Abstract
Background
Adenoviremia adversely affects prognosis in the post-hematopoietic stem cell transplant (HSCT) setting.
Methods
We sought to determine retrospectively the cutoff load of adenovirus in the stool as a predictor of adenoviremia, in children who underwent an allogeneic HSCT. The prevalence of sapovirus, norovirus and astrovirus in the stool was also studied.
Results
The study cohort consisted of 117 patients, of which 71 (60%) had diarrhea. Adenovirus was detected in the stool in 39 out of 71 (55%) patients. Age ≤ 10 years (P = 0.05; odds ratio, 2.57; 95% confidence interval: 0.98–6.75), and male sex (P = 0.04; odds ratio 2.67; 95% confidence interval: 1.02–6.99) increased risk for detection of adenovirus in stool on univariate analysis. Co-infections with enteric pathogens were infrequent. Viral load > 106 copies / gram stool predicted adenoviremia with a sensitivity and specificity of 82%. Sapovirus, norovirus, and astrovirus were detected in 3, 4 and one patient, respectively.
Conclusions
Quantitative detection of adenovirus in stool may have implications for pre-emptive therapy. Testing for other enteric viruses may have implications for infection control.
Keywords: adenovirus, stool, pediatric, transplant
INTRODUCTION
Viruses are an important cause of diarrhea in otherwise healthy children. While rotavirus and norovirus are the main pathogens, adenovirus is frequently detected followed by sapovirus and astrovirus.1 Adenovirus is an important cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT).2 Patients with diarrhea and increasing stool viral load may be at risk for adenoviremia. Sustained adenoviremia is a specific and sensitive indicator of adenoviral disease after T cell-replete HSCT.3 The purpose of our study was to define a viral load cutoff in stool to predict adenoviremia in children with diarrhea post-HSCT.
PATIENTS AND METHODS
This retrospective study cohort consisted of 117 patients who underwent HSCT over a four-year period (January 2009 – May 2012) at St. Jude Children’s Research Hospital (SJCRH). The study was approved by the SJCRH Institutional Review Board. Adenovirus was detected with a quantitative real-time polymerase chain reaction (PCR) assay4 using a 7500 Real-Time PCR System (Applied Biosystems®, now part of ThermoFisher Scientific, Waltham, MA). Quantiative co-linearity was previously shown with this assay for all non-enteric strains.4 Laboratory developed, real-time PCR methods were used to detect sapovirus, norovirus GI / GII (Cepheid, Sunnyvale, CA), and astrovirus. Assays were performed on stool samples previously stored for quality assurance purposes. Approximately one gram of stool was extracted using the QIAamp® Fast DNA Stool Mini Kit on the QIAcube automated extraction system (Qiagen, Hilden, Germany). Following nucleic acid extraction, DNA was eluted in 200μL of buffer and stored at 4°C until PCR amplification.
Adenovirus was tested on blood samples sent once weekly for clinical adenovirus testing. Qualitative testing results for adenovirus in stool was available for clinical use; quantitative values were calculated retrospectively using calibration curves run concomitantly and used for standard-of-care quantitative testing in blood samples.
Diarrhea was defined as change in stool consistency or frequency. Stool samples were sent for microbiologic evaluation for all patients with diarrhea. The day of onset of infection was defined as the day when the first adenovirus-positive diagnostic sample was collected. The first positive stool test and concomitant blood test was used for retrospective viral load analysis. Co-pathogens included those detected up to 6 weeks prior to, and 6 weeks after the date of collection of the first and last adenovirus-positive stool sample, respectively.
Transplant-related variables were abstracted from a prospectively collected database that included patient demographics, underlying diagnosis, remission status, donor and product type, cytomegalovirus donor/recipient status, conditioning regimen, antiviral prophylaxis, and presence or absence of grade II–IV graft-versus-host disease (GVHD). Presence of blood in stool, abdominal pain, vomiting, weight change (%), laboratory parameters, and death due to diarrheal infection or disseminated adenovirus disease were noted.
