Abstract
Magnetic resonance imaging (MRI) is highly sensitive in identifying residual breast cancer following neoadjuvant chemotherapy (NAC), and consequently is a commonly used imaging modality in locally advanced breast cancer patients. In these patients, tumor response is an important prognostic indicator. However, discrepancies between MRI findings and surgical pathology are well documented. Overestimation of residual disease by MRI may result in greater surgery than is actually required while underestimation may result in insufficient surgery. Thus, it is important to understand when MRI findings are reliable and when they are less accurate. MRI most accurately predicts pathology in triple negative, Her2 positive and hormone receptor negative tumors, especially if they are of a solid imaging phenotype. In these cases, post-NAC MRI is highly reliable for surgical planning. Hormone receptor positive cancers and those demonstrating non mass enhancement show lower concordance with surgical pathology, making surgical guidance more nebulous in these cases. Radiologists and surgeons must assess MRI response to NAC in the context of tumor subtype. Indiscriminate interpretations will prevent MRI from achieving its maximum potential in the pre-operative setting.
Keywords: Breast, Magnetic resonance imaging, Neoadjuvant chemotherapy, Biomarkers, Phenotypes
Core tip: Following neoadjuvant chemotherapy, breast magnetic resonance imaging (MRI) most accurately predicts surgical pathology in triple negative, Her2 positive and hormone receptor negative tumors, especially if they are of a solid imaging phenotype. In these cases, post-neoadjuvant chemotherapy (NAC) MRI is highly reliable for surgical planning. Hormone receptor positive cancers and those demonstrating non mass enhancement show lower concordance with surgical pathology, making surgical guidance more nebulous in these cases. Radiologists and surgeons must assess MRI response to NAC in the context of tumor subtype.
INTRODUCTION
Breast cancer is a heterogeneous disease consisting of many different tumor subtypes, each with its own biology, prognosis, and treatment options. These subtypes are characterized by distinct molecular profiles, proliferation rates, and tumor receptors, including estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). In today’s paradigm of personalized medicine, biomarker profiles allow tailoring treatment strategies to the individual tumor. Current treatment of locally advanced breast cancers includes chemotherapy, hormone therapy [if hormone receptor (HR) positive] and surgical resection. Increasingly, chemotherapy is given prior to surgery. Neoadjuvant chemotherapy (NAC) offers advantages in terms of adding prognostic information and improving surgical options. Tumor response to NAC is an important prognostic indicator. Patients who have a pathologic complete response (pCR) following NAC have improved overall survival, disease-free survival and recurrence-free survival[1-6]. NAC can also facilitate breast-conserving surgery in patients whose initial presentation may have warranted mastectomy[7-9]. Even if patients still have residual disease, especially if they need radiation, breast conservation will have fewer complications than mastectomy and radiation. As treatments improve and responses to NAC become more common, a new challenge arises - accurately determining the extent of surgical resection needed to excise residual tumor. Magnetic resonance imaging (MRI) is highly sensitive in identifying residual disease following NAC, with multiple studies demonstrating it to be more accurate than mammography, ultrasound or physical examination[10-16]. Consequently, MRI is a commonly used imaging modality in locally advanced breast cancer patients.
In these patients, pre-operative MRI is an important addition to the decision-making armamentarium. The appearance of breast cancer on MRI can be classified by its morphology into phenotypic categories[17], which are associated with response to NAC and ability to offer breast-conserving surgery[17,18]. Overall, MRI has been shown to be the most sensitive imaging modality by which to follow a patient’s response to NAC and to be more sensitive than clinical examination[11-16,19-23]. While an excellent test, MRI is far from perfect. Discrepancies between MRI findings and surgical pathology are well documented. Overestimation of residual disease by MRI may result in greater surgery than is actually required (larger lumpectomies, wider margins, mastectomy)[1,24]. Underestimation may result in insufficient surgery, resulting in positive margins and re-excisions[1]. Thus, it is important to understand when MRI findings [particularly radiologic complete responses (rCR)] are reliable and when they are less accurate.
