Abstract
For typically developing infants, the last trimester of fetal development extending into the first post-natal months is a period of rapid brain development. Infants who are born premature face significant risk of brain injury (e.g., intraventricular or germinal matrix hemorrhage and periventricular leukomalacia) from complications in the perinatal period and also potential long-term neurodevelopmental disabilities because these early injuries can interrupt normal brain maturation. Neuroimaging has played an important role in the diagnosis and management of the preterm infant. Both cranial US and conventional MRI techniques are useful in diagnostic and prognostic evaluation of preterm brain development and injury. Cranial US is highly sensitive for intraventricular hemorrhage IVH and provides prognostic information regarding cerebral palsy. Data are limited regarding the utility of MRI as a routine screening instrument for brain injury for all preterm infants. However, MRI might provide diagnostic or prognostic information regarding PVL and other types of preterm brain injury in the setting of specific clinical indications and risk factors. Further development of advanced MR techniques like volumetric MR imaging, diffusion tensor imaging, metabolic imaging (MR spectroscopy) and functional connectivity are necessary to provide additional insight into the molecular, cellular and systems processes that underlie brain development and outcome in the preterm infant. The adult concept of the “connectome” is also relevant in understanding brain networks that underlie the preterm brain. Knowledge of the preterm connectome will provide a framework for understanding preterm brain function and dysfunction, and potentially even a roadmap for brain plasticity. By combining conventional imaging techniques with more advanced techniques, neuroimaging findings will likely be used not only as diagnostic and prognostic tools, but also as biomarkers for long-term neurodevelopmental outcomes, instruments to assess the efficacy of neuroprotective agents and maneuvers in the NICU, and as screening instruments to appropriately select infants for longitudinal developmental interventions.
Keywords: White matter injury of prematurity, Neuroimaging biomarkers, Cranial US, MRI
Introduction
Advances in neonatal care have improved survival rate of very preterm and extremely low-birth-weight (ELBW) infants [1–3]. However, while the majority of these infants survive the neonatal period, they are at significant risk for long-term neurodevelopmental disabilities ranging from cerebral palsy (and other motor deficits) to cognitive-behavioral and learning impairments. Moreover, data now show that children who are born preterm might continue to lag behind term-born peers well into adolescence and adulthood [4–6]. Neuroimaging has played an important role in the diagnostic evaluation and monitoring of preterm infants. Because of its simplicity, cranial US is usually the first imaging modality to be performed and has been found to provide significant diagnostic and prognostic information. MRI more precisely depicts changes in the brain parenchyma and might be more sensitive than cranial US to detection of certain patterns of brain injury in preterm infants [7–11]. CT is an alternative to cranial US and MRI that can exclude some types of intracranial hemorrhage and calcification (TORCH infection), but it should not be utilized routinely because of the exposure to ionizing radiation, particularly when MRI is available.
Recently, there has been an explosion in the application of advanced MR neuroimaging techniques in the study of preterm neonates including volumetric imaging, diffusion imaging, MR spectroscopy, functional MR, resting-state connectivity and perfusion imaging. However, the utility of these techniques for diagnosis and prognosis in evaluation of preterm brain injury remains unknown, limiting their usefulness in clinical care. In neuroscience, advanced neuroimaging techniques have allowed for the development of the human connectome. The human connectome is a “comprehensive structural description of the network of elements and connections forming the [adult] human brain” that has been applied to not only understanding the normal brain but also understanding disease processes like epilepsy and schizophrenia [12–15]. Advanced neuroimaging techniques, particularly volumetric imaging, diffusion tensor imaging and resting-state connectivity, are likely to provide a framework for the preterm infant connectome as well as an understanding of the maturational processes that occur across neural systems starting in the perinatal period and extending through childhood into adulthood.
Because of a range of perinatal factors, including intrauterine complications (e.g., problems with the placenta, infection), vascular complications (e.g., congenital heart lesions, patent ductus arteriosis, impairments in cerebral autoregulation), pulmonary complications (e.g., premature lung development, persistent pulmonary hypertension), and systemic complications (e.g., infection, genetic syndromes, metabolic syndromes, other organ system disorders), preterm infants are at risk for a wide range of intracranial complications. These include hemorrhages (e.g., intraventricular and germinal matrix, intraparenchymal, subdural), ischemic/inflammatory injuries (e.g., infarcts, periventricular leukomalacia), hydrocephalus (congenital or secondary to hemorrhage), congenital abnormalities (e.g., malformations) and even tumors or other intracranial pathological processes (e.g., meningitis, encephalitis, abscess). For this review, we will focus on the two most prominent pathologies in the preterm infant: germinal matrix/intraventricular hemorrhage (GMH/IVH) and periventricular leukomalacia (PVL). We will (1) review the use of both cranial US and MRI in the evaluation of IVH and PVL; (2) review the predictive value of the most common brain abnormalities detected by cranial US and MRI for adverse neurodevelopmental outcome in preterm neonates (tables 1–3); (3) review the recent results of advanced MR imaging techniques applied to preterm brain development and brain injury; (4) provide insight into integrating neuroimaging techniques into both clinical practice and research protocols aimed at improving outcomes, and (5) provide a perspective about the importance of mapping the preterm brain connectome. Recent review articles summarize the role of neuroimaging biomarkers in evaluation of the preterm brain at different ages as well as the updated neuroscience of preterm brain injury [16–18].
