Abstract
Oil-in-water emulsions have gained consideration as vaccine adjuvants in recent years due to their ability to elicit a differentiated immunogenic response compared to traditional aluminum salt adjuvants. Squalene, a cholesterol precursor, is a natural product with immunostimulatory properties, making it an ideal candidate for such oil-in-water emulsions. Particle size is a key parameter of these emulsions and its relationship to stability and adjuvanticity has not been extensively studied. This study evaluates the effect of particle size on the stability and immunogenicity of squalene emulsions. We investigated the effect of formulation parameters such as surfactant concentration on particle size, resulting in particles with average diameter of 80 nm, 100 nm, 150 nm, 200 nm, or 250 nm. Emulsions were exposed to shear and temperature stresses, and stability parameters such as pH, osmolarity, size, and in-depth visual appearance were monitored over time. In addition, adjuvanticity of different particle size was assessed in a mouse model using Respiratory Syncytial Virus Fusion protein (RSV-F) as a model antigen. Temperature dependent phase separation appeared to be the most common route of degradation occurring in the higher particle sizes emulsions. The emulsions below 150 nm size maintained stability at either 5°C or 25°C, and the 80 nm diameter ones showed no measurable changes in size even after one month at 40°C. In vivo studies using the emulsions as an adjuvant with RSV F antigen revealed that superior immunogenicity could be achieved with the 80 nm particle size emulsion.
Keywords: adjuvants, emulsions, vaccines, RSV, squalene, stability, surfactants
Introduction
Efficacious vaccination is clearly the most effective method to control infectious diseases,1 and has saved millions of lives in the last 2 centuries. Vaccination works by educating the immune system to recognize and mount a response against an invading pathogen. Vaccines are typically made up of weakened/inactivated forms of the pathogen, or its constituents.2 In the last 2 decades, there has been a push to develop recombinant subunit protein-based vaccines due to the ease of manufacturing and characterization, as well as potential safety benefits. Unfortunately, subunit vaccines typically are not sufficiently immunogenic of themselves and often require an adjuvant to boost their immunogenicity.3
Traditionally, aluminum salts such as Alhydrogel® and Adjuphos® are used as vaccine adjuvant in order to boost an immune response in the presence of an antigen. While aluminum salts remain the most widely used adjuvants in human vaccines to date, the immune response to aluminum salt adjuvants may not confer the needed type of immune response or boost cellular immunogenicity. Within the last 2 decades, emulsion-based vaccine adjuvants have been shown to provide an alternative to aluminum salt adjuvants in terms of specific vaccine immunogenicity.3 The most common emulsions used as adjuvants are oil-in-water emulsions, where the oil acts as the solute in the water phase and forms isolated droplets, stabilized by emulsifying agents.
Squalene, a common metabolic intermediate in plants and animals, is a popular choice for oil-in-water emulsions due to its physical and immunostimulatory properties.4-8 Currently, there are 2 squalene emulsion adjuvants approved for clinical use in Europe: MF59®9-11 (Novartis Vaccines & Diagnostics), and AS03 11,12 (GSK Biologics; AS03 is also approved in the US for the use with a 2009 pandemic flu vaccine). Both these emulsions contain a squalene content of around 2–4% (w/w) with additional emulsifying agents. MF59 was the first oil-in-water emulsion on the market and is produced using microfluidization and contains sorbitan trioleate and polysorbate-80 (PS80) as surfactants. MF59 has a particle size of around 160 nm. AS03 is another oil-in-water emulsion that utilizes squalene. AS03 contains α-tocopherol (vitamin E) and PS80 as a surfactant. AS03 has a particle size of about 150 nm. GLA-SE (IDRI) is currently in clinical trials, consisting of a squalene emulsion in combination with GLA, which is a synthetic form of MPLA and a potent immunopotentiator.13,14 It has been suggested that emulsions with particle sizes ranging from 100–200 nm are efficiently taken up by dendritic cells, and hence stimulate the immune system.15 However, no significant work has been published on the effect of particle size on the stability and immunogenicity of these emulsions.
In this paper, we investigated the properties of squalene emulsions of different particle sizes, with the goal of understanding the effect of particle size on stability and immunogenicity. Stability was monitored over the course of 30 days at temperatures ranging from 5°C – 60°C; key parameters studied included particle size, pH, osmolarity and changes in visual appearance. Additionally, we studied the immunogenicity profile of the emulsion at 3 particle sizes (approximately 80 nm, 150 nm and 250 nm) in mice in the context of RSV vaccination. RSV is a negative-stranded RNA virus that causes significant respiratory disease in infants, immune-compromised, and older adults.16 A number of studies have shown that immune responses specific to RSV F are key for a potential RSV vaccine.17 In the present study, we used RSV F as a model antigen to test the immunogenicity of our different emulsion preparations. Adjuvant-induced serum cytokines were monitored in addition to RSV F-specific serum antibody titers and cell mediated immune response.
