Abstract
The racial diversity and gender distribution of HIV-infected patients make it essential to confirm the safety and efficacy of raltegravir in these populations. A multicenter, open-label, single-arm observational study was conducted in a diverse cohort of HIV-infected patients (goals: ≥25% women; ≥50% blacks in the United States), enrolling treatment-experienced patients failing or intolerant to current antiretroviral therapy (ART) and treatment-naive patients (limited to ≤20%). All patients received raltegravir 400 mg b.i.d. in a combination antiretroviral regimen for up to 48 weeks. A total of 206 patients received study treatment at 34 sites in the United States, Brazil, Dominican Republic, Jamaica, and South Africa: 97 (47%) were female and 153 (74%) were black [116 (56%) in the United States]. Of these, 185 patients were treatment experienced: 97 (47%) were failing and 88 (43%) were intolerant to current therapy; 21 patients (10%) were treatment naive. Among treatment-intolerant patients, 55 (63%) had HIV-1 RNA<50 copies/ml at baseline. Overall, 15% of patients discontinued: 13% of men, 18% of women, 14% of blacks, and 17% of nonblacks. At week 48, HIV RNA was <50 copies/ml in 60/94 (64%) patients failing prior therapy, 61/80 (76%) patients intolerant to prior therapy, and 16/21 (76%) treatment-naive patients. Response rates were similar for men vs. women and black vs. nonblack patients. Drug-related clinical adverse events were reported by 8% of men, 18% of women, 14% of blacks, and 9% of nonblacks. After 48 weeks of treatment in a diverse cohort of HIV-infected patients, raltegravir was generally safe and well tolerated with potent efficacy regardless of gender or race.
Introduction
At the end of 2009, women represented more than half of the 33.3 million people with HIV infection worldwide.1 The majority (68%) of people with HIV infection live in sub-Saharan Africa, where 60% of all cases are in women, and levels of new HIV infections are higher among women.1 In the United States in 2010, women accounted for 21% of all diagnoses of HIV infection, sand blacks/African-Americans continued to experience the most severe HIV burden, accounting for 46% of infections,2 even though they represent only 14% of the U.S. population. The rate of new HIV infections is 15 times higher in black women than in white women and 6.5 times higher in black men than in white men.3 Both gender and race influence HIV-1 RNA levels and the rate of decline in CD4 cell counts over time,4 the principal measures used to provide prognostic information and guide treatment decisions. Guidelines for the treatment of HIV infection are based largely on data from clinical trials,5 yet women and racial minorities have been underrepresented in clinical trials of HIV therapy.6–9
Raltegravir (ISENTRESS) is an HIV integrase inhibitor that has been shown to be generally safe and well tolerated, with potent antiretroviral activity in treatment-experienced patients10–14 and treatment-naive patients16–20 with HIV-1 infection. Phase III studies have demonstrated the durable antiretroviral effect and favorable long-term safety profile of raltegravir in both treatment-experienced21 and treatment-naive patients22; however, only 15% of the patients in these studies were women and only 12% were black. Given that HIV-infected patients include increasing numbers of women and persons from diverse racial and ethnic backgrounds, additional data are needed on the efficacy and safety of raltegravir in these patient populations.
The REALMRK study was conducted to evaluate the safety, tolerability, and antiretroviral activity of raltegravir in a diverse cohort of HIV-infected patients who were primarily treatment experienced and included substantial numbers of women and black patients. This report summarizes data on all study participants through the end of the 48-week treatment period and the 14-day posttreatment follow-up period. In addition, this study utilized population pharmacokinetic (PK) methods to explore the impact of PK variability on the safety and efficacy of raltegravir in this diverse patient population.
Materials and Methods
Study design
REALMRK (Protocol 055; NCT #00764946) was a multicenter, open-label, single-arm study conducted at 34 sites (28 in the United States, two in Brazil, two in South Africa, one in Jamaica, and one in the Dominican Republic) in approximately 200 HIV-infected patients ≥16 years old. The protocol was approved by the Institutional Review Board or Ethical Review Committee at each site and conducted in accordance with Good Clinical Practice guidelines. All participants provided written informed consent. The study enrolled treatment-experienced patients who were failing or intolerant to other licensed antiretroviral therapy (ART), as well as treatment-naive patients; however, because raltegravir was not approved for use in treatment-naive patients when the study began, enrollment was monitored to ensure that no more than 20% of enrolled patients were treatment naive. In addition, the protocol specified that the study population was to include at least 25% women and 50% African-Americans (black patients enrolled in the United States).
Specific recruitment strategies were used to ensure that the enrollment goals were achieved. Potential study sites were queried regarding their ability to enroll women (at least two) and black patients (at least four), and enrollment of men and nonblack patients was limited by the IVRS managed enrollment system. A 2-year enrollment period was planned in order to have sufficient time to find the appropriate patients. Recent studies have found higher discontinuation rates in women than in men23,24; therefore, we also used specific retention strategies such as selecting patients who could meet the study's time commitment and supporting their continued participation through educational programs and events, follow-up phone calls between study visits, and reimbursement for travel and childcare expenses.
All patients were assigned to raltegravir 400 mg b.i.d. in combination with other antiretroviral agents for 48 weeks. At baseline, the site investigator selected the other antiretroviral agents for each patient on the basis of current treatment guidelines, the patient's prior treatment history (including reasons for discontinuing prior antiretroviral agents), the results of HIV-1 genotypic and phenotypic antiretroviral resistance testing at screening, and prior genotypic and phenotypic antiretroviral resistance testing (including history of mutations), if available. After a screening visit, patients who met enrollment criteria returned to the study center for scheduled procedures on day 1 (initiation of therapy); weeks 4, 8, 16, 24, 36, and 48; and 14 days after completion of study treatment for a follow-up visit. If a patient discontinued prior to study completion, all final visit procedures were to be performed at the time of discontinuation. Medication adherence was monitored with diary cards and tablet counts. At the day 1 visit, each patient was given a diary card to record study drug administration, including their background antiretroviral therapy. Completed diary cards were to be returned at each scheduled visit and reviewed with the study coordinator for completeness and accuracy. All containers of study drug were to be returned at each visit so that the number of tablets remaining could be determined and recorded.
HIV-1 RNA levels were measured at a central laboratory using the standard COBAS Amplicor HIV-1 Monitor assay (version 1.5; Roche Diagnostics, Branchburg, NJ) with a lower limit of quantification of 400 copies/ml and the Ultrasensitive Amplicor HIV-1 Monitor assay (version 1.5; Roche Diagnostics) with a lower quantification limit of 50 copies/ml. Blood samples were collected from all patients to determine resistance to antiretroviral agents at screening, weeks 24 and 48, and if applicable, at early discontinuation and to confirm virologic failure. In patients demonstrating viral rebound (HIV RNA≥50 copies/ml after initial response with HIV RNA<50 copies/ml; or >1.0 log10 increase in HIV RNA above nadir level) or nonresponse (confirmed decrease of <1.0 log10 from baseline in HIV RNA and HIV RNA ≥50 copies/ml starting at week 24 or beyond; or decrease of <1.0 log10 from baseline in HIV RNA and HIV RNA ≥50 copies/ml at early discontinuation), resistance to raltegravir and other ART in the treatment regimen was assessed if the viral load exceeded 400 copies/ml, the nominal limit of detection for the genotypic and phenotypic resistance assays utilized in this study (performed by Monogram Biosciences, South San Francisco, CA).