Table 1.
Comparisons between MRI and US
| No. of Infants | Inclusion Criteria in weeks | Gestational Age in weeks | Timing of Imaging | HUS Finding Outcome | Study |
|---|---|---|---|---|---|
| 32 | 30, CUS and MRI on the same day. | 27 (23–30) | Between birth and term | CUS accurately predicted the presence of GMH, IVH, and hemorrhagic parenchymal infarction on MRI. However, its ability to predict the presence of DEHSI and small petechial hemorrhages in the WM on T2 weighted images is not as good, but improves on scans performed at >/=7 days after birth. In addition, normal WM echogenicity on CUS is not a good predictor of normal WM signal intensity on MR | Maalouf et al. (8) |
| 40 | >32, US and MRI on the same day. | 28.6 (25.1–31.9) | 33.2 weeks (27.3–45.1) | WM changes on sequential CUS were predictive of WM changes on MRI. Severely abnormal WM on CUS/MRI was predictive of adverse outcome, and normal-mildly abnormal WM of favorable outcome. Moderately abnormal WM on CUS/MRI was associated with variable outcome. | Leijser et al. (35) |
| 72 | >27 | 25 (23–27) | Term equivalent age. | CUS is highly effective in detecting severe lesions of the white matter in preterm infants, but MRI seems to be necessary for the diagnosis of less severe damage. MRI performed at about the third week of life is highly predictive of the final diagnosis at term. | Horsch et al. (36) |
| 51 | >34 | 29.3 + 2.2 | Term equivalent age. | Parenchymal lesions in MRI, excluding subependymal haemorrhages, predicted CP with a sensitivity of 82% and a specificity of 97%, the corresponding figures for CUS being 58% and 100%, respectively. | Valkama et al. (110) |
| 61 | Preterm NICU admission. | 29.6–32.6 weeks | Early, 32, and late MRI, 40. | Early MRI was superior to cranial US in detecting cysts earlier, especially in preterm infants with c-PVL. | Roelants-van Rijn et al. (114) |
| 167 | ≥30 or less | 27+ 2 | Term equivalent age. | Any WM abnormality on MRI and any abnormality on CUS to predict any neurodevelopmental impairement. MRI: sensitivity (84%) and specificity (34%). CUS sensitivity (11%) and specificity (95%). | Woodward et al. (109) |
Abbr: GA: gestational age, CUS, cranial ultrasound; MRI, magnetic resonance imaging; GMH, germinal matrix hemorrhage; IVH, intraventricular hemorrhage; WM, white matter; DEHSI, diffuse excessive high signal intensity.
Table 3.
MR Imaging parameters that have been correlated with outcome in the preterm
| No. of Infants | Inclusion Criteria in GA weeks | Gestational age in weeks | Timing of Imaging in weeks | Finding/Outcome | Study |
|---|---|---|---|---|---|
| 119 | 30 | 27 GA (23–29) | Serial MRI scans from birth to term. | Diffuse white matter abnormalities and post-hemorrhagic ventricular dilation are common at term and seem to correlate with reduced developmental quotients median birth weight of 880 g (370–1606 g). | Dyet et al, [47] |
| 38 | 34 | 31 | 40 (38–44) | Higher diffusion coefficient values in the white matter apparent at term-equivalent age in preterm infants without overt lesions are associated with poorer developmental performance in later childhood. | Krishnan ML [69] |
| 12 | Infants with grade 4. | 25 – 36 | 40 | Asymmetric involvement of the PLIC is an early predictor of future hemiplegia | De Vries et al [80] |
| 61 | Preterm NICU admission. | 29.6–32.6 | Early, 32, and late MRI, 40. | Late MRI was useful for assessment of the PLIC, as this finding helped to predict hemiplegia in those with unilateral parenchymal injury and to a lesser degree diplegia or quadriplegia in those with bilateral parenchymal injury. | Roelants-van Rijn et al [81] |
| 137 | 33 week and birthweight < 1800 g | Not specified | 34–42 | Significant reduction of fractional anisotropy in the posterior limb of the internal capsule in neurologically abnormal infants (including those with cerebral palsy) | Arzoumanian et al [82] |
| 24 | 32 weeks and >1500g | 28.4 | 37 | Strong negative correlation between FA and GMFCS among preterm infants with abnornal FA in the PLIC and no correlation among perterm infants with normal FA. | Rose Et al [83] |
| 78 | Preterm infants with VLBW | 27.6 | Term- equivalent age | Children with abnormal neurodevelopment had more abnormalities on MRI and reduced FA in the splenium of the CC and right PLIC. | Rose et al 78] |
| 10 | Healthy term and stable preterm infants | Not specified | 35.4 ± 1.1 | WM volumes in the sensorimotor and midtemporal regions correlated strongly with measures of neurodevelopmental outcome. | Peterson BS et al [73] |
| 119 | <32 weeks with birth weight <1500 g | 27.7 ± 2.2 (23–32) | 40.2 ± 0.3 | Infants with significantly reduced cortical GM and deep nuclear GM volumes and increased CSF volume volumes exhibited moderate to severe neurodevelopmental disability at 1 year of age | Inder et al [70] |
| 83 | 32 weeks gestation | 27.6 ± 2.1 | 40.3 ± 1.2 (37.4 – 42.9) | Cerebellar volume showed a weak correlation with cognitive and motor development, although this was principally mediated by WM injury. | Shah et al [104] |
Abbr: MRI, magnetic resonance imaging; PLIC, posterior limb of the internal capsule; NICU, neonatal intensive care unit; FA, fractional anisotropy; GMFCS, gross motor function classification system; CC, corpus callosum; WH, white matter; GM, gray matter; CSF, cerebrospinal fluid.