The general question of the accuracy of an rCR to predict a pCR may be overly broad - accuracy needs to be considered in the context of tumor subtype. Literature has shown that the accuracy of post-NAC MRI is related to tumor subtype, with the strongest evidence arising from multi-institutional trials like I-SPY[18] and Translational Breast Cancer Research Consortium Trial 017[25], as well as additional support from multiple single-institution studies[26-29]. A smaller literature base suggests that MRI phenotype is also related to the accuracy of MRI in the post-NAC, pre-operative setting.
In this manuscript, we review the evidence for accuracy of post-NAC MRI findings and focus on how best to use MRI in this setting, specifically for the evaluation of extent of disease and pCR. In particular, this review will evaluate the association between the diagnostic performance of MRI in the post-NAC setting and the biomarker profile of the tumor, as well as the association between pre-NAC phenotypic tumor appearance on MRI and diagnostic accuracy. A clear understanding of these relationships can be valuable in setting appropriate treatment goals and expectations[18]. In much the same way each breast cancer requires a tailored treatment strategy, a strategy for tailored imaging interpretation should also be employed and would enable more accurate recommendations to be made for individual patients.
ASSOCIATIONS WITH MRI PHENOTYPE
The relationship between phenotypic MRI appearance of breast cancers and response to NAC has been studied[17,29]. Although phenotypic categorizations vary slightly, in general, phenotypes tend to focus on the separation of solid and well-contained unifocal (Figure 1A) and multifocal masses from more diffuse and infiltrative non-mass enhancement (NME) (Figure 1B)[17,18,29]. These phenotypes impact NAC response, with well-defined mass phenotypes more likely to have a response sufficient to allow for breast conserving surgery[17,18]. Well-defined masses also show higher concordance between MRI and surgical pathology, with an rCR in the setting of solid phenotypes (particularly hormone-negative tumors) predictive of a corresponding pCR at surgery[18]. On the other hand, MRI is less accurate in predicting pCR in tumors presenting as non-mass/diffuse enhancement, with larger discrepancies between post-NAC MRI and surgical pathology[18].
Figure 1.
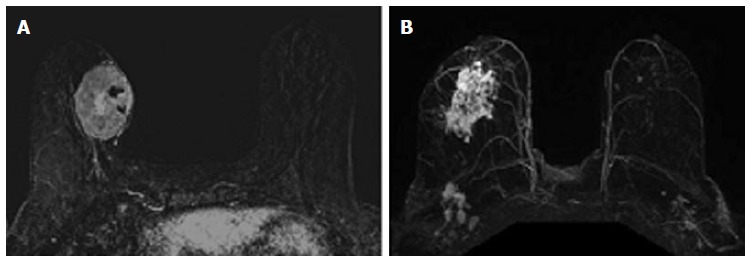
Magnetic resonance imaging phenotypes solid unifocal mass (A) and more diffuse non-mass enhancement (B).
Studies have also suggested that these differing phenotypic appearances have particular patterns of response to NAC[17,24,29-31]. Locally advanced malignancies presenting as a mass lesion often shrink in a concentric pattern to a smaller mass. Following NAC, NME often diminishes to a scattered pattern of residual disease that can extend throughout the original area of involvement, though as small foci that are difficult to detect on MRI. Residual infiltrating single cells will likely not be visible on MRI.
The associations between MRI accuracy and phenotype are likely confounded by tumor biomarker status. Comparisons of MRI phenotypes relative to tumor biomarker profiles[18,24,28,29,32,33] have shown a number of trends, with an association between unifocal mass presentation and triple negative tumors (TN: ER negative, PR negative, Her2 negative) (Figure 2). Multifocal mass presentation is more common in HER2+ (and questionably in HR positive) tumors. Although they do not have a characteristic phenotypic presentation, HR positive cases, especially ER positive tumors, are more likely to present as non mass/diffuse enhancement compared to other subtypes (Figures 3 and 4). Although these relationships have been demonstrated, all phenotypes are seen in all biomarker profiles[18].
Figure 2.

Forty-two years old woman with triple negative right breast cancer. A: Baseline axial T1-weighted post-gadolinium fat-saturated magnetic resonance image demonstrates a 3.6 cm unifocal mass in the upper outer quadrant; B: Post-neoadjuvant chemotherapy magnetic resonance imaging demonstrates complete resolution of the mass seen previously. Surgical pathology demonstrates biopsy site changes and expected changes related to chemotherapy with no evidence of residual cancer.
