Introduction
The transformation of sickle cell disease (SCD) from obscurity in Africa to visibility in America over the past 100 years is intertwined with politics and race relations unique to America.1 Parallel to the development of a conventional scientific understanding of the disease and the evolution of disease control strategies, SCD also developed socio-politically. Initially thought to be a disease exclusively affecting a minority group, it was brought on the political agenda through concerted efforts made primarily by the community that identified closely with the people who suffered from it. The socio-political development that propelled investments in research into the disease’s origins, treatment, and models of care resulted in considerable improvements in life expectancy of people with SCD over the past nine decades.2
In this article, we explore the timeline of scientific attention to SCD and the published literature, available from various online sources including the National Library of Medicine’s PubMed. In our online search, we used the key words and phrases: sickle cell disease history; history of sickle cell disease in United States; and prevalence of sickle cell disease. References found in retrieved articles and books provided additional sources of information aligned with our search.
Factors Influencing the Development of the SCD Knowledge Base
Beyond the scientific breakthroughs (Figure 1), several factors in the United States influenced the efforts to understand SCD as we know it today. First, a 1934 report indicated that Memphis led the nation with an 11% infant mortality rate. By the 50s–60s, noteworthy racial disparities in overall mortality rates among children3 and the estimates of high mortality in the first two years of life among children with SCD4 could no longer be ignored. Instead, researchers began to acknowledge SCD as a public health challenge in the United States.
“The most significant feature of sickle cell anemia is not its characteristic bizarre deformation of erythrocytes but the fact that it is apparently the only known disease completely confined to a single race.” JAMA, 1947
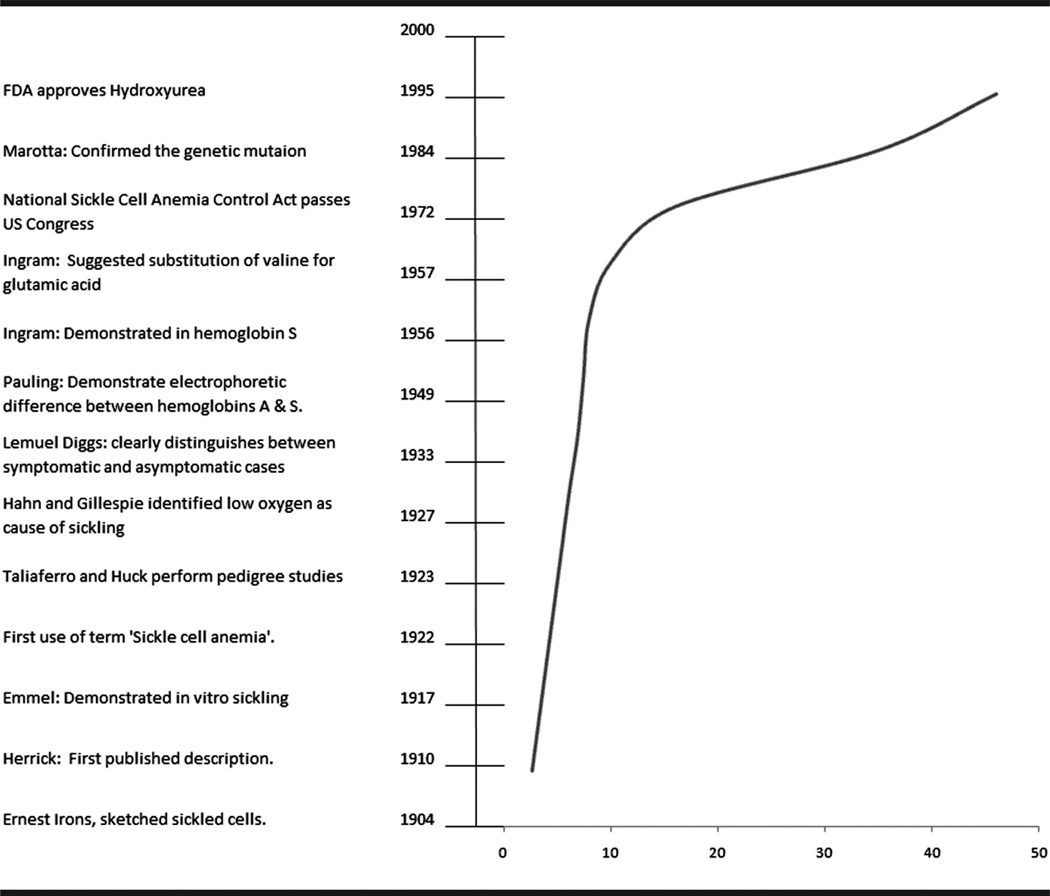
Fig 1.