Technical overview of cranial US and MRI of the preterm brain
Cranial US provides a rapid and inexpensive assessment of the brain in preterm infants. It does not use radiation and can be performed at the bedside. For these reasons, cranial US is the modality of choice for serial examinations. Cranial US has been shown to be sensitive to germinal matrix/intraventricular hemorrhage, hydrocephalus and, to a certain extent, patterns of white matter injury and posterior fossa hemorrhage in the preterm infant. The standard sagittal and coronal views through the anterior fontanel provide assessments of the lateral ventricle, choroid plexus and periventricular white matter [19–21]. Additional views allow further visualization of the posterior fossa and brainstem via the mastoid (posterolateral) fontanel and of the trigones and occipital horns of the lateral ventricles via the posterior fontanel [22–24]. In clinical settings, one limitation of cranial US has been its limited sensitivity to punctate white matter lesions or focal white matter necrosis, which have become the more common presentation of periventricular leukomalacia (PVL) and are more readily detected using conventional MRI (Figs. 1, 2 and 3) [25, 26]. At the same time, cranial US can demonstrate secondary changes associated with PVL and other forms of perinatal white matter injury, including ventriculomegaly, enlargement of sulci and increased subarachnoid spaces, thereby providing useful diagnostic and prognostic information in the appropriate clinical setting (Fig. 4). Inter-rater reliability of cranial US has been found to be excellent for assessment of ventriculomegaly, cystic PVL and diffuse volume loss but might be lower for more subtle patterns of perinatal white matter injury [27, 28].
Fig. 1.
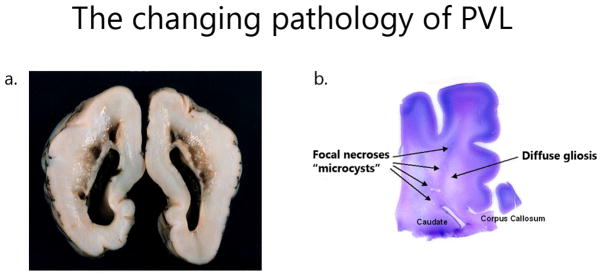
The evolving neuropathology of periventricular leukomalacia in the preterm brain. a Gross autopsy of cavitary periventricular leukomalacia. b H&E/LFB stain of the preterm brain shows both the focal injury (microcysts) and the diffuse component (diffuse white matter gliosis) of more recent periventricular leukomalacia autopsy findings
Fig. 2.
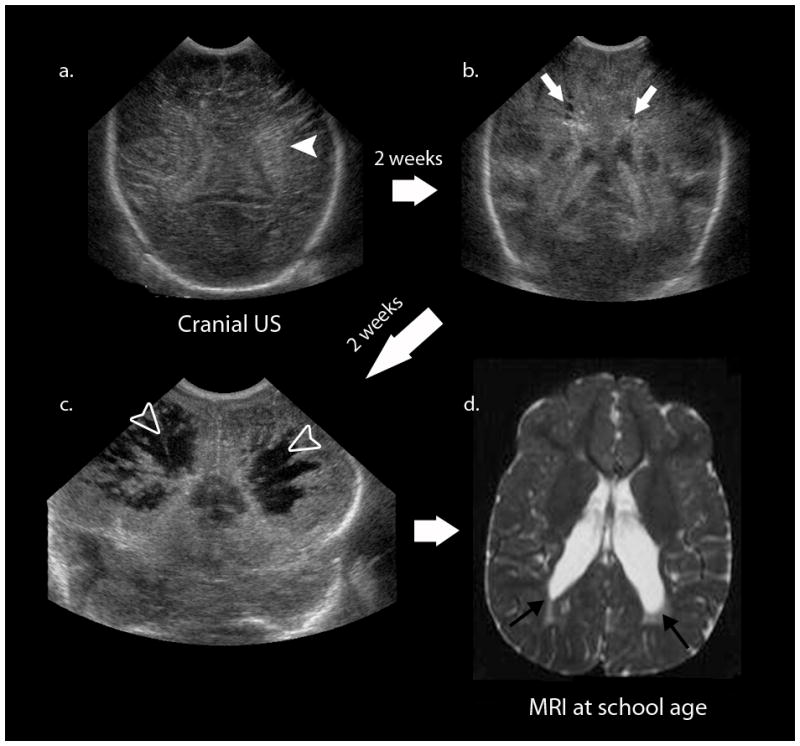
The classic evolution of cystic periventricular leukomalacia on cranial US. The first image (a) shows diffuse increased echogenicity (white arrowhead) followed serially by two other studies 2 weeks apart (b, c), which show the development of hypoechoic cystic leukomalacia (white arrows) and severe cavitary periventricular leukomalacia (open white arrowheads). The longterm sequelae of these neonatal findings are cerebral palsy and spastic diplegia. d Axial T2-W MR image during follow-up examination of this patient at school-age shows enlargement of the lateral ventricles at the level of the trigone extending caudally (black arrows) with adjacent region of periventricular T2 hyperintensity
Fig. 3.