Statistical Analysis
The number of HSCT recipients with diarrhea, who had adenovirus, sapovirus, norovirus, and astrovirus detected in their stool samples was determined. For each specific virus, in each patient the first positive test was counted for estimating the virus-specific incidence. Both proportions and 95% confidence interval are reported. Receiver operating characteristic (ROC) curves, area under the curve (AUC) of the ROC, sensitivity, and specificity were calculated to determine adenoviral load threshold in the stool that best predicted detection of virus in blood using the maximum Youden index method.5 Adenoviral load was classified as high (or low) if its level in stool sample was above (or below) the threshold for cutoff. Fisher’s exact test (categorical) and Wilcoxon Rank Sum test (quantitative) were used to compare patients with diarrhea with or without adenovirus detected in stool, and patients with stool adenoviral load above and below threshold. Univariate logistic regression model was used to test associations in patients with diarrhea with or without adenovirus detected in stool, and patients with stool adenoviral load above and below threshold, with other co-variates. All reported P-values are 2-sided and considered significant if < 0.05. Statistical analyses were performed with SAS software version 9.3 and R-2.13.2.
RESULTS
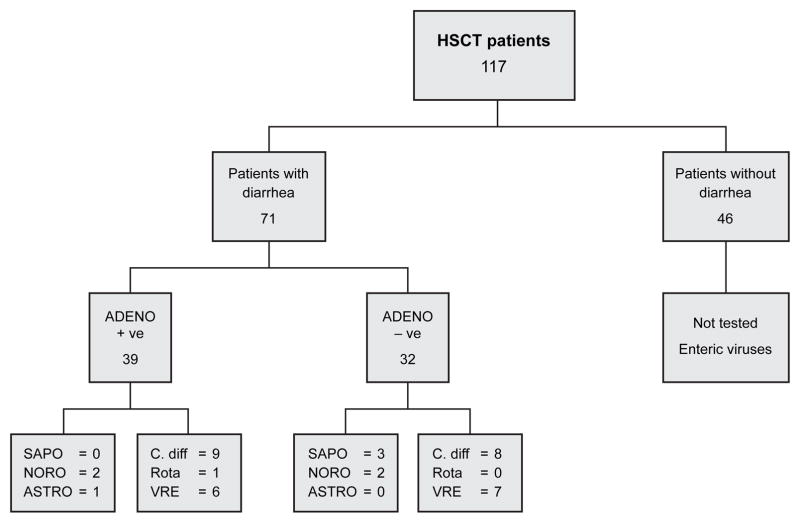
Of the 117 patients in the cohort, 71 (60%) patients had diarrhea (Figure 1). Demographics and characteristics of the 71 patients with diarrhea with (39, 55%) or without (32, 45%) adenovirus detected in stool are presented in Table 1. Age ≤ 10 years (P = 0.05; odds ratio, 2.57; 95% confidence interval: 0.98–6.75), and male sex (P = 0.04; odds ratio, 2.67; 95% confidence interval: 1.02–6.99) increased risk for detection of adenovirus in stool on univariate analysis. Children with adenovirus detected in stool were more likely to need parenteral nutrition (P = 0.04; odds ratio, 2.94; 95% confidence interval: 1.06–8.14). Other variables including race (P = 0.60), cytomegalovirus donor / recipient status (P = 0.57) were not significant. There were 16 (41%), 16 (41%), and 7 (18%) patients who had adenovirus detected in stool 0–30, 31–100 and > 100 days post-HSCT.
Figure 1.
Number of hematopoietic stem cell transplant (HSCT) patients with or without diarrhea, with or without adenovirus (adeno), sapovirus (sapo), norovirus (noro), astrovirus (astro), C. difficile (C. diff), rotavirus (rota), and vancomycin-resistant enterococcus (VRE) detected in stool.
Table 1.
