Abstract
It is obvious that natural selection operates at the level of individuals and collections of individuals. Nearly two decades ago we showed that in multi-individual colonies of protochordate colonial tunicates sharing a blood circulation, there exists an exchange of somatic stem cells and germline stem cells, resulting in somatic chimeras and stem cell competitions for gonadal niches. Stem cells are unlike other cells in the body in that they alone self-renew, so that they form clones that are perpetuated for the life of the organism. Stem cell competitions have allowed the emergence of competitive somatic and germline stem cell clones. Highly successful germline stem cells usually outcompete less successful competitors both in the gonads of the genotype partner from which they arise and in the gonads of the natural parabiotic partners. Therefore, natural selection also operates at the level of germline stem cell clones. In the colonial tunicate Botryllus schlosseri the formation of natural parabionts is prevented by a single-locus highly polymorphic histocompatibility gene called Botryllus histocompatibility factor. This limits germline stem cell predation to kin, as the locus has hundreds of alleles. We show that in mice germline stem cells compete for gonad niches, and in mice and humans, blood-forming stem cells also compete for bone marrow niches. We show that the clonal progression from blood-forming stem cells to acute leukemias by successive genetic and epigenetic events in blood stem cells also involves competition and selection between clones and propose that this is a general theme in cancer.
Keywords: evolution, stem cell competition, natural selection, Botryllus schlosseri, cancer
Stem cells are defined as cells that can undergo self-renewal upon division, and, in all tissues of the vertebrate body, only stem cells self-renew, with few exceptions (1). Blood-forming stem cells (called hematopoietic stem cells, or HSCs) are a good example of tissue stem cells, as they have been isolated prospectively, transplanted into mice and humans whose own blood-forming system had been destroyed by radiation, and by themselves fully restore blood formation for life to those recipients (1). Blood-forming stem cells are estimated to be only 1 in 20,000 bone marrow cells, the blood-forming organ in both mice and in humans. Prospective isolation of mammalian blood-forming stem cells has provided much of what we know about stem cells in general (1). Here we present experiments and data that show that stem cells in the germline and soma are not homogeneous within a stem cell type (e.g., mouse germline spermatogenic stem cells and mouse and human blood-forming stem cells). The competitions in the germline are present in both urochordates and mice and, by extension, humans. The competitions in somatic stem cells such as blood-forming stem cells underlie not only changing needs for particular blood cell types in aging, but also the slow progression from normal blood-forming stem cells to the malignant blood cells that drive leukemia, leukemia stem cells.
Clonal Competition Among Germline and Among Somatic Stem Cells in a Colonial Marine Species
Stem cells, which are present in all metazoan species, are best understood as units of natural selection when examining their role in somatic tissue development and gonad development during the life cycle of colonial species such as the Urochordate, Botryllus schlosseri, a “protochordate” species. The life cycle of this protochordate is outlined in Fig. 1 (2). Following sexual reproduction it undergoes blastula, gastrula, and neurula stages of development, giving rise to a chordate tadpole with a head (containing eyes), tail, notochord, neural tube, and segmented musculature—hence, a protochordate. The newly developed tadpole escapes from the maternal colony, lands on a subtidal surface, and metamorphoses into an invertebrate by the loss of chordate structures by apoptosis, followed by macrophage digestion of the dying cells. B. schlosseri lives in temperate zones throughout the world, and the newly metamorphosed individual, called an oozoid (developed from an egg) has a deceptively simple appearance belying a complex organism with oral and atrial (anal) siphons bounding a digestive tract, gill slits, a hormone-producing endostyle, a two-chambered heart, and a connecting intracorporeal and extracorporeal blood vascular system that extends out into the gelatinous tunic that covers the organism. Upon metamorphosis, one to three buds evaginate from the body wall of the oozoid to initiate the asexual reproduction cycle. The bud, which includes both germline and somatic tissue stem cells, progresses in a week to the adult body plan (Fig. 1) shared with the individual from which it was derived. Each new body, called a blastozooid, maintains a vascular circulation in the tunic connecting it to the parent zooid and to all other newly developed blastozooids in the same tunic; the blood vessels form terminal blunt-ended ampullae at the edge of the tunic. The adult developing organism in the second week enlarges all of the body’s systems. The adult survives through a third week, at the end of which all organs in the body of the blastozooid undergo apoptosis, again to be cleared by blood-borne phagocytic cells.
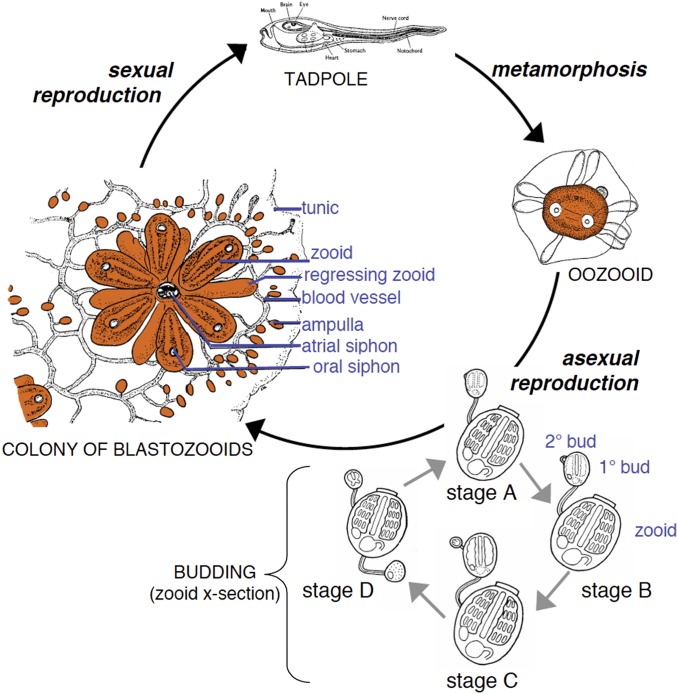
Fig. 1.
