Abstract
Background
Exercise stimulates bone remodeling and improves insulin sensitivity (Si), even without associated weight loss. Osteocalcin (OCN), a bone-derived protein, is associated with improved Si.
Purpose
We examined how daily physical activity is associated with OCN and Si.
Methods
Physical activity was measured through questionnaires completed in Minneapolis from 2010 to 2012. A physical activity score (PAQsum) was calculated to quantify physical activity (range 1–5). OCN and bone specific alkaline phosphatase (BAP) were measured by ELISA. Si was measured by the insulin modified frequently sampled IV glucose tolerance test.
Results
The mean PAQsum value was 2.4 ± 0.8 in 47 participants (12–17.9 years old). PAQsum was positively associated with OCN (p = 0.006). Participants with PAQsum < 2 had significantly lower OCN levels compared to participants with PAQsum > 2 (p < 0.02). Obesity did not modify the association between PAQsum and OCN. There was no statistically significant association between PAQsum and Si or between OCN and Si, even after adjustment for percent body fat.
Conclusions
OCN is higher in more physically active individuals. More research is needed to clarify the relationship between OCN, physical activity and Si.
Abbreviations: OCN, osteocalcin; PAQsum, physical activity score; Si, insulin sensitivity; BAP, bone specific alkaline phosphatase; HOMA-IR, HOMA-Insulin Resistance; FSIGT, frequently sampled intravenous glucose tolerance test
Keywords: Osteocalcin, Adolescents, Obesity, Physical activity
Highlights
-
•
We examined the influence of physical activity on OCN.
-
•
Moderate physical activity is associated with higher levels of OCN.
-
•
Obesity did not impact the positive association between physical activity and OCN.
-
•
OCN may play a role in the insulin sensitizing effects of physical activity.
Introduction
It is estimated that over 300 million adults worldwide will be living with diabetes in 2030 (Mathers and Loncar, 2006). Since β-cell dysfunction can begin more than 10 years before type 2 diabetes presents (Hussain et al., 2007), and type 2 diabetes is being diagnosed in children and adolescents, childhood interventions are critically important in preventing diabetes and its long-term complications, particularly for populations with known risk factors.
Exercise decreases the risk for type 2 diabetes by mitigating insulin resistance (Andersen et al., 2006, Ekelund et al., 2007, Jago et al., 2008, King et al., 1987, Kirwan et al., 2000, Parrett et al., 2011, Rizzo et al., 2008, Rochefort et al., 2011, Ruiz et al., 2006, Schmidt et al., 2008, Simmons et al., 2008). Lower levels of physical activity observed in children between the ages of 9 and 15 years old are associated with higher markers of insulin resistance as measured by HOMA-Insulin Resistance (HOMA-IR) and higher fasting insulin levels (Jago et al., 2008). Compared to other cardiovascular disease risk factors in children, including systolic blood pressure, total cholesterol, triglycerides and fasting insulin, HOMA-IR has the strongest association with physical inactivity (Andersen et al., 2006). Changes in insulin resistance with physical activity are not solely mediated by weight loss, since physical activity can improve insulin sensitivity with or without weight loss (Boulé et al., 2001, Duncan et al., 2003, Nassis et al., 2005). In addition, physical activity modulates insulin sensitivity independent of body mass index (BMI) (Brage et al., 2004, Ekelund et al., 2007, Rizzo et al., 2008, Ruiz et al., 2006). Adiposity is positively correlated with insulin resistance (Rizzo et al., 2008), but even in the context of significant adiposity (BMI > 25) physical activity is still protective against metabolic disease (Parrett et al., 2011, Rizzo et al., 2008, Simmons et al., 2008).