Demographics and characteristics of patients with diarrhea with or without adenovirus detected in stool
| Characteristic | All patients (n=71) | Patients AdV stool (n=39) | Patients No AdV stool (n=32) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 9.6 (6.1) | 8.3 (5.10) | 11.3 (6.9) | 0.07 |
| Median (Range) | 9.0 (1.0–24) | 8.0 (1.5 – 18.0) | 12.0 (1.0–24.0) | |
| Male sex | 36 (51) | 24 (62) | 12 (38) | 0.06 |
| Diagnosis heme malignancy | 61 (86) | 34 (87) | 27 (84) | 0.74 |
| Remission prior to transplant | 39 (55) | 23 (59) | 16 (50) | 0.48 |
| Total body irradiation | 14 (20) | 7 (18) | 7 (22) | 0.77 |
| Reduced-intensity conditioning | 29 (41) | 20 (51) | 9 (28) | 0.06 |
| T-cell depletion | 31 (44) | 14 (36) | 17 (53) | 0.16 |
| Donor Classification | 0.20 | |||
| Matched-related donor | 7 (10) | 5 (13) | 2 (6) | |
| Matched-unrelated donor | 30 (42) | 19 (49) | 11 (35) | |
| Haplo-identical donor | 32 (45) | 15 (38) | 17 (53) | |
| Cord | 2 (3) | 0 (0) | 2 (6) | |
| Product Type | 0.08 | |||
| Bone marrow | 37 (52) | 24 (62) | 13 (41) | |
| Peripheral blood | 32 (45) | 15 (38) | 17 (53) | |
| Cord | 2 (3) | 0 (0) | 2 (6) | |
| Viral prophylaxis | 0.85 | |||
| ACV | 59 (83) | 33 (85) | 26 (81) | |
| Inpatient status | 51 (72) | 30 (77) | 21 (66) | 0.42 |
| GVHD (Grade II–IV) | 25 (35) | 14 (36) | 11 (34) | 1.00 |
| Nutrition | 0.04 | |||
| Parenteral+ enteral | 47 (66) | 30 (77) | 17 (53) | |
| Enteral | 24 (34) | 9 (23) | 15 (47) | |
| Ultrasound evidence of colitis | 14 (20) | 11 (28) | 3 (9) | 0.07 |
| Vomiting | 16 (23) | 16 (41) | 0 (0) | <0.001 |
| Blood in stool | 5 (7) | 2 (5) | 3 (9) | 0.65 |
| Use of steroids at time of infection | 22 (31) | 14 (36) | 8 (25) | 0.44 |
| ALC (cells/μL) | 0.85 | |||
| Mean (SD) | 435.9 (870) | 492.7 (1075) | 366 (530) | |
| Median (range) | 84 (0.0–5293) | 76 (0.0–5293) | 119 (0.0–2200) | |
| ANC (cells/μL) | 0.55 | |||
| Mean (SD) | 1844 (2385) | 1703 (2387) | 2016 (2410) | |
| Median (range) | 1100(0–11600) | 1100 (0–11600) | 1250 (0–9400) | |
Data are number (%) unless otherwise indicated. Abbreviations : AdV, adenovirus; ACV, acyclovir; GVHD, graft-versus-host disease; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; SD, standard deviation.
The median stool viral load was 5.2 (range 2–11.3) log10 copies/gram stool. Stool viral load > 106 copies/gram stool predicted adenoviremia with a sensitivity of 82% (95% confidence interval: 48%–98%), and specificity of 82% (95% confidence interval: 63%–94%). Stool viral load > 105 copies/gram stool predicted adenoviremia with a sensitivity of 82% and specificity of 61%. Stool viral load > 107 copies/gram stool predicted adenoviremia with a sensitivity of 73% and specificity of 86%.
Stool viral load > 106 copies/gram stool was used as the threshold for cutoff using the maximum Youden index method, and was seen in 15 (38%) patients. Adenoviremia was detected in 11 (28%) patients, 9 of whom had stool viral loads above threshold. The median time for detection of adenoviremia was 37 (range 3–337) days. The median blood viral load was 2.6 (range < 2 – 6.78) log10 copies/mL. All patients with adenoviremia had adenovirus detected in the stool. Adenoviremia did not precede detection of adenovirus in the stool in any patient. The incidence of viremia in individuals with stool viral load above threshold was 60% (9 out of 15) versus 8% (2 out of 24) in patients with stool viral load below threshold (P = .002; odds ratio, 16.5; 95% confidence interval: 2.79–97.68). The median time between detection of stool viral load above threshold and first observation of viremia was 6.5 (range 0–25) days.
Age (P = 0.24), race (P = 0.54), sex (P = 0.14), donor type (P = 0.54), GVHD (P = 0.67), other sites of infection (P = 0.79), concomitant use of steroids (P = 0.79), T-cell depletion (P = 0.79), co-infections (P = 0.32), C. difficile infection (P = 0.72), and absolute lymphocyte count (P = 0.48) were not associated with stool viral load above threshold on univariate analysis.
Co-infections with C. difficile, rotavirus or vancomycin-resistant enterococcus (VRE) were seen in 17, one and 13 patients, respectively, not significantly different in patients with or without adenovirus detected in stool (Figure 1). Co-infection with C. difficile and VRE was seen in 3 patients with adenovirus, and in 2 patients where no adenovirus was detected in stool. Sapovirus, astrovirus, norovirus and rotavirus were co-pathogens only with adenvovirus (Figure 1).
Of the 39 patients with adenovirus detected in stool, one died due to invasive infection. He received a haplo-identical HSCT for relapsed acute lymphoblastic leukemia, had a course complicated by acute skin GVHD, and developed adenoviremia less than two months post-transplant. Despite initiating cidofovir therapy three days prior to the positive blood test, the patient progressed to liver failure, coagulopathy, hepato-renal syndrome, and succumbed to the infection.
Four other patients had evidence of adenoviral disease other than colitis. Three patients had adenovirus detected in nasophayngeal wash with symptoms of upper respiratory tract infection. In 2 patients diarrhea followed these symptoms after 4 and 12 weeks, viral loads were 105 and 103 copies/gram stool respectively, and was not detected in blood. In the third patient respiratory symptoms coincided with diarrhea, viral load was 1011 copies/gram stool, and blood viral load 5 log10 copies/mL. All are presently alive and well. The fourth patient had altered mental status, adenovirus was detected in the cerebrospinal-fluid, followed by diarrhea 2 weeks later with 1011 copies/gram stool, and blood viral load 2 log10 copies/mL. She died of an invasive fungal sinus infection three weeks later.
Stool samples of 71 patients with diarrhea were analyzed with real-time PCR to detect sapovirus, norovirus, and astrovirus. This included the original 39 patients, as well as 32 additional HSCT patients from the initial cohort, who did not have a positive adenoviral stool test. Sapovirus was detected in 3 patients (4.2%), norovirus in 4 patients (5.7%), and astrovirus in one patient (1.4%).
The first patient with sapovirus infection was an 8 year old boy who developed diarrhea day +122 following a haplo-identical HSCT for acute lymphoblastic leukemia. Diarrhea lasted 3 weeks. Four months later two twins with severe combined immunodeficiency who were 7 months of age, developed diarrhea within a day of each other during conditioning therapy for a haplo-identical transplant. These twins were housed in separate rooms in strict isolation, and cared for by different care-givers. Diarrhea lasted 3 days. None of these patients had adenovirus detected in stool.
The 4 patients with norovirus infection were 6 months to 5 years of age, recipients of haplo-identical (3) or matched-unrelated donor transplants (1). Norovirus infection developed at a median of 38 days post-transplant. Diarrhea lasted less than 5 days in all patients. Two patients had adenovirus detected in stool at 5 × 1010 and 2 × 1011 copies/gram stool. The first patient had adenoviremia of 2.5 log10 copies/mL.
DISCUSSION
Our finding that stool viral load > 106 copies/gram stool predicts adenoviremia corroborates previous reports.1,6 These data support the use of quantitative rather than qualitative PCR for adenoviral stool testing in HSCT patients, which would allow earlier initiation of antiviral therapy in patients with high stool viral loads with cidofovir, a potentially nephrotoxic drug. High levels of adenoviremia correlate with a fatal outcome in children after HSCT.7,8 Adenovirus-associated mortality was minimized in our cohort as has been in other studies with routine monitoring, and pre-emptive use of cidofovir with detection of adenoviremia.8 Novel cellular therapy approaches have been developed to treat adenoviremia.9 However they are limited by a turn-around time of several weeks. The median time between detection of stool viral load above threshold and first observation of viremia was 6.5 (range 0–25) days. Hence, a surveillance program using quantitative detection of stool viral load may identify patients at high-risk for adenoviremia, who may benefit from these approaches.
Patients with low stool viral loads may be monitored closely. Experience with an oral lipid formulation of cidofovir (brincidofovir, previously CMX001, Chimerix Inc., Durham, NC), has been reported in retrospective studies.10 It is presently undergoing clinical trials. Cells take up the inactive drug, and the lipid conjugate is cleaved by phospholipase C, which allows it to be converted to active drug inside the cell obviating any nephrotoxicity.11 Future studies may explore its role as pre-emptive therapy in patients with low stool viral loads.
Co-infections with more than one virus was seen in 13.5% of specimens from otherwise healthy children with acute gastroenteritis.1 Norovirus was the most frequently detected pathogen in mixed infections in combination with adenovirus or astrovirus.1 Co-infections were uncommon in our study. Isolation of viruses from stool in HSCT recipients was infrequent (8%) in a prospective surveillance study, and co-infections were not seen.12
Children less than 10 years of age, and males were at increased risk of detection of adenovirus in the stool. Higher incidence of adenovirus infections in young children has been corroborated in other studies.13 Male predominance has been noted as risk factor for adenovirus-induced acute hemorrhagic cystitis in children.14 Lymphopenia 2,15, and GVHD 2,16 have been previously noted as important risk factors for disseminated adenoviral disease. There were more children less than 10 years of age in our cohort who had severe lymphopenia (ALC < 200 cells/μL) and GVHD, although this was not statistically significant (P = 1.00). The increased use of parenteral nutrition in children where adenovirus was detected may be related to the significantly higher incidence of vomiting in this group. None of the variables predicted infection with a high stool viral load.
Adenoviruses are divided into 6 subspecies and 51 serotypes. Types A31, C1 and C2 have been noted to be predominant in pediatric HSCT patients.2,7,8,17 Multiple serotypes including A31 have been detected by sequential analysis. Infection is most likely due to viral reactivation in the epithelial and lymphoid cells of the intestine rather than from exogenous spread.
Sapoviruses belong to the calciviridae family and were detected in 2 of 58 symptomatic pediatric oncology inpatients in a prospective study.18 Both were on low dose chemotherapy. Diarrhea resolved within 10 days. Nosocomial transmission is expected as was seen in our twins. Sapovirus infections have previously not been reported in children or adults undergoing HSCT. Inclusion of sapovirus in routine testing panels may enable early institution of contact precautions.
The cumulative incidence of norovirus infection was 12.9% at 2 years post-transplantation in a retrospective study of 55 pediatric HSCT recipients.19 In a case series of 13 pediatric HSCT recipients, the median duration of norovirus excretion was 150 days.20 These patients required intensive nutritional support, and clearance of norovirus from the gut was associated with donor T-cell recovery. Norovirus was detected in only 6% of patients in our series, and diarrhea was limited.
Infection by astrovirus in immune-compromised children has been described in case reports.21 Astrovirus increases epithelial barrier permeability causing disruption of tight junctions which contributes to diarrhea, and may allow enteric viruses to gain access to the blood stream.22
Limitations of the study include lack of asymptomatic controls, or children without diarrhea to evaluate incidence of shedding and stool viral loads, and analysis of adenovirus subtypes.
Quantitative detection of adenovirus in the stool may have implications for preemptive therapy. Testing for other enteric viruses may help direct care and have implications for infection control. Future prospective studies may evaluate if co-infection with other enteric viruses or C. difficile increases risk for adenoviremia in patients with low stool viral loads, compare stool viral loads in asymptomatic patients, and study adenovirus subtypes in cases and controls.
Acknowledgments
Funding sources: This work was supported by 5R25CA02394 from the National Cancer Institute (NCI), NCI Cancer Center CORE Support Grant P30 CA 21765 and by the American Lebanese Syrian Associated Charities.
The authors thank the staff from the microbiology laboratory at SJCRH for developing and performing the real-time PCR assays.
Footnotes
Conflict of interest disclosure: The authors declare no competing financial interests.
References
- 1.Chhabra P, Payne DC, Szilagyi PG, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis. 2013;208:790–800. doi: 10.1093/infdis/jit254. [DOI] [PubMed] [Google Scholar]
- 2.Lion T, Kosulin K, Landlinger C, et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24:706–714. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- 3.Erard V, Huang ML, Ferrenberg J, et al. Quantitative real-time polymerase chain reaction for detection of adenovirus after T cell-replete hematopoietic cell transplantation: viral load as a marker for invasive disease. Clin Infect Dis. 2007;45:958–965. doi: 10.1086/521851. [DOI] [PubMed] [Google Scholar]
- 4.Gu Z, Belzer SW, Gibson CS, Bankowski MJ, Hayden RT. Multiplexed, real-time PCR for quantitative detection of human adenovirus. J Clin Microbiol. 2003;41:4636–4641. doi: 10.1128/JCM.41.10.4636-4641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Jeulin H, Salmon A, Bordigoni P, Venard V. Diagnostic value of quantitative PCR for adenovirus detection in stool samples as compared with antigen detection and cell culture in hematopoietic stem cell transplant recipients. Clin Microbiol Infect. 2011;17:1674–1680. doi: 10.1111/j.1469-0691.2011.03488.x. [DOI] [PubMed] [Google Scholar]
- 7.Schilham MW, Claas EC, van Zaane W, et al. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem cell transplantation. Clin Infect Dis. 2002;35:526–532. doi: 10.1086/341770. [DOI] [PubMed] [Google Scholar]
- 8.Mynarek M, Ganzenmueller T, Mueller-Heine A, et al. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: results of a routine monitoring program. Biol Blood Marrow Transplant. 2014;20:250–256. doi: 10.1016/j.bbmt.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21:2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18:731–738. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Painter W, Robertson A, Trost LC, Godkin S, Lampert B, Painter G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug available against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56:2726–2734. doi: 10.1128/AAC.05983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti S, Collingham KE, Stevens RH, Pillay D, Fegan CD, Milligan DW. Isolation of viruses from stools in stem cell transplant recipients: a prospective surveillance study. Bone Marrow Transplantation. 2000;25:277–282. doi: 10.1038/sj.bmt.1702164. [DOI] [PubMed] [Google Scholar]
- 13.Van Tol MJD, Kroes ACM, Schinkel J, et al. Adenovirus infection in paediatric stem cell transplant recipients: increased risk in young children with a delayed immune recovery. Bone Marrow Transplantation. 2005;36:39–50. doi: 10.1038/sj.bmt.1705003. [DOI] [PubMed] [Google Scholar]
- 14.Kondo M, Kojima S, Kato K, Matsuyama T. Late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation in children. Bone Marrow Transplant. 1998;22:995–998. doi: 10.1038/sj.bmt.1701482. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti S, Mautner V, Osman H. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 16.Watson T, MacDonald D, Song X, et al. Risk factors for molecular detection of adenovirus in pediatric hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2012;18:1227–1234. doi: 10.1016/j.bbmt.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Kroes ACM, de Klerk EPA, Lankester AC, et al. Sequential emergence of multiple adenovirus serotypes after pediatric stem cell transplantation. J Clin Virol. 2007;38:341–347. doi: 10.1016/j.jcv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Moser O, Luck S, Dilloo D, Eis-Hubinger AM, Simon A. Sapovirus as a gastrointestinal pathogen in febrile pediatric patients with cancer. J Med Virol. 2011;83:2233–2236. doi: 10.1002/jmv.22219. [DOI] [PubMed] [Google Scholar]
- 19.Robles JDF, Cheuk DKL, Ha SY, Chiang AKS, Chan GCF. Norovirus infection in pediatric hematopoietic stem cell transplant recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2012;18:1883–1889. doi: 10.1016/j.bbmt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Saif MA, Bonney DK, Bigger B, et al. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: A cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplantation. 2011;15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 21.Wunderli W, Meerbach A, Guengoer T. Astrovirus infection in hospitalized infants with severe combined immunodeficiency after allogeneic hematopoietic stem cell transplantation. PLoS One. 2011;6:e27483. doi: 10.1371/journal.pone.0027483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser LA, Carter M, Schultz-Cherry S. Astrovirus increases epithelial barrier permeability independently of viral replication. J Virol. 2007;81:11937–11945. doi: 10.1128/JVI.00942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]