Life cycle of B. schlosseri. B. schlosseri reproduces both through sexual and asexual (budding) pathways, giving rise to virtually identical adult body plans. Upon settlement, the chordate tadpole, the product of sexual reproduction, metamorphoses into an invertebrate founder individual, an oozooid. This oozoid undergoes asexual reproduction through budding that proceeds through four stages (A–D) to create a colony (Left) of genetically identical individuals (blastozooids, also known as zooids). This budding process continues weekly throughout the life of the colony producing multiple individuals (buds that grow into zooids). Each individual has anatomical features that include atrial and oral siphons as well as a simple tube-like heart, intestines, and a branchial sac. The individuals are connected via a network of blood vessels that are embedded within a gelatinous matrix (termed “tunic”) and terminate in finger-like protrusions (ampullae). Reproduced with permission from ref. 2.
Colonies of Multiple Clonally Derived Individuals Can Form Natural Parabiotic Chimeras in the Wild
A single clonal precursor can give rise to an entire colony by the successive budding cycles that begin every week. When one colony grows adjacent to another, they touch, and the ampullae extend into the tunic of the other, developing a tip-to-side contact between blood vessels. This phenomenon of tip-to-side adhesion leads to the beginning of an anastomosis between extracorporeal blood vessels of two colonies. Within a day the extracorporeal vasculature between the two either results in a semipermanent anastomosis of the blood vessels, resulting in the sharing of blood contents between these natural parabionts, or results in a broadly based inflammatory and cytotoxic reaction, producing a permanent scar between the two adjacent colonies (3).
A Highly Polymorphic Gene Locus Mediates Colony Vascular Fusion or Rejection
In 1982 we showed that the genetics of this fusion/rejection phenomenon is governed by a single, highly polymorphic locus with over 100 alleles in the Monterey, California, Marina. The rules of fusion or rejection mimic those of natural killer cell recognition: sharing of one allele or two alleles leads to vascular fusion, whereas sharing of no alleles leads to rejection (3). We have identified the histocompatibility gene, named it BHF (for Botryllus histocompatibility factor), and sequenced several alleles; it exists in an extended haplotype of genes that likely govern several different types of allorecognition responses (4, 5).
Natural Parabionts Exchange Somatic and Germline Stem Cells That Compete Between Genotypes for Somatic and Germline Niches
Using individual specific genetic markers such as dinucleotide repeats, line elements, or allelic single-gene polymorphisms, we found that vascular fusion allowed not only circulating blood cells, but also circulating germline and somatic stem cells to pass from one colony to another with which the colony is fused (Fig. 2). We assessed the germline and individual somatic organs in a long-term chimeric colony formed by fusion of two genetically distinct original colonies. From this we sampled individuals, buds, blood, and sperm from one end to the other (for anatomical distribution of buds, blood, and testes, see Fig. S1). We found that all of the gametes in the colony were genetically identical, indicating that they arose from the germline stem cells of only one chimeric partner, which had effectively outcompeted the germline stem cells of the other partner (Fig. 3). Similarly, all of the sampled somatic tissue was derived from both of the chimeric partner colonies, suggesting a parallel somatic stem cell competition. If colony 1 wins the germline competition with colony 2, every subclone of colony 1 will win the germline competition with colony 2 [Fig. 4 and Stoner and Weissman (7)]. If colony 2 wins the germline competition with colony 3, then, predictably, colony 1 beats both colonies 2 and 3 in the germline competition (7, 8). In Fig. 4, taken from experiments reported in Stoner et al. (8), colony F wins the germline competition with colony G, but usually loses the somatic competition with G. In the germline competition, F beats B, but in the soma, B beats F (8). Similarly, in each replicate, G beats B in both germline and soma. These hierarchies hold true when we form a trichimera of the three colonies (F, B, and G), wherein F wins all of the germline and G wins all of the soma.
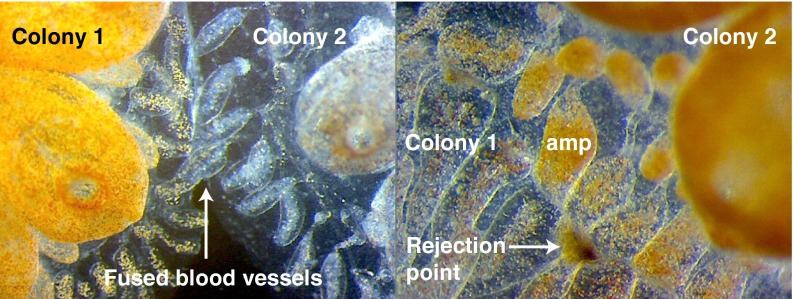
Fig. 2.
Fusion/rejection between B. schlosseri colonies is governed by the BHF gene. Colonies that share an allele will fuse (Left), whereas colonies that do not will reject (Right). Rejection is an inflammatory response of blood cells in ampullae and results in a fibrotic scar that blocks vascular anastomoses. Major anatomical features are indicated (amp, ampullae, the colony’s blind-ended vasculature). Adapted with permission from ref. 4.