Plasma samples for measurement of raltegravir concentrations were collected in all patients at day 1 (predrug) and weeks 4, 8, 16, 24, 36, and 48. At weeks 24 and 48, samples were collected before the morning dose; other samples were collected irrespective of dosing time. Times of sample collection and the prior dose were recorded. Plasma samples were analyzed for raltegravir concentrations at PharmaNet Canada, Inc. (Quebec, Canada). The analytical method for the determination of raltegravir in human plasma involves isolation, via 96-well liquid–liquid extraction, of the analyte and internal standard from plasma, followed by HPLC-MS/MS analysis.25 The lower limit of quantitation for the plasma assay was 2 ng/ml (4.5 nM) and the linear calibration range was 2 to 1,000 ng/ml.
Statistical methods
The primary treatment period for the efficacy analyses was October 14, 2008 to February 25, 2011. The primary efficacy endpoint was the proportion of patients with plasma HIV RNA <50 copies/ml at week 48. Secondary efficacy endpoints included the proportion of patients with plasma HIV RNA <400 copies/ml and the change from baseline in CD4 cell counts at week 48. Efficacy analyses included all patients who took at least one dose of study medication and had at least one postbaseline evaluation. The Treatment-Related Discontinuation=Failure (TRD=F) approach was the primary method for handling missing HIV RNA values. For the change from baseline in CD4 cell count, baseline values were carried forward for patients who discontinued prior to week 48 due to lack of efficacy [Observed Failure (OF) approach]. The study objectives were addressed via estimation using descriptive statistics for the total study population as well as by gender (male, female) and race (black, nonblack) with patients grouped by treatment experience as follows: treatment experienced, failing current therapy; treatment experienced, intolerant to current therapy; treatment naive. No hypothesis testing was performed.
All patients who took at least one dose of study medication were included in the safety analyses. Adverse events occurring during the study or within 14 days after discontinuation were included. Adverse-event terms were adapted from the Medical Dictionary for Regulatory Activities (MedDRA version 13.0). Adverse events were considered drug related if judged by the investigator as definitely, probably, or possibly related to any of the study drugs. The intensity of clinical adverse events was graded by the investigator as mild, moderate, or severe. Severity of laboratory abnormalities was graded according to the 1992 DAIDS toxicity guidelines for adults.
For the sparse PK sampling data, three summary measures of the observed concentration data for each patient were calculated to characterize raltegravir pharmacokinetics: GM-C12h (the geometric mean of all samples for an individual patient collected between 11 and 13 h after dosing), GM-All (the geometric mean of all samples for an individual patient, regardless of time of sample collection), and Cmin (the minimum concentration value of all samples for an individual patient, regardless of collection time).
Results
Demographics
Of the 206 patients who received study treatment, 97 (47%) were women, 153 (74%) were black [116 (56%) in the United States; 37 (18%) ex-United States], and 44 (21%) were Hispanic or Latino (Table 1). The median age of 45 years (range 20–79) was similar across subgroups defined by gender and race. A disproportionate number (39/53; 74%) of nonblack patients were male. At the time of enrollment, most patients (90%) were ART experienced; 47% were failing current therapy and 43% were intolerant of current therapy. The small subgroup of treatment-naive patients (N=21, 10%) had a median age 8–10 years younger than treatment-experienced failing and intolerant patients, respectively, and a disproportionate number (67%) were male. ART-experienced patients who were intolerant of their most recent therapy had lower HIV RNA levels and higher CD4 counts at baseline than patients who were failing their most recent therapy and those who were treatment naive (Table 1). Antiretroviral agents selected by the investigator as background therapy during the study included emtricitabine/tenofovir (53%), ritonavir (41%), darunavir (26%), and lopinavir/ritonavir (21%).
Table 1.
Patient Baseline Characteristics by Treatment Experience
| Treatment experienced—failing prior therapy (N=97) | Treatment experienced—intolerant to prior therapy (N=88) | Treatment naive (N=21) | Total (N=206) | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 51 (52.6) | 44 (50.0) | 14 (66.7) | 109 (52.9) |
| Female | 46 (47.4) | 44 (50.0) | 7 (33.3) | 97 (47.1) |
| Race, n (%) | ||||
| White | 19 (19.6) | 17 (19.3) | 6 (28.6) | 42 (20.4) |
| Black | 70 (72.2) | 69 (78.4) | 14 (66.7) | 153 (74.3) |
| Native American | 1 (1.0) | 1 (1.1) | 0 (0.0) | 2 (1.0) |
| Multiracial | 7 (7.2) | 1 (1.1) | 1 (4.8) | 9 (4.4) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 27 (27.8) | 12 (13.6) | 5 (23.8) | 44 (21.4) |
| Region, n (%) | ||||
| North America | 76 (78.4) | 85 (96.6) | 20 (95.2) | 181 (87.9) |
| Southern Africa | 10 (10.3) | 2 (2.3) | 1 (4.8) | 13 (6.3) |
| South America | 11 (11.3) | 1 (1.1) | 0 (0.0) | 12 (5.8) |
| Age (years) | ||||
| Mean (SD) | 44.0 (9.2) | 46.9 (9.0) | 38.5 (10.1) | 44.7 (9.5) |
| Median (min, max) | 45.0 (20.0, 73.0) | 47.0 (22.0, 79.0) | 37.0 (23.0, 60.0) | 45.0 (20.0, 79.0) |
| CD4 cell count (cells/μl) | ||||
| Mean (SD) | 217.7 (180.9) | 417.5 (272.5) | 184.6 (112.0) | 299.7 (242.0) |
| Median (min, max) | 190.0 (5.0, 1073.0) | 374.5 (4.0, 1274.0) | 168.0 (2.0, 387.0) | 236.0 (2.0, 1274.0) |
| Plasma HIV RNA1 (log10 copies/ml) | ||||
| Mean (SD) | 4.1 (1.1) | 2.5 (1.4) | 4.8 (1.1) | 3.5 (1.5) |
| Median (min, max) | 4.2 (1.4, 5.9) | 1.7 (1.4, 5.5) | 4.9 (1.7, 5.9) | 3.8 (1.4, 5.9) |
| Plasma HIV RNA1 (copies/ml) | ||||
| Mean (SD) | 94,903.2 (182,204) | 26,147.7 (65,843.3) | 229,668 (263,742) | 79,270.0 (166,784) |
| Median (min, max) | 15,100 (25, 750,001) | 49 (25, 327,000) | 85,700 (49, 750,001) | 6,440 (25, 750,001) |
| Hepatitis infection, n (%) | ||||
| Hepatitis B or C positive2 | 13 (13.4) | 12 (13.6) | 2 (9.5) | 27 (13.1) |
| Viral subtype, n (%) | ||||
| Clade B | 75 (77.3) | 22 (25.0) | 18 (85.7) | 115 (55.8) |
| Non-clade B | 13 (13.4) | 2 (2.3) | 2 (9.5) | 17 (8.3) |
| Missing | 9 (9.3) | 64 (72.7) | 1 (4.8) | 74 (35.9) |
| Baseline plasma HIV RNA,3n (%) | ||||
| ≤50 copies/ml | 2 (2.1) | 55 (62.5) | 1 (4.8) | 58 (28.2) |
| >50 copies/ml | 95 (97.9) | 33 (37.5) | 20 (95.2) | 148 (71.8) |
| >100,000 copies/ml | 20 (20.6) | 9 (10.2) | 9 (42.9) | 38 (18.4) |
| ≤100,000 copies/ml | 77 (79.4) | 79 (89.8) | 12 (57.1) | 168 (81.6) |
| Baseline CD4 cell counts, n (%) | ||||
| ≤50 cells/mm3 | 16 (16.5) | 5 (5.7) | 3 (14.3) | 24 (11.7) |
| >50 and ≤200 cells/mm3 | 36 (37.1) | 16 (18.2) | 9 (42.9) | 61 (29.6) |
| >200 cells/mm3 | 45 (46.4) | 67 (76.1) | 9 (42.9) | 121 (58.7) |
RNA Amplicor Ultrasensitive assay values reported as “<50 copies/ml HIV RNA detected” were imputed by 50 copies/ml, and values reported as “<50 copies/ml HIV RNA not detected” were imputed as 25 copies/ml.
