Abstract
Studies have shown high intussusception rates in Spain. We performed a hospital-based retrospective observational study of the intussusception risk following rotavirus vaccinations among infants in Valencia, a region of Spain with an annual birth cohort of approximately 48,000 children, during 2007–2011, using a self-controlled case series design. We performed medical record review of all cases using Brighton Collaboration´s case definition and assessed the positive predictive value (PPV) of the intussusception diagnosis code. Among 151 hospitalized cases discharged as intussusception, we confirmed 136 as Brighton Collaboration's Levels 1 or 2, resulting in a PPV of 93% (95% CI: 87%–96%). Three confirmed cases occurred within days 1–7 following the first rotavirus vaccination. The incidence rate ratio was 9.0 (95% CI: 0.9–86.5) (crude) and 4.7 (95% CI:0.3–74.1)(age adjusted). In this first study in Europe, the intussusception risk point estimate was comparable to other studies, although results were not statistically significant, maybe due to limited power. The high PPV found will facilitate implementation of a larger study without requiring medical record review. Our finding of very few vaccinated cases despite a thorough 5-year investigation in a country that, according to previous studies, may have a large background rate of intussusception is reassuring and should contribute to deliberations about the need to include rotavirus vaccines in the official Spanish calendars.
Keywords: intussusception, positive predictive value, rotavirus vaccines, self-controlled case-series (SCCS) method, vaccine safety
Abbreviations
- CI
Confidence Interval
- CMBD
Spanish hospital discharge database
- IRRs
Incidence Rate Ratios
- PPV
Positive Predictive Value
- RV1
Rotarix® (GlaxoSmithKline Biologicals Rixensart Belgium)
- RV5
RotaTeq® (Merck & Co. Inc. West Point PA USA)
- SCCS
Self-Controlled Case Series
- SIV
Valencia´s Vaccine Information System
Manuscript
Two oral live-attenuated rotavirus vaccines are currently available in the global market: a monovalent human vaccine, Rotarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium), indicated as a 2-dose series in infants between the ages of 6–12 and 24 weeks,1 and a pentavalent bovine-human reassortant vaccine, RotaTeq® (Merck & Co., Inc., West Point, PA, USA), indicated as a 3-dose series starting at age 6–12 weeks and ending at age ≤32 weeks.2 Previously, an observed association between Rotashield®, the first licensed rotavirus vaccine, and intussusception, caused its withdrawal from the US market in 1999, only 9 months after licensure.3,4 The pre-licensure clinical trials for both Rotarix® (RV1) and RotaTeq® (RV5), designed to exclude an association with intussusception of similar magnitude than that found for Rotashield®, did not find an association.5,6 However, after licensure, an increased risk, although of much lower magnitude, was found with both vaccines in Australia, Mexico, Brazil, and the United States.7-10
Both vaccines, RV1 and RV5, have been available in Spain since August 2006 and January 2007, respectively. Rotavirus vaccines in Spain are not funded by the National Health System, but are recommended by scientific societies and most pediatricians, and paid for by parents. Due to the incidental finding of circovirus DNA contamination affecting both vaccines, the Spanish Medicines Agency suspended RV5 distribution during June-November 2010, and RV1 distribution since March 2010.11 As of this publication, RV1 remains suspended in Spain.
Intussusception risk varies across population and regions,12 and some studies have shown high background rates of intussusception in Spain;13,14 a potential association with rotavirus vaccination could cause concern. Thus, our aim was to investigate such association in the Valencia Region, Spain.
We performed a hospital-based retrospective observational study during January 1, 2007 – December 31, 2011. Intussusception risk following rotavirus vaccinations was assessed using a self-controlled cases series (SCCS) design.15 The SCCS is a case-only design that uses individuals who had the event of interest during the study period; we applied the vaccinated cases only SCCS approach, which uses only subjects who were exposed to a rotavirus vaccine.16 Therefore, inference is done within individuals, and time-fixed confounders are controlled implicitly by design. Unvaccinated individuals and individuals for which vaccination status is unknown are excluded. The observation period (follow-up person-time) for each individual is divided in predefined risk and non-risk periods based on exposure. Therefore, the event of interest falls either into a risk or a non-risk period.15 In our case, the risk periods were defined as days 1–7, and 8–21, post-vaccination, following each dose of any rotavirus vaccine. The comparison (non-risk) period was days 22–42 following each dose. The study included assessment of the positive predictive value (PPV) of the intussusception code used in the Spanish hospital discharge database, CMBD.17
Almost all the Valencia Region population (>98%) is covered by the public health system,18 and all users have a unique identification number that allows linking all health care databases and all medical records.