Figure 3.
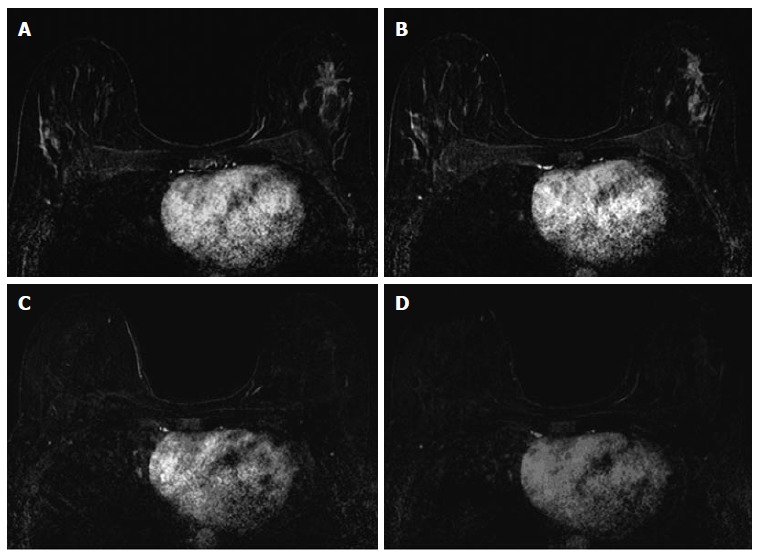
Thirty-seven years old woman with HR+ left breast cancer. A and B: Baseline axial T1-weighted post-gadolinium fat-saturated magnetic resonance image demonstrates a speculated mass and contiguous non-mass enhancement extending posteriorly for a total of 7 cm of disease in the upper outer breast; C and D: Post-neoadjuvant chemotherapy magnetic resonance imaging demonstrates decrease in size and degree of enhancement of prior findings. Surgical pathology demonstrates 6.9 cm of invasive ductal carcinoma.
Figure 4.
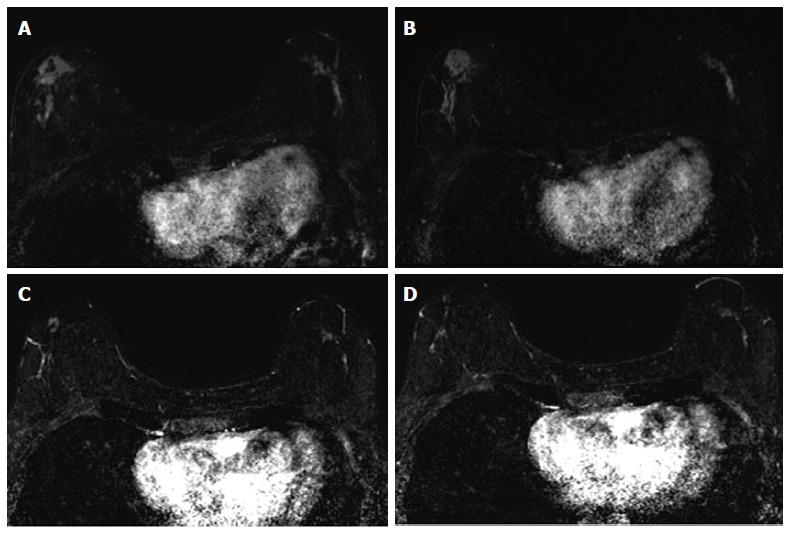
Sixty-four years old woman with bilateral HR+ breast cancer. A and B: Baseline axial T1-weighted post-gadolinium fat-saturated magnetic resonance image demonstrates 3.2 cm irregular mass and contiguous non-mass enhancement (NME), spanning up to 7.2 cm, in the right central outer breast and 3.5 cm of clumped linear NME in the central outer left breast; C and D: Post-neoadjuvant chemotherapy magnetic resonance imaging demonstrates decrease in size of the right breast mass and NME. NME in the left breast demonstrates only mild improvement. Surgical pathology demonstrates 4.3 cm of residual disease on the right and 3.7 cm of disease on the left.