Results and Discussion
Emulsion preparation and emulsion stability
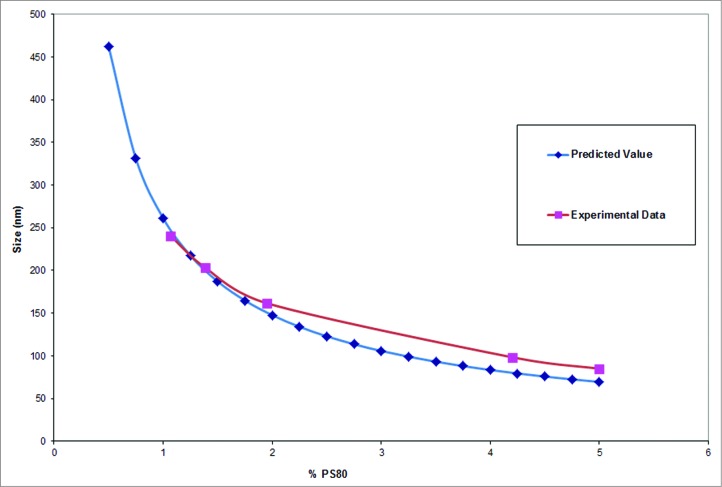
Squalene emulsions were prepared using a micro-fluidization process with PS80 as surfactant. The average particle size of the emulsions was varied by changing the concentration of the surfactant during the micro-fluidization process. Particle size was followed as a function of surfactant concentration, to determine the specific concentration of surfactant required to obtain an emulsion of a consistent particle size. After initial experimentations titrating PS80 concentrations from 0.5% to 5%, a trend line was fitted to the data correlating surfactant concentration to the particle size (Fig. 1). Emulsions of particle size ranging from 80 nm to 250 nm were obtained using different concentrations of PS80 in the microfluidization process. Conceptually, homogenization and microfluidization disperse the oil into tiny droplets which spontaneously begin to fuse and phase separate in the absence of a surfactant. In the presence of polysorbate-80, the oil droplets become coated with it, and are stabilized in solution. The particle size of the emulsion is inversely proportional to the concentration of the surfactant, as higher concentrations of surfactants are required to coat the larger surface area attributable to a larger number of smaller particles. The smallest particle size achievable is related to the pressure and the shear forces to which the emulsion is subjected. In our experiment, the smallest particle size achievable under the specified experimental conditions was 70–80 nm, although it may be possible to achieve smaller particles by changing other environmental and formulation parameters. Emulsions were prepared at average particle sizes of 80 nm, 100 nm, 150 nm, 200 nm and 250 nm. It was found that emulsions tend to have slightly higher polydispersity at larger particle sizes. This is potentially due to the heterogeneous micro-environment that exists at lower surfactant concentrations, as the smaller emulsion particles may be coated with surfactant first, followed immediately by the bigger particles. This may not be the case when surfactant is available in excess. The addition of PS80 after the microfluidization process (For the B1 and the B2 groups) did not change the particle size of the emulsions. This may be due to complete coating of the oil with the surfactant, and the addition of the excess surfactant does not break up the larger emulsion particles into smaller ones. Prior studies also indicate such changes in particle size with increases in surfactant concentrations.22,23 A critical upper surfactant concentration was not reached in the studies performed. Though stability studies were conducted in formulations containing PS80 at concentrations as high as 5% and squalene concentrations as high as 20%, these only represent preferred manufacturing concentrations. These concentrations would be diluted down to 4% squalene and 1% PS80 to make them more acceptable for use as vaccine adjuvants.
Figure 1.
Particle size of the emulsion can be modulated using the concentration of the surfactants. The presence of 1% PS80 yielded an emulsion with a particle size of about 250 nm, the particle size decreases with increasing concentration of PS80.
Stability study
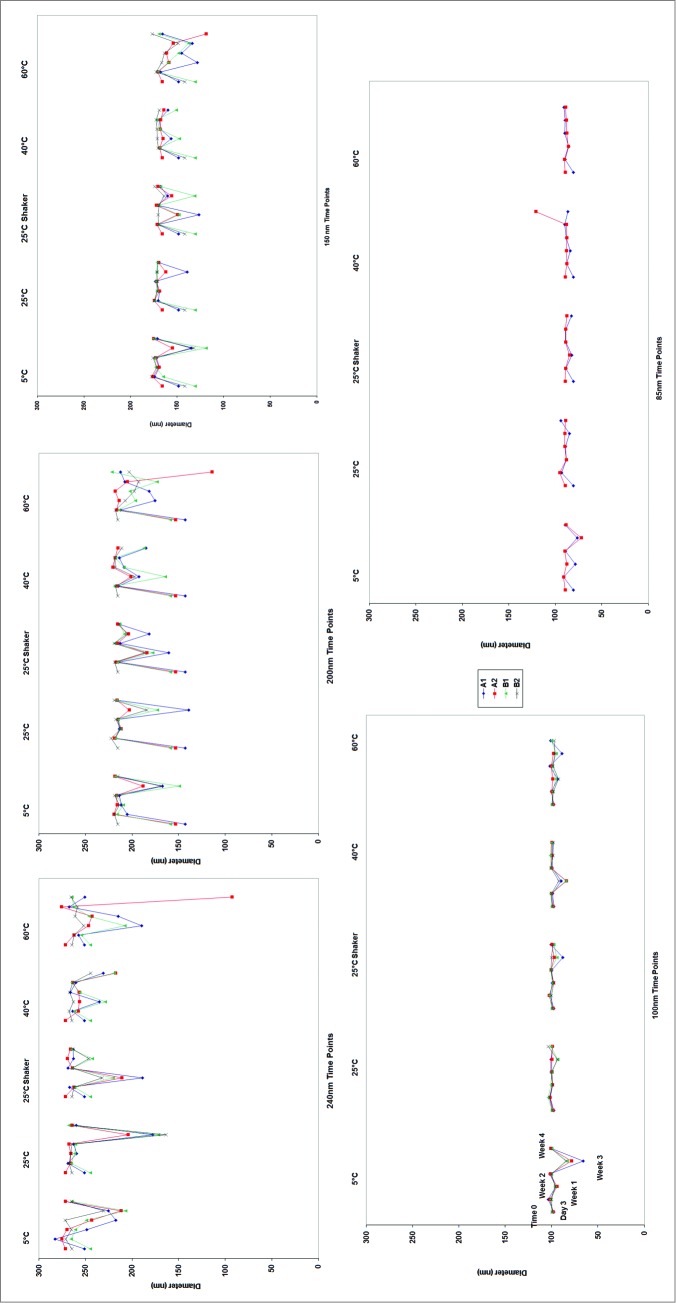
To assess stability, 5 emulsions with particle sizes ranging from 80 nm to 250 nm were exposed to temperatures up to 60°C for 30 days to assess their thermal and kinetic stability. Key parameters such as size, pH and osmolarity were monitored as a function of time and temperature. Figure 2 shows the changes in particle size as measured by dynamic light scattering over the time period. By day 3, the emulsions stored at 60°C began to show early signs of phase separation. Tiny droplets of oil were found on top of the A1 (20% Squalene and the initial (and lower) concentrations of PS80) emulsions at 250 nm, 200 nm, 150 nm, and 100 nm. This small degree of phase separation in the emulsions with the bigger particle sizes made it difficult to accurately measure particle size due to a high degree of light scattering from the oil droplets on the top of the emulsion samples. This is evident by the high variability in the data shown in Figure 2. This high degree of variability was also observed in samples stored at 5°C, indicating the phase separation may not be solely dependent on temperature. By the end of the first week, this trend was also observed with the A2 (4% Squalene with the initial concentration of PS80) emulsions as the same particle sizes. During this time, the B1 and B2 emulsions stored at 60°C, and the 80 nm samples from the A1 and A2 emulsions remained stable. This indicates that emulsions with larger particle sizes and relatively lower concentration of PS80 were less stable. The emulsions with 4% Squalene appeared marginally more stable than those with 20% Squalene.