More recent case of periventricular leukomalacia. a, b Cranial US shows slightly increased diffuse periventricular echogenicity (white arrowheads) relative to the choroid plexus (black arrow) and no other abnormality. c, d Coronal SPGR MR (in plane resolution 0.625 mm × 0.625 mm with slice thickness of 1.5 mm) performed the same day as cranial US with a neonatal head coil shows multiple foci of high T1 signal (white arrows) in the deep/periventricular white matter that likely represent focal white matter necrosis or non-cystic PVL. e These lesions (white arrows) demonstrate low T2 signal intensity surrounded by T2 hyperintensity, which could represent diffuse injury or unmyelinated white matter. f The trace image from the diffusion weighted MRI scan shows that the focal lesions (white arrow) are diffusion restricting. The mean diffusivity and fractional anisotropy maps are not shown.
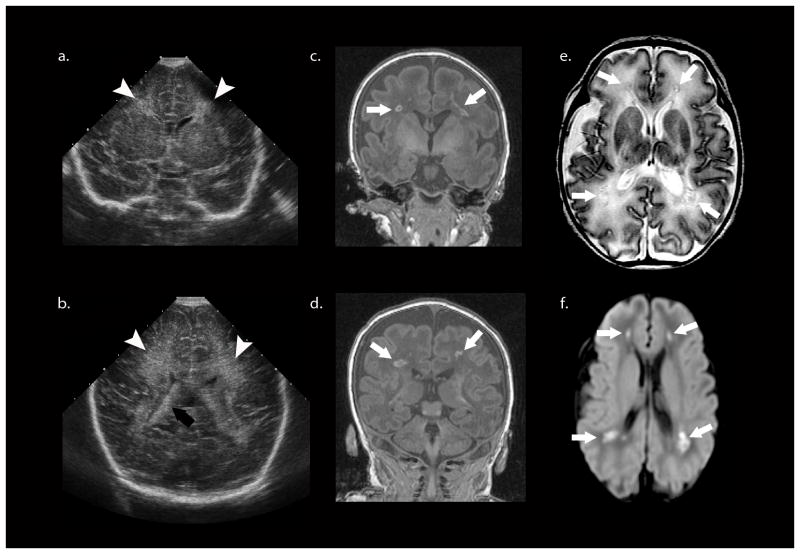
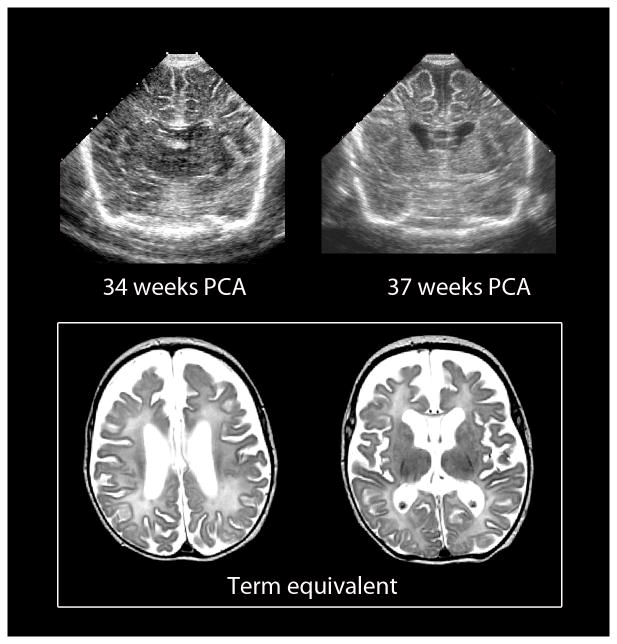
Fig. 4.
Chronic diffuse white matter injury in the preterm infant can be detected by both cranial US and MRI. Images of serial cranial US performed at 34 and 37 weeks’ gestation (top) show progressive enlargement of the lateral ventricles and concomitant increases in the sulci and subarachnoid spaces. The corresponding MR performed at term-equivalent age (bottom) shows similar findings likely related to diffuse white matter hypoplasia
Recent advancements in MR technology, including MR compatible monitoring equipment and neonatal isolettes, have allowed for less stressful transport and a safer environment for imaging even for the most extremely preterm infants. Specifically designed neonatal head coils provide higher spatial resolution, better gray-white contrast and better visualization of the brain stem and posterior fossa compared to cranial US and conventional MRI at 1.5T with a standard adult head coil [29, 30]. Standard clinical MR sequences (e.g., axial T1 or T1 FLAIR, FSE T2) readily demonstrate punctate white matter lesions or focal white matter necroses as well as diffuse changes involving the developing cerebral white matter (see below).