Evidence of hepatitis B surface antigen, hepatitis C antibody, and HCV RNA (by HCV Taqman quantitative test).
Patients with missing results were excluded.
Disposition
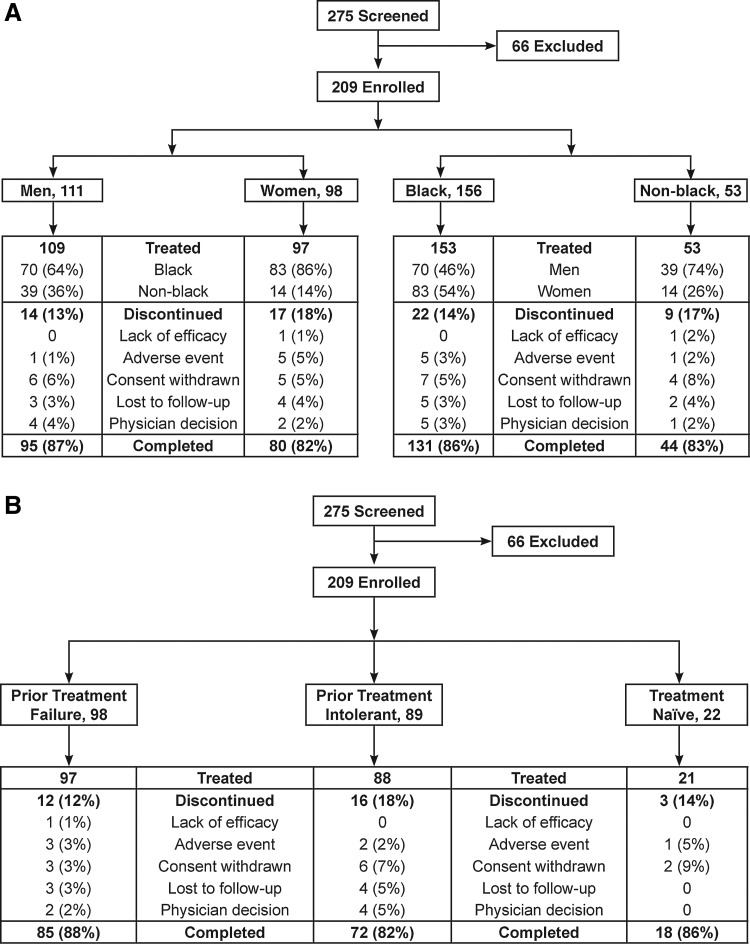
Overall, 175 patients (85%) completed the study: 87% of men and 82% of women; 86% of black patients and 83% of nonblack patients (Fig. 1A). Discontinuation due to an adverse event was less common in men (1/109, 1%) than in women (5/97, 5%) but was similar for black patients (5/153, 3%) and nonblack patients (1/53, 2%). Other reasons for discontinuation are shown in Fig. 1A. The study was completed by 87% of patients who failed their most recent therapy, 81% of patients intolerant of their most recent therapy, and 82% of previously untreated patients (Fig. 1B). Discontinuation rates due to adverse events were similar across the treatment-experienced categories (3%, 2%, and 5%, respectively). Medication adherence ≥90% was reported by 95% (195/206) of all treated patients, including 94% (103/109) of males, 95% (92/97) of females, 95% (145/153) of black patients, and 94% (50/53) of nonblack patients.
FIG. 1.
Patient disposition by gender and race (A) and by prior treatment experience (B).
Efficacy
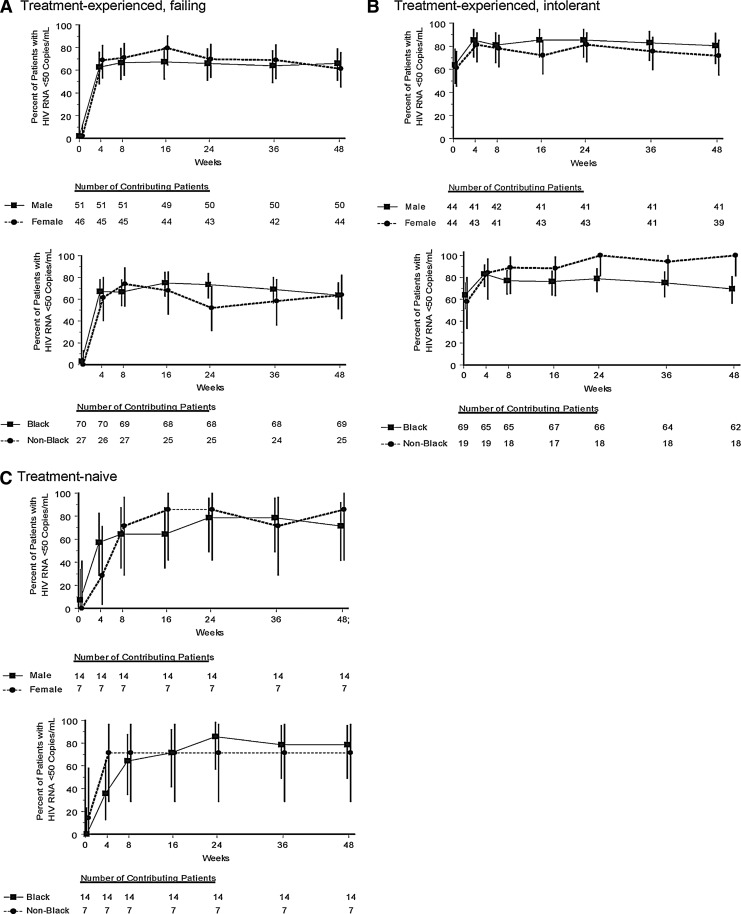
After 48 weeks of treatment with raltegravir, an HIV-1 RNA level <50 copies/ml was achieved in 64% of treatment-experienced patients failing their most recent therapy prior to the current study (Table 2) and in 76% of treatment-experienced patients intolerant of their prior therapy (of whom, 63% had HIV-1 RNA <50 copies/ml at baseline). Among the small number of treatment-naive patients followed in this study, 76% had an HIV-1 RNA level <50 copies/ml at the end of the study. In all three treatment categories, response rates were similar between men and women (Fig. 2A–C). Response rates were also similar between black and nonblack patients who were failing prior therapy (Fig. 2A) and those who were treatment naive (Fig. 2C). Among treatment-experienced patients intolerant of prior therapy, an HIV-1 RNA level <50 copies/ml was achieved in 69% of black patients and 100% of nonblack patients (Fig. 2B). Overall virologic response rates were 69% for black men [46/67, 95% CI (56, 79)] and 71% for black women [52/73, 95% CI (59, 81)], compared with 81% for nonblack men [30/37, 95% CI (65, 92)] and 75% for nonblack women [9/12, 95% CI (43, 95)]. The mean change in CD4 cell count from baseline to week 48 was 134 cells/μl in patients failing prior therapy, 64 cells/μl in patients intolerant to prior therapy, and 193 cells/μl in treatment-naive patients (Table 2). Within each treatment-experienced category, the mean change in CD4 count was similar between men and women and between black and nonblack patients (Table 2).