Potential intussusception cases among resident infants aged 6–42 weeks, irrespective of their rotavirus vaccination status, were identified from CMBD through the ICD-9-CM code 560.0 in any diagnosis position. A review of hospitalization and primary care medical records of all potential cases was carried out using the standardized Brighton Collaboration case definition for intussusception (see the Table S1 showing the Brighton criteria in the Appendix).19 First intussusception episodes considered confirmed (Brighton Level 1-Level 2) were included in the analyses. In our study, the event date was the date of onset of symptoms.
The exposure of interest was vaccination with any dose of rotavirus vaccines. Rotavirus vaccination status was obtained from the regional vaccine information system, SIV.20 Lack of information or inconsistencies were confirmed through phone consultation with parents and verbal verification of the information in the child´s vaccination card.
Dose-specific incidence rate ratios (IRRs), and their 95% confidence intervals (CI), were assessed to investigate the association between confirmed intussusception cases and rotavirus vaccines within the 2 predefined risk periods. The IRR is estimated by within-individual comparisons of the incidence of the event of interest in risk and control periods using person-time denominators. Given the strong confounding effect of age, analyses were adjusted using, as reference, intussusception-hospitalized rates by age in unvaccinated Valencia Region´s children during 2001–2011.10 Specific rates by age were estimated using data from CMBD and from the Spanish Statistical Office database (www.ine.es); the pattern was modeled using spline function (Fig. 1).
Figure 1.
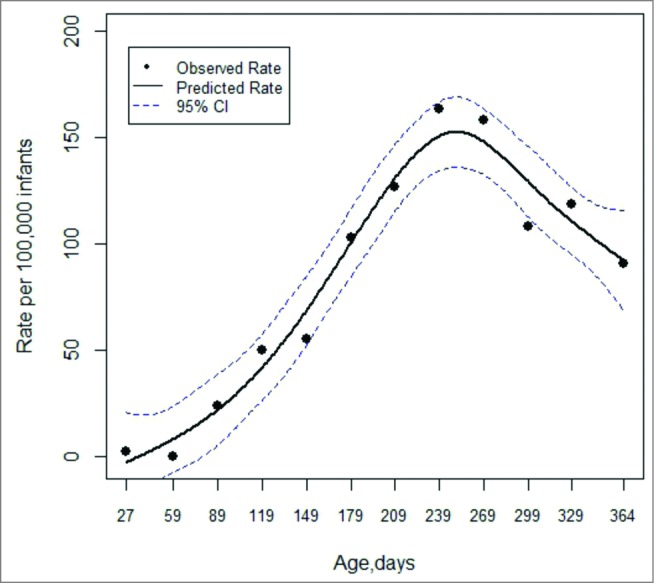
Intussusception-hospitalized rates by age in unvaccinated Valencia Region´s children aged less than 12 months during 2001–2011.
The PPVs were assessed for the different categories of Brighton Collaboration case classification: (1) Level 1, (2) Level 1-Level 2, and (3) Levels 1-Level 2-Level 3. They were stratified for discharge code position and calendar year.
All statistical tests were 2-sided. Statistical significance was defined as p < 0.05. Analyses were performed using Stata/SE 13.1 (StataCorp LP Texas, USA), R 3.0.3 (Foundation for Statistical Computing, Vienna, Austria), SAS 9.2 (SAS Institute, Inc.), and SAS macros developed by Bart Spiessens.
The study was conducted according to the existing legislation including the Good Epidemiological Practices (CIOMS 2009), the Helsinki Declaration (Seoul 2008) and the Law 14/2007, of 3 July, on Biomedical Research, and was approved by the Ethics Research Committee of the Dirección General de Salud Pública/Centro Superior de Investigación en Salud Pública, which provided a waiver to access personal information and contact parents.