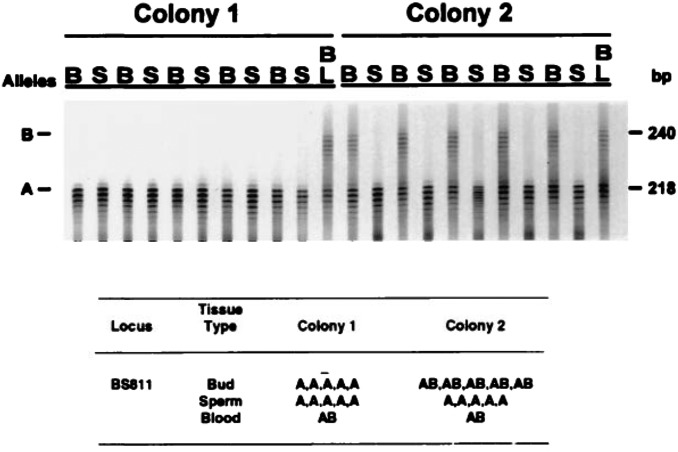
Fig. 3.
An autoradiograph and interpretative table that show the results of genotypic sampling of buds (B), sperm (S), and blood (BL) from a field chimera of B. schlosseri in which we observed germ cell or somatic cell parasitism (G/SCP, the phenomenon whereby the stem cells of one chimeric partner take over the tissue of the other chimeric partner in a colony). The tissues were typed using microsatellite locus BS811 (6). Based on the spatial pattern of genotypes within the somatic tissues of the chimera at the time of collection, it is presumed that the initial genotypes for the two colonies were AA (colony 1) and AB (colony 2). After fusion, both the somatic and gametic tissues of colony AA appear to remain unchanged. In contrast, whereas the somatic tissues of colony AB retained their original genotype, its gametic tissues were almost completely replaced by tissues having genotype AA. This replacement is interpreted as an example of G/SCP. Reproduced with permission from ref. 7.
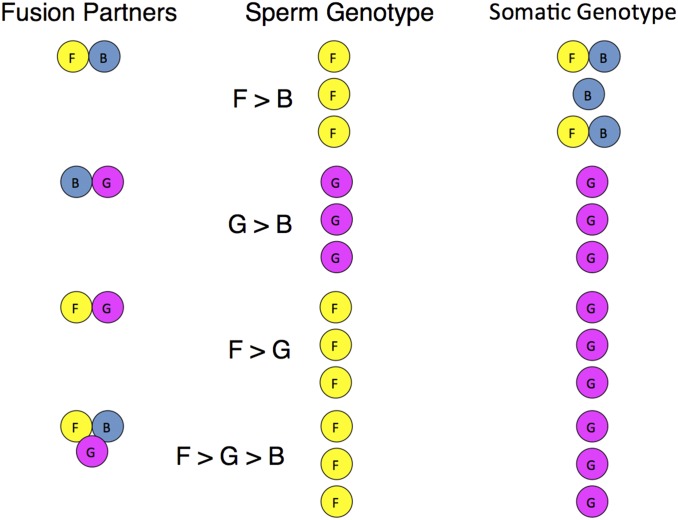
Fig. 4.
Hierarchies of germline stem cell and somatic stem cell competition. Three genetically distinct of colonies (strains F, B, and G) with shared BHF alleles were allowed to fuse in all pair-wise combinations and also all together to form chimeric colonies. (n = 3, Fusion Partners). After 3 mo, the sperm (Middle) and somatic tissue (Right) were analyzed to identify which strains contributed to each tissue. In most cases, a single germline “winner” outcompeted the germline loser, and the outcomes of this competition to dominate spermatogenesis followed a hierarchical relationship. The allelic markers shown here identify the majority of cells in the testis. Both testis and ovaries from each colony gave rise to gametes that were tested in crosses. If the germline allelic marker in the testis assay won, the sperm and eggs from that colony came from the winner (F dominated over G, which dominated over B) (8).
We have recorded several instances wherein the budding process ceases in one of the colonies, leading over time to the resorption of that colony and the preservation of the other (8) (9). However, this resorption is of the soma only, as the surviving colony often carries only the germline of the resorbed colony. These asexually transmitted germline competition winners also can be predicted by sexual reproduction; breeding of colonies shows that the asexually inherited germline winners also carry heritable elements specifying the germline predatory behavior (i.e., ability to win in germline competitions) (8). The germline and somatic competitions result from the action of highly enriched somatic and germline stem cells because when these cells are taken from a germline winning strain (e.g., A) and injected intravascularly into a germline losing strain, they take over the production of gametes (10). Most importantly, all germline and somatic stem cell competitions are blocked by rejection, so the spread of predatory germline stem cells is limited to kin, insofar as only kin are likely to share a histocompatibility allele (3).
If we think of these germline competitions as always favoring the germline winner, then several issues of interest arise. First, if often the soma of one can provide a fit body that incubates the germline of the other, how are the genes that provide superior body fitness passed on to the next generation? Second, it is apparent that one role of the histocompatibility locus is in preventing passage of germline stem cells into another colony. Such a role prevents predatory germline stem cells from an unrelated BHF-incompatible colony from capturing the germline, as particularly successful germline stem cells without the histocompatibility barrier could spread and tend to make large regions containing colonial tunicates that have more homogeneous genomes. The ultimate of competition could be akin to cancer, which if it spreads throughout a species could dominate large regions and result in species extinctions. We propose that the prevention of the spread of predatory germline stem cells provides sufficient drive for high polymorphism in the histocompatibility locus of B. schlosseri (3, 8, 11). The high polymorphism of BHF also results in high genomic diversity between colonies of Botryllus.
The vertebrate major histocompatibility complex (MHC) is apparently primarily involved in host–pathogen protection via T-cell immunity to MHC-presented intracellular foreign molecules. That would have been an interesting scenario to consider if the B. schlosseri histocompatibility gene was related to the vertebrate MHC, but the recently cloned BHF gene has no sequence similarity to vertebrate MHC (4).