Table 2.
Virologic and Immunologic Outcomes at Week 48 by Treatment Experience and Gender/Race
| Treatment experienced—failing prior therapy | Treatment experienced—intolerant to prior therapy | Treatment naive | ||||
|---|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | n/N | % (95% CI) | |
| Proportion of Patients with Plasma HIV RNA <50 copies/mla | ||||||
| Total | 60/94 | 63.8 (53.3, 73.5) | 61/80 | 76.3 (65.4, 85.1) | 16/21 | 76.2 (52.8, 91.8) |
| Male | 33/50 | 66.0 (51.2, 78.8) | 33/41 | 80.5 (65.1, 91.2) | 10/14 | 71.4 (41.9, 91.6) |
| Female | 27/44 | 61.4 (45.5, 75.6) | 28/39 | 71.8 (55.1, 85.0) | 6/7 | 85.7 (42.1, 99.6) |
| Black (U.S.+ex-U.S.) | 44/69 | 63.8 (51.3, 75.0) | 43/62 | 69.4 (56.3, 80.4) | 11/14 | 78.6 (49.2, 95.3) |
| Nonblack | 16/25 | 64.0 (42.5, 82.0) | 18/18 | 100.0 (81.5,100.0) | 5/7 | 71.4 (29.0, 96.3) |
| Baseline HIV RNA ≤50 copies/mL* | 44/50 | 88.0 (75.7, 95.5) | ||||
| Baseline HIV RNA >50 copies/mL* | 17/30 | 56.7 (37.4, 74.5) | ||||
| Treatment experienced—failing prior therapy | Treatment experienced—intolerant to prior therapy | Treatment naive | ||||
|---|---|---|---|---|---|---|
| N | Mean change (95% CI) | N | Mean change (95% CI) | N | Mean change (95% CI) | |
| Change from Baseline in CD4 Cell Countb(cells/μl) | ||||||
| Total | 89 | 134 (107, 160) | 76 | 64 (34, 93) | 19 | 193 (117, 268) |
| Male | 48 | 111 (77, 145) | 38 | 55 (15, 94) | 13 | 146 (59, 232) |
| Female | 41 | 161 (120, 202) | 38 | 73 (27, 119) | 6 | 294 (140, 448) |
| Black (U.S.+ex-U.S.) | 66 | 141 (110, 173) | 61 | 62 (27, 98) | 12 | 209 (109, 309) |
| Nonblack | 23 | 113 (62, 164) | 15 | 69 (17, 122) | 7 | 164 (13, 316) |
Missing values handled with Treatment-Related Discontinuation=Failure (TRD=F) approach.
Among treatment experienced patients intolerant to prior therapy.
Missing values handled with Observed Failure (OF) approach.
FIG. 2.
Percent of patients with HIV-1 RNA <50 copies/ml [Treatment-Related Discontinuation=Failure (TRD=F)] by gender (top panels) and by race (bottom panels) in treatment-experienced patients failing prior therapy (A), in treatment-experienced patients intolerant of prior therapy (B), and in treatment-naive patients (C).
Resistance
Virologic failure was confirmed in 48 (23%) of 206 treated patients by week 48. Genotypic and phenotypic resistance testing was performed for 38 patients. Eleven patients had viral isolates that displayed primary raltegravir resistance mutations, including one in Y143, six in Q148, and six in N155 (two patients had variants of both Q148 and N155). These 11 patients were treatment experienced, and nine had failed their most recent ART. Among the 27 patients with virologic failure whose viruses did not display a primary raltegravir resistance mutation (143, 148, or 155), two patients had viruses with other known raltegravir resistance mutations. A viral isolate from a previously treatment-naive patient exhibited the I203I/M mutation but retained phenotypic sensitivity to raltegravir. Of note, virus isolated from one nonresponder had the L74I integrase polymorphism present in both baseline and virologic failure isolates; L74I is not known to confer reduced susceptibility to raltegravir, consistent with the observation that the patient's baseline virus was sensitive to raltegravir. However, virus isolated from this patient after virologic failure also had a treatment-emergent integrase mutation, F121C, which has been shown to confer raltegravir resistance in phenotypic assays (data on file at Merck).
Pharmacokinetics
Raltegravir PK parameters calculated from sparse sampling were consistent with expectations based on prior studies of raltegravir 400 mg b.i.d., regardless of patients' gender or race (Table 3). Logistic regression models were used to explore the potential association between raltegravir PK parameter values and antiretroviral responses (Table 4). One statistically significant (p<0.05) association was observed between virologic failure and GM-All. The average GM-All was slightly lower in patients with virologic failure, but there was significant overlap in GM-All values between this group and patients without virologic failure. No threshold value of GM-All below which most patients fail was evident from the data. There were also trends toward associations between all of the efficacy endpoints and GM-C12h, although none of these relationships was statistically significant.
Table 3.
Summary Statistics for Raltegravir Sparse Pharmacokinetic Parameters by Gender and Race
| PK parameter | N | LS mean (95% CI)a | %CVb | Median | Min | Max |
|---|---|---|---|---|---|---|
| Female | ||||||
| GM all (nM) | 91 | 338 (273, 419) | 147 | 330 | 14 | 7,039 |
| GM C12 (nM) | 60 | 331 (251, 437) | 137 | 311 | 57 | 6,394 |
| Cmin (nM) | 91 | 99 (76, 128) | 196 | 96 | 6 | 4,566 |
| Male | ||||||
| GM all (nM) | 105 | 381 (312, 466) | 134 | 344 | 5 | 5,800 |
| GM C12 (nM) | 58 | 282 (212, 374) | 165 | 274 | 7 | 15,291 |
| Cmin (nM) | 105 | 83 (65, 105) | 197 | 80 | 5 | 5,800 |
| Black | ||||||
| GM all (nM) | 146 | 353 (298, 418) | 121 | 302 | 29 | 5,800 |
| GM C12 (nM) | 90 | 285 (227, 357) | 139 | 274 | 7 | 6,394 |
| Cmin (nM) | 146 | 93 (76, 115) | 189 | 89 | 6 | 5,800 |
| Nonblack | ||||||
| GM all (nM) | 50 | 385 (288, 514) | 203 | 409 | 5 | 7,039 |
| GM C12 (nM) | 28 | 385 (257, 578) | 186 | 336 | 62 | 15,291 |
| Cmin (nM) | 50 | 80 (56, 114) | 221 | 86 | 5 | 1,154 |
| Female vs. male comparison | GMR (90% CI) | |||||
| GM all female/GM all male | 0.89 (0.69, 1.13) | |||||
| GM C12h female/GM C12h male | 1.17 (0.84, 1.64) | |||||
| Cmin female/Cmin male | 1.20 (0.89, 1.61) | |||||
| Black vs. nonblack comparison | GMR (90% CI) | |||||
| GM all black/GM all nonblack | 0.92 (0.69, 1.22) | |||||
| GM C12h black/GM C12h nonblack | 0.74 (0.50, 1.09) | |||||
| Cmin black/Cmin nonblack | 1.17 (0.83, 1.64) | |||||
Back-transformed from log scale. LS mean, geometric least-squares mean.