Utility of CUS and MRI in relation to major neuropathologies of the preterm infant: IVH and PVL
As noted above, the two most common neuropathological complications of prematurity are germinal matrix/intraventricular hemorrhage (GMH/IVH) and periventricular leukomalacia (PVL). It is important to note that although the neuroimaging correlates of GMH/IVH are well established on both cranial US and MRI, there remains controversy regarding the precise neuroimaging correlate of periventricular leukomalacia, particularly with regard to the more diffuse component of the white matter injury (see below). Accordingly, we will subdivide our discussion of white matter injury of prematurity into focal injuries, including cystic and non-cystic or punctate white matter lesions, and diffuse injuries, including diffuse extensive high signal intensity (DEHSI) in the white matter and white matter hypoplasia. For the sake of consistency, we will use the term white matter injury of prematurity (focal and diffuse) as the neuroimaging correlate of PVL for the remaining portions of this review (except when in reference to neuropathology).
Germinal matrix and intraventricular hemorrhage
The germinal matrix, which includes the ventricular and subventricular zones, is a transient region present in fetal (and for preterm infants, early post-natal) development. It is a site of active cell proliferation and migration and is therefore highly vascularized. Premature and low-birth-weight newborns are at risk for hemorrhages originating in the germinal matrix, with an incidence of 20–25% of preterm infants and as high as 45% of the very smallest (500 to 749 grams) [1, 31].
Clinically, germinal matrix hemorrhages (GMH) and intraventricular hemorrhages (IVH) convey a risk for complications (e.g., hydrocephalus, further hemorrhage or infarction) and even death as blood spreads throughout the ventricular system. The classic grading system of GMH/IVH (Papile grading system) classifies as grade I if there is germinal matrix hemorrhage without extension into the ventricular system, grade II if there is intraventricular hemorrhage without ventricular dilatation, grade III if there is intraventricular hemorrhage with ventricular dilatation, and grade IV if there is intraparenchymal hemorrhage [32]. Long-term, infants who survive grades III or IV intraventricular hemorrhages are at risk for adverse outcome, including cerebral palsy and other neurodevelopmental disabilities [33–35]; however, grades I and II IVH do not appear to convey a significantly increased risk of adverse outcome, although there is some controversy about this [36]. In a large multi-center study, grades III and IV intraventricular hemorrhage were present in 27.3% of the infants with monoplegia, 66.7% with hemiplegia, 35.2% with diplegia, 58.8% with triplegia, 48.1% with quadriplegia and 39.3% with hypotonic cerebral palsy. Additionally, 14.2% of infants without gross motor dysfunction also displayed grades III or IV IVH [33].
Both cranial US and MRI have detected germinal matrix or intraventricular hemorrhage. Cranial US demonstrates unilateral or bilateral hyperechoic hemorrhagic material in the caudothalamic groove and the lateral ventricles in neonates with GMH or IVH. MRI typically shows increased signal intensity on T1-weighted images and decreased signal intensity on T2-weighted images in the hemorrhagic regions, but the signal intensity varies considerably and blood products can have any combination of signal intensities on T1- and T2-weighted imaging. Peripheral susceptibility effects from hemosiderin and ferritin deposition are clearly depicted on gradient-echo and susceptibility-weighted imaging. Neonates with acute large intraventricular hemorrhages might have a fluid-fluid level in the lateral ventricle. Hydrocephalus and parenchymal atrophy can be seen as a complication of high-grade GMH/IVH. MRI is also excellent in delineating complications of post-hemorrhagic hydrocephalus including “trapped fourth ventricle” (Fig. 5).
Fig. 5.