Mouse and human studies have demonstrated that serum OCN, a protein expressed by osteoblasts during bone mineralization (Lian et al., 1998, Pi and Quarles, 2013), is associated with higher insulin sensitivity (Fernández-Real et al., 2009, Ferron et al., 2008, Hwang et al., 2009, Kanazawa et al., 2009, Lee et al., 2007, Lee and Karsenty, 2008, Lian et al., 1998, Pittas et al., 2009, Reinehr and Roth, 2010). As plasma OCN levels increase, fasting and post-challenge serum glucose levels (Hwang et al., 2009, Lee et al., 2007, Pittas et al., 2009) and HOMA-IR significantly decrease (Hwang et al., 2009, Pittas et al., 2009, Reinehr and Roth, 2010). The carboxylated and uncarboxylated forms of OCN in plasma have also been studied and both are found to be inversely related to fasting and post-challenge glucose levels after adjustment for age and BMI. The uncarboxylated form of OCN is associated with improved β-cell function and the carboxylated form is associated with improved insulin sensitivity (Si) (Hwang et al., 2009). Moreover, daily injections of uncarboxylated OCN increase insulin sensitivity in mice (Ferron et al., 2012). OCN also appears to enhance β-cell function based on HOMA-%B (Hwang et al., 2009) and β-cell proliferation (Ferron et al., 2008, Kanazawa et al., 2009, Lee et al., 2007). In addition, higher OCN levels in adults with type 2 diabetes mellitus are associated with better glycemic control (Okazaki et al., 1997). Interestingly, total OCN levels increase with endurance, aerobic and strength exercise interventions (Cooper, 1999, Fernández-Real et al., 2009, Kim et al., 2015). Since both OCN and physical activity are associated with higher insulin sensitivity, research is needed to clarify the relationship between OCN, physical activity and Si. Although OCN increases with aerobic and resistance exercise interventions, it is unknown whether OCN levels are related to routine daily physical activity.
This study examined whether OCN levels were associated with daily physical activity in adolescents. It is hypothesized that higher levels of daily physical activity would be associated with higher OCN levels and better insulin sensitivity. If confirmed, this observation would further advance the understanding of the relationship between OCN, insulin sensitivity and physical activity.
Methods
Participants
Obese (BMI > 95%) and normal weight children, age 8 to 17 years old, were recruited to study OCN and Si from Fairview University Clinics and the community via posted advertisements. Adolescents with diabetes, those taking medication that altered insulin sensitivity, secretion or β-cell mass, individuals concurrently participating in an intervention trial, or individuals who were pregnant were excluded. Overweight children (BMI z score of 1–1.49) were excluded from the study while normal (BMI − 1 to 0.99) and obese (BMI 1.5 or greater) children were included. This study was approved by the University of Minnesota Institutional Review Board. Written informed consent was obtained from all parents/guardians, and assent from all participants.
Measurements
Following a minimum 10-hour fast, all participants underwent an insulin modified frequently sampled intravenous glucose tolerance test with 32 blood samples over 3 h (FSIGT) (Bergman et al., 1979, Boston et al., 2005), phlebotomy, physical examination for pubertal stage by Tanner (Marshall and Tanner, 1969, Marshall and Tanner, 1970), anthropometrics, and body composition by dual-energy x-ray absorptiometer (GE Lunar iDXA, enCORE software version 13.6; General Electric Medical Systems, Madison, WI). Total OCN was measured in serum using the MicroVue Osteocalcin EIA kit from Quidel Corporation (San Diego, CA) (intra-assay CV 3.1%). Bone-specific alkaline phosphatase (BAP), another marker of bone formation, was measured in serum using the MicroVue BAP EIA kit from Quidel Corporation (San Diego, CA).
Physical activity was measured through questionnaires completed by subjects, with the assistance of their parent/guardian. These questionnaires were completed at the time of initial assessment from 2010 to 2012. The Physical Activity Questionnaire for Older Children was completed by participants less than 14 years old and the Physical Activity Questionnaire for Adolescents was for participants aged 14–17 years old. In these questionnaires, the participant must recall how often they completed an activity in the last week and the responses are quantified as a score of 1 through 5 (“no” activity = 1 and “7 times or more” = 5). A physical activity composite score or Physical Activity Questionnaire summation score (PAQsum) is calculated as the average response reported, excluding questions left unanswered. A PAQsum of 1 indicates low physical activity, whereas a score of 5 indicates high physical activity. PAQsum is a reliable and valid measure of physical activity in children that is used to differentiate between active and inactive children (Crocker et al., 1997, Kowalski et al., 1997a, Kowalski et al., 1997b).