ASSOCIATIONS WITH TUMOR BIOMARKERS
Extent of disease evaluation
The impact of tumor biomarkers on the accuracy of MRI for detecting the extent of disease must be considered when interpreting post-NAC MRI in anticipation of surgical resection. In addition to the individual status of receptors, biomarkers can categorize tumors into different subtypes. Tumor subtypes include luminal (ER/PR positive, Her2 negative), Her2 positive, and basal (ER/PR/Her2 negative; analogous to TN.) Multiple studies have demonstrated that in the post-NAC setting, the MRI assessment of extent of residual disease is most accurate in tumors that are either TN (Figure 2) or Her2 positive.
McGuire et al[26] retrospectively reported their institutional experience and found that MRI was most accurate in estimating pathologic size of residual disease in the Her2 positive and TN subtypes. Additionally, they found that MRI was more likely to underestimate the amount of residual disease in the luminal subtype (ER/PR positive, Her2 negative) when compared with TN or Her2 positive tumors. In a study done by Loo et al[29], MRI findings correlated well with the pathologic findings in the TN and Her2 positive breast cancers, but not with ER positive or Her2 negative breast cancers. Kuzucan et al[24] evaluated only Her2 negative cancers, and report similar findings-higher concordance between post-NAC tumor size on MRI and pathologic size in HR negative tumors compared to HR positive tumors. In Kuzucan’s study, MRI accuracy was also increased in tumors expressing high levels of the proliferation marker Ki-67 (defined as > 40% positive). A study by Kim et al[34], which investigated TN cancer, also found that Ki-67 affects the diagnostic accuracy of MRI, with higher correlation between MRI and residual tumor size at surgery in Ki-67 positive patients.
In I-SPY, a multicenter neoadjuvant trial with serial MRIs over the course of therapy, there were the fewest discrepancies between the post-NAC MRI tumor size and pathologic size in Her2 positive, HR negative, and TN tumors[18]. Overall, 38% of patients analyzed had a size discrepancy of at least 2 cm between MRI and surgical pathology, with two thirds of these discrepancies being an overestimation of disease on MRI. These size discrepancies were significantly more common in HR+/Her2- tumor subtypes. Additionally, size discrepancies differed by MRI phenotype; among the solid phenotypes, underestimation of disease by 1.5 cm or more was rare. These Her2+, HR-, and TN tumors were also the tumor subtypes most likely to have a substantial response to NAC. The experience at our institution is in accordance with other published reports. In cases of Her2 positive, HR negative, and TN tumors, if there is residual disease on MRI, it is highly likely that there will be residual disease in the surgical specimen. Underestimation of disease in these subtypes is rare, particularly in the triple negative group where no false negative MRI’s were seen.
pCR evaluation
Apart from measuring residual disease for the purposes of surgical planning, the ability of MRI to predict a pathologic complete response (pCR), a surrogate for improved outcome, is of particular importance in breast cancer management. Attaining pCR gives prognostic information that can be used for decision making, including the type of surgical procedure and/or reconstruction to recommend, and is also used as an immediate endpoint in evaluating the efficacy of NAC. Data show that pCR is associated with improved outcomes, and is more predictive when assessed by individual tumor subtype than for all subtypes combined[1,35]. A non-invasive method to accurately determine whether or not a pCR had been achieved would potentially change how trials are designed, and could eventually change surgical management of breast cancer.
While MRI accuracy depends on both its positive predictive value (PPV), and its negative predictive value (NPV), the NPV becomes the most important variable if the goal is to spare a patient invasive treatment in the setting of an rCR. That is, one must be able to trust that a negative MRI is a true negative in order to safely omit surgical resection or other treatment. In the reported papers looking at the accuracy of MRI for predicting pCR in the post-NAC setting, one must note that relatively high accuracy is possible with a low NPV. This can occur when MRI has a very high PPV, ultimately leading to high accuracy despite low NPV. In tumor subtypes that are less likely to respond to NAC, such as luminal tumors, the likelihood of residual disease is high, resulting in high PPV. However, the chance of a false negative is also highest in this group, so despite high apparent accuracy (driven by PPV), an rCR should be interpreted with caution (Figures 3 and 4).