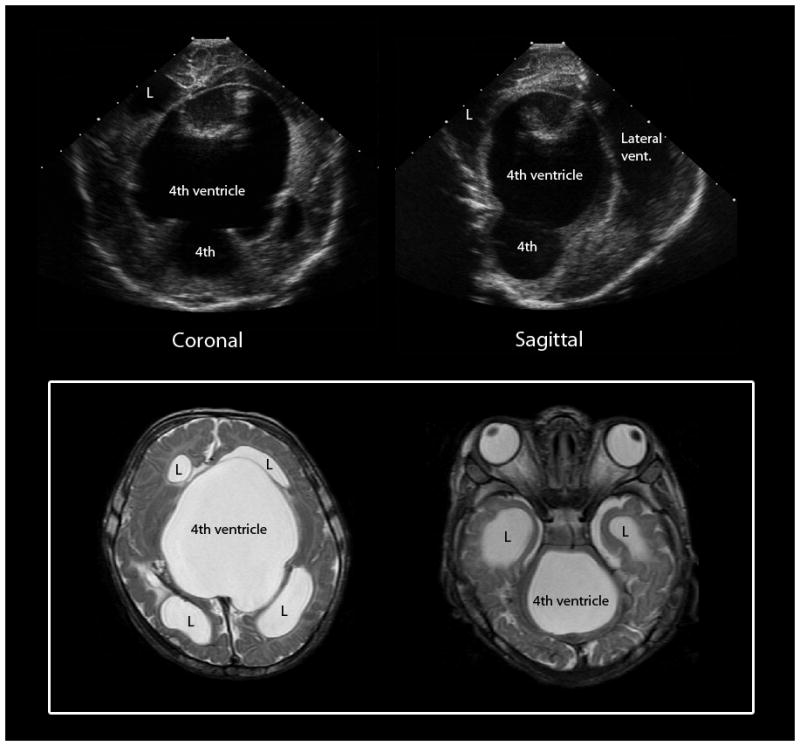
Trapped 4th ventricle, a complication of post-hemorrhagic hydrocephalus. Cranial US images (top) show a large cystic structure extending from the posterior fossa supratentorially through the tentorial incisura. Axial T2-weighted MR images (bottom) confirm an enlarged 4th ventricle with blocked outlet at the level of the aqueduct of Sylvius and the bilateral foramen of Lushka. L = lateral ventricle
Ventricular dilatation (IVH -- post hemorrhagic)
Ventricular dilatation can be secondary to either (or both) of the most common neuropathological complications of prematurity: GMH/IVH and white matter injury of prematurity with subsequent white matter hypoplasia (see discussion below under diffuse white matter injury of prematurity). Severe ventricular dilatation in the context of IVH results in damage to the periventricular microvasculature as well as adjacent neural tissue, leading to axonal injury and impaired myelin formation in the regions surrounding the lateral ventricles, whereas ventricular dilatation associated with white matter injury of prematurity can reflect ex-vacuo changes resulting from more widespread damage involving the pre-oligodendrocytes and developing axons. It has been shown that cranial US has good sensitivity to ventriculomegaly and excellent correlation with 3D MRI [37, 38]. Several methods to quantify ventriculomegaly from cranial US have been described, including the ventricular index, the diagonal width and ventricular height. The ventricular index is defined as the distance in millimeters between the midline and lateral border of the smaller lateral ventricle in the coronal plane at the level of the foramen of Monro [39]. The diagonal width is the width in millimeters of frontal horns in the coronal plane at the level of the caudate nucleus [40]. Ventricular height is measured at the level of the apex of the thalamus in a parasagittal plane, just posterior to the foramen of Monro [41]. The ventricular–brain ratio is defined as the ratio of the added widths of both ventricular mid-bodies at the border of the frontal horns and the mid-bodies to the width of both brain hemispheres on coronal US at the level of the foramen of Monro [42]. Simplified criteria to assess the degree of ventriculomegaly have been proposed, considering it mild if there is no third ventricular dilatation, moderate if there is third ventricle and temporal horn dilatation and severe if there is cortical thinning [43].
In severe post-hemorrhagic ventricular dilatation, the loss of axodendritic connections leads to adverse neurodevelopmental outcomes [44–46]. Using cranial US data, one study reported that 40% of infants with ventriculomegaly developed cerebral palsy (CP) and that infants with ventricular dilatation were 17 times more likely to have quadriparesis or hemiparesis [47]. A ventricular-brain ratio above 0.35 was found to be a sensitive predictor of developmental impairment [42]. MRI data have been more mixed. One study demonstrated an association between abnormal dilation of the occipital horns and CP and poorer developmental outcome at 1 year of age (assessed using the Denver and Bayley scales of infant development) [48]. Other studies have shown that ventricular dilatation without IVH was not associated with adverse developmental outcome [42, 49]. Finally, in several of the studies of outcome related to ventricular dilation, both IVH cases and diffuse white matter hypoplasia cases from diffuse white matter injury of prematurity are combined as one group, which limits the interpretation of the findings.
Periventricular leukomalacia and focal white matter injury of prematurity
Neuropathologically, periventricular leukomalacia (PVL) consists of two basic components: (1) focal necroses, often in periventricular regions, involving the loss of all cellular elements, and (2) a diffuse, cell-specific lesion involving the loss of early differentiating oligodendrocytes (i.e. premyelinating oligodendrocytes, pre-OLs) with accompanying astrogliosis and microgliosis [17]. Classically, the focal necroses were associated with subsequent cyst formation in the developing periventricular white matter (i.e. cystic PVL); however, with advances in care of preterm and extremely low birth weight infants, large cavitated cysts are rare (i.e. approximately 3–5% of very low-birth-weight infants) [50], so that the most common neuropathological finding involves the diffuse injury to the pre-OLs with or without microcyst formation. Finally, neuropathological studies have also shown that PVL, at least the more classic cystic form, is associated with neuronal loss and gliosis in the thalamus, strongly suggesting more widespread effects on developing corticothalamic pathways in patients with PVL.