For the FSIGT, two peripheral intravenous lines were inserted in a superficial vein in each arm 30 min prior to blood draws. A dose of 0.3 g/kg body weight (50% dextrose) was manually injected over 1 min. Time zero was taken at the end of the dextrose infusion. Blood samples were collected every 1–20 min for 180 min after dextrose infusion. Insulin 0.05 units/kg body weight was given as an IV push at time + 20 min. Insulin sensitivity (Si) was determined by the MINMOD method for interpretation of FSIGT (Bergman et al., 1979, Boston et al., 2005). Si measures the capacity of insulin to promote the clearance of glucose and inhibit endogenous glucose production.
Statistical analysis
Descriptive statistics are presented as means (standard deviation) for continuous variables and as number (percentages) for nominal variables. All skewed variables (total OCN, BAP, Si) were log transformed for analysis. The association between total OCN and PAQsum was analyzed using linear regression with and without adjustment for age, sex, Tanner stage, and BAP; the interaction of obesity with PAQsum association with OCN was evaluated as well. The association between total OCN and Si was analyzed using linear regression with adjustment for age, sex, and Tanner stage. Tanner stage was categorized as either < 3 or ≥ 3 for analysis, and prepubertal subjects (Tanner stage 1) were excluded. Individuals were separated by PAQsum categories (PAQsum < 2, 2–3, and > 3) and the respective OCN and Si levels were tested by ANOVA. For all statistical analysis an alpha level of less than 0.05 was used to determine statistical significance. The analysis was conducted between 2013 and 2015 after all data was collected.
Results
Fifty-four (26 obese and 28 non-obese) participants were enrolled in the study. Seven participants were excluded from analysis: 3 with polycystic ovarian syndrome, 1 with incomplete PAQ data, and 3 with Tanner stage 1. Characteristics of the remaining 47 participants are described in Table 1. PAQsum ranged from 1 to 4 among participants. There was no significant difference in PAQsum between sexes (p = 0.9) or Tanner stage (p = 0.2).
Table 1.
Subject characteristics and comparison of obese to normal weight subjects.
| All (n = 47) |
Obese (n = 22) |
Normal weight (n = 25) |
|
|---|---|---|---|
| Age, years | 14.9 ± 1.7 | 15.0 ± 1.7 | 14.9 ± 1.7 |
| BMI, percentile⁎ | 74 ± 28 | 99 ± 1 | 53 ± 22 |
| Body fat, %⁎ | 36 ± 11 | 45 ± 6 | 27 ± 7 |
| PAQsum | 2.4 ± 0.8 | 2.3 ± 0.9 | 2.4 ± 0.8 |
| Gender, female | 26 (55) | 11 (50) | 15 (60) |
| Tanner stage | |||
| 2–3 | 8 (17) | 5 (23) | 3 (12) |
| 4–5 | 39 (83) | 17 (77) | 22 (88) |
| OCN, ng/mL | 14 (11–18) | 16 (13–20) | |
| BAP, U/L | 64 (46–88) | 61 (45–82) | |
| Si⁎, (mU/L)− 1 min− 1 | 1.5 (1.2–1.8) | 4.1 (3–5) |
Values are mean ± SD for non-skewed variables, geometric mean (95% confidence interval) for skewed variables, or N(%) for categorical variables.
BMI = body mass index; PAQsum = Physical Activity Questionnaire summation score; OCN = osteocalcin; BAP = bone specific osteocalcin; Si = insulin sensitivity.
Study completed in Minneapolis 2010–2012.
Significant differences (p < 0.05) between obese vs. normal weight subjects.
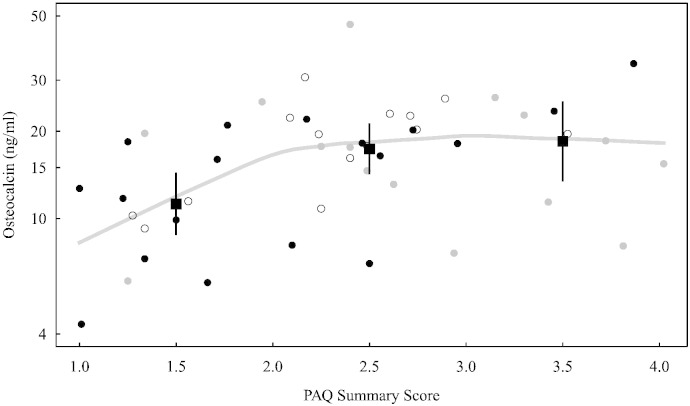
PAQsum was significantly associated with both OCN (correlation r = 0.39, p = 0.006 (Fig. 1)) and BAP (r = 0.30, p = 0.043). PAQsum remained significantly associated with OCN after adjustment for age, sex, Tanner stage (p = 0.022) and BAP (p = 0.046) and percent body fat (p = 0.044). Participants with low physical activity had significantly lower OCN levels compared to participants with either moderate or high physical activity (Table 2). There was a plateau in OCN levels at a PAQsum score of greater than 2 (Fig. 1). There was no interaction between obesity and the association of PAQsum with OCN.