%CV=100×sqrt[exp(s2)−1], where s2 is the observed variance on the natural log-scale.
Note: Raltegravir was administered with background antiretroviral therapy.
min, minimum; max, maximum; CI, confidence interval; CV, coefficient of variation; PK, pharmacokinetic; GM, geometric mean.
Table 4.
Sparse Pharmacokinetic Parameters as a Predictor for Antiretroviral Responses at Week 48
| na | Na | Odds ratio (95% CI)b | p-valueb | |
|---|---|---|---|---|
| HIV RNA<400 at week 48c | ||||
| GM all (nM) | 152 | 185 | 1.246 (0.510, 3.040) | 0.630 |
| GM C12h (nM) | 98 | 115 | 2.578 (0.740, 8.978) | 0.137 |
| Cmin (nM) | 152 | 185 | 0.691 (0.321, 1.489) | 0.345 |
| HIV RNA<50 at week 48 | ||||
| GM all (nM) | 137 | 184 | 1.332 (0.615, 2.882) | 0.467 |
| GM C12h (nM) | 88 | 114 | 2.379 (0.841, 6.725) | 0.102 |
| Cmin (nM) | 137 | 184 | 0.988 (0.517, 1.888) | 0.971 |
| Virologic failured | ||||
| GM all (nM) | 42 | 185 | 0.428 (0.187, 0.977) | 0.044 |
| GM C12h (nM) | 22 | 115 | 0.393 (0.132, 1.166) | 0.092 |
| Cmin (nM) | 42 | 185 | 0.684 (0.349, 1.340) | 0.269 |
N, number of patients with both PK and efficacy data. n, number of patients (out of N) with events.
Logistic regression with the following covariates: baseline HIV RNA (log10 copies/ml) and PK parameters (in log10 scale).
For HIV RNA <400 at week 48, a reliable estimate of odds ratio with corresponding p-value cannot be obtained due to the low number of subjects who failed this criterion.
Virologic failure was defined as (1) nonresponders who had a confirmed <1.0 log10 decrease from baseline plasma HIV RNA and HIV RNA >50 copies/ml starting at week 24 or beyond, or (2) viral rebound starting at week 24 or beyond, which was defined as (a) HIV RNA >50 copies/ml (on two consecutive measurements at least 1 week apart) after initial response with HIV RNA <50 copies/ml, or (b) >1.0 log10 increase in HIV RNA above nadir level (on two consecutive measurements at least 1 week apart).
Note: (1) Patients who prematurely discontinued assigned treatment due to lack of efficacy were considered as failures (OF). (2) Raltegravir (400 mg b.i.d.) was administered with an optimized background therapy.
Safety
Clinical adverse events were reported in 72% of all patients who received raltegravir: 75% of men vs. 69% of women and 70% of black vs. 79% of nonblack patients. Clinical adverse events were considered related to raltegravir (alone or in combination with background ART) in 13% of patients overall; the most common drug-related clinical adverse events were nausea (4%), diarrhea (2%), and vomiting (2%). Drug-related clinical adverse events were reported in 8% of men and 18% of women. The difference was largely attributable to the broad category of gastrointestinal (GI) disorders, which were considered drug related in 6% of men vs. 10% of women, although rates of the most common drug-related GI events were the same in men and women (nausea 4%, vomiting 2%, and diarrhea 2%). Clinical adverse events related to raltegravir were reported in 14% of black patients and 9% of nonblack patients. Rates of drug-related adverse events were similar in black men (7%) and nonblack men (10%) but were higher in black women (19%) than in nonblack women (7%). None of the most common drug-related adverse events was reported by nonblack women (Table 5).
Table 5.
Adverse Events and Laboratory Abnormalities by Gender and Race, n (%)
| Male (n=109) | Female (n=97) | |||
|---|---|---|---|---|
| Black (N=70) | Nonblack (N=39) | Black (N=83) | Nonblack (N=14) | |
| Clinical adverse events (AEs) | 52 (74.3) | 30 (76.9) | 55 (66.3) | 12 (85.7) |
| Drug-relateda AE | 5 (7.1) | 4 (10.3) | 16 (19.3) | 1 (7.1) |
| Serious AE | 9 (12.9) | 3 (7.7) | 7 (8.4) | 2 (14.3) |
| Serious and drug-relateda AE | 1 (1.4) | 0 | 3 (3.6) | 0 |
| Discontinued due to AE | 1 (1.4) | 0 | 3 (3.6) | 1b (7.1) |
| Most common drug-relateda clinical AEs | ||||
| Abdominal discomfort | 0 | 0 | 2 (2.4) | 0 |
| Diarrhea | 1 (1.4) | 1 (2.6) | 2 (2.4) | 0 |
| Nausea | 2 (2.9) | 2 (5.1) | 4 (4.8) | 0 |
| Vomiting | 1 (1.4) | 1 (2.6) | 2 (2.4) | 0 |
| Myalgia | 0 | 0 | 2 (2.4) | 0 |
| Headache | 1 (1.4) | 0 | 2 (2.4) | 0 |
| Laboratory AEs | 5 (7.1) | 6 (15.4) | 11 (13.3) | 1 (7.1) |
| Drug-relateda AE | 2 (2.9) | 1 (2.6) | 1 (1.2) | 0 |
| Discontinued due to AE | 0 | 0 | 1 (1.2) | 0 |
| Laboratory abnormalities, grade 2 and higher | ||||
| Absolute neutrophil count | 8/70 (11.4) | 0 | 5/81 (6.2) | 1/13 (7.7) |
| Hemoglobin | 0 | 0 | 1/81 (1.2) | 1/13 (7.7) |
| Platelet count | 0 | 1/39 (2.6) | 0 | 0 |
| Fasting LDL cholesterol | 6/56 (10.7) | 1/27 (3.7) | 7/65 (10.8) | 0 |
| Fasting total cholesterol | 8/58 (13.8) | 5/29 (17.2) | 9/69 (13.0) | 1/11 (9.1) |
| Fasting triglycerides | 2/58 (3.4) | 2/29 (6.9) | 2/69 (2.9) | 0 |
| Fasting glucose | 6/59 (10.2) | 1/29 (3.4) | 6/67 (9.0) | 1/12 (8.3) |
| Total bilirubin | 6/70 (8.6) | 3/39 (7.7) | 4/81 (4.9) | 2/13 (15.4) |
| Serum creatinine | 1/70 (1.4) | 1/39 (2.6) | 3/81 (3.7) | 1/13 (7.7) |
| Aspartate aminotransferase | 2/70 (2.9) | 4/39 (10.3) | 2/81 (2.5) | 0 |
| Alanine aminotransferase | 1/70 (1.4) | 4/39 (10.3) | 3/81 (3.7) | 0 |
| Alkaline phosphatase | 0 | 0 | 2/81 (2.5) | 0 |
| Creatine kinase | 8/70 (11.4) | 3/39 (7.7) | 0 | 0 |
Determined by investigator to be possibly, probably, or definitely related to raltegravir alone or in combination with background ART.