Serial cranial US has demonstrated the evolving nature of focal white matter injury of prematurity in preterm and extremely low-birth-weight infants. Typically, focal white matter injury of prematurity first appears as a focal echogenic lesion on cranial US and can develop cystic changes at approximately 3–4 weeks of age. The increased periventricular echogenicity on cranial US is sometimes reversible, but if it persists for more than 7 days, it is considered white matter injury (PVL) grade 1. As disease progresses, the periventricular areas of increased echogenicity on cranial US evolve into small localized cysts (grade 2) and then into extensive periventricular cystic changes predominately involving the frontoparietal and occipital regions (grade 3). With more severe insult, the cystic changes on cranial US extend to involve the subcortical white matter (grade 4) [51]. The cystic changes are often not detected after the first 4–6 weeks following the injury as coagulation necrosis and cellular loss evolve over time [41]. Cranial US is highly reliable for detection of cystic WM injury but has significant limitations for noncystic WM injury [25, 26]. Moreover, as noted earlier non-cystic WM injury is considerably more common than cystic and this difference has increased with the continued advancements in neonatal care. White matter injury with or without volume loss has been strongly associated with spastic diplegia or quadriparesis and cerebral palsy [47, 52–54]. A study reported that 52% of infants with parenchymal echolucency on cranial US developed CP and these infants were 24 times more likely to have quadriparesis and 29 times more likely to have hemiparesis [47}
MR findings in premature neonates with focal white matter injury include increased signal intensity on T1-weighted images and decreased signal intensity on T2-weighted images involving the periventricular white matter, which can progress to periventricular cysts with or without focal or punctuate hemorrhages. The neuropathological correlate of these punctate lesions is not clearly understood. In our clinical practice, we have seen these lesions at any stage of gestation, including term-equivalency. Thus, these lesions are not specific to preterm infants. We have also observed these punctate lesions in a variety of neonates including term neonates with congenital heart disease and primary pulmonary hypertension and in neonates treated with ECMO. We have also observed these lesions in neonates with hypoxic ischemic injury and different kinds of infection. These punctate lesions are best depicted on 3D volumetric SPGR sequences and are usually found in the periventricular white matter, particularly posteriorly near the trigones of the lateral ventricles, and in the corona radiata. Sequential assessments by MRI in our practice have revealed different long-term courses for these lesions (Figs. 6, 7 and 8). In some instances, punctate lesions are not visualized at all on follow-up imaging and are instead replaced by normal-appearing unmyelinated white matter. In other instances, we are able to visualize a glial scar. The number of punctate lesions might also decrease with serial scanning [55]. In some situations, the punctate lesions co-exist with cystic lesions, and it is not entirely clear that these lesions can progress to cystic lesions. Cystic lesions are defined as areas with signal intensity identical to that of CSF in all sequences. Hemorrhage shows increased signal intensity on T1-weighted images and susceptibility effect on gradient-echo or susceptibility-weighted images [56]. In rare cases, intraparenchymal hemorrhagic or cystic lesions are as severe as to involve both periventricular and subcortical white matter.
Fig. 6.
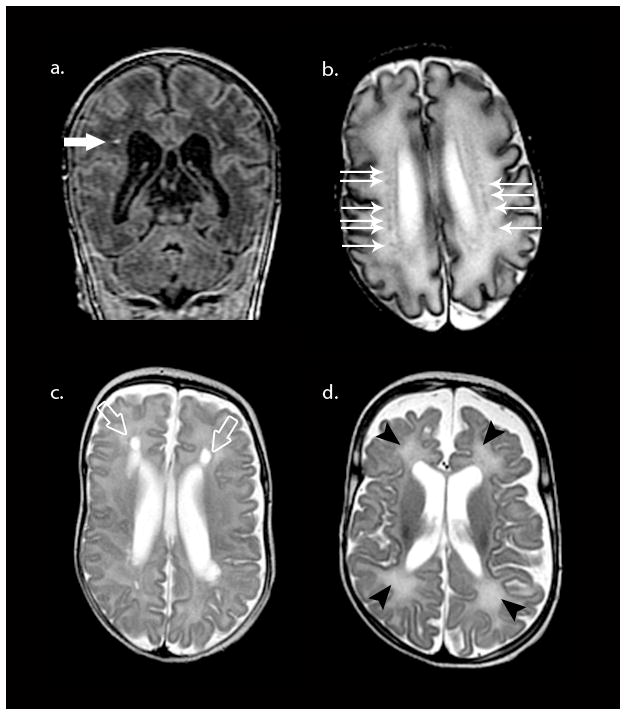
Spectrum of PVL lesions in the preterm. Top row depicts non-cavitated, focal white matter necrosis or punctate white matter lesion as hyperintense on T1-weighted coronal SPGR (large white arrow in a) and hypointense on axial T2 image (smaller white arrows in b). Bottom row depicts cystic PVL (open arrows in c) and diffuse excessive high signal intensity (DEHSI) on T2-weighted MRI (black arrowheads in d) with associated volume loss
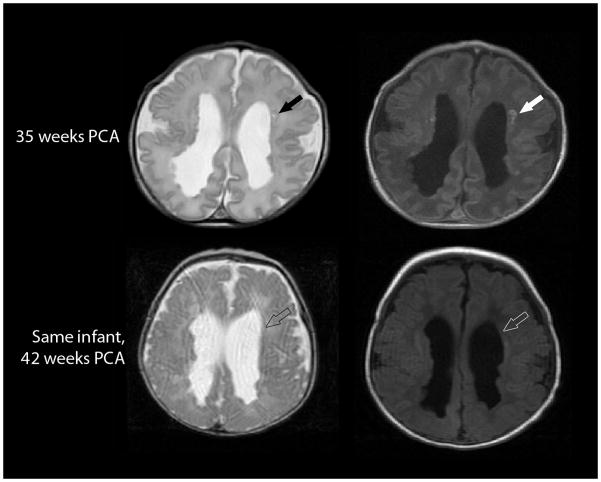
Fig. 7.