Just as the accuracy of MRI in predicting extent of disease differs by tumor subtype, it appears that the ability of MRI to accurately predict pCR also differs by tumor subtype[24]. The NPV of MRI for predicting pCR differs by tumor subtype, highest in HR negative/Her2 positive tumors and triple negative (Figure 2) tumors[18,24-26,36]. In our report of I-SPY patients, when the post-NAC MRI underestimated residual disease (which occurred 4.3% of the time), all the discordant cases were either HR positive (Figures 3 and 4) or had diffuse phenotypes (Figure 4)[18].
Recently, several groups have reported on the accuracy of post-NAC MRI for correctly identifying pCR. Of note, some groups define pCR as the absence of any invasive tumor cells (the preferred definition of the FDA)[37], while others require the absence of both invasive and in situ disease - this definition must be noted when interpreting study findings, as residual in situ disease may lead to higher local recurrence rates[38].
One retrospective multicenter study of 746 women undergoing NAC found overall NPV for MRI of 47% and accuracy of 74% for predicting pCR[25]. The NPV for MRI varied by tumor subtype, and was highest amongst HR-/Her2+ tumors (62%) and TN tumors (60%). The overall accuracy was highest for HR+/Her2 negative tumors, likely because the PPV in this group was 91%. This likely reflects the fact that because this subtype is the least likely to respond to NAC, the pretest probability for having residual disease is higher.
Single institution studies have shown similar results. Chen et al[27] demonstrated the vast difference in MRI accuracy by tumor subtype, with accurate prediction of pCR in 95% of Her2 positive tumors, but only in 50% of Her2 negative tumors. Kim et al[34] found MRI accurately predicted pCR in 91% of TN cases. Kuzucan et al[24] focused on Her2 negative patients, and also found higher accuracy in HR negative tumors, with a PPV of 88% and NPV of 88%. In HR positive tumors, MRI had a PPV of 100% but an NPV of only 56%. The authors noted that the higher NPV in the HR negative tumors may have been related to a higher prevalence of solid tumor phenotypes, acknowledging that tumor phenotype impacts MRI accuracy and response to NAC[24]. In Ko et al[28]’s 2013 report, overall PPV was 89.6% and NPV was 83.8%. Of the five false negative MRI’s in their study, 3 were ER positive, 2 were Her2 positive, and 3 initially appeared as non mass enhancement. The most recent report, by Bufi et al[36] in 2014, shows the highest NPV rates to date. In the TN tumor subtypes, they report NPV of 100%. In the Her2 positive subtype, they report NPV of 100% using diffusion weighted imaging, suggesting that newer advanced MRI techniques may improve accuracy of MRI in different subtypes[36].
CONCLUSION
MRI most accurately predicts pathology in TN, Her2 positive and HR negative tumors, especially if they are of a solid imaging phenotype. In these cases, post-NAC MRI is highly reliable for surgical planning. Hormone receptor positive cancers and those demonstrating NME demonstrate lower concordance with surgical pathology, making surgical guidance more nebulous in these cases.
While MRI may not yet meet the necessary NPV threshold to safely allow for omission of surgical treatment, this may be feasible for specific tumor subtypes in the future. It is unclear whether or not the differential accuracy of MRI by tumor subtype is mediated by tumor phenotype, tumor response to NAC, or biological differences that affect imaging, or possibly by all of these factors. Regardless, it is clear at this point that radiologists and surgeons must assess MRI response to NAC in the context of tumor subtype. If imaging interpretations are not made in this context, pre-operative MRI will continue to be limited by both overestimation and underestimation of residual disease. The same way each breast cancer requires a tailored treatment strategy, tailored interpretation strategies should also be employed. Future work on redefining thresholds for enhancement interpretation based on tumor biology and on the development of receptor subtype-based imaging protocols may improve accuracy in the future.
With the understanding that pCR predicts recurrence free survival, if an rCR can confidently predict pCR (as in TN and Her2 positive tumors), then an rCR can predict recurrence free survival. As an imaging predictor for such important outcomes, MRI interpreted in the context of tumor subtype would be a tremendous asset in decision-making and patient counseling.