Timeline of major scientific events in the history of sickle cell disease and life expectancy at birth of persons with sickle cell disease in the United States
Historians believe that many victims of SCD, who succumbed to infectious diseases like pneumonia and tuberculosis during 1930s–1940s when SCD was still largely unrecognized and unknown, had the cause of death ascribed to the more visible and better known infectious complication of the underlying disease, rather than the disease itself.1 Physicians from the 1930s and 1940s often called SCD the “great masquerader” because of attribution of SCD-related deaths to more visible infectious complications.1,5
Almost 3 decades later, in 1971, President Nixon’s inclusion of the disease in his health message to Congress paved the way for the National Sickle Cell Anemia Control Act of 1972 (Public law no. 92–294), which brought $500,000 to Memphis for research on SCD. Such high-level public engagement of SCD has not been seen in other countries with a high burden of SCD, except for the royal decree for premarital screening in the Kingdom of Saudi Arabia in 2003 (The Royal Decree no. 3 Issued on 7th day of the 11th month in the year 1424 of the Islamic calendar). Political engagement for a cause, even more so for an invisible chronic disease condition such as SCD that does not cause instant mortality (for example as in cholera), is not a top-down paradigm. In most instances, political sensitization is only possible through bottom-up public advocacy that is achieved after attaining the highest level of awareness about the disease in the community.
Community compliance to disease control strategies requires the highest level of community participation. As in any public health crisis, the community role in SCD in the United States progressed through the stages of denial, stigmatization, acknowledgment, and advocacy. In the 1930s, the lyrics of blues guitarist Lizzie Douglas in “Memphis Minne-jitis Blues” helped to elevate positive social dialogues on the discourse of SCD in America, particularly among the African American community. Movies by African American celebrities Bill Cosby (To all my Friends on Shore, 1972) and Sidney Poitier (A Warm December, 1974) that highlighted the lives of people with SCD afforded the highest point of social visibility for SCD in America.
History of Scientific Inquiry of SCD
While political and community engagement are the enabling factors to foster movements for disease control, the foundation of any disease control strategy lies in scientific inquiry. Well before Western medical literature acknowledged SCD, tribal terms existed in Africa that reflected the onomatopoeia of the pain and agony associated with sickle cell crisis.1 The earliest text that is generally thought to be a description of an SCD patient in the United States is found in the work of Dr. R. Lebby, published in 1846. In the Southern Journal of Medical Pharmacology, entitled “Case of Absence of the Spleen,” referenced by Bloom,6 Lebby discussed autopsy findings of a runaway slave who was tried and executed for murder. The credit for discovering SCD, however, goes to James Herrick and his intern, Ernest Irons, who used the power of laboratory science and microscopes to make the previously invisible disease visible to the Western world.7 This well-known 1910 publication by James Herrick represents a summary of findings from the follow-up of one of his patients over a period of 3 years, from 1904 to 1907. Documentation of subsequent cases of SCD followed rather slowly, though a case report describing what is now considered the second case of SCD was published by Benjamin Earl Washburn, a medical student at University of Virginia,8 just three months after Herrick’s paper, and the third case in 1915 from Washington University Medical School.9
The fact that this third patient’s three siblings had died from severe anemia coupled with the demonstrated “sickling” of the patient’s (as well as her asymptomatic father’s) blood, raised suspicion that SCD might be of genetic origin. Victor Emmel observed sickle-shaped red cells over a period of several hours after placing a drop of blood in a ring of ‘petrolatum’ (petroleum jelly), covered by a cover slip creating an air-tight chamber.10 He noted transformation of a large number of normal biconcave disk-shaped red blood cells into elliptical sickle shaped cells. Emmel’s work led to invention of the first simple diagnostic test for the disease. Similarities in the sickling phenomena observed in the first four reported cases led Mason to name the disease “sickle cell anemia.”11 Later, researchers like Daland and Castle also proposed quicker and simpler methods for demonstrating sickling in vitro.12
Advancements in technology that provided researchers an opportunity to study the disease in greater detail made SCD the first disease to be identified as a molecular disease.13 Specific differences between normal and sickle cell hemoglobins were later identified,14,15 eventually leading to the recognition of the genetic mutation responsible for this difference in 1977.16 Sickle cell disease serves as a recognized paradigm for understanding genetic diseases. However, scientific breakthroughs that led to specific treatments for people with SCD have appeared only in the ninth decade after Herrick’s report.
Management of SCD
The only notable breakthrough in the management of SCD has been the finding in the United States that hydroxyurea therapy increases the synthesis of fetal hemoglobin.17 Fetal hemoglobin binds more strongly and efficiently to oxygen, making it somewhat “resistant” to sickling. Hydroxyurea indirectly decreases the frequency of painful “sickling” crises in patients with sickle cell anemia by inducing fetal hemoglobin production. A randomized, double-blind, placebo controlled clinical trial demonstrated the efficacy of hydroxyurea, making it the first effective medical treatment in reduction of symptoms and improvement in hematological indicators for SCD. This trial led to decreased utilization of hospital services in SCD.17,18 With early detection through newborn screening and prevention of infection via administration of penicillin, noteworthy increases in survival of people with SCD, particularly young children, have been noted.19 Following the 1987 Consensus Statement from the National Institutes of Health,20 SCD was included in the newborn screening program in many US states.21,22 Recently, CDC and the National Heart, Lung and Blood Institute (NHLBI) have jointly launched a surveillance and research program for inherited blood diseases that will allow prospective research and evaluations of SCD services.23
Advancements in the United States were further propelled by reframing SCD as a health disparity not only in regard to outcomes among persons affected but also in terms of public and private support for research and care of persons with SCD as compared to cystic fibrosis.24 Inequalities among similarly situated disorders in research, clinical care, advocacy and overall public support based on socioeconomic characteristics of the affected populations led to the call for a SCD Summit in June 2006.25 One of the numerous outcomes of the Summit was to challenge federal entities to work collaboratively to develop a comprehensive public health agenda for SCD. By including SCD in its portfolio of blood disorders, the CDC created an enhanced focus on population issues in the areas of policy and legislation, surveillance, epidemiology and health services research and prevention research.
While the progress made so far is laudable, much remains to be achieved in terms of palliative and curative care for the thousands of Americans who suffer from SCD today. Although estimates reported in contemporary literature vary26; one estimate by NIH indicates that one in 12 African Americans and one in every 100 Hispanic Americans carry the sickle cell trait.27 The total number of Americans with SCD is estimated to be about 100,000,26 leading to hospitalizations at an estimated cost of $488 million annually in 2004 US dollars.28 The latest legislation, the Sickle Cell Treatment Act of 2003, was signed into law in 2004 and provides funding to learn best practices and to expand specialized treatment programs in the 40 SCD treatment centers established by the 1972 Sickle Cell Act. This initiative is intended to close the gaps in provision of uniform care for people affected with the disease. Similarly, the parallel restructuring of NHLBI’s SCD research agenda29 to basic, translational, and clinical research is expected to close the research gaps in SCD.
Conclusion
In the 1940s, SCD was thought to be completely confined to African Americans. Since then, it has come to be recognized as an American public health challenge. Because of this recognition, the American socio-political and scientific community established national policies and research agendas while engaging communities to help fight the disease. These activities have greatly contributed to the understanding of SCD—an understanding that is now poised to have a global benefit for all who have SCD.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Wailoo K. Dying in the City of the Blues: Sickle Cell Anemia and the Politics of Race and Health. Chapel Hill, NC: University of North Carolina Press; 2001. [Google Scholar]
- 2.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 3.Singh GK, Yu SM. US childhood mortality, 1950 through 1993: Trends and socioeconomic diffferentials. Am J Public Health. 1996;86(4):505–512. doi: 10.2105/ajph.86.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diggs LM. Anatomic lesions in sickle cell disease. In: Abramson HBJ, Wethers DL, editors. Sickle Cell Disease: Diagnosis, Management, Education, and Research. St. Louis: C. V. Mosby; 1973. pp. 189–229. [Google Scholar]
- 5.Winsor T, Burch GE. Sickle cell anemia, “a great masquerader” easily recognizable with routine use of diagnostic parameter. JAMA. 1945;129(12):793–796. [Google Scholar]
- 6.Bloom M. Understanding Sickle Cell Disease. Jackson. Jackson, MS: University Press of Mississippi; 1995. [Google Scholar]
- 7.Herrick JB. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. 1910. Yale J Biol Med. 2001;74(3):179–184. [PMC free article] [PubMed] [Google Scholar]
- 8.Savitt TL. The second reported case of sickle cell anemia. Charlottesville, Virginia, 1911. Va Med Q. 1997;124(2):84–92. [PubMed] [Google Scholar]
- 9.Serjeant GR. The emerging understanding of sickle cell disease. Br J Haematol. 2001;112(1):3–18. doi: 10.1046/j.1365-2141.2001.02557.x. [DOI] [PubMed] [Google Scholar]
- 10.Emmel VE. A study of the erythrocytes in a case of severe anemia with elongated and sickle-shaped red blood corpuscles. Arch Intern Med. 1917;XX(4):586–598. [Google Scholar]
- 11.Mason VR. Sickle cell anemia. JAMA. 1922;79(16):1318–1320. doi: 10.1001/jama.254.14.1955. [DOI] [PubMed] [Google Scholar]
- 12.Daland GA, Castle WB. A simple and rapid method for demonstrating sickling of the red blood cells; the use of reducing agents. J Lab Clin Med. 1948;33(9):1082–1088. [PubMed] [Google Scholar]
- 13.Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 14.Ingram VM. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- 15.Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180(4581):326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- 16.Marotta CA, Wilson JT, Forget BG, Weissman SM. Human beta-globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. J Biol Chem. 1977;252(14):5040–5053. [PubMed] [Google Scholar]
- 17.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 18.Charache S, Dover GJ, Moore RD, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79(10):2555–2565. [PubMed] [Google Scholar]
- 19.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J Pediatr. 2009;154(4):541–545. doi: 10.1016/j.jpeds.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 20.Newborn Screening for Sickle Cell Disease and Other Hemoglobinopathies. [Last accessed: November 15, 2012];NIH Consens Statement. 1987 Apr 6–8; http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsnihcdc&part=A3041#A3076. [PubMed]
- 21.National Newborn Screening Status Report Updated 09/06/12. [Last accessed: November 15, 2012]; http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/nbsdisorders.pdf. [Google Scholar]
- 22.Benson JM, Therrell BL., Jr History and current status of newborn screening for hemoglobinopathies. Semin Perinatol. 2010;34(2):134–144. doi: 10.1053/j.semperi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.National Heart, Lung, and Blood Institute. [Last accessed: November 15, 2012];Surveillance and Research Program for Inherited Blood Diseases. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2691.
- 24.Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: a question of equity and quality. Pediatrics. 2006;117(5):1763–1770. doi: 10.1542/peds.2005-1611. [DOI] [PubMed] [Google Scholar]
- 25.Hassell K, Pace B, Wang W, et al. Sickle cell disease summit: from clinical and research disparity to action. Am J Hematol. 2009;84(1):39–45. doi: 10.1002/ajh.21315. [DOI] [PubMed] [Google Scholar]
- 26.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 27.National Human Genome Research Institute. [Last accessed: November 15, 2012];Learning about Sickle Cell Disease. http://www.genome.gov/10001219.
- 28.Steiner CA, Miller JL. Sickle Cell Disease Patients in U.S. Hospitals. Washington, DC: Agency for Healthcare Research and Quality; 2004. [Last accessed: November 15, 2012]. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb21.pdf. [PubMed] [Google Scholar]
- 29.NHLBI. [Last accessed: November 15, 2012];Report of the National Heart, Lung, and Blood Advisory Council Subcommittee Review of the NHLBI Sickle Cell Disease Program. http://www.nhlbi.nih.gov/resources/docs/scd_program.htm.