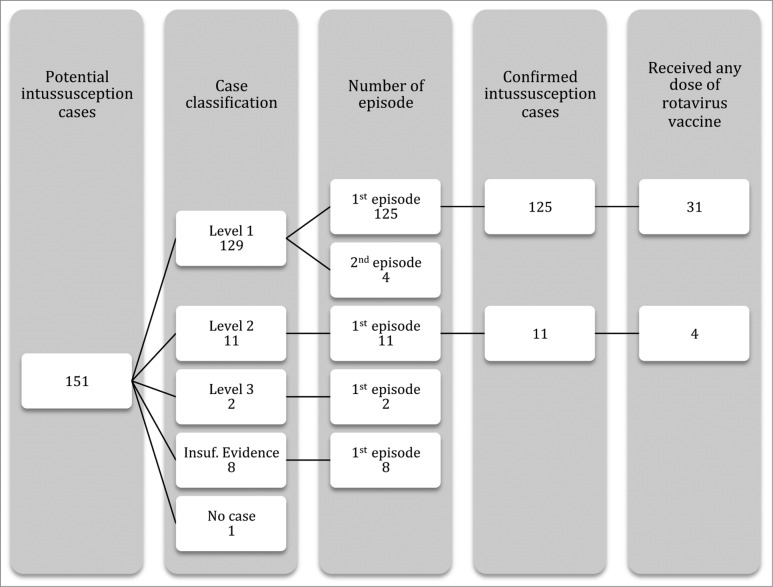
The Valencia Region has an annual birth cohort of around 48,000 infants among a total population of approximately 5,000,000 inhabitants. Within the population of interest, infants aged 6 to 42 weeks discharged from hospitals of the Valencia Region´s public health system, 151 potential hospitalized-intussusception cases were identified after having excluded duplicate episodes (children transferred to a reference hospital). Cases occurred in 147 infants, since 4 infants (3%) had a second episode. Medical records were available for all cases. Among first episodes, 125 cases (85.0%) were classified as Level 1, 11 (7.5%) as Level 2, 2 (1.4%) as Level 3, 8 (5.4%) as Insufficient Evidence, and one case (0.7%) was discarded. Of 136 confirmed intussusception cases, 35 (26%) occurred in rotavirus-vaccinated children (Fig. 2). Among them, 14 (40%), and 21 (60%) had received RV1 and RV5, respectively.
Figure 2.
Flowchart of hospitalized-intussusception cases.
The PPV of the diagnosis code for hospitalized intussusception cases (any discharge diagnosis position) was 93% (95% CI: 87%–96%) for Level 1-Level 2 of diagnosis certainty. No differences by discharge diagnosis position or by calendar year were found (Table 1).
Table 1.
Positive predictive values according to The Brighton Collaboration case classification by discharge code position, and by calendar year
| Positive Predictive Values | ||||
|---|---|---|---|---|
| Casesn | Level 1% (95% CI) | Level 1- Level 2% (95% CI) | Level 1-Level 2-Level 3 % (95% CI) | |
| Discharge code position | ||||
| 1st | 148 | 86.5 (79.9–91.5) | 93.2 (87.9–96.7) | 94.6 (89.6–97.6) |
| 1st or 2nd | 149 | 86.6 (80.0–91.6) | 93.3 (88.0–96.7) | 94.6 (89.7–97.7) |
| Any | 151 | 85.4 (78.8–90.6) | 92.7 (87.3–96.3) | 94.0 (89.0–97.2) |
| Calendar year1 | ||||
| 2007 | 44 | 81.8 (67.3–91.8) | 90.9 (78.3–97.5) | 95.5 (84.5–99.4) |
| 2008 | 30 | 86.7 (69.3–96.2) | 93.3 (77.9–99.2) | 93.3 (77.9–99.2) |
| 2009 | 25 | 84.0 (63.9–95.5) | 92.0 (74.0–99.0) | 92.0 (74.0–99.0) |
| 2010 | 28 | 89.3 (71.8–97.7) | 92.9 (76.5–99.1) | 92.9 (76.5–99.1) |
| 2011 | 24 | 87.5 (67.6–97.3) | 95.8 (78.9–99.9) | 95.8 (78.9–99.9) |
Any diagnosis position.
Abbreviations: Positive Predictive Value (PPV); Confidence interval (CI).
Three intussusception cases occurred within days 1–7 following first dose of a rotavirus vaccine (two after RV1 and one after RV5) resulting in a crude IRR point estimate of 9.0 (95% CI: 0.9–86.5), and in an age-adjusted estimate of 4.7 (95% CI:0.3–74.1) within this risk window. The number of cases occurring within 1–7, 8–21, and 22–42 days following each dose, and dose-specific IRRs are shown in Table 2.
Table 2.