Evolution of a focal non-cystic PVL on axial T2-weighted (left) and axial T1-weighted (right) MRI in a 34-week gestation preterm infant imaged at 35 weeks postconceptional age (top row) and 42 weeks postconceptional age (bottom row). The T1 hyperintense punctate non-cystic lesions evidenced on the early scan (solid arrows) could not be visualized on the later scan (open arrows). (Of note, these studies also demonstrate numerous nodules along the surface of the lateral ventricles, presumed to be heterotopias.)
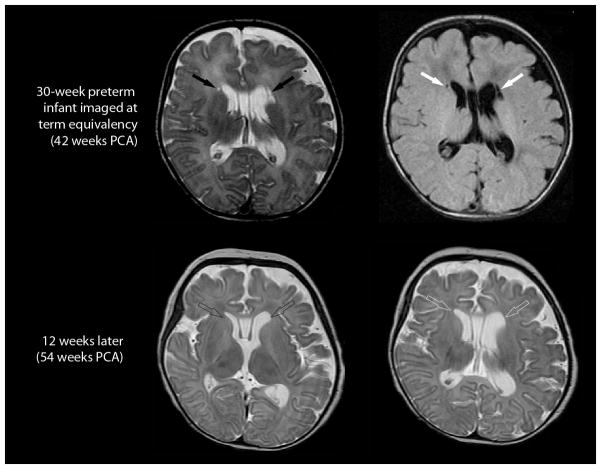
Fig. 8.
Evolution of a focal cystic PVL in an infant born at 30 weeks PCA. Imaging at term-equivalency (42 weeks PCA; top row, axial T2-weighted image on the left and axial FLAIR image on the right) demonstrates . . focal cystic periventricular lesions (solid arrows) abutting the frontal horns of the lateral ventricles. Comparable cuts on a follow-up MRI conducted 12 weeks later (bottom, both T2-weighted sequences), shows no evidence of cystic lesions adjacent to the lateral ventricles (open arrows). Instead, the follow-up MRI shows enlargement of the lateral ventricles and associated white matter volume loss.
Clinically, diffusion-weighted (DWI) and diffusion tensor imaging (DTI) are useful for determining the acuity of focal periventricular white matter lesions, with the focal white matter necrosis demonstrating restricted diffusion and corresponding low ADC (diffusion weighted) or mean diffusivity (diffusion tensor) during the acute epoch following insult. This could be related to cellularity or cytotoxic edema. At this stage, the lesions are typically not well visualized on cranial US [25, 26]. Later, preterm infants with punctate lesions in the periventricular white matter might show evidence of reduced white and gray matter volume and myelin at term-equivalent age compared to preterm infants without punctate lesions [57]. The neuropathological correlate of these punctate lesions is unknown but likely represents an inflammatory response to a focal area of injury (axonal or pre-oligodendrocyte) in the white matter [55] (personal communication, Dr. H.C Kinney). Recently, DTI was used to examine the microstructure of the developing white matter in preterm infants with and without punctate white matter lesions, and this study found that punctate lesions were associated with altered microstructure in the white matter of the corticospinal tracts [58]. Studies have looked specifically at the correlation of punctate white matter lesions and neurodevelopmental outcomes. Cornette et al. [59] found that isolated punctate white matter lesions were associated with a favorable outcome at 30 months of age if no other associated white matter lesions were noted. Dyet et al. [49] also found no difference in neurodevelopmental outcome between preterm infants with and without punctate lesion images at term-equivalent age and followed to both 18 and 36 months of age. However, Miller et al. [60] found that focal white matter lesions detected at 32 weeks and near-term were associated with poorer outcomes at 36 months.
Diffuse white matter disease of prematurity
As noted above, neuropathologically, diffuse white matter disease of prematurity is characterized by cell-specific damage to the myelinating oligodendrocytes and gliosis and later by white matter hypoplasia (Figs. 4, 6 and 8). However, the precise neuroimaging correlate of these neuropathological findings remains to be determined.
On cranial US, diffuse white matter injury is visualized as areas of hyperintense signal in the periventricular white matter relative to the choroid plexus. It is important to note that preterm neonates can have mildly increased periventricular echogenicity, which does not reflect any pathological process and should not be confused with white matter injury. These areas of hyperintensity are homogeneous, symmetrical and less echogenic than those of the choroid plexus. To be considered abnormal, the periventricular white matter should be as echogenic or more echogenic than the choroid plexus. To confirm the presence of a structural abnormality rather than an artifact, the diffuse periventricular hyperechogenicity and the cysts should be seen in both coronal and parasagittal planes. Finally, although the diffuse periventricular hyperechogenicity is often presumed to be the neuroimaging correlate of the diffuse neuropