial growth factor receptor carrying the deletion of the C-tail tyrosine motifs), named Erb-2, and gHADA carrying the indicated substitution in the RGD motif were previously described (29, 33, 51). Plasmids encoding αv-, β6-, and β8-integrin were provided by S. Blystone and S. L. Nishimura (52, 53). The plasmid encoding Strep-tagged αv-integrin, named αvSTREP, was previously described (30).
Strep-Tagged Forms of gD and Nectin1.
To enable retention to resin, gD and nectin1 were tagged with the Strep epitope. To generate Strep-Tag gD (gDSTREP), gDN plasmid (54) was digested with BglII-HindIII, and the N-terminal part of enhanced green fluorescent protein (EGFP) was removed from the construct. Two annealing oligonucleotides encoding the One-Strep-Tag 5′-GGA AGA TCT CTG GCT GGA GCC ACC CGC AGT TCG AGA AAG GTG GAG GTT CCG GAT CGG GAG GTG GAT CG- 3′ and 5′- CCC AAG CTT CCC GGA TCC TCA TTT TTC GAA CTG CGG GTG GCT CCA CGA TCC ACC TCC CGA TCC GGA ACC T- 3′ were ligated to BglII-HindIII–digested gD. To generate His-One-Strep-Tag nectin1 (nectin1STREP) we followed a similar strategy. Plasmid NectC (54) was digested with BglII-XhoI, and the C-terminal part of EGFP was substituted with the annealing oligonucleotides encoding the His-One-Strep-Tag epitopes digested with the same enzymes. The two synthetic oligonucleotides used were 5′- GGA AGA TCT AGC GGA GGT GGA CAT CAT CAC CAT CA CAT AGC GGA GGT GGA AGC GCT TGG AGC CAC CCG CAG TTC GAG AAA GGT GGA GGT TCC GAG GGT- 3′ and 5′- CCG CTC GAG TCA TTT TTC GAA CTG CGG GTG GCT CCA CGA TCC ACC TCC CGA TCC ACC TCC GGA ACC TCC ACC TTT CTC GAA CTG CGG GTG GCT CCA AGC G-3′.
β6N1 Construct and Characterization.
The β6N1 chimera contains the ectodomain of β6-integrin fused to the transmembrane and C-tail of nectin1. To generate the β6N1 chimera, the ectodomain of nectin1 was removed from plasmid pCF18HNK (21), which carries the entire nectin1 ORF, through digestion with BamHI and HpaI, and replaced with β6-integrin ectodomain. The latter was amplified from β6-integrin plasmid by means of oligonucleotides 5′- GAA CTG GGA TCC ATG GGG ATT GAA CTG CTT TGC C- 3′ and 5′- CAT GGG GTT AAC TGG AGG CTT CGG ACA ATC- 3′ and digested with the same enzymes. J cells were transfected with nectin1, plus or minus plasmids encoding αv+β6-integrin or αv+β6N1, as described (49). Forty-eight hours later, cells were exposed to BFLA or wortmannin (all from Sigma Aldrich) for 1 h at 37 °C and then infected with R8012 (3 PFU per cell) for 90 min in the presence of inhibitors. The viral inoculum was removed, and cells were overlaid with medium containing inhibitors for 6–8 h. J cells transfected with nectin1 plus or minus plasmids encoding αv+β6-integrin or αv+β6N1 were infected with R8102 at increasing multiplicity of infection for 90 min at 37 °C. After infection, cells were overlaid with DMEM and harvested 16–18 h after infection. The extent of infection was assessed 24 h later through β-galactosidase (β-Gal) activity at 405 nm by means of O-nitrophenyl–β-galactopyranoside (ONPG).
Pulldown Experiments.
