For over 100 years it has been known that a blue light photoreceptor regulates growth and development in plants under low-light conditions. These photoreceptors were termed cryptochomes (CRYs) because of their importance in cryptogamic plants and the cryptic aspects of their function and photochemistry (1, 2). Despite decades of progress into their structure and photochemistry (discussed below), several elements of CRY’s photocycle and signaling mechanisms remain fiercely debated. These debates center on sequence conservation within the CRY/photolyase family (CPF). Despite high sequence similarities, CPF members demonstrate functions ranging from DNA repair enzymes (photolyases) to blue light-regulated growth, development, and circadian rhythms in diverse organisms (CRYs) (3). In all cases, CPF function hinges upon a bound flavin adenine dinucleotide (FAD) cofactor that undergoes interconversion between several redox states (Fig. 1). Currently, the nature of the ground and excited states of FAD, the presence and role of secondary pigments, and the requirement of a conserved sequence of three Trp residues (Trp triad) remain controversial (3, 4). Debates stem from the difficulties of resolving contradictions between in vitro photochemical experiments and in vivo biological function (4). These conflicts are further exacerbated by apparent differences within the CPF family. For instance, the Arabidopsis thaliana AtCRY1 and AtCRY2 proteins reportedly differ in the requirement of the Trp triad for function: the Trp triad is not required for signaling in CRY2 (5), but was reported to be required for CRY1 function in vivo (6). In PNAS, Gao et al. elegantly demonstrate that the Trp triad is indeed not required for in vivo function of AtCRY1 and that photochemical activation of Trp triad mutants is not dependent upon ATP or other metabolites (7). In this manner, Gao et al. clarify two conflicts in CRY chemistry and function regarding the biological role of the Trp triad. Thereby, they present a more unified understanding of CRY photochemistry.
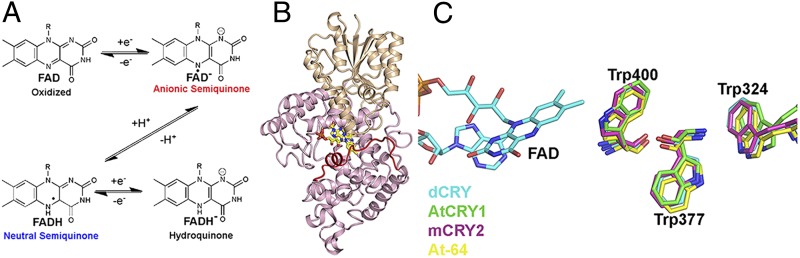
Fig. 1.
CRY structure and chemistry. (A) CRY proteins are believed to exist in an oxidized FAD ground state. Photoactivation generates the one-electron reduced anionic or neutral semiquinones. Further reduction is possible to the two-electron hydroquinone that functions as the ground state in photolyases. (B) Structure of dCRY (PDB ID code 4GU5). The PHR is composed of an N-terminal αβ domain (orange) and a C-terminal helical domain (pink). The CCE (red) docks to a solvent-exposed cleft adjacent to the FAD (yellow) in the dark. (C) The Trp triad is conserved in dCRY and AtCRY1 as well as photolyases (Arabidopsis thaliana 6-4 photolyase: At-64) and light independent mouse CRY2 (mCRY2).
CRY Structure and Signaling Mechanisms
In A. thaliana, AtCRY1 and AtCRY2 function as UV-A/blue light photoreceptors to regulate hypocotyl elongation and photoperiodic control of floral initiation, respectively (8). In addition, these proteins play a wide role in plant physiology, where they affect circadian rhythms, stress responses, and programmed cell death, among others. The wide-ranging function of CRY proteins in plants has led to significant interest in their photochemical mechanisms and downstream signal transduction. Currently, much of our understanding of AtCRY function stems from structural and chemical studies of the broader CPF.
Structural and biochemical studies of CPF proteins indicate that CRYs can be differentiated from photolyases based on two primary elements. First, CRYs are distinguished by their inability to recognize and repair cyclobutane pyrimidine dimers or 6-4 DNA lesions. Second, in addition to the conserved N-terminal photocatalytic core (photolyase homology region; PHR), CRYs contain a C-terminal extension (CCE) to the PHR that is species-specific and often dictates protein function (Fig. 1B). For example, in crystal structures of Drosophila CRY (dCRY) the CCE docks to a solvent-exposed cleft adjacent to the photocatalytic FAD under dark-state conditions (9). Photo- or chemical reduction of the FAD then releases the CCE to engage interaction partners (Timeless) and target CRY for degradation (10). Although only structures of the isolated PHR domain of AtCRY1 are currently available, biochemical studies suggest plant CRYs conserve a similar mechanism.