Figure 2.
Changes in the particle size of the emulsions as a function of time and temperature. It was observed that the 80 nm emulsion showed the least change in particle size.
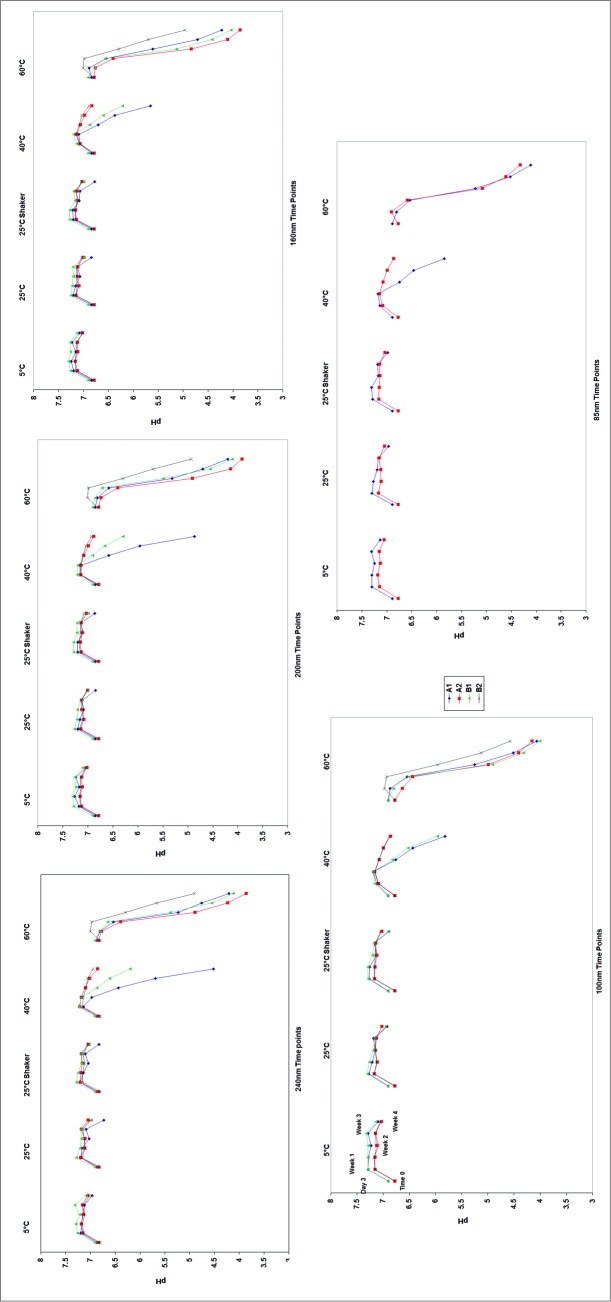
Over the course of the 4 week study, the emulsions placed at 60°C experienced a significant drop in pH. Figure 3 compares the pH drop in the 5 stability chambers. The largest drop in pH occurred at 60°C, followed by 40°C. A pH drop was observed at all particle sizes, with the largest drop occurring in the 250 nm emulsions (a final pH of 4.5, compared to pH 5.8 for the 100 nm emulsion). At 5°C and 25°C, however, such drops in pH were not observed. Temperature may have an effect on squalene oxidation rates, resulting in a more rapid pH shift at higher temperatures. Temperature dependent degradation of PS80 has also been observed. The peroxides released from the degradation of PS80 may potentially oxidize the squalene. Such degradation was not monitored specifically, but its effects are mirrored by changes in pH.
Figure 3.
Changes in the pH of the emulsion solutions as a function of time and temperature. It was observed that the 80 nm emulsion showed the least change in pH after one month of incubation at 60°C.
At the end of the 4 weeks, the osmolality readings show that the greatest variability occurred at 60°C, similar to the pH and size readings. This was common among all particle sizes, with greater variability occurring in the 250 nm emulsions.
Pictures of the different emulsions are shown in Figure 4 at 2 weeks (A) and 4 weeks (B). Phase separation was observed when the emulsions were stored at 60°C in the emulsions with the higher particle sizes (>100 nm). No changes were observed in the emulsions with the 80 nm particles in any of the stability conditions.
Figure 4.
Pictures of the emulsion reveal phase separation of the emulsion when stored at 60°C for up to 2 weeks (A) and one month (B). No phase separation was observed in the 80 nm emulsion particles as observed in samples with particle size of 150 nm or higher.
From the data obtained, it can be inferred that smaller particle size (≤100 nm) squalene emulsions are more stable than the emulsions with the larger particle sizes. A general trend was observed in which emulsions with smaller particles demonstrated better stability. Lastly, spiking in PS80 after microfluidization to obtain higher surfactant concentration increased emulsion stability of the larger particles, potentially due to the availability of additional surfactant to stabilize the larger emulsion particles.