Footnotes
Conflict-of-interest statement: Authors have no conflicts of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 8, 2015
First decision: March 20, 2015
Article in press: May 18, 2015
P- Reviewer: Cosmi E, Dreau D, Huang C, Lee HH, Wang PH, Yokoyama Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, Beets-Tan RG, Wildberger JE. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4:163–175. doi: 10.1007/s13244-013-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, Brufsky AM, Lembersky BC, Ahrendt GM. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116:1431–1439. doi: 10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 4.Mersin H, Yildirim E, Berberoglu U, Gülben K. The prognostic importance of triple negative breast carcinoma. Breast. 2008;17:341–346. doi: 10.1016/j.breast.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 6.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 7.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;(30):96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Fisher ER, Wickerham DL, Wolmark N, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, Miller MJ, Whelan T, Pierce LJ, Esserman LJ, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008;26:791–797. doi: 10.1200/JCO.2007.15.0326. [DOI] [PubMed] [Google Scholar]
- 10.Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, Ames FC, Babiera GV, Feig BW, Hunt KK, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243:257–264. doi: 10.1097/01.sla.0000197714.14318.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with x-ray mammography and palpation. J Magn Reson Imaging. 2001;13:868–875. doi: 10.1002/jmri.1124. [DOI] [PubMed] [Google Scholar]
- 12.Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, Samulski TV, Dewhirst MW. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181:1275–1282. doi: 10.2214/ajr.181.5.1811275. [DOI] [PubMed] [Google Scholar]
- 13.Akazawa K, Tamaki Y, Taguchi T, Tanji Y, Miyoshi Y, Kim SJ, Shimazu K, Ueda S, Yanagisawa T, Okishiro N, et al. Potential of reduction in total tumor volume measured with 3D-MRI as a prognostic factor for locally-advanced breast cancer patients treated with primary chemotherapy. Breast J. 2008;14:523–531. doi: 10.1111/j.1524-4741.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- 14.Montemurro F, Martincich L, De Rosa G, Cirillo S, Marra V, Biglia N, Gatti M, Sismondi P, Aglietta M, Regge D. Dynamic contrast-enhanced MRI and sonography in patients receiving primary chemotherapy for breast cancer. Eur Radiol. 2005;15:1224–1233. doi: 10.1007/s00330-005-2656-6. [DOI] [PubMed] [Google Scholar]
- 15.Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat. 2002;72:145–152. doi: 10.1023/a:1014856713942. [DOI] [PubMed] [Google Scholar]
- 16.Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, Halpern E, Moore R, Kuter I, Taghian A. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 17.Esserman L, Kaplan E, Partridge S, Tripathy D, Rugo H, Park J, Hwang S, Kuerer H, Sudilovsky D, Lu Y, et al. MRI phenotype is associated with response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol. 2001;8:549–559. doi: 10.1007/s10434-001-0549-8. [DOI] [PubMed] [Google Scholar]
- 18.Mukhtar RA, Yau C, Rosen M, Tandon VJ, Hylton N, Esserman LJ. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Ann Surg Oncol. 2013;20:3823–3830. doi: 10.1245/s10434-013-3038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, Weatherall PT, Lehman CD, Newstead GM, Polin S, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham DC, Jones RC, Jones SE, Cheek JH, Peters GN, Knox SM, Grant MD, Hampe DW, Savino DA, Harms SE. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996;78:91–100. doi: 10.1002/(SICI)1097-0142(19960701)78:1<91::AID-CNCR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Gilles R, Guinebretière JM, Toussaint C, Spielman M, Rietjens M, Petit JY, Contesso G, Masselot J, Vanel D. Locally advanced breast cancer: contrast-enhanced subtraction MR imaging of response to preoperative chemotherapy. Radiology. 1994;191:633–638. doi: 10.1148/radiology.191.3.8184039. [DOI] [PubMed] [Google Scholar]