Fig. 1.
Osteocalcin and PAQ summary score for 47 participants in Minneapolis 2010–2012. Classified by tertile of insulin sensitivity (Si): black dots indicate those with lowest Si, gray dots indicate medium Si, and open circles indicate highest Si. The curve is a nonparametric estimate (lowess) of mean osteocalcin. Mean osteocalcin within each PAQ summary group (1–2, 2–3, 3–4) is indicated by filled squares with 95% confidence intervals.
Table 2.
Comparison of OCN, insulin sensitivity, and adiposity by PAQsum tertiles.
| Low activity < 2 (n = 15) |
Moderate activity 2–3 (n = 23) |
High activity > 3 (n = 9) |
|
|---|---|---|---|
| Age, years | 15.8 ± 1.6a | 14.5 ± 1.7b | 14.4 ± 1.4b |
| OCN, ng/mL | 11 (9–14)a | 17 (14–21)b | 18 (14–25)b |
| BAP, U/L | 43 (30–63)a | 72 (53–97)b | 78 (48–125)ab |
| Si, (mU/L)− 1 min− 1 | 2.1 (1–3) | 3.1 (2–4) | 2.6 (2–4) |
| BMI, percentile | 79 ± 31 | 71 ± 26 | 75 ± 29 |
| Body fat, % | 40 ± 10a | 32 ± 10b | 36 ± 12ab |
Values are mean ± SD or geometric mean (95% confidence interval) or N(%).
Letters indicate significant differences (p < 0.05) between groups. No letters indicate no significant differences.
BMI = body mass index; PAQsum = Physical Activity Questionnaire summation score; OCN = osteocalcin; BAP = bone specific osteocalcin; Si = insulin sensitivity.
Study completed in Minneapolis 2010–2012.
Although there were higher levels of Si in the moderate activity (PAQsum 2–3) group compared to the low activity (PAQsum < 2) group (p = 0.08) and there was no further improvement in Si in the high activity group. After controlling for sex, age, and Tanner score, there was no statistically significant association between Si and PAQsum, whether or not percent body fat was included as an adjustor (Table 2). Si was also not positively associated with OCN after adjustment for age, sex, percent body fat, and Tanner stage (p = 0.555).
Discussion
Moderate to high levels of unstructured physical activity were associated with an elevated level of OCN compared to a low level of physical activity. This association of OCN with physical activity was independent of another marker of bone formation (BAP) suggesting a unique role for OCN in the enhancement of Si through physical activity. Previous research has identified a positive correlation between OCN and exercise interventions (Cooper, 1999, Fernández-Real et al., 2009, Reinehr and Roth, 2010, Rochefort et al., 2011); however, the unique contribution of this study was to demonstrate an association between moderate and high levels of unstructured daily physical activity and elevated OCN levels in older children. Higher levels of OCN observed in the moderate and high physical activity groups represent another potentially beneficial effect of physical activity and exercise, further supporting the importance of promoting physical activity in children and adolescents.
The association between serum OCN and physical activity has not been well characterized. Research has shown that children and adults who complete exercise interventions, ranging from 1 month to 1 year, all demonstrate significant increases in OCN levels (Cooper, 1999, Fernández-Real et al., 2009, Reinehr and Roth, 2010, Rochefort et al., 2011). Acute increases in OCN were found as soon as two days after a triathlon (Guadalupe-Grau et al., 2010). Two different pediatric studies both demonstrated OCN increases in parallel with insulin sensitivity after exercise intervention (Reinehr and Roth, 2010, Rochefort et al., 2011). Both interventional studies provided participants with trainers or coaches who created structured exercise programs. None of these studies addressed how unstructured play, activity during free time or the levels of daily physical activity affect OCN levels. The PAQsum measurement of physical activity does not quantify the time spent in vigorous activities but does identify how physically active a child has been in the week prior. Therefore, this study demonstrates that OCN is positively associated with even unstructured daily physical activity.