Risk estimates for confirmed intussusception cases after Rotarix®/RotaTeq® vaccination, vaccinees-only SCCS approach
| Vaccine dose | Risk period(days post-vaccination) | Number of cases | Non-risk period(days post-vaccination) | Number of cases | IRR (95% CI)(crude) | IRR (95% CI)(age-adjusted) |
|---|---|---|---|---|---|---|
| Dose 1 | ||||||
| 1–7 | 3 | 22–42 | 1 | 9.0 (0.9–86.5) | 4.7 (0.3–74.1) | |
| 8–21 | 1 | 1.5 (0.1–24.0) | 0.8 (0.1–13.9) | |||
| Dose 2 | ||||||
| 1–7 | 1 | 22–42 | 2 | 1.5 (0.1–16.5) | 1.6 (0.1–32.3) | |
| 8–21 | 3 | 2.3 (0.4–13.5) | 3.9 (0.3–44.0) | |||
| Dose 3 | ||||||
| 1–7 | 0 | 22–42 | 1 | 3.0 (0.2–48.0) | Non interpretable1 | |
| 8–21 | 0 | Non interpretable1 | Non interpretable1 |
The observation period for each vaccine dose ends at day 42 post-vaccination.
Non-interpretable IRR due to very small numbers.
Abbreviations: Intussusception (IS); Confidence interval (CI).
In this first post-licensure analytical study of the intussusception risk following rotavirus vaccination in Europe, we investigated intussusception cases among infants discharged from all hospitals of the Valencia Region´s public health system, which covers >98% of the approximately 5 million population of the region, for a 5-year period. Therefore, it is unlikely that a significant proportion of the total cases of intussusception would have been seen in private hospitals. Also, vaccines administered in all public and some private health centers are recorded in SIV, regardless of their inclusion in the official immunization schedule. A recent study has shown that, among all rotavirus vaccine doses distributed during 2009–2012, most (86%) were registered in SIV as administered in children aged less than one year.21 Nonetheless, because of these concerns, we chose a SCCS approach restricted only to vaccinated cases. This method implicitly controls for time-fixed confounders, and, because of the restriction to vaccinees, also minimizes potential misclassification bias, and eliminates the need to address the healthy vaccinee effect and the contraindication to vaccination issue found in some studies. An additional strength of this study is that intussusception diagnoses were confirmed through medical record review of all potential cases hospitalized.
Our finding of an elevated intussusception risk point estimate during the first week following administration of the first dose of rotavirus vaccines was comparable in magnitude to estimates from similar studies performed in the United States, Australia, and Mexico.7-10 Despite having analyzed the whole population of a large region for 5 years, our findings were not statistically significant, maybe due to lack of study power. This is understandable given the rarity of intussusception and the low rotavirus vaccine coverage in the region during the study period (full-schedule coverage estimated for children aged under one year as ranging between 24% to 49%, based on distributed vaccine data). Because of the small size of the total vaccinated population, and our need to evaluate rotavirus vaccine safety for the region (regardless of specific vaccines used) both vaccines were evaluated jointly. Our finding of very few vaccinated cases despite a thorough multi-year investigation in a country which, according to previous studies, may have a large background rate of intussusception,13,14 is reassuring, and provides information that should contribute to deliberations about the need to include rotavirus vaccines in the official Valencian and Spanish calendars.
This study has also shown that a high quality investigation of the safety of childhood vaccinations using Valencia´s healthcare databases is possible. The high (93%) PPV found for a discharge diagnosis of intussusception has opened the door to the implementation of a larger study without the need to perform medical record reviews. Thus, we plan to continue the study for additional years, and invite also participation from other Spanish regions. Moreover, following verification of the PPV of the discharge diagnosis codes in candidate sites, the study could be extended to include other European databases, thus gaining substantial power for the analysis of this rare event, even if vaccination coverage remains low. Such database integration will substantially improve the capacity for timely post-licensure assessments of the safety of any new vaccines introduced in Europe.
Acknowledgments
Hector S. Izurieta, US Food and Drug Administration, for advise in study design, analysis and review of the manuscript, Martin Kulldorff, Harvard Medical School and Harvard Pilgrim Health Care Institute, for advice regarding study methodology, Marian Martín-Navarro and Mónica López-Lacort, FISABIO-Public Health, for assistance in data collection and contributions to the statistical analysis, respectively.
Disclosure of Potential Conflicts of Interest
SPV, JDD, and JPB are working at FISABIO-Public Health, institution that has ongoing research contracts with GlaxoSmithKline Biologicals, Sanofi Pasteur MSD, and Merck & Co., Inc. JDD, JPB and RGP have received support for grants and travel grants from GlaxoSmithKline Biologicals, Sanofi Pasteur MSD, and Merck & Co., Inc. SPV has received travel grants for Scientific Congress from GlaxoSmithKline Biologicals and Sanofi Pasteur MSD. SR has no conflicts of interest.