If we take this to the next level, one can consider that super competitors could evolve to the stage where they approach malignancy or develop the capacity for self-renewal, migration, survival, etc. (Fig. 5). This brings the question of stem cells as units of selection back to noncolonial organisms such as humans and mice. Is there evidence for somatic or germline stem cell competition in the life span of the individual? Is it possible for winners and losers to appear simultaneously? Do separate clonal stem cell lineages with common tissue fates such as spermatogenesis or hematopoiesis exist in humans and mice? How does the phenomenon of stem cell competition within an individual relate to the development of cancers?
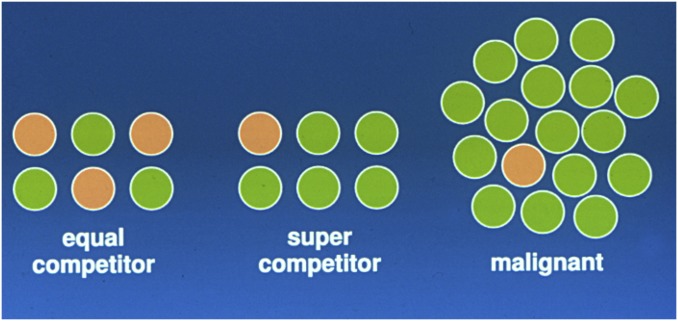
Fig. 5.
Model of clonal frequencies of germline (or somatic) stem cells in an individual. (Left) Equally competitive stem cells coexist. (Center) Supercompetitor stem cells expand at the expense of normal stem cells, but the supercompetitor stem cells + normal stem cells do not exceed the stem cell frequency for that type of stem cell. (Right) The heritable changes in a stem cell lineage allow the clone to expand dramatically and beyond the frequency limitation of the previous part. Here the stem cell resembles a cancer stem cell.
Clonal Competitions of Mouse Germline Stem Cells in the Formation of Reproductively Competent Gonads
To develop general methods to identify clonal populations of cells, mainly stem cells, in vivo in the mouse we developed tetrachimeras and Rainbow mouse models (12). In the tetrachimera model, we developed color-coded mouse embryonic stem (ES) cells, each of which constitutively expressed a differently colored fluorescent protein of red, blue, or green. Embryonic stem cells are derived from the inner cell mass of mouse preimplantation blastocyst using proteins and conditions that allow self-renewal and expansion of the cells at a stage before conversion to the next stage of development that occurs after implantation into the uterus—the epiblast stage in which the primitive mesoderm, ectoderm, and endoderm, as well as incipient germline stem cells, are formed as developmental alternates. We injected 5 of each colored ES cell (15 total) into the uncolored blastocysts of a mouse, which we then implanted into the uterus of another mouse. Whenever a large number of same-color (e.g., green) cells were adjacent to each other, we could conclude that they were derived from a single cell of that color—a stem or clonal precursor. We limited our analyses to tetrachimeras with all four colors in the skin, blood, and many other tissues.
In the adult testis of these mice, the number of independent colors in germline clones was two or three, which by Poisson distribution is consistent with only four germline stem cells separating from the epiblast somatic cells (13). The adult testis contained dozens of adjacent seminiferous tubules of the same color, demonstrating that single germline stem cells are capable of inhabiting many adjacent seminiferous tubules. In each mouse, the two to three colors in the left testis were the same in the right testis; these had expanded from four epiblast germline stem cells to ∼4,000 germline stem cells that then entered the genital ridges bilaterally (Fig. 6) (13). Upon entry, each seminiferous tubule was a mixture of the total number of the different color cells that exist; that is, they were polyclonal. Polyclonal seminiferous tubules exist as such until 14–21 d after birth (p14–21), when the vast majority of these cells undergo apoptosis, and the survivors become the clonal germline stem cells for many adjacent tubules, a single color per cluster of tubules. Therefore, the germline of mice contains competing germline stem cells, most of which are lost by apoptosis, culling out most of the germline stem cell progeny (Fig. 7). This is similar to the competition between germline stem cells in the colonial tunicates described above.
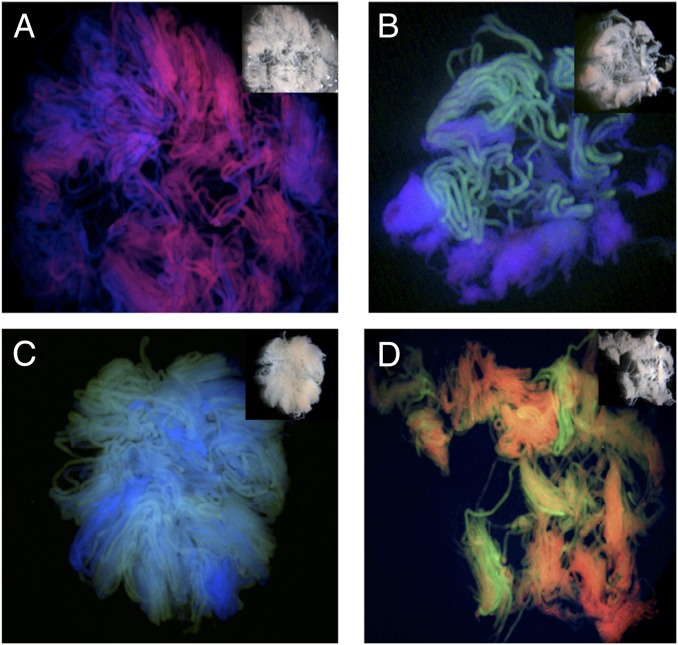
Fig. 6.
Many adjacent seminiferous tubules within a testis derive from a single germline stem cell. To define how many germline stem cells ultimately contribute to the seminiferous tubules of the adult testes, a clonal analysis of stem cell progeny was performed. Tetrachimeric mice harbor cells that are permanently labeled with one of four marks: green (eGFP), blue (eCFP), red (mRFP1), or fluorescently uncolored. The dissected and untangled seminiferous tubules from these tetrachimeras show that each tubule is a single color and that multiple adjacent tubules are all of the same color (i.e., derived from a single germline stem cell). Images are representative of 32 chimeric testes analyzed; examples are of (A) a testes with only blue or red tubules; (B and C) a testes with only blue or green tubules; and (D) a testes with only red and green tubules. Reproduced with permission from ref. 13.
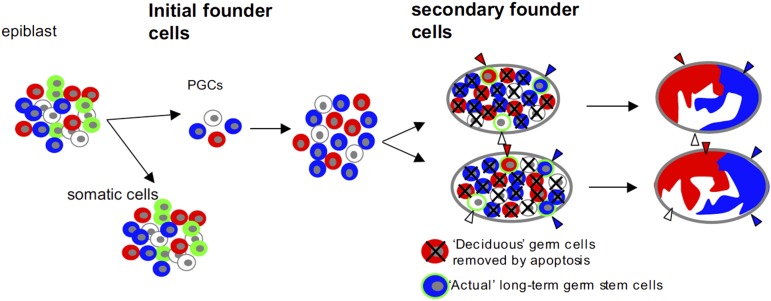
Fig. 7.
Our model of two-step oligoclonal development of testis germ cells. Male germline starts from four cells. They proliferate, migrate, and split to bilateral genital ridges. However, germ cells actually contributing adult spermatogenesis during the reproductive period originate from a small number of a second founder population that initially seed onto genital ridges. The rest of the germ cells (deciduous germ cells) are removed, most likely by apoptosis, before the reproductive period. Reproduced with permission from ref. 13.
Speculation on the Basis of Germline Stem Cell Loss During the Germline Competitions
What happens to cause the death of so many germline stem cells? We propose that genetic changes occur in most of the cells, and most will not give rise to viable germline-derived sperm. Others have noted that the time period in which we see the maximal cell death coincides with the movement within the germline of transposable DNA elements, many of which are the survivors of horizontally transmitted retroviruses that have invaded the germline (13, 14). During this movement, copies of particular retrotransposons are made and insert throughout the genome, creating, for the most part, interruptions of genes and, perhaps, enhancers retained in the transposable element. [This movement is modulated in mice by small RNA elements called Miwi (14).] We propose that those retrotransposon movements by insertion, and by repair of the genes from which they came, mostly cause mutations that lead to reproductively incompetent sperm. It should be apparent that retrotransposons that move in the germline are perhaps the principal basis for mutations upon which selection acts. This hypothesis fits with the finding that these insertions are expressed in the germline at the same time as the gene AIRE, which is expressed in the testis and thymus (6). AIRE in thymic medullary epithelial and dendritic cells allows opening and limited transcription and translation of virtually every ORF in the body (6, 15). [This allows developing T cells with anti-self high-affinity antigen receptors to recognize self-proteins from other parts of the body on the surface of the medullary epithelial and medullary dendritic cells; this results in negative selection, probably mediated by apoptosis and programmed removal of potentially autoimmune T-cell clones (16).] In both developing T cells and gametes, therefore, mechanisms exist for the removal of cells that cannot function correctly. Our model is shown in Fig. 7. It is interesting to note that mice that lack the enzyme AIRE are infertile, indicating that AIRE may be important in revealing nonfunctional gene products in the precursors of sperm, the detection of which in a cell might lead to death of that cell. Enforced expression of the anti-apoptosis protein Bcl2 in the germline leads to a failure of the germline cells to die, but these animals are almost completely infertile (17, 18).
Are Somatic Stem Cells also Competitive?
Here we studied the blood-forming (i.e., hematopoietic) system. Each HSC can give rise to all blood cell types, as well as self-renew to retain a stem cell pool (Fig. 8) (19). However, the HSC population is diverse, with some HSCs forming a roughly equal proportion of myeloid elements (platelets, red blood cells, granulocytes, monocytes, and macrophages) and lymphocytes (B lymphocytes, T lymphocytes, NK lymphocytes). In contrast to these balanced HSCs, others have a significant bias of production toward myeloid over lymphoid cells (20). As shown in Fig. 9, young mice and humans have mainly balanced HSCs, with a few myeloid-biased HSCs in the pool, whereas aged mice and humans have a predominance of myeloid-biased HSCs. There are two possible explanations for these findings: either balanced HSCs mature to myeloid-biased HSCs via hundreds of changes in their gene expression profiles, or all HSCs retain their balanced vs. biased gene expressions present early in life, but aging selects for biased HSCs over balanced HSCs. Current data favor, but do not yet prove, that clonal selection of HSCs with determined differentiation fates occurs with aging (20). Newborn lymphocytes have clonally determined antigen receptors, and encounters of the antigens on microbes lead to massive expansion of the clone and differentiation of many of these daughter cells to memory lymphocytes, which have a very long life span. Because the organisms that have lymphocyte-based adaptive immune responses existed long before trains, planes, and cars, young animals with a limited geography of movement by puberty had probably encountered a high proportion of the total possible microbial pathogens in that geography, and with time the proportion of naive to memory lymphocytes goes down. The process could trigger the selection, with age, of myeloid-biased HSCs over balanced HSCs, reflecting the need to provide acute inflammatory responses for early protection, followed by the production of immune effector lymphocytes to rid the body of the pathogen. In that view, old mice and humans are living mainly on memory in the immune system, as we have sadly discovered for other bodily functions. With global travel, however, microbes that are benign to a resident population (with appropriate memory) will be highly pathogenic to naive travelers entering the pathogen’s geographic reach.
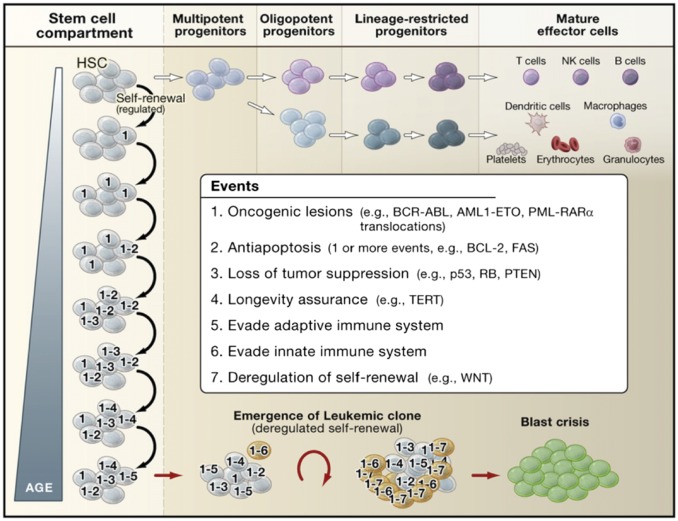
Fig. 8.
Model of leukemic progression. In this model, HSCs serve as the reservoir for the accumulation of the genetic and epigenetic events that eventually lead to blast crisis and leukemia. Stem cell self-renewal and differentiation enable heritable mutations acquired in the stem cell compartment to be propagated to both self-renewing progeny and downstream progenitors over the lifetime of the organism. Stem cells with heritable lesions act as substrates for additional hits, which in turn can promote selection of preleukemic clones if lesions imparting a growth or survival advantage are acquired. In this model, seven events are listed as the full complement of events required for the progression of preleukemic clones to leukemia, but the actual number of events may vary depending upon the type of cancer and the nature of the lesions involved as it is possible that multiple oncogenic properties could be conferred upon a preleukemic clone through the acquisition of a single hit. Although the mutagenic events accrue in stem cells, the eventual emergence of leukemic clones occurs at the stage of progenitor cells downstream of HSCs that acquire the capacity for unlimited self-renewal. In CML, this can be the granulocyte/macrophage progenitor, whereas, in acute myeloid leukemia, the leukemic clone can emerge at the level of a multipotent progenitor or at a stage further downstream depending upon the nature of the oncogenic lesions involved. Reproduced with permission from ref. 19.
Fig. 9.
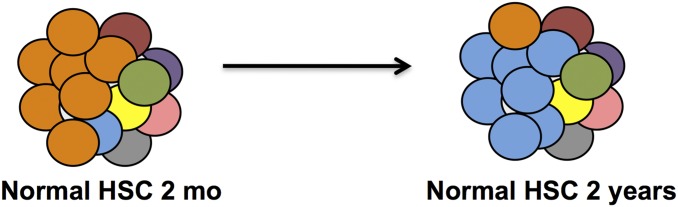
Model of aging HSCs. In young humans and mice, the HSC pool is dominated by cells that have a balance of myeloid and lymphoid lineage fates (orange circles). In older humans and mice, HSCs with a bias toward myeloid fates dominate (blue circles). Adapted from data presented in ref 20.
Stem Cell Competitions also Are the Hallmark of Cancer Development Through Cancer Stem Cells
Many events are required for normal tissue stem cells to become cancer stem cells, the self-renewing subset of cells within a cancer that show disordered differentiation. Most of these events, genetic modifications (mutations, translocations, etc.) and epigenetic changes (heritable expression of genes, e.g., increased expression of oncogenes and decreased expression of tumor suppressor genes), do not endow self-renewal, but increase survival and clonal competitiveness with the remaining normal tissue stem cells. For example, when we examined closely the gene expression of the myeloid-biased blood-forming stem cells, we noted of the 32 most highly expressed genes that act selectively as signal transduction and transcription factors for myeloid cell functions, 17 are also proto-oncogenes in human acute myelogenous leukemia (19). [Acute myelogenous leukemia is the most malignant of the cancers of the blood system myeloid (granulocytes and monocytes) lineages; it can have various subtypes that are classified by the morphology of the affected overabundant cells, the genetic pathways that give rise to it, and the clinical course of the diseases.] The emergence of myelogenous leukemias reflects, as in the colonial tunicates, that clones of blood-forming stem cell winners are being selected primarily for their competitive capacity, and most of the genes that give competitiveness tend to lead to overproduction of the cell type. We therefore looked directly at the stepwise development of myelogenous leukemias in their transition from HSCs to leukemia stem cells (LSCs).
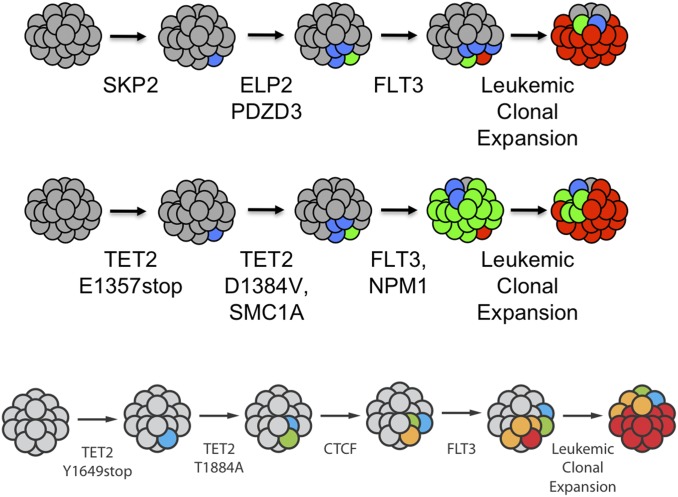
Early phases of myelogenous leukemias, exemplified by human chronic myelogenous leukemia (CML) and other myeloproliferative neoplasms, exist in a proliferative phase wherein the cells with genetic alterations outcompete their normal counterparts, but do not undergo the extreme proliferation and spread that the acute (or, in CML, myeloid blast crisis) phase cells do. Because most of the “events” do not endow self-renewal, we proposed that the preleukemic stages would have to occur in self-renewing cells, which are HSCs (Fig. 8) (21). Diseases at this early stage in humans are chronic myelogenous leukemia, myeloproliferative neoplasms, and myelodysplastic syndrome. We found that the later emergence of acute leukemia LSCs from preleukemic cells involved cells that were not at the HSC stage, but at the next step down from HSC, called multipotent progenitors (MPP) a stage in which the single cells are multipotent to make all blood cell types, but do not self-renew (Fig. 8). In the myeloid blast crisis stage of CML, a more differentiated progenitor such as the granulocyte–monocyte progenitor (GMP) stage contains the LSCs (22–24). The LSCs in the acute leukemias had undergone more genetic and epigenetic events to achieve virtually unregulated proliferation and migration that would soon kill the host. We prospectively purified HSCs and each downstream progenitor from patients with disease at the myeloproliferative stage and the acute leukemia stage (21–27). In every case the single HSC that had sustained the first mutation expanded by clonal competition at the “expense” of normal HSCs, but the total frequency of HSCs was not increased (23, 25); the early mutant cells were outcompeting the normal ones. We analyzed 21 leukemias to test the hypothesis most stringently: We used high-throughput DNA sequencing of the acute myeloid leukemia cells to determine mutations in the leukemia and then prepared DNA probes to interrogate single residual HSCs for the presence of the n mutations found in the leukemia, or the n − 1, or n − 2, or n − 3, etc., to determine the nature and the order of the preleukemic mutations that each added to the competitive advantage of the HSC clones that carried them (26, 27). Several results are shown in Fig. 10. In each of the patients studied, we could find the founder HSC clone with a single mutation, the subsequent stage with two mutations, etc., up to all but one of the mutations in clones that still could be isolated with HSC markers. In the 21 cases analyzed, most of the initiating mutations affected the ability of the cell to differentiate or to undergo epigenetic changes from the self-renewing stem cell to downstream progeny. The collection of mutations in the preleukemia cell were all present in the HSC clone (identified immunophenotypically), but in all, the last mutation occurred in the blood-forming daughter cell at the MPP or GMP stage, not at the HSC stage (23, 26, 27). The final mutations in these clones were of genes that cause massive proliferation—in the β-catenin pathway, the mutated receptor flt3 gene, or the Ras family genes (N and K) (27). The conclusion, therefore, is that the progression in preleukemia occurs in self-renewing stem cells until a more differentiated daughter cell with increased proliferative capacity (enabled by the final genetic mutation) breaks free from the proliferative constraints on the cell. However, the progression does not stop there.
Fig. 10.
Single-cell analysis identifies sequential acquisition of mutations in preleukemic HSCs. The schematic in each row shows models for the proposed clonal evolution of acute myeloid leukemia in each of three different patients. Adapted with permission from ref. 26.
A Critical Step in Cancer and Leukemia Progression Is the Up-Regulation of the CD47 “Don’t Eat Me” Signal for Phagocytic Macrophages
Preleukemic cells have sustained mutations that signal the suicide pathways in the cell, the type that result in programmed cell death (PCD). As we had shown two decades ago, PCD is accompanied by programmed cell removal (PrCR) of the dying, but not yet ruptured, cell that expresses prophagocytic (“eat me”) signals recognized by tissue macrophages, the “eating” cells of the body (28). In this way, the inflammatory contents of ruptured cells are released inside of macrophages instead of in tissue spaces. Early in the progression the most competitive of the clones block PCD by any of a number of mechanisms, but these cells have not yet defeated PrCR. From the masses of these leukemic but local cells, clones that had up-regulated cell surface CD47, which we showed to be a “don’t eat me” signal that counters PrCR, had a competitive advantage and could spread (29–31). We showed that CD47 is up-regulated on all highly malignant human and mouse cancers, as well as in the leukemias (32). Before acquisition of the CD47 “don’t eat me” signal, the evolving preleukemic and leukemic cells are restricted in their migration by surrounding macrophages that eat and kill them. With the up-regulation of CD47, these cells now are free to migrate according to their inherent potential throughout the body, causing leukemia or cancer spread, which is called metastasis. Because cancers arise only from tissues that have tissue stem cells, we propose this to be a general principle: only the tissue stem cells can accumulate the preleukemic or precancerous mutations that, taken together, with the final step of unregulated self-renewal for the proliferation and the final epigenetic events of preventing macrophages from eating them, will be at the root of the development of all human cancers.
We have shown that antibodies to CD47 that block the “don’t eat me” signal to the SIRPα receptor on macrophages result in the macrophages eating the live cancer cells (29–32). These antibodies eliminate human tumors transplanted into immune-deficient mice. A clinical trial is currently ongoing to test the potential clinical utility of these antibodies as cancer therapeutics. This follows the line of logic that we proposed from our studies of colonial tunicates in the mid-1990s, showing that what we learn in one species about behaviors—here the colonial state wherein stem cells between distinct individuals can compete for somatic and germline stem cell niches—can lead to new insights and interpretations of how normal stem cells work and how they lead to cancers. All of this demonstrates that natural selection operates at the level of “colonies” of individuals as units, at the level of individuals as units, and at the level of competing normal and neoplastic stem cells.
Supplementary Material
Acknowledgments
This is a summary of work by many individuals in the laboratory, who are cited in the references. A key finding was of the colonial tunicate stem cell competitions, discovered by the late Douglas Stoner in the laboratory. Others who developed the Botryllus model include Virginia Scofield, Baruch Rinkevich, Diana Laird, Tony DeTomaso, Aaron Newman, and for many years, Ayelet Voskoboynik. Our studies of germline and somatic tetrachimeras were carried out by Hiroo Ueno. Normal and neoplastic hematopoiesis studies were the work of Gerald Spangrude, Shelly Heimfeld, Christa Muller-Sieburg, Koichi Akashi, Motonari Kondo, David Traver, Sean Morrison, Tannishta Reya, Michael Clarke, Catriona Jamieson, Derrick Rossi, Jun Seita, Isabel Beerman, Markus Manz, and many others. Clonal progressions in leukemias also were studied by Max Jan, Ryan Corces-Zimmerman, and Ravi Majeti. And of course, the earliest citation on the possibility that natural selection acts in colonial tunicates at the level of the colony and also at the level of the individual comes from John Steinbeck, a former Stanford student who accompanied Ed Ricketts in 1941 on a specimen-finding adventure, described in The Log from the Sea of Cortez, published in 1955.
There are colonies of pelagic tunicates which have taken shape like the finger of a glove. Each member of the colony is an individual animal, but the colony is another individual animal, not like the sum of its individuals . . . So a man of individualistic reason, if he must ask, “Which is the animal?” must abandon his particular kind of reason and say, “Why, it’s two animals and they aren’t alike any more than the cells of my body are like me. I am much more than the sum of my cells, and, for all I know, they are much more than the division of me (33).
Funding for this research was provided by the NIH (Grants R01 GM100315, R01 AG037968, and R01 CA86017); the California Institute for Regenerative Medicine (Grants RC1-00354, DR1-01485, and RT2-02060); the Virginia and D. K. Ludwig Fund for Cancer Research; the Thomas and Stacey Siebel Foundation; and the Smith Family Fund.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution IX: Clonal Reproduction: Alternatives to Sex,” held January 9–10, 2015, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/ILE_IX_Clonal_Reproduction.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505464112/-/DCSupplemental.
References
- 1.Weissman IL. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Laird DJ, Weissman IL. Telomerase maintained in self-renewing tissues during serial regeneration of the urochordate Botryllus schlosseri. Dev Biol. 2004;273(2):185–194. doi: 10.1016/j.ydbio.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Scofield VL, Schlumpberger JM, West LA, Weissman IL. Protochordate allorecognition is controlled by a MHC-like gene system. Nature. 1982;295(5849):499–502. doi: 10.1038/295499a0. [DOI] [PubMed] [Google Scholar]
- 4.Voskoboynik A, et al. Identification of a colonial chordate histocompatibility gene. Science. 2013;341(6144):384–387. doi: 10.1126/science.1238036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voskoboynik A, et al. The genome sequence of the colonial chordate, Botryllus schlosseri. eLife. 2013;2:e00569. doi: 10.7554/eLife.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller CE, et al. Expression of Aire and the early wave of apoptosis in spermatogenesis. J Immunol. 2008;180(3):1338–1343. doi: 10.4049/jimmunol.180.3.1338. [DOI] [PubMed] [Google Scholar]
- 7.Stoner DS, Weissman IL. Somatic and germ cell parasitism in a colonial ascidian: Possible role for a highly polymorphic allorecognition system. Proc Natl Acad Sci USA. 1996;93(26):15254–15259. doi: 10.1073/pnas.93.26.15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoner DS, Rinkevich B, Weissman IL. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc Natl Acad Sci USA. 1999;96(16):9148–9153. doi: 10.1073/pnas.96.16.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinkevich B, Weissman IL. A long-term study on fused subclones in the ascidian Botryllus schlosseri: The resorption phenomenon (Protochordata: Tunicata) J Zool. 1987;213(4):717–734. [Google Scholar]
- 10.Laird DJ, De Tomaso AW, Weissman IL. Stem cells are units of natural selection in a colonial ascidian. Cell. 2005;123(7):1351–1360. doi: 10.1016/j.cell.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Buss LW. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc Natl Acad Sci USA. 1982;79(17):5337–5341. doi: 10.1073/pnas.79.17.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11(4):519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Turnbull BB, Weissman IL. Two-step oligoclonal development of male germ cells. Proc Natl Acad Sci USA. 2009;106(1):175–180. doi: 10.1073/pnas.0810325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmell MA, et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell. 2007;12(4):503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 16.Akashi K, Kondo M, Weissman IL. Two distinct pathways of positive selection for thymocytes. Proc Natl Acad Sci USA. 1998;95(5):2486–2491. doi: 10.1073/pnas.95.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domen J, Weissman IL. Hematopoietic stem cells and other hematopoietic cells show broad resistance to chemotherapeutic agents in vivo when overexpressing bcl-2. Exp Hematol. 2003;31(7):631–639. doi: 10.1016/s0301-472x(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16(9):2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci USA. 2010;107(12):5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissman I. Stem cell research: Paths to cancer therapies and regenerative medicine. JAMA. 2005;294(11):1359–1366. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA. 2000;97(13):7521–7526. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 24.Majeti R, et al. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci USA. 2009;106(9):3396–3401. doi: 10.1073/pnas.0900089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang WW, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci USA. 2013;110(8):3011–3016. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jan M, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science Transl Med. 2012;4(149):149ra118. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA. 2014;111(7):2548–2553. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao MP, Majeti R, Weissman IL. Programmed cell removal: A new obstacle in the road to developing cancer. Nat Rev Cancer. 2012;12(1):58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 32.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinbeck J. The Log of the Sea of Cortez. Penguin Books; New York: 1955. pp. 136–137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.