This patient discontinued due to an adverse event in the poststudy period.
A total of 28 serious clinical adverse events were reported in 21 patients (10%); in four patients (2%), these events were considered drug related. Serious clinical adverse events occurred in 11% of men, 9% of women, 10% of black patients, and 9% of nonblack patients. The most common serious adverse events were various infections, as would be expected in patients immunocompromised due to HIV. Cryptococcal meningitis was diagnosed in two patients and pneumonia (including a case due to Pneumocystis jirovecii) was diagnosed in three patients. All other serious clinical events were single instances. Two patients died on the study as a result of serious clinical events: one patient with deep vein thrombosis and one with malignant bone neoplasm; these events were considered unrelated to the study medication. One patient had a serious event of rhabdomyolysis, which was considered related to raltegravir: CPK was mildly elevated at randomization (160 IU/liter) and on day 60 (199 IU/liter). On day 72, the patient went to the emergency room with calf pain and dark urine; CPK was 8,141 IU/liter (grade 4), and raltegravir was stopped. About 2 months later (day 127) CPK had fallen to 231 IU/liter (grade 0). The patient's urine toxicology screen on day 72 was positive for cocaine, which can cause rhabdomyolysis.
Laboratory adverse events occurred in 11% of all treated patients (10% of men, 12% of women, 10% of blacks, and 13% of nonblacks) and were considered drug related in 2%. One patient had a serious laboratory adverse event (Grade 4 elevation of ALT), which led to discontinuation and was considered unrelated to the study drug. No deaths occurred as a result of a laboratory adverse event. Increased ALT and AST (grade 2 or higher) were slightly more common in men (5% and 6%, respectively) than women (3% and 2%, respectively) and were less common in black patients (both 3%) than in nonblack patients (both 8%). Increased creatine kinase (grade 2 or higher) was more common in men (10%) than women (0%) but occurred with similar frequency in blacks (5%) and nonblacks (6%). Rates of increased serum cholesterol (grade 2 or higher) were similar between men (12%) and women (10%) and between blacks and nonblacks (both 11%). The proportion of patients with laboratory abnormalities of Grade 2 or higher is displayed by gender and race in Table 5.
Discussion
Raltegravir 400 mg b.i.d. is approved for the treatment of HIV-1 infection in combination with other ART. The HIV-infected population includes increasing numbers of women and patients from diverse racial and ethnic backgrounds, but these groups are often underrepresented in clinical trials of new HIV therapy. In the phase III development program for raltegravir, 15% of study patients were female and 12% were black; therefore, the goal of this study was to increase our knowledge about the efficacy and safety of raltegravir in these patients by enrolling at least 25% women and at least 50% African-Americans. The 206 patients who participated in REALMRK included 97 women (47%) and 153 black patients (74%), of whom 116 were African-American. The study population was further diversified by enrolling patients with different categories of treatment experience, including 47% who were failing their most recent ART, 43% who were intolerant of their most recent ART, and 10% who were treatment naive. Baseline HIV-1 RNA levels and CD4 counts varied with prior treatment experience. Despite the diversity of the population, the 48-week completion rate was high overall (85%) and within subgroups defined by gender and race. This retention rate is comparable to, if not better than, other contemporary HIV trials in diverse patient populations.23,24
At completion of the study treatment, 64% of patients who were prior treatment failures had HIV-1 RNA levels <50 copies/ml, as did 76% of previously intolerant patients (including both those who were suppressed and those who were not suppressed at the time of study initiation) and 76% of treatment-naive patients. At week 48, mean CD4 cell counts had increased in all patient subgroups, although the CD4 response was lower in patients intolerant to prior therapy than in prior treatment failures and treatment-naive patients; this difference was most likely related to the higher baseline CD4 cell count (418 cells/μl) in the previously intolerant group. By week 48, 23% of patients had experienced virologic failure. Across all of the populations (by treatment history) studied here, fewer than 50% of patients failing a raltegravir-based regimen had viruses displaying raltegravir resistance after virologic failure. This observation is consistent with reports of low raltegravir resistance rates in patients outside the clinical trial setting.26
Virologic response rates were generally similar between men and women regardless of prior treatment experience. Previous studies of raltegravir have also shown similar response rates between men and women. In the BENCHMRK studies (prior treatment failures), 64% of men (249/387) and 64% of women (36/56) had HIV RNA <50 copies/ml at week 48.13 In STARTMRK (treatment-naive patients), virologic response rates were 89% for men (183/206) and 96% for women (45/47) at week 96.19 In SWITCHMRK (treatment-experienced patients, all of whom had HIV-1 RNA levels <50 copies/ml for at least 3 months on their prior regimens), response rates were 88% for men (233/265) and 97% for women (60/62) at week 24.27
Other clinical trials that have examined gender-based differences in response to HIV therapy include an open-label study of darunavir/ritonavir in treatment-experienced patients (the GRACE study23), two open-label studies of tipranavir/ritonavir vs. ritonavir-boosted comparator protease inhibitors in treatment-experienced patients (the RESIST studies28), and an open-label study of atazanavir/ritonavir vs. lopinavir/ritonavir in treatment-naive patients (the CASTLE study24). In the RESIST studies,28 the proportion of patients with viral load<50 copies/ml was not significantly different between men and women at week 48. In the GRACE study (48 weeks)23 and the CASTLE study (96 weeks),24 virologic response rates were lower in women than in men, but this difference was attributed to higher discontinuation rates among women. In our study, the discontinuation rate was slightly higher in women (18%) than in men (13%), and the potential impact of this difference on virologic response rates was adjusted by counting only treatment-related discontinuations as failures in the efficacy analyses. CD4 increases tended to be higher in women than in men in our study, which is consistent with findings in RESIST28 and GRACE.23
In our study, virologic response rates were similar between black and nonblack patients who were either prior treatment failures (both 64%) or treatment naive (79% vs. 71%). Among patients who were intolerant of their prior therapy, the response rate was lower in black patients (69%) than in nonblack patients (100%). Overall response rates were numerically slightly lower among black patients, more so for men than women, but similar to those in nonblack patients due to the wide confidence intervals. Comparisons of this nature should be made cautiously given the potential imbalance of important prognostic factors within each subgroup. In previous studies of raltegravir, subgroup analyses have shown virologic response rates to be similar in black and nonblack patients, respectively: in BENCHMRK,13 62% (39/63) vs. 63% (214/339) at week 48; in STARTMRK,19 89% (25/28) vs. 90% (203/225) at week 96; and in SWITCHMRK,27 86% (44/51) vs. 90% (249/276) at week 24.
Several cohort studies have compared response to ART by race/ethnicity and found no difference in CD4 response29,30 or virologic response29,31 based on race. However, more recent studies have found associations between virologic response and race. An increased risk for virologic failure in black patients was found in two ACTG clinical trials32,33 and in a military cohort study.34 Another military cohort study found that African-Americans were significantly less likely to achieve viral suppression than European-Americans,35 and a longitudinal study in the southeastern United States found that minority racial/ethnic groups discontinued ART faster and were more likely to experience virologic failure.36 Results from the REALMRK study, together with the subgroup analyses of the Phase III studies, suggest that raltegravir has similar efficacy in black and nonblack patients.
Raltegravir PK parameters calculated from sparse sampling were consistent with expectations based on prior studies of raltegravir 400 mg b.i.d.,37 regardless of patient gender or race. Raltegravir administered in combination with ART for 48 weeks was generally well tolerated, with relatively few serious drug-related adverse events (2%) and discontinuations due to adverse events (3%). Drug-related adverse events were more common in women than in men and in black women compared with nonblack women. Discontinuation due to an adverse event was also more common in women than in men, and four of the five women who discontinued due to an adverse event were black. A trend for higher discontinuation rates in women due to adverse events has been noted with other antiretroviral agents.23,38,39 Raltegravir-based regimens were well tolerated by patients who were intolerant of their prior ART: 81% of these patients completed the study, and only 2% withdrew because of adverse events.
The REALMRK study was neither powered nor designed to detect differences based on gender or race; it was designed specifically to generate further safety and efficacy data in populations that are traditionally underrepresented in clinical trials. To address anticipated difficulties in conducting an HIV treatment trial in the patient populations we sought to include, we implemented specific recruitment and retention strategies that enabled us to enroll a high proportion of women (47%) and black patients (74%) and to achieve retention rates >80%. In this diverse cohort of HIV-infected, ART-experienced or naive patients, 48 weeks of therapy with raltegravir 400 mg b.i.d. in combination with background ART showed sustained antiretroviral activity and was generally safe and well tolerated, regardless of gender or race, and is thus a useful component of antiretroviral therapy in a broad patient population.
Acknowledgments
We thank all the patients and their caregivers who contributed to this study. We also thank Michael Miller (Merck Research Laboratories) for his expert review of the resistance sections of the article. Portions of the data were presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, September 17–20, 2011.
Principal investigators by country: Brazil: B. Grinstejn, E. Martins Netto; Dominican Republic: Y. Donastorg; Jamaica: P. Figueroa; South Africa: M. Botes, S. Miller; United States: H. Albrecht, D. Barker, R. Bedimo, R. Campo, K. Casey, P. Cook, G. Rieg, E. Daar, E. DeJesus, J. Gathe, B. Gilliam, S. Gupta, M. Goldman, D. Hagins, R. Hao, P. Kadlecik, P. Kumar, F. Marquez, K. Mounzer, K. Mullane, R. Myers, O. Osiyemi, D. Parks, G. Perez, R. Polland, S. Santiago, P. Sax, R. Tandon, P. Skrik, K. Squires, M. Thompson, M. Yin, R. Corales, R. Liporace.
This study was designed, managed, and analyzed by the sponsor in conjunction with external investigators. Authors had access to all study data upon request. This report was principally drafted by Drs. Squires, Bekker, Lu, Sklar, and Ms. Strohmaier. The presentation was critically reviewed multiple times and subsequently approved by each co-author in its essentially final form. The article underwent formal review by the sponsor. The opinions expressed in the article represent the collective views of the authors and do not necessarily reflect the official position of Merck.
Author Disclosure Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., provided all funding for the conduct of this study.
K.E.S. has received grant/research support (to Thomas Jefferson University) from Biocryst, Gilead, GSK, Merck, and Janssen; has served on advisory boards for Abbott, Gilead, Merck, Janssen, Tobira, and ViiV; as a consultant for GSK, Merck, and Tobira; and on a DSMB for Pfizer.
L.-G.B. and B.C. served on the scientific advisory committee for the REALMRK study.
J.J.E. has been an investigator for Merck, GlaxoSmithKline/ViiV, and Bristol-Myers Squibb on research grants to the University of North Carolina, and has served as a paid consultant or speaker for Merck, Bristol-Myers Squibb, Gilead, GlaxoSmithKline/ViiV, Tibotec/Janssen, and Abbott.
J.K.R. has been an investigator and a paid consultant for Merck, Gilead, Abbott, Bristol-Myers Squibb, Boehringer-Ingelheim, Bionor, Tibotec, GlaxoSmithKline, Pfizer, ViiV, Vertex, and Roche.
F.M. served as a site principal investigator for the REALMRK study.
P.K. has been an investigator for Merck, GSK, Janssen, and Bristol-Myers Squibb, and has served as a paid consultant for Bristol-Myers Squibb, ViiV Healthcare, and Janssen, and as a speaker for Janssen, ViiV Healthcare, and Boehringer-Ingelheim.
M.T. has served as site principal investigator for Bristol Myers Squibb, Boehringer-Ingelheim, Cepheid, Gilead Sciences, GeoVax, Katketsuken, Kowa Research Institute, Merck Research Laboratories, Peregrine Pharmaceuticals, Pfizer, Roche Molecular Systems, Theratechnologies, Tibotec Therapeutics, Tobira Therapeutics, and ViiV Healthcare; Clinical Trial Design Consultant for Kowa Research Institute and GeoVax; prevention research consultant for Gilead Sciences; Data Safety Monitoring Boards for Tibotec Therapeutics and GlaxoSmithKline/ViiV Healthcare.
R.E.C. has received research grants from Merck and served on advisory boards for Merck.
K.M. has been an investigator for Merck, Gilead Sciences, BMS, GSK, Tibotec, and has served as a speaker for GS, Merck, BMS, and Tibotec.
K.M.S., C.L., A.R., B.E.J., M.R., B.-Y.T.N., and P.S. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., and may own stock and/or stock options in the company.
References
- 1.UNAIDS Report on the Global AIDS Epidemic 2010. [Mar 1;2012 ]. www.unaids.org/globalreport/Global_report.htm www.unaids.org/globalreport/Global_report.htm
- 2.Centers for Disease Control and Prevention: HIV Surveillance Report. www.cdc.gov/hiv/topics/surveillance/resources/reports/ 2010;22 Published March 2012. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Fact sheet: HIV among African Americans. Nov, 2011.