Nectin1STREP, αvSTREP, and gDSTREP pulldown experiments were carried out with lysates of transfected cells using Strep-Tactin Sepharose (IBA, Solutions for Life Sciences), as described (17). J cells were cotransfected with gB, gH, gL, and gD (WT or gDSTREP), nectin1 (WT or nectin1STREP), and the indicated combinations of αvSTREP-, β6-, β8-, and β6N1-integrin. Twenty-four hours later, Strep-Tag pulldown experiments were performed (17). In coculture experiments, receptor-expressing target cells were cocultured with donor cells expressing the viral glycoproteins. Donor J cells were transfected with gD, gB, gH, and gL or as otherwise indicated. Target J cells were transfected with nectin1STREP or WT-nectin1 plus αvSTREP- and β6- or αvSTREP- and β6-integrin alone. Twenty-four hours later, the two cell populations were coseeded and cultured for a further 24 h. Strep-Tag pulldown experiments were performed as described (17). In all cases, the total amount of transfected DNA was made equal by using the Erb-2 expression plasmid. Proteins retained by Strep-Tactin Sepharose were separated by SDS polyacrylamide gel electrophoresis (SDS/PAGE), western blotted (WB) with appropriate antibodies, and developed by means of ChemiDoc XRS+ using Image Lab Software (Biorad).
Integrin Silencing and RT-PCR.
SW480 cells were transfected with One target plus siRNAβ6-integrin, siRNAβ8-integrin, or both (Dharmacon, ON-TARGET plus, smart pool), as described (49). Control cells were transfected with siRNA to E.coli-polA_0054, synthesized by IBA (20). Extent of silencing was analyzed by RT-PCR using TaqMan gene expression assay (Applied Biosystems), as described (20).
Flow Cytometry and Immunofluorescence Assay and Analysis of Media.
J cells were transfected with nectin1, nectin1 plus αv- and β6-integrin, or αv- and β6-integrin alone. Twenty-four hours later, cells were exposed for 90 min at 4 °C with HSV-1 or ΔgB-KΔT at 1 PFU per cell. When indicated, HSV-1 was preincubated for 1 h at 37 °C with a polyclonal neutralizing serum to gD (PAb R8) or monoclonal antibodies to gH 52S, 53S, or LP11. Cells were then shifted to 37 °C for 30 min. A replicate sample of J cells transfected with WT-nectin1 plus αv- and β6- integrin was treated with 30 nM BFLA (Sigma Aldrich) for 1h before HSV-1 infection and during infection. SW480 cells were silenced starting 72 h before infection. Cell monolayers were fixed with 4% paraformaldehyde and incubated with 53S or 52S MAbs, followed by fluorescein isothiocyanate-conjugated anti-mouse secondary antibody. Control cells were incubated with secondary antibody. Cytofluorimetric analyses were performed by means of AccuriC6 (Becton Dickinson). Cell culture medium was concentrated fivefold, devoid of serum IgGs by Protein A-Sepharose as appropriate, and subjected to SDS/PAGE. Glycoproteins were identified by WB with appropriate Abs.
For immunofluorescence assay (IFA), J cells were transfected with nectin1 or nectin1 plus αvβ6-integrin; αv- and β6-integrin plasmids were transfected at threefold excess relative to nectin1. Twenty-four hours later, cells were sorted for nectin1 by means of MAb R1.302 followed by anti-mouse IgG Micro Beads (Miltenyi Biotec). Forty-eight hours later, cells were infected with K26-GFP (10 PFU per cell) for 30 min at 37 °C, fixed with 4% paraformaldehyde and reacted with MAbs 53S, 52S, or VIII-62, followed by anti-mouse DyLight 549 secondary antibody (Jackson). Pictures were taken in a Nikon Eclipse Ni microscope and analyzed by “NIS Elements AR” software from Nikon. Relative quantification of GFP (green) (K26) fluorescence and of red immunofluorescence to gL, 52S, and 53S MAbs is detailed in the legend to Fig. 5.
Rescue of Infection with gH−/− Virions by Soluble Forms of gH.
gH−/− virions (5 μL corresponding to 30 PFU equivalents per cell) were mixed with soluble gHt/gL (33), or soluble gHt/gL plus soluble gDΔ290t-299t (48) (300 nM each) and added to the cells, grown in 24 wells. BSA was used as negative control. After 150 min absorption at 37 °C, the inoculum was removed, and cells were overlaid with DMEM containing 1% FBS. The extent of infection was assessed by means of ONPG. When indicated, the infected cell medium was analyzed by WB as detailed above.