- 22.Trecate G, Ceglia E, Stabile F, Tesoro-Tess JD, Mariani G, Zambetti M, Musumeci R. Locally advanced breast cancer treated with primary chemotherapy: comparison between magnetic resonance imaging and pathologic evaluation of residual disease. Tumori. 1999;85:220–228. doi: 10.1177/030089169908500402. [DOI] [PubMed] [Google Scholar]
- 23.Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kühn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002;12:1711–1719. doi: 10.1007/s00330-001-1233-x. [DOI] [PubMed] [Google Scholar]
- 24.Kuzucan A, Chen JH, Bahri S, Mehta RS, Carpenter PM, Fwu PT, Yu HJ, Hsiang DJ, Lane KT, Butler JA, et al. Diagnostic performance of magnetic resonance imaging for assessing tumor response in patients with HER2-negative breast cancer receiving neoadjuvant chemotherapy is associated with molecular biomarker profile. Clin Breast Cancer. 2012;12:110–118. doi: 10.1016/j.clbc.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK, Hudis CA, Hylton NM, McGuire K, Meric-Bernstam F, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119:1776–1783. doi: 10.1002/cncr.27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuire KP, Toro-Burguete J, Dang H, Young J, Soran A, Zuley M, Bhargava R, Bonaventura M, Johnson R, Ahrendt G. MRI staging after neoadjuvant chemotherapy for breast cancer: does tumor biology affect accuracy? Ann Surg Oncol. 2011;18:3149–3154. doi: 10.1245/s10434-011-1912-z. [DOI] [PubMed] [Google Scholar]
- 27.Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta RS, Nalcioglu O, Su MY. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112:17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- 28.Ko ES, Han BK, Kim RB, Ko EY, Shin JH, Hahn SY, Nam SJ, Lee JE, Lee SK, Im YH, et al. Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann Surg Oncol. 2013;20:2562–2568. doi: 10.1245/s10434-013-2925-6. [DOI] [PubMed] [Google Scholar]
- 29.Loo CE, Straver ME, Rodenhuis S, Muller SH, Wesseling J, Vrancken Peeters MJ, Gilhuijs KG. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol. 2011;29:660–666. doi: 10.1200/JCO.2010.31.1258. [DOI] [PubMed] [Google Scholar]
- 30.Bahri S, Chen JH, Mehta RS, Carpenter PM, Nie K, Kwon SY, Yu HJ, Nalcioglu O, Su MY. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Ann Surg Oncol. 2009;16:1619–1628. doi: 10.1245/s10434-009-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomida K, Ishida M, Umeda T, Sakai S, Kawai Y, Mori T, Kubota Y, Mekata E, Naka S, Abe H, et al. Magnetic resonance imaging shrinkage patterns following neoadjuvant chemotherapy for breast carcinomas with an emphasis on the radiopathological correlations. Mol Clin Oncol. 2014;2:783–788. doi: 10.3892/mco.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009;250:638–647. doi: 10.1148/radiol.2503081054. [DOI] [PubMed] [Google Scholar]
- 33.Chen JH, Agrawal G, Feig B, Baek HM, Carpenter PM, Mehta RS, Nalcioglu O, Su MY. Triple-negative breast cancer: MRI features in 29 patients. Ann Oncol. 2007;18:2042–2043. doi: 10.1093/annonc/mdm504. [DOI] [PubMed] [Google Scholar]
- 34.Kim MJ, Kim EK, Park S, Moon HJ, Kim SI, Park BW. Evaluation with 3.0-T MR imaging: predicting the pathological response of triple-negative breast cancer treated with anthracycline and taxane neoadjuvant chemotherapy. Acta Radiol. 2014:Sep 16; Epub ahead of print. doi: 10.1177/0284185114548507. [DOI] [PubMed] [Google Scholar]
- 35.Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, DeMichele A, Gray JW, Conway-Dorsey K, Lenburg ME, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132:1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bufi E, Belli P, Di Matteo M, Terribile D, Franceschini G, Nardone L, Petrone G, Bonomo L. Effect of breast cancer phenotype on diagnostic performance of MRI in the prediction to response to neoadjuvant treatment. Eur J Radiol. 2014;83:1631–1638. doi: 10.1016/j.ejrad.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 37. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm370449.htm.
- 38.Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, von Minckwitz G, Brennan ME, Ciatto S. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105:321–333. doi: 10.1093/jnci/djs528. [DOI] [PubMed] [Google Scholar]