Authors' Contributions
All authors contributed to the study design. SPV coordinated the study, acquired the data, and review the cases. SPV, HSI and SR analyzed the data. SPV drafted the manuscript. All authors were involved in interpretation of the results, the critical revision of drafts, and approved the final version of the manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Rotarix: Product information. European Medicines Agency , 2009 [Google Scholar]
- 2.RotaTeq: Product information. European Medicines Agency , 2008 [Google Scholar]
- 3.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, et al.. Intussusception among infants given an oral rotavirus vaccine. N Eng J Med 2001; 344:564-72; PMID:11207352; http://dx.doi.org/ 10.1056/NEJM200102223440804 [DOI] [PubMed] [Google Scholar]
- 4.Kramarz P, France EK, Destefano F, Black SB, Shinefield H, Ward JI, et al.. Population-based study of rotavirus vaccination and intussusception. Pediat Infect Dis J 2001; 20:410-6; PMID:11332666; http://dx.doi.org/ 10.1097/00006454-200104000-00008 [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al.. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Eng J Med 2006; 354:23-33; PMID:16394299; http://dx.doi.org/ 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al.. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Eng J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/ 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- 7.Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE; PAEDS/APSU Study Group. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29:3061-6; PMID:21316503; http://dx.doi.org/ 10.1016/j.vaccine.2011.01.088 [DOI] [PubMed] [Google Scholar]
- 8.Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, Bines J, McIntyre PB. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clin Infect Dis 2013; 57:1427-34; PMID:23964090; http://dx.doi.org/ 10.1093/cid/cit520 [DOI] [PubMed] [Google Scholar]
- 9.Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, et al.. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Eng J Med 2011; 364:2283-92; PMID:21675888; http://dx.doi.org/ 10.1056/NEJMoa1012952 [DOI] [PubMed] [Google Scholar]
- 10.Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M. Intussusception risk after rotavirus vaccination in U.S. infants. N Eng J Med 2014; 370:503-12; PMID:24422676 [DOI] [PubMed] [Google Scholar]
- 11.Bouzon Alejandro M, Diez Domingo J, Martinon-Torres F. Circovirus and impact of temporary withdrawal of rotavirus vaccines in Spain. Hum Vaccin 2011; 7:798-9; PMID:21715979; http://dx.doi.org/ 10.4161/hv.7.7.15683 [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One 2013; 8:e68482; PMID:23894308; http://dx.doi.org/ 10.1371/journal.pone.0068482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bines JE, Ivanoff B. Acute intussusception in infants. Incidence, clinical presentation and management: a global perspective Vaccines and Biologicals. Geneva: World Health Organization, 2002 [Google Scholar]
- 14.Bringue Espuny X, Ibars Valverde Z, Martinez Alonso M, Morales Bara I, Sole Mir E. [Intestinal invagination: change in its incidence from 1987 to 2008]. Cir Pediatr 2010; 23:206-10; PMID:21520551 [PubMed] [Google Scholar]
- 15.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006; 25:1768-97; PMID:16220518; http://dx.doi.org/ 10.1002/sim.2302 [DOI] [PubMed] [Google Scholar]
- 16.Maclure M, Fireman B, Nelson JC, Hua W, Shoaibi A, Paredes A, et al.. When should case-only designs be used for safety monitoring of medical products? Pharmacoepidemiol Drug Safety 2012; 21 Suppl 1:50-61; PMID:22262593; http://dx.doi.org/ 10.1002/pds.2330 [DOI] [PubMed] [Google Scholar]
- 17.Ministerio de Sanidad Servicios Sociales e Igualdad Explotación del registro de altas CMBD del Sistema Nacional de Salud. Spain, 2014. [Google Scholar]
- 18.Ministerio de Sanidad Servicios Sociales e Igualdad Informe Anual del Sistema Nacional de Salud 2011. Spain, 2013. [Google Scholar]
- 19.Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, et al.. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004; 22:569-74; PMID:14741146; http://dx.doi.org/ 10.1016/j.vaccine.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 20.Pastor Villalba E, Martín-Ivorra R, Alguacil-Ramos AM, Portero Alonso A, Barberà Català MA, Pons Sánchez C, Lluch Rodrigo JA. Sistema de Información Vacunal (SIV). In: Valenciana Generalitat, de Sanitat Conselleria, eds. Valencia (Spain: ) 2009. [Google Scholar]
- 21.Pastor-Villalba E, Martín-Ivorra R, Alguacil-Ramos AM, Portero-Alonso A, Lluch Rodrigo J. [Exhaustividad en la declaración en el sistema de información vacunal (SIV) de vacunas no incluidas en el calendario sistemático infantil de la Comunidad Valenciana. Años 2009 a 2012]. P04. 7th Congress of the Spanish Association of Vaccinology Cáceres (Spain) 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.