VIRUS ELISA (VIR-ELISA).
HSV-1 virions (1 × 108 PFU per well) were immobilized onto 96 wells by means of bicarbonate buffer, pH 8, at 4 °C overnight. They were then reacted with a suspension of J cells expressing αvβ6-integrin plus nectin1, or nectin1 alone, for 1 h at 37 °C. Cell suspensions were removed, and virions were reacted with MAbs 52S or 53S, followed by peroxidase-conjugated secondary antibody. Immunoreactivity was measured by means O-phenyilendiamine substrate and reading of the optical density at 490 nm.
Acknowledgments
We thank Drs. Gary H. Cohen and Roselyn Eisenberg (University of Pennsylvania), Scott Blystone (State University of New York Upstate Medical University), Helena Browne (Cambridge, United Kingdom), and Prashan Desai (Johns Hopkins University) for generous gifts of reagents. This work was supported by the ERC (European Research Council) Advanced Grant 340060 under the FP7/2007-2013 agreement, by Grant IG14535 from the Italian Association for Cancer Research (AIRC), by Roberto and Cornelia Pallotti grants from the Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, by the Italian Ministry for Education, University and Research (“Progetti di Ricerca di Interesse Nazionale”), and by the University of Bologna Ricerca Fondamentale Orientata (RFO).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506846112/-/DCSupplemental.
References
- 1.Campadelli-Fiume G, et al. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev Med Virol. 2007;17(5):313–326. doi: 10.1002/rmv.546. [DOI] [PubMed] [Google Scholar]
- 2.Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci. 2008;65(11):1653–1668. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krummenacher C, Carfí A, Eisenberg RJ, Cohen GH. Entry of herpesviruses into cells: The enigma variations. Adv Exp Med Biol. 2013;790:178–195. doi: 10.1007/978-1-4614-7651-1_10. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9(5):369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krummenacher C, et al. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 2005;24(23):4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfí A, et al. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8(1):169–179. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc Natl Acad Sci USA. 2010;107(52):22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backovic M, et al. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proc Natl Acad Sci USA. 2010;107(52):22635–22640. doi: 10.1073/pnas.1011507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdary TK, et al. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol. 2010;17(7):882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atanasiu D, et al. Regulation of herpes simplex virus gB-induced cell-cell fusion by mutant forms of gH/gL in the absence of gD and cellular receptors. MBio. 2013;4(2):e00046–13. doi: 10.1128/mBio.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böhm SW, et al. Structure-based functional analyses of domains II and III of pseudorabies virus glycoprotein H. J Virol. 2015;89(2):1364–1376. doi: 10.1128/JVI.02765-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero S, et al. Analysis of a membrane interacting region of herpes simplex virus type 1 glycoprotein H. J Biol Chem. 2008;283(44):29993–30009. doi: 10.1074/jbc.M803092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianni T, Menotti L, Campadelli-Fiume G. A heptad repeat in herpes simplex virus 1 gH, located downstream of the alpha-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J Virol. 2005;79(11):7042–7049. doi: 10.1128/JVI.79.11.7042-7049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldwein EE, et al. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 15.Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci USA. 2009;106(8):2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocchi F, et al. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc Natl Acad Sci USA. 2004;101(19):7445–7450. doi: 10.1073/pnas.0401883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianni T, Amasio M, Campadelli-Fiume G. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL in part through the C-terminal profusion domain. J Biol Chem. 2009;284(26):17370–17382. doi: 10.1074/jbc.M109.005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campadelli-Fiume G, Menotti L, Avitabile E, Gianni T. Viral and cellular contributions to herpes simplex virus entry into the cell. Curr Opin Virol. 2012;2(1):28–36. doi: 10.1016/j.coviro.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol. 2010;84(23):12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9(12):e1003806. doi: 10.1371/journal.ppat.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J Virol. 2004;78(22):12268–12276. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79(11):6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77(9):5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108(3):407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 25.