Biological studies of AtCRY1/2 indicate that the CCE is both necessary and sufficient for signal transduction in vivo. The functional role of the CCE was elegantly demonstrated in a series of experiments where the isolated CCE was fused to a carrier protein. These studies showed that when the CCE is fused to dimerization domains, the chimera proteins exhibit constitutive activity (11, 12). These findings led to a conclusion that the CCE retains the required signaling elements, whereas the PHR domain imparts light-regulated control of CCE function and dimerization. Solution studies of AtCRY proteins confirmed that the PHR domain dictates light-regulated control of CCE folding and release. Limited proteolysis assays demonstrate that the CCE is sequestered by the PHR in the dark but is released upon photoactivation (13). Combined, these elements provided a tentative model of AtCRY regulation. Upon photoactivation, the CCE is released from the core PHR, allowing it to engage the COP1 associated signaling components and in this manner inhibiting the activity of COP1 (8, 11). Thus, a consensus model of CRY activation in both animals and plants involves the blue light-induced release of the CCE to engage downstream targets and initiate signaling (3). In contrast, how CCE release is coupled to CRY chemistry is unclear.
CRY Photochemistry
Most knowledge of CRY photochemistry stems from decades of research into the mechanism of photolyases. Photolyase activation involves two cofactors, the catalytic FAD in a two-electron reduced FADH– state and an antennae cofactor that can vary in identity (i.e., MTHF, HDF, flavins). Activation of photolyases is achieved by absorption of UV-A light by the antennae cofactor, which induces electron transfer from FADH– to the DNA lesion (3, 8). Importantly, a second electron transfer pathway exists in photolyases and is composed of a series of three Trp residues (Trp triad) forming a pathway from the catalytic FAD to the protein surface (Fig. 1C) (3). The Trp triad is not required for DNA repair (14), but is believed to be important in maintaining population of the FADH– state.
Characterization of recombinant plant and animal CRYs suggested key differences from the consensus photolyase mechanism. First, CRY proteins purify with bound oxidized FAD (1). Second, CRY proteins typically do not bind an antennae pigment, and CRY structures indicate key residue substitutions in the antennae binding pockets that would occlude cofactor recognition (9, 15). Thus, researchers proposed that CRY proteins function independently of an antennae pigment and use oxidized FAD as the ground state. In addition, in vitro studies of CRY photochemistry indicated that photoreduction of oxidized FAD to either the neutral (FADH) or anionic (FAD–) semiquinone required the presence of the Trp triad and external electron donors (4, 9). These studies led to a proposed mechanism for CRY activation, whereby, blue light excitation of an oxidized FAD ground state induced electron transfer through the Trp triad to generate a semiquinone signaling state (Fig. 1).
These initial proposed mechanisms were contentious because they contrasted with expected results from in vivo cellular conditions. For example, cellular reduction potentials are sufficient to support bound FAD in the
Gao et al. clarify two conflicts in CRY chemistry and function regarding the biological role of the Trp triad.
reduced semiquinone or hydroquinone states, thereby contradicting an oxidized FAD ground state (16). In addition, characterization of dCRY and AtCRY2 indicated that the Trp triad was not required for in vivo function (5, 17). These results contrasted with both the in vitro photochemical mechanisms requiring the Trp triad, as well as data suggesting the Trp triad was required for AtCRY1 function in vivo (6). Researchers contended that the in vitro data reflected artifacts resulting from oxygen-rich conditions under which CRY proteins were purified.
Several recent studies have attempted to reconcile the differences between in vitro photochemical experiments and the contrasting in vivo data. Spectroscopic studies of CRY proteins in a cellular system confirmed an oxidized FAD ground state under cellular conditions (18). These proteins, including Trp triad mutants, rapidly photoreduce in the presence of blue light to generate semiquinone intermediates (18). The ability to rescue photoreduction efficiency in a cellular system led to a hypothesis that cellular metabolites, such as ATP and NADH, may contribute to photoreduction in CRY proteins. Indeed supplementation of recombinant CRYs with ATP rescued photoreduction deficiencies of Trp triad mutants (4). These studies seemed to reconcile key elements of CRY photochemistry. Namely, they confirmed that under cellular conditions, CRYs exist as oxidized FAD (ground state). The oxidized FAD ground state can then be reduced to generate semiquinone intermediates (signaling state), although alternative models still exist (16). Furthermore, the studies indicated that the presence of cellular metabolites are likely responsible for rescuing defects in Trp triad mutants in vivo. Combined, these studies provided a plausible model to explain deviations between in vitro and in vivo experiments; however, such studies could not confirm a functional role of metabolites in CRY photochemistry in vivo, nor could they explain the requirement of the Trp triad in AtCRY1 signaling. In PNAS, Gao et al. definitively show that the Trp triad is not required for CRY1 signaling in vivo; moreover, they elegantly demonstrate inconsistencies with the metabolite model of CRY electron transfer processes (7). Moving forward, we now have a unified platform to decipher how fundamental CRY photochemistry regulates CCE recognition and release.