Although this study did not find that the relationship between OCN and physical activity was affected by BMI, OCN has been negatively associated with BMI and fat mass (Boucher Berry et al., 2012, Fernández-Real et al., 2009, Foresta et al., 2010, Hwang et al., 2009, Kanazawa et al., 2009, Kindblom et al., 2009, Pittas et al., 2009, Reinehr and Roth, 2010), even after adjusting for differences in physical activity (Pittas et al., 2009, Reinehr and Roth, 2010), and changes in BMI after exercise training have been shown to be significantly correlated with changes in OCN (Kim et al., 2015). Obese children have higher insulin resistance and leptin concentrations with decreased serum OCN and adiponectin levels in comparison to normal weight children (Reinehr and Roth, 2010). Since leptin is elevated in patients with high adiposity and has an inverse relationship with OCN, leptin has been suggested to mediate the relationship between OCN, insulin sensitivity and adiposity (Gravenstein et al., 2011). Interestingly, lean adults have demonstrated a stronger association between OCN and insulin sensitivity than obese adults (Fernández-Real et al., 2009) suggesting that these associations are important to evaluate in both normal and obese individuals. While muscle mass, leptin and adiposity may affect the relationship between physical activity and OCN, the results of this study demonstrate that OCN and physical activity uphold a significant association independent of BMI. Therefore, the benefits of physical activity on OCN levels span across all BMI and suggest that all adolescents and children, from normal weight to obese, may experience a beneficial effect from even mild to moderate physical activity.
This study was limited by the relatively small sample size and indirect measurement of physical activity — the PAQ does not measure caloric expenditure, intensity or duration of physical activity. This study reported total serum OCN and did not clarify how uncarboxylated osteocalcin may have unique relationships between physical activity and insulin sensitivity or evaluate the role of leptin in these relationships. However it is strengthened by the direct measurement of FSIGT versus the use of surrogate measures of insulin resistance such as fasting insulin. In addition, the participants in this study completed the PAQ during summer break and therefore questions about recess and after school activities in the PAQ were excluded in those individuals. Despite these limitations, this is the first study to evaluate the relationship of OCN with daily physical activity levels and explore the role of OCN in the insulin sensitizing effects of physical activity.
In conclusion, unstructured daily activity during free time has a positive relationship with OCN. Unstructured play is the most practical intervention to modulate OCN, promote health and possibly delay or prevent the onset of obesity related complications in all children. This study provides further support for initiatives which aim to integrate more physical activity into the daily routines of children to improve health outcomes and prevent diabetes. Studies have established that OCN is associated with increased insulin sensitivity but more research is needed to investigate how unstructured play, as well as bone focused interventions, may affect OCN, leptin and insulin sensitivity.
Conflict of interest statement
LEP, WT, BF, SC, and ASK have nothing to declare. BN receives grant support from Medtronic (NCT01991470).
Financial disclosure
No financial disclosures were reported by the authors of this paper.
Acknowledgments
We gratefully acknowledge the study participants and parents as well as Jane Kennedy, R.N., who made this project possible. This project was supported by Grant Number K23AR057789 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), by the Irvine McQuarrie Clinical Research Scholar Award, and by Grant Number 8UL1TR000114-02 from the National Center for Advancing Translational Sciences (NCATS) and Translational Science Institute (CTSI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH.
Contributor Information
Saydi E. Chahla, Email: chah0004@umn.edu.
Brigitte I. Frohnert, Email: brigitte.frohnert@ucdenver.edu.
William Thomas, Email: thoma003@umn.edu.
Aaron S. Kelly, Email: kelly105@umn.edu.
Brandon M. Nathan, Email: natha039@umn.edu.
Lynda E. Polgreen, Email: lpolgreen@labiomed.org.
References
- Andersen L.B., Harro M., Sardinha L.B. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- Bergman R.N., Ider Y.Z., Bowden C.R., Cobelli C. Quantitative estimation of insulin sensitivity. Am. J. Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- Boston R.C., Moate P.J., Stefanovski D., Sumner A.E., Bergman R.N. AKA-glucose: a program for kinetic and epidemiological analysis of frequently sampled intravenous glucose tolerance test data using database technology. Diabetes Technol. Ther. 2005;7:298–307. doi: 10.1089/dia.2005.7.298. [DOI] [PubMed] [Google Scholar]
- Boucher Berry C., Speiser P.W., Carey D.E. Vitamin D, osteocalcin, and risk for adiposity as comorbidities in middle school children. J. Bone Miner. Res. 2012;27:283–293. doi: 10.1002/jbmr.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulé N.G., Haddad E., Kenny G.P., Wells G.A., Sigal R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- Brage S., Wedderkopp N., Ekelund U. Objectively measured physical activity correlates with indices of insulin resistance in Danish children. The European Youth Heart Study (EYHS) Int. J. Obes. Relat. Metab. Disord. 2004;28:1503–1508. doi: 10.1038/sj.ijo.0802772. [DOI] [PubMed] [Google Scholar]
- Cooper D.M. Exercise, growth, and the GH-IGF-I axis in children and adolescents. Curr. Opin. Endocrinol. Diabetes. 1999;6(2):106–111. [Google Scholar]
- Crocker P.R., Bailey D.A., Faulkner R.A., Kowalski K.C., McGrath R. Measuring general levels of physical activity: preliminary evidence for the physical activity questionnaire for older children. Med. Sci. Sports Exerc. 1997;29:1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- Duncan G.E., Perri M.G., Theriaque D.W., Hutson A.D., Eckel R.H., Stacpoole P.W. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557–562. doi: 10.2337/diacare.26.3.557. [DOI] [PubMed] [Google Scholar]
- Ekelund U., Griffin S.J., Wareham N.J. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care. 2007;30:337–342. doi: 10.2337/dc06-1883. [DOI] [PubMed] [Google Scholar]
- Fernández-Real J.M., Izquierdo M., Ortega F. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J. Clin. Endocrinol. Metab. 2009;94:237–245. doi: 10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- Ferron M., Hinoi E., Karsenty G., Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M., McKee M.D., Levine R.L., Ducy P., Karsenty G. Vol. 50. 2012. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice; pp. 568–575. (Bone, Interactions Between Bone, Adipose Tissue and Metabolism). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C., Strapazzon G., De Toni L. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J. Clin. Endocrinol. Metab. 2010;95:3502–3506. doi: 10.1210/jc.2009-2557. (jc.2009–2557) [DOI] [PubMed] [Google Scholar]
- Gravenstein K.S., Napora J.K., Short R.G., Ramachandran R., Carlson O.D., Metter E.J. Cross-Sectional Evidence of a Signaling Pathway from Bone Homeostasis to Glucose Metabolism. J Clin Endocrinol Metab. 2011;96(6):E884–E890. doi: 10.1210/jc.2010-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe-Grau A., Ara I., Dorado C. Osteocalcin as a negative regulator of serum leptin concentration in humans: insight from triathlon competitions. Eur. J. Appl. Physiol. 2010;110:635–643. doi: 10.1007/s00421-010-1550-3. [DOI] [PubMed] [Google Scholar]
- Hussain A., Claussen B., Ramachandran A., Williams R. Prevention of type 2 diabetes: a review. Diabetes Res. Clin. Pract. 2007;76:317–326. doi: 10.1016/j.diabres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Hwang Y.-C., Jeong I.-K., Ahn K.J., Chung H.Y. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab. Res. Rev. 2009;25:768–772. doi: 10.1002/dmrr.1045. [DOI] [PubMed] [Google Scholar]
- Jago R., Wedderkopp N., Kristensen P.L. Six-year change in youth physical activity and effect on fasting insulin and HOMA-IR. Am. J. Prev. Med. 2008;35:554–560. doi: 10.1016/j.amepre.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Yamaguchi T., Yamamoto M. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009;94:45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- Kim Y.-S., Nam J.S., Yeo D.-W., Kim K.R., Suh S.-H., Ahn C.W. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin. Endocrinol. (Oxf.) 2015;82:686–694. doi: 10.1111/cen.12601. [DOI] [PubMed] [Google Scholar]