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101(43):15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J Virol. 2011;85(24):13214–13223. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci USA. 2009;106(48):20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azab W, Lehmann MJ, Osterrieder N. Glycoprotein H and α4β1 integrins determine the entry pathway of alphaherpesviruses. J Virol. 2013;87(10):5937–5948. doi: 10.1128/JVI.03522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72(12):9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianni T, Campadelli-Fiume G. The epithelial αvβ3-integrin boosts the MYD88-dependent TLR2 signaling in response to viral and bacterial components. PLoS Pathog. 2014;10(11):e1004477. doi: 10.1371/journal.ppat.1004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Showalter SD, Zweig M, Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72(9):7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gianni T, et al. Herpes simplex virus glycoproteins H/L bind to cells independently of alphaVbeta3 integrin and inhibit virus entry, and their constitutive expression restricts infection. J Virol. 2010;84(8):4013–4025. doi: 10.1128/JVI.02502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. doi: 10.1128/JVI.01597-14. Chesnokova LS, Ahuja MK, Hutt-Fletcher LM (2014) Epstein-Barr virus glycoprotein gB and gHgL can mediate fusion and entry in trans, and heat can act as a partial surrogate for gHgL and trigger a conformational change in gB. J Virol 88(21):12193–12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrester A, et al. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gompels U, Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 37.Gilmore TD. NF-kappa B, KBF1, dorsal, and related matters. Cell. 1990;62(5):841–843. doi: 10.1016/0092-8674(90)90257-f. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson L, et al. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66(4):2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roop C, Hutchinson L, Johnson DC. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67(4):2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roizman B, Zhou G. The 3 facets of regulation of herpes simplex virus gene expression: A critical inquiry. Virology. 2015;479-480:562–567. doi: 10.1016/j.virol.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu IM, et al. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319(5871):1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 42.Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol. 2006;4(1):67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullen MM, Haan KM, Longnecker R, Jardetzky TS. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol Cell. 2002;9(2):375–385. doi: 10.1016/s1097-2765(02)00465-3. [DOI] [PubMed] [Google Scholar]
- 44.Longnecker R, Neipel F. Introduction to human gamma-herpesviruses. In: Arvin A, et al., editors. Human Herpesviruses. Cambridge Press; UK: 2007. pp. 341–359. [PubMed] [Google Scholar]
- 45.Vanarsdall AL, Johnson DC. Human cytomegalovirus entry into cells. Curr Opin Virol. 2012;2(1):37–42. doi: 10.1016/j.coviro.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori Y. Recent topics related to human herpesvirus 6 cell tropism. Cell Microbiol. 2009;11(7):1001–1006. doi: 10.1111/j.1462-5822.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 47.Cai WZ, Person S, Warner SC, Zhou JH, DeLuca NA. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987;61(3):714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicola AV, Willis SH, Naidoo NN, Eisenberg RJ, Cohen GH. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70(6):3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gianni T, Leoni V, Campadelli-Fiume G. Type I interferon and NF-κB activation elicited by herpes simplex virus gH/gL via αvβ3 integrin in epithelial and neuronal cell lines. J Virol. 2013;87(24):13911–13916. doi: 10.1128/JVI.01894-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avitabile E, Lombardi G, Campadelli-Fiume G. Herpes simplex virus glycoprotein K, but not its syncytial allele, inhibits cell-cell fusion mediated by the four fusogenic glycoproteins, gD, gB, gH, and gL. J Virol. 2003;77(12):6836–6844. doi: 10.1128/JVI.77.12.6836-6844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rovero S, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165(9):5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 52.Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J Cell Biol. 1994;127(4):1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson T, et al. Integrin alphavbeta8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J Virol. 2004;78(9):4533–4540. doi: 10.1128/JVI.78.9.4533-4540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avitabile E, Forghieri C, Campadelli-Fiume G. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J Virol. 2007;81(20):11532–11537. doi: 10.1128/JVI.01343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]