Animal study
Next, we investigated whether the difference in particle size influenced the immunogenicity of a model antigen combined with different size emulsions. Emulsions at 80, 150, and 250 nm particle size were selected for in vivo immunogenicity evaluation in combination with recombinant RSV F in the presence or absence of CpG 2395, a toll-like-receptor 9 (TLR9) agonist. TLRs are membrane-bound receptors that detect danger signals or endogeneous processes and stimulate immune responses through induction of pro-inflammatory cytokines.24 The commercially-available oil-in-water emulsion AddaVax (160 nm size), an approximation of MF59, was used as a comparator, also in the presence or absence of CpG. There was no significant change in the particle size of the emulsions in the presence of CpG (data not shown).
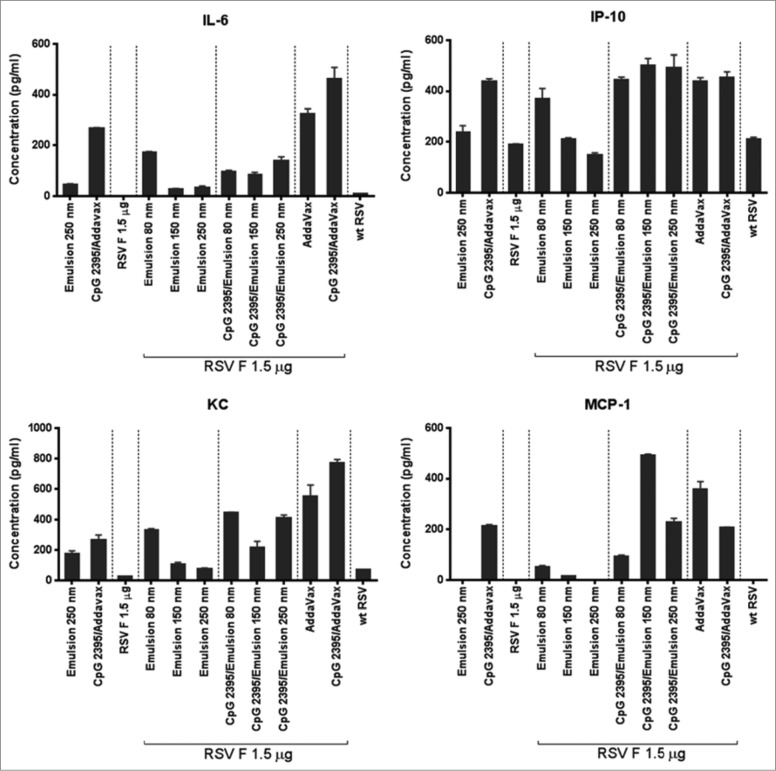
The measurement of early systemic innate immune response to vaccine adjuvant reflects the magnitude of the initial adjuvant activity.25 To this end, the concentration of adjuvant-induced protein cytokines IL-6, MCP-1, KC, and IP-10 were evaluated in mouse sera 6 h post immunization. In the absence of CpG 2395, the expression level of the cytokines appeared to be inversely correlated to the particle size (see Table 2 for correlation coefficients), with the highest induction observed in the animals immunized with the 80 nm particles (Fig. 5). In the presence of CpG 2395, the particle size did not influence the cytokine expression levels.
Table 2.
Pearson coefficient correlation measured between particle emulsion size and cytokine expression levels. No statistical significance could be measured as the samples were ran in duplicate of pooled sera per group
| IL-6 | IP-10 | KC | MCP-1 | |
|---|---|---|---|---|
| Pearson Corr Coef | −0.8326 | −0.9648 | −0.909 | −0.9748 |
Figure 5.
Adjuvant-induced serum cytokine. Levels of IL-6, IP-10, KC, and MCP-1 were measured in the serum of animals 6 h after vaccine injection. The data is presented as a mean of experimental replicates ±SD.
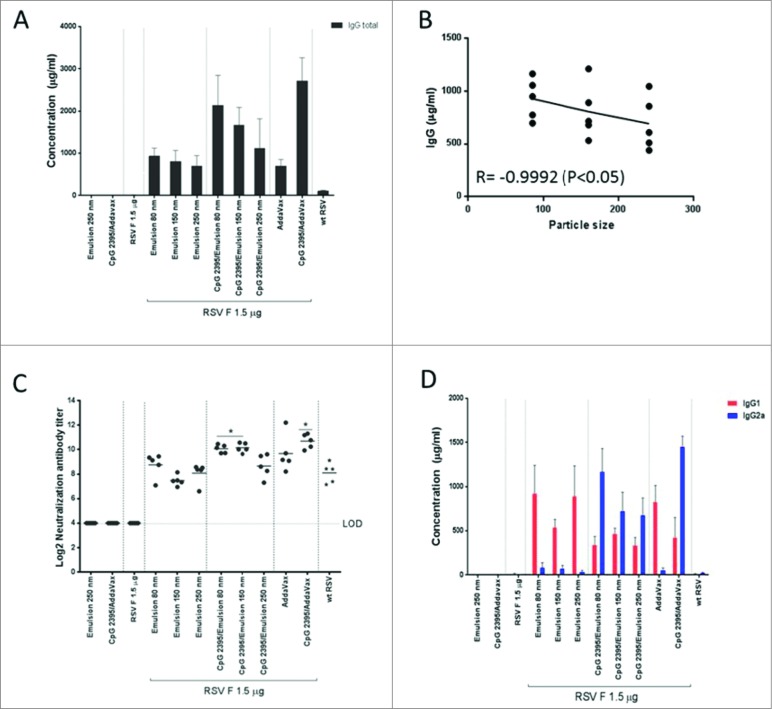
To assess the impact of vaccine composition on the induced humoral immune response, anti-RSV F antibody titers and virus neutralizing antibody titers were measured in sera from immunized animals (Fig. 6). Anti-RSV F IgG antibody titers (Fig. 6A) inversely correlated with the particle size, especially when CpG 2395 was added to the inocula (Fig. 6B). In addition, lower neutralization titers were observed with the largest particle size of 250 nm (Fig. 6C). Emulsions alone induced primarily IgG1 type antibodies, indicative of a T-helper 2 (Th2) biased immune response consistent with previous observations using emulsion alone adjuvant.26 The magnitude of this response was not dependent on the particle size (Fig. 6D). The TLR9 ligand CpG 2395, a well-defined IgG2a inducer,27 shifted the response toward a Th1 bias that was especially prominent when immunization occurred with small particle size emulsions.
Figure 6.
RSV F-specific serum antibody titers (A) Serum total IgG titers were measured 4 days post-challenge by ELISA. (B) Scatter plot of the correlation between total IgG titers and Emulsion particle size without CpG. (R = Pearson correlation coefficient). (C) Serum neutralizing antibody titers were measured at day 28 (2 weeks after boost). (D) The RSV F-binding IgG1 and IgG2a antibody titers were assessed by ELISA. LOD: lower limit of detection. Data bars represent the mean of 5 animals ±SD.
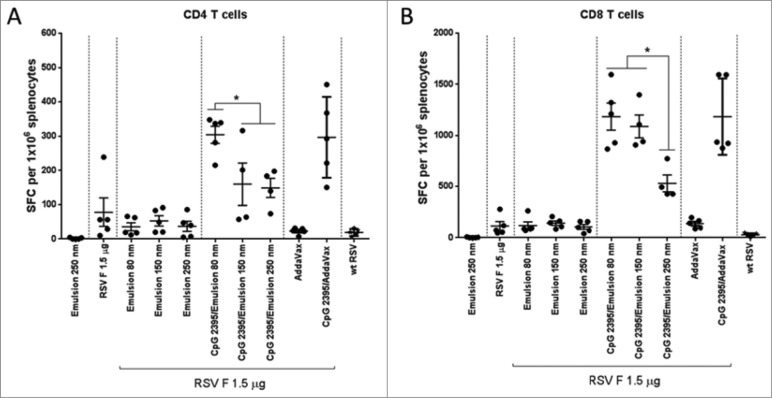
Cellular immunity was determined via INF-γ ELISPOT assay. Splenocytes from immunized mice were stimulated ex vivo with RSV F specific CD4 and CD8 peptides (Fig. 7). The number of CD4 and CD8 T cells secreting INF-γ was as expected low in the animals immunized with RSV F and emulsion alone, and was not affected by the particle size. The addition of CpG 2395 strongly increased the number of RSV F specific CD4 and CD8 T cells. However, in the animals immunized with the smallest particle size formulation, the numbers of INF-γ secreting cells were comparable to the RSV F + CpG 2395/Addavax formulation. Administration of RSV F with the 250 nm emulsion in combination with CpG 2395 resulted in the induction of fewer IFN-γ secreting CD4 and CD8 cells compared to the smaller size emulsions. The Th1/Th2 bias in immunized animals was assessed by IFN-γ/IL-5 measurement in the supernatant from splenocytes restimulated with an RSV F overlapping peptide pool (Fig. 8). High IL-5 levels and very low levels of IFN-γ were detected in the splenocytes supernatants from animals immunized with the RSV F antigen alone or in combination with the emulsion, suggesting a clear Th2 bias. The addition of CpG to the emulsion shifted the bias toward Th1 with level of IFN-γ higher than the upper limit of detection for the animals immunized with the 2 smallest sizes of particles. Overall, the data confirmed the ELISPOT observations regarding the T cell activation and confirmed the Th1/Th2 bias deduced from the anti-RSV F IgG1/IgG2a ratios. Future studies, investigating antigen uptake by antigen presenting cells, are required to determine whether 250 nm particles are less efficiently processed by APCs.
Figure 7.
Cell mediated immune responses to immunodominant RSV F peptides, spleens were isolated at 4 days post-challenge and splenocytes were stimulated with peptides, representing RSV F-specific (A) CD4 and (B) CD8 T-cell epitopes. The number of IFN-γ secreting cells per 106 splenocytes was determined by ELISPOT. Group means with SD of 5 mice per group are shown. Statistical analysis by ANOVA/Dunnett's multiple comparison test (*, P<0.05).
Figure 8.
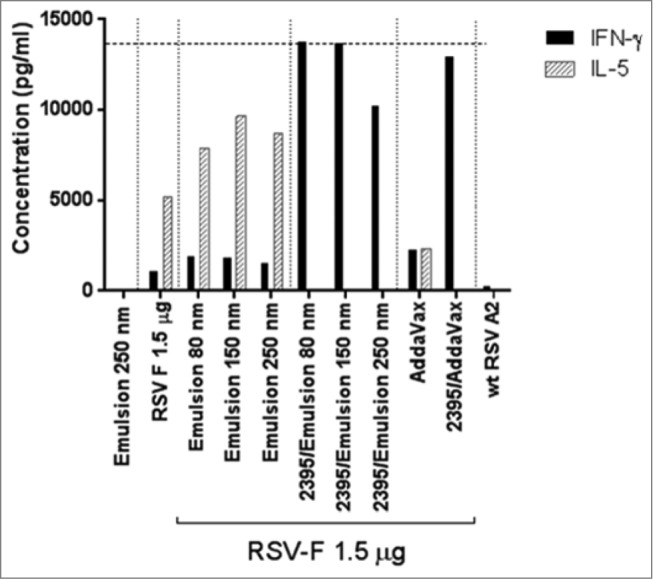
IFN-γ/IL-5 levels in supernatants of RSV F restimulated splenocytes from immunized animals, spleens were isolated 4 days post-challenge and splenocytes were stimulated with a RSV F overlapping peptide pool. The concentrations of IFN-γ and IL-5 in the supernatant 48 h post-stimulation were determined by Luminex on pooled supernatants from each group. Mean of duplicate measurements are shown. The horizontal dotted line is the upper limit of detection.
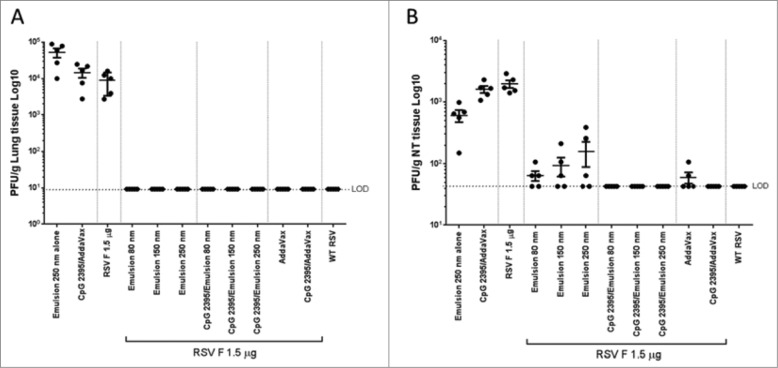
After RSV challenge no virus was detected in lungs from animals immunized with RSV F in the presence of adjuvant (Fig. 9A). Viral titers were also assessed in nasal turbinates for which achieving protection is more challenging than in the lungs as it requires both humoral and cell mediated immunity.28 Superior protection was achieved with RSV F + emulsion 80 nm (Fig. 9B). The addition of CpG in the formulation conferred complete protection from virus replication in the nasal turbinates independent of emulsion particle size.
Figure 9.
Lung and nasal turbinates viral loads 4 days post-infection. Lungs and (B) nasal turbinates (NT) were harvested, homogenized, and infectious RSV was detected by plaque assay. Group means with SD of 5 mice per group are shown.
Materials and Methods
Materials
Squalene to make the emulsions was obtained commercially from Arco Organics. PBS (pH 7.2; Life Technologies) as buffer and PS80 (Sigma-Aldrich) used as a surfactant to stabilize the emulsion were also obtained from commercial sources.
Generation of emulsion
Process
100 g of each emulsion were made with 20 g Squalene and up to 5 g Polysorbate 80 as a surfactant with PBS making up the remaining mass. The resulting liquid was homogenized using a Silverson L5M-A standard mixer (Silverson, MA) at 7000 RPM for 5 minutes. The emulsion was then passed through a Microfluidics M-110P microfluidizer (Microfluidics, MA) at 28,000 psi, where the emulsion underwent 10 passes. The resulting emulsion was then stored at 4°C for further testing.
Particle Size Variability
Emulsions with varying amount of PS80 were prepared and the particle size of the emulsions was measured using dynamic light scattering. Five particle sizes were chosen for emulsion stability tests: 250 nm, 200 nm, 150 nm, 100 nm, and 80 nm. Each emulsion was prepared and divided into 4 groups as shown in Table 1. The purpose for (A1) and (B1) are to monitor emulsion stability during the manufacturing process, and to determine whether the addition of the surfactant after microfluidization would affect particle stability. Groups (A2) and (B2) are made to monitor emulsion stability in the dosage concentrations at which the emulsion adjuvant would be administered in vivo.
Table 1.
List of the groups tested for stability of emulsions along with their size, oil and surfactant concentrations
| Particle Size | Groups | [Squalene] (w/w) | [PS80] (w/w) |
|---|---|---|---|
| 250 nm | A1 | 20% | 1% |
| 250 nm | A2 | 4% | 0.2% |
| 250 nm | B1 | 20% | 5% |
| 250 nm | B2 | 4% | 1% |
| 200 nm | A1 | 20% | 1.4% |
| 200 nm | A2 | 4% | 0.3% |
| 200 nm | B1 | 20% | 5% |
| 200 nm | B2 | 4% | 1% |
| 150 nm | A1 | 20% | 2% |
| 150 nm | A2 | 4% | 0.4% |
| 150 nm | B1 | 20% | 5% |
| 150 nm | B2 | 4% | 1% |
| 100 nm | A1 | 20% | 4% |
| 100 nm | A2 | 4% | 0.8% |
| 100 nm | B1 | 20% | 5% |
| 100 nm | B2 | 4% | 1% |
| 85 nm | A1 | 20% | 5% |
| 85 nm | A2 | 4% | 1% |
These emulsions were then placed in stability chambers at 5°C, 25°C, 40°C, and 60°C and monitored over the course of a month. In addition, samples were also placed on an orbital shaker at 120 rpm (at 25°C) to assess the impact on shear stress on the emulsion stability. Emulsions in 40°C and 60°C stability chambers were used to simulate accelerated degradation conditions. The emulsions were sampled at time zero, day 3, and then once a week for 4 weeks. A sample would be removed and tested for changes in pH, osmolality, and particle size via Dynamic Light Scattering. Additionally, the visual appearance of the emulsions were observed and recorded based on stability and phase separation.
Animal studies
Study design
Pathogen-free, 7–8 weeks-old Balb/c mice were purchased from Harlan (Indianapolis, IN). All animal procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee.
The fusion F protein of RSV A2 was expressed in Chinese Hamster Ovary (CHO) cells (ATCC, Manassas, VA) to >95% purity as previously described.18
Mice were randomly assigned into 12 groups (n = 5 mice per group), primed at day 0 and boosted at day 14 intramuscularly (i.m.) with 1.5 μg of RSV F and different combinations of emulsions +/− 20 μg of CpG 2395 (Invivogen, San Diego, CA), and AddaVax (Invivogen) +/− 20 μg of CpG 2395 (Invivogen). The 1.5 μg dose was based on previous observations showing that RSV F + AddaVax/CpG 2395 immunization induces complete protection against RSV A2 challenge. Mice were challenged intranasally (i.n.) at day 28 with 106 pfu of RSV A2. Placebo groups were immunized with squalene emulsion (250 nm) and CpG 2395/AddaVax alone. The RSV infection control group was infected at day 0 and challenged at day 28 i.n. with 106 pfu of RSV A2. All groups were retro-orbitally bled at day 0 (6 h post priming) and at day 28 before viral challenge. All animals were euthanized at day 35, blood was harvested for serum IgG isotyping, and spleens were harvested for analysis of cellular immune responses, and lungs and nasal turbinates for RSV A2 titer measurement by plaque assay.
Micro-neutralization assay
Serum samples at day 35 were heat inactivated at 56°C for 45 minutes. In 96-well plates the control antibody (Synagis®) was serially diluted by 3 fold increments (starting at 8 μg/ml) in cell culture media (minimal essential medium (MEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (all from Invitrogen)) for a final volume of 50 µl. In duplicate, the test sera (starting dilution 1:2) were serially diluted by 3-fold increments in cell culture media for a final volume of 50 µl. Each serum dilution was mixed with 50 µl RSV A2 at 500 pfu per well. Following 2 hour incubation at 37°C with 5% CO2, 2.5 × 104 HEp-2 cells in 100 µl volume were added to each well. Cells plus virus and cell only wells served as controls. After 3 days of incubation at 37°C with 5% CO2, the cell culture medium was removed and the monolayer was fixed with chilled 80% acetone. RSV replication was visualized by immunostaining with an HRP-labeled 1331H monoclonal antibody. The reciprocal log2 of the IC50 was determined for each serum sample using Prism GraphPad software.
Serum IgG, IgG1, IgG2a ELISA
RSV F-specific IgG antibody titers were measured in serum at day 32. High binding 96-well plates were coated with RSV F at 100 ng/well. Control antibodies (purified 1331H for total IgG and IgG2a and purified 1308F for IgG119 were serially diluted by 3-fold increments starting from a concentration of 1 μg/ml in sample diluent (PBS with 1% BSA and 0.05% Tween 20). Samples were diluted in sample diluent at 1:100 for non-immunized animals, at 1:105 and 1:106 for RSV A2 immunized animals and at 1:106 and 1:107 for RSV F + adjuvant immunized animals. Bound total IgG, IgG1 or IgG2a were detected with the appropriate HRP-labeled antibody (HRP-labeled goat anti-mouse IgG, HRP-labeled goat anti-mouse IgG1 antibody, HRP-labeled goat anti-mouse IgG2a antibody). The serum antibody titers were calculated based on the standard curves to determine μg/ml of each antibody type (SoftMax Pro 5.4).
Viral plaque assay
Lungs and nasal turbinates were placed in cold balanced Hanks salt solution supplemented with 1X sucrose phosphate in tissue homogenization tube (MP Biomedicals) and homogenized using an MP FastPrep24 instrument (MP Biomedicals).
Clarified supernatant were serial diluted and placed onto sub-confluent HEp-2 cells in 24-well plates. After 90 min of incubation, supernatants were removed and cells were overlaid with MEM+FBS+Pen/Strep supplemented with 0.75% methylcellulose. After 5 days, the medium was removed and the cells were fixed with methanol. Plaques were visualized by immunodetection as previously described.20
Cytokine quantification
Serum harvested post 6 h of immunization and supernatants from splenocytes stimulated with a RSV F overlapping peptide pool for 48 h were evaluated for the presence of cytokines in a Luminex-based cytokine profiling assay (Millipore, Billerica, MA). Custom kits including INF-γ, IL-5, IL-6, IP-10, MCP-1, and KC were used according to the manufacturer protocol and read using a Bio-Rad Luminex 200 reader (Bio-Rad, Hercules, CA).
Cell mediated immunity
Splenocytes were isolated as previously described.21 From this preparation, the number of mouse splenocytes secreting gamma interferon (IFN-γ) was determined by enzyme-linked immunospot (ELISPOT) assay (BD Biosciences, San Diego, CA) according to the manufacturer's recommendations. For the in vitro stimulation, splenocytes from individual mice (5×105/well) were incubated with a RSV F specific CD8 peptide (KYKNAVTEL), or 2 RSV F specific CD4 peptides (GWYTSVITIELSNIKE and VSVLTSKVLDLKNYI) at a concentration of 1 μg/ml per peptide (JPT, Berlin, Germany). Controls included splenocytes that were stimulated with Cell Stimulation Cocktail (eBioscience, San Diego, CA) or mock stimulated. Following 20 h of incubation in the presence of peptide at 37°C in a humidified incubator, the ELISPOT assay was completed and spots were counted by an ImmunoSpot ELISPOT assay reader (Cellular Technology Ltd., Cleveland, OH). For analysis, the spot counts in medium control wells were subtracted from the specific spot count after peptide stimulation, and the difference is reported as the number of spot-forming cells (SFC) per 1 × 106 splenocytes.
Conclusion
The impact of the particle size of oil-in-water emulsions on the stability and the adjuvanticity was explored. The stability of the emulsions was assessed by changes in particle size, pH, visual inspection and osmolarity of the emulsion solutions exposed to different stresses. It was observed that the emulsions with the smaller particle sizes were more stable than their larger counterparts. The emulsions with an average particle size of 80 nm were found to be stable even after 30 days at 60°C, whereas the 250 nm emulsions phase showed signs of phase separation in this time frame. Overall, it was observed that stability was inversely proportional to the particle size of the emulsion under the given formulation parameters. Immunogenicity assessment of the differently sized emulsions, using RSV F as a model antigen, revealed that the 80 nm particle sized emulsion trended toward better humoral and cellular immune response compared to the larger sized emulsions. This implies that smaller emulsion size particles in addition to be more stable could also provide higher immunogenicity and represent a better adjuvant choice than larger emulsion particles. Nevertheless, further studies are needed to determine whether this observation is specific to this antigen or holds true for other antigens as well. Future investigations will be needed to address whether this improved immunogenicity is due to preferential antigen uptake by antigen presenting cells and/or due to the enhanced stability observed with the 80 nm sized particles.
Acknowledgments
The authors would like to thank Dr. Steve Bishop and Dr. Gail Wasserman for the support of the project. The authors would also like to thank all members of the Laboratory Animal Resources for their in vivo study support, especially Krystal Nacel and Leigh Hostetler, as well as Li Yu for her valuable help with the statistical analyses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fenner F. Smallpox: emergence, global spread, and eradication. Hist Philos Life Sci, 1993. 15(3): p. 397-420; PMID:7529932 [PubMed] [Google Scholar]
- 2.Vogel FR, Caillet C, Kusters IC, Haensler J. Emulsion-based adjuvants for influenza vaccines. Expert Rev Vaccines, 2009. 8(4): p. 483-92; PMID:19348563; http://dx.doi.org/ 10.1586/erv.09.5 [DOI] [PubMed] [Google Scholar]
- 3.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine, 2010. 28 Suppl 3: p. C25-36; PMID:20713254; http://dx.doi.org/ 10.1016/j.vaccine.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 4.Channon HJ. The Biological Significance of the Unsaponifiable Matter of Oils: Experiments with the Unsaturated Hydrocarbon, Squalene (Spinacene). Biochem J, 1926. 20(2): p. 400-8; PMID:16743673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang ZR, Lin YK, Fang JY. Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules, 2009. 14(1): p. 540-54; PMID:19169201; http://dx.doi.org/ 10.3390/molecules14010540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klucker MF, Dalençon F, Probeck P, Haensler J. AF03, an alternative squalene emulsion-based vaccine adjuvant prepared by a phase inversion temperature method. J Pharm Sci, 2012. 101(12): p. 4490-500; PMID:22941944; http://dx.doi.org/ 10.1002/jps.23311 [DOI] [PubMed] [Google Scholar]
- 7.Suli J, Benísek Z, Eliás D, Svrcek S, Ondrejková A, Ondrejka R, Bajová V. Experimental squalene adjuvant. I. Preparation and testing of its effectiveness. Vaccine, 2004. 22(25-26): p. 3464-9; http://dx.doi.org/ 10.1016/j.vaccine.2004.02.023 [DOI] [PubMed] [Google Scholar]
- 8.Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules 2009. 14(9): p. 3286-312; PMID:19783926; http://dx.doi.org/ 10.3390/molecules14093286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines, 2003. 2(2): p. 197-203; http://dx.doi.org/ 10.1586/14760584.2.2.197 [DOI] [PubMed] [Google Scholar]
- 10.Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine, 2008. 26(26): p. 3209-22; http://dx.doi.org/ 10.1016/j.vaccine.2008.03.093 [DOI] [PubMed] [Google Scholar]
- 11.Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines, 2013. 12(7): p. 747-58; http://dx.doi.org/ 10.1586/14760584.2013.811188 [DOI] [PubMed] [Google Scholar]
- 12.Garcon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines, 2012. 11(3): p. 349-66; http://dx.doi.org/ 10.1586/erv.11.192 [DOI] [PubMed] [Google Scholar]
- 13.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods, 1999. 19(1): p. 103-7; PMID:10525445; http://dx.doi.org/ 10.1006/meth.1999.0834 [DOI] [PubMed] [Google Scholar]
- 14.Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis, 2012. 205(3): p. 466-73; PMID:22147791; http://dx.doi.org/ 10.1093/infdis/jir769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol, 1995. 6: p. 277-96; PMID:7551221; http://dx.doi.org/ 10.1007/978-1-4615-1823-5_10 [DOI] [PubMed] [Google Scholar]
- 16.Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol 2004. 17(2): p. 165-81; PMID:15279697; http://dx.doi.org/ 10.1089/0882824041310513 [DOI] [PubMed] [Google Scholar]
- 17.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine, 2013. 31 Suppl 2: p. B209-15; http://dx.doi.org/ 10.1016/j.vaccine.2012.11.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherukuri A, Stokes KL, Patton K, Kuo H, Sakamoto K, Lambert S, Stillman E, Moore ML, Lee S. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immun Ageing, 2012. 9(1): p. 21; PMID:23031690; http://dx.doi.org/ 10.1186/1742-4933-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol, 1989. 63(7): p. 2941-50; PMID:2470922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore ML, Chi MH, Luongo C, Lukacs NW, Polosukhin VV, Huckabee MM, Newcomb DC, Buchholz UJ, Crowe JE Jr, Goleniewska K, et al.. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol, 2009. 83(9): p. 4185-94; PMID:19211758; http://dx.doi.org/ 10.1128/JVI.01853-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cayatte C, Schneider-Ohrum K, Wang Z, Irrinki A, Nguyen N, Lu J, Nelson C, Servat E, Gemmell L, Citkowicz A, et al.. Cytomegalovirus vaccine strain towne-derived dense bodies induce broad cellular immune responses and neutralizing antibodies that prevent infection of fibroblasts and epithelial cells. J Virol, 2013. 87(20): p. 11107-20; PMID:23926341; http://dx.doi.org/ 10.1128/JVI.01554-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanana GD, Sheth BB. Particle size reduction of emulsions by formulation design. I: Effect of polyhydroxy alcohols. J Parenter Sci Technol, 1993. 47(3): p. 130-4; PMID:8360805 [PubMed] [Google Scholar]
- 23.Chanana GD, Sheth BB. Particle size reduction of emulsions by formulation design-II: effect of oil and surfactant concentration. PDA J Pharm Sci Technol, 1995. 49(2): p. 71-6; PMID:7780748 [PubMed] [Google Scholar]
- 24.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine, 2011. 29(17): p. 3341-55; PMID:20713100; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L, Jin H, Woo J. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS One, 2012. 7(12): p. e51618; http://dx.doi.org/ 10.1371/journal.pone.0051618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudner BC, Ronconi V, Casini D, Tortoli M, Kazzaz J, Singh M, Hawkins LD, Wack A, O'Hagan DT. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020). Pharm Res, 2009. 26(6): p. 1477-85; http://dx.doi.org/ 10.1007/s11095-009-9859-5 [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Gerth AJ, Peng SL, CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol, 2004. 34(5): p. 1483-7; http://dx.doi.org/ 10.1002/eji.200324736 [DOI] [PubMed] [Google Scholar]
- 28.Crowe JE Jr., Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol, 2001. 167(7): p. 3910-8; http://dx.doi.org/ 10.4049/jimmunol.167.7.3910 [DOI] [PubMed] [Google Scholar]