- 4.Anastos K. Gange SJ. Lau B, et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24:218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Oct 14, 2011. [Mar 1;2012 ]. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf pp. 1–167.www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 6.Zorrilla CD. Tamayo Agrait VM. Why do we need the GRACE (Gender, Race & Clinical Experience) study? Future HIV Ther. 2007;1:357–363. [Google Scholar]
- 7.Sullivan PS. McNaghten AD. Begley E. Hutchinson A. Cargill VA. Enrollment of racial/ethnic minorities and women with HIV in clinical research studies of HIV medicines. J Natl Med Assoc. 2007;99:242–250. [PMC free article] [PubMed] [Google Scholar]
- 8.Gifford AL. Cunningham WE. Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346:1373–1382. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 9.Vidaver RM. Lafleur B. Tong C, et al. Women subjects in HNIH-funded clinical research literature: Lack of progress in both representation and analysis by sex. J Womens Health Gend Based Med. 2000;9:495–504. doi: 10.1089/15246090050073576. [DOI] [PubMed] [Google Scholar]
- 10.Grinsztejn B. Nguyen B-Y. Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: A Phase II randomized controlled trial. Lancet. 2007;369:1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 11.Gatell JM. Katlama C. Grinsztejn B, et al. Long-term efficacy and safety of the HIV integrase inhibitor raltegravir in patients with limited treatment options in a Phase II study. J Acquir Immune Defic Syndr. 2010;53:456–463. doi: 10.1097/qai.0b013e3181c9c967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steigbigel RT. Cooper DA. Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Eng J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 13.Cooper D. Steigbigel RT. Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Eng J Med. 2008;359:355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 14.Steigbigel RT. Cooper DA. Teppler H, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with resistant HIV infection: Week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50:605–612. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markowitz M. Morales-Ramirez JO. Nguyen BY, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43:509–515. doi: 10.1097/QAI.0b013e31802b4956. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz M. Nguyen B-Y. Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naïve patients with HIV-1 infection: Results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 17.Markowitz M. Nguyen BY. Gotuzzo E, et al. Sustained antiretroviral efficacy of raltegravir as part of combination antiretroviral therapy in treatment-naive HIV-1 infected patients: 96-week data. J Acquir Immune Defic Syndr. 2009;52:350–356. doi: 10.1097/QAI.0b013e3181b064b0. [DOI] [PubMed] [Google Scholar]
- 18.Lennox JL. DeJesus E. Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naïve patients with HIV-1 infection: A multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 19.Lennox JL. DeJesus E. Berger DS, et al. Raltegravir-based compared to efavirenz-based regimens in treatment-naive HIV-1 infected patients: Efficacy, durability, subgroup, safety, and metabolic analyses through 96 weeks of follow-up. J Acquir Immune Defic Syndr. 2010;55:39–48. doi: 10.1097/QAI.0b013e3181da1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockstroh JK. Lennox JL. DeJesus E, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53(8):807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 21.Eron JJ. Cooper DA. Steigbigel RT. Clotet B. Wan H. Zhao J, et al. Exploratory analysis in the BENCHMRK studies at week 192: Late outcomes based on early virologic response. Presented at the 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. Jul, 2011. [Abstract MOPE225] [Google Scholar]
- 22.DeJesus E. Rockstroh J. Lennox J. Saag M. Lazzarin A. Wan H, et al. Raltegravir (RAL)-based therapy demonstrates superior virologic suppression and immunologic response compared with efavirenz (EFV)-based therapy, with a favorable metabolic profile through 4 years in treatment-naïve patients: 192 week (Wk) results from STARTMRK. Presented at the 49th Annual Meeting of the Infectious Disease Society of American; Boston, MA. Oct, 2011. [Abstract 30623] [Google Scholar]
- 23.Currier J. Bridge DA. Hagins D, et al. Sex-based outcomes of darunavir-ritonavir therapy: A single-group trial. Ann Intern Med. 2010;153:349–357. doi: 10.1059/0003-4819-153-6-201009210-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squires KE. Johnson M. Yang R, et al. Comparative gender analysis of the efficacy and safety of atazanavir/ritonavir and lopinavir/ritonavir at 96 weeks in the CASTLE study. J Antimicrob Chemother. 2011;66:363–370. doi: 10.1093/jac/dkq457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merschman SA. Vallano PT. Wenning LA. Matuszewski BK. Woolf EJ. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:15–24. doi: 10.1016/j.jchromb.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Geretti AM. Fearnhill E. Ceccherini-Silberstein F, et al. Prevalence and patterns of raltegravir resistance in treated patients in Europe [abstract] Antivir Ther. 2010;15(Suppl 2):A62. [Google Scholar]
- 27.Eron JJ. Young B. Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): Two multicentre, double-blind, randomised controlled trials. Lancet. 2010;375:396–407. doi: 10.1016/S0140-6736(09)62041-9. [DOI] [PubMed] [Google Scholar]
- 28.Walmsley SL. Squires K. Weiss L, et al. Multidrug-experienced HIV-1 infected women demonstrated similar virological and immunological responses to tipranavir/ritonavir compared with men. AIDS. 2009;23:429–431. doi: 10.1097/QAD.0b013e3283229f81. [DOI] [PubMed] [Google Scholar]
- 29.Jensen-Fangel S. Pedersen L. Pedersen C, et al. The effect of race/ethnicity on the outcome of highly-active antiretroviral therapy for human immunodeficiency virus type 1-infected patients. Clin Infect Dis. 2002;35:1541–1548. doi: 10.1086/344769. [DOI] [PubMed] [Google Scholar]
- 30.Giordano TP. Wright JA. Hasan MQ, et al. Do sex and race/ethnicity influence CD4 cell response in patients who achieve virologic suppression during antiretroviral therapy? Clin Infect Dis. 2003;37:433–437. doi: 10.1086/376638. [DOI] [PubMed] [Google Scholar]
- 31.Anastos K. Schneider MF. Gange SJ, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39:537–544. [PubMed] [Google Scholar]
- 32.Gulick RM. Ribaudo HJ. Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: A randomized controlled trial. JAMA. 2006;296:769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 33.Riddler SA. Haubrick R. DiRienzo G, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartzell JD. Spooner K. Howard R, et al. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr. 2007;44:411–416. doi: 10.1097/QAI.0b013e31802f83a6. [DOI] [PubMed] [Google Scholar]
- 35.Weintrob AC. Grandits GA. Agan BK, et al. Virologic response differences between African Americans and European Americans initiating highly active antiretroviral therapy with equal access to care. J Acquir Immune Defic Syndr. 2009;52:574–580. doi: 10.1097/QAI.0b013e3181b98537. [DOI] [PubMed] [Google Scholar]
- 36.Pence BW. Ostermann J. Kumar V, et al. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- 37.Brainard DM. Wenning LA. Stone JA. Wagner JA. Iwamoto M. Clinical pharmacology profile of raltegravir, an HIV-1 integrase strand transfer inhibitor. J Clin Pharmacol. 2011;51:1376–1402. doi: 10.1177/0091270010387428. [DOI] [PubMed] [Google Scholar]
- 38.Nicastri E. Leone S. Angeletti C, et al. Sex issues in HIV-1 infected persons during highly active antiretroviral therapy: A systematic review. J Antimicrob Chemother. 2007;60:724–732. doi: 10.1093/jac/dkm302. [DOI] [PubMed] [Google Scholar]
- 39.Kempf MC. Pisu M. Dumcheva A. Westfall AO. Kilby JM. Saag MS. Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr. 2009;52:336–341. doi: 10.1097/QAI.0b013e3181b628be. [DOI] [PMC free article] [PubMed] [Google Scholar]