Acknowledgments
This work was funded by National Institutes of Health Grant R15GM109282 (to B.D.Z.).
Footnotes
The author declares no conflict of interest.
See companion article on page 9135.
References
- 1.Lin C, et al. Association of Flavin Adenine Dinucleotide with the Arabidopsis Blue Light Receptor CRY1. Science. 1995;269(5226):968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- 2.Gressel J. Blue-light photoreception. Photochem Photobiol. 1979;30(6):749–754. [Google Scholar]
- 3.Zoltowski BD, Gardner KH. Tripping the light fantastic: Blue-light photoreceptors as examples of environmentally modulated protein-protein interactions. Biochemistry. 2011;50(1):4–16. doi: 10.1021/bi101665s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelhard C, et al. Cellular metabolites enhance the light sensitivity of Arabidopsis cryptochrome through alternate electron transfer pathways. Plant Cell. 2014;26(11):4519–4531. doi: 10.1105/tpc.114.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, et al. Arabidopsis cryptochrome 2 (CRY2) functions by the photoactivation mechanism distinct from the tryptophan (trp) triad-dependent photoreduction. Proc Natl Acad Sci USA. 2011;108(51):20844–20849. doi: 10.1073/pnas.1114579108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeugner A, et al. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J Biol Chem. 2005;280(20):19437–19440. doi: 10.1074/jbc.C500077200. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, et al. Trp triad-dependent rapid photoreduction is not required for the function of Arabidopsis CRY1. Proc Natl Acad Sci USA. 2015;112:9135–9140. doi: 10.1073/pnas.1504404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X, Liu H, Klejnot J, Lin C. The cryptochrome blue light receptors. Arabidopsis Book. 2010;8:e0135. doi: 10.1199/tab.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoltowski BD, et al. Structure of full-length Drosophila cryptochrome. Nature. 2011;480(7377):396–399. doi: 10.1038/nature10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaidya AT, et al. Flavin reduction activates Drosophila cryptochrome. Proc Natl Acad Sci USA. 2013;110(51):20455–20460. doi: 10.1073/pnas.1313336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HQ, et al. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell. 2000;103(5):815–827. doi: 10.1016/s0092-8674(00)00184-7. [DOI] [PubMed] [Google Scholar]
- 12.Sang Y, et al. N-Terminal Domain-Mediated Homodimerization Is Required for Photoreceptor Activity of Arabidopsis CRYPTOCHROME 1. Plant Cell. 2005;17(5):1569–1584. doi: 10.1105/tpc.104.029645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partch CL, Clarkson MW, Ozgür S, Lee AL, Sancar A. Role of structural plasticity in signal transduction by the cryptochrome blue-light photoreceptor. Biochemistry. 2005;44(10):3795–3805. doi: 10.1021/bi047545g. [DOI] [PubMed] [Google Scholar]
- 14.Kavakli IH, Sancar A. Analysis of the role of intraprotein electron transfer in photoreactivation by DNA photolyase in vivo. Biochemistry. 2004;43(48):15103–15110. doi: 10.1021/bi0478796. [DOI] [PubMed] [Google Scholar]
- 15.Brautigam CA, et al. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101(33):12142–12147. doi: 10.1073/pnas.0404851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozturk N, Selby CP, Zhong D, Sancar A. Mechanism of photosignaling by Drosophila cryptochrome: Role of the redox status of the flavin chromophore. J Biol Chem. 2014;289(8):4634–4642. doi: 10.1074/jbc.M113.542498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oztürk N, Song SH, Selby CP, Sancar A. Animal type 1 cryptochromes. Analysis of the redox state of the flavin cofactor by site-directed mutagenesis. J Biol Chem. 2008;283(6):3256–3263. doi: 10.1074/jbc.M708612200. [DOI] [PubMed] [Google Scholar]
- 18.Hoang N, et al. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008;6(7):e160. doi: 10.1371/journal.pbio.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]