- Kindblom J.M., Ohlsson C., Ljunggren Ö. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J. Bone Miner. Res. 2009;24:785–791. doi: 10.1359/jbmr.081234. [DOI] [PubMed] [Google Scholar]
- King D.S., Dalsky G.P., Staten M.A., Clutter W.E., Van Houten D.R., Holloszy J.O. Insulin action and secretion in endurance-trained and untrained humans. J. Appl. Physiol. 1987;63:2247–2252. doi: 10.1152/jappl.1987.63.6.2247. [DOI] [PubMed] [Google Scholar]
- Kirwan J.P., del Aguila L.F., Hernandez J.M. Regular exercise enhances insulin activation of IRS-1-associated PI3-kinase in human skeletal muscle. J. Appl. Physiol. 2000;88:797–803. doi: 10.1152/jappl.2000.88.2.797. [DOI] [PubMed] [Google Scholar]
- Kowalski K.C., Crocker Peter R.E., Kowalski N.P. Convergent validity of the physical activity questionnaire for adolescents. Pediatr. Exerc. Sci. 1997;9:342–352. [Google Scholar]
- Kowalski K.C., Crocker P.R.E., Faulkner R.A. Validation of the physical activity questionnaire for older children. Pediatr. Exerc. Sci. 1997;9:74–186. [Google Scholar]
- Lee N.K., Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol. Metab. 2008;19:161–166. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Lee N.K., Sowa H., Hinoi E. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J.B., Stein G.S., Stein J.L., van Wijnen A.J. Osteocalcin gene promoter: unlocking the secrets for regulation of osteoblast growth and differentiation. J. Cell. Biochem. Suppl. 1998;30–31:62–72. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<62::AID-JCB10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassis G.P., Papantakou K., Skenderi K. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Totsuka Y., Hamano K. Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J. Clin. Endocrinol. Metab. 1997;82:2915–2920. doi: 10.1210/jcem.82.9.4258. [DOI] [PubMed] [Google Scholar]
- Parrett A.L., Valentine R.J., Arngrímsson S.A., Castelli D.M., Evans E.M. Adiposity and aerobic fitness are associated with metabolic disease risk in children. Physiol. Appliquée Nutr. MétabolismeAppl. Physiol. Nutr. Metab. 2011;36:72–79. doi: 10.1139/H10-083. [DOI] [PubMed] [Google Scholar]
- Pi M., Quarles L.D. Novel bone endocrine networks integrating mineral and energy metabolism. Curr. Osteoporos. Rep. 2013;11:391–399. doi: 10.1007/s11914-013-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas A.G., Harris S.S., Eliades M., Stark P., Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J. Clin. Endocrinol. Metab. 2009;94:827–832. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T., Roth C.L. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int. J. Obes. 2010;2005(34):852–858. doi: 10.1038/ijo.2009.282. [DOI] [PubMed] [Google Scholar]
- Rizzo N.S., Ruiz J.R., Oja L., Veidebaum T., Sjöström M. Associations between physical activity, body fat, and insulin resistance (homeostasis model assessment) in adolescents: the European Youth Heart Study. Am. J. Clin. Nutr. 2008;87:586–592. doi: 10.1093/ajcn/87.3.586. [DOI] [PubMed] [Google Scholar]
- Rochefort G.Y., Rocher E., Aveline P.C. Osteocalcin–insulin relationship in obese children: a role for the skeleton in energy metabolism. Clin. Endocrinol. (Oxf.) 2011;75:265–270. doi: 10.1111/j.1365-2265.2011.04031.x. [DOI] [PubMed] [Google Scholar]
- Ruiz J.R., Rizzo N.S., Hurtig-Wennlöf A., Ortega F.B., Wärnberg J., Sjöström M. Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am. J. Clin. Nutr. 2006;84:299–303. doi: 10.1093/ajcn/84.1.299. [DOI] [PubMed] [Google Scholar]
- Schmidt M.D., Cleland V.J., Thomson R.J., Dwyer T., Venn A.J. A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann. Epidemiol. 2008;18:378–386. doi: 10.1016/j.annepidem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Simmons R.K., Griffin S.J., Steele R., Wareham N.J., Ekelund U. Increasing overall physical activity and aerobic fitness is associated with improvements in metabolic risk: cohort analysis of the ProActive trial. Diabetologia. 2008;51:787–794. doi: 10.1007/s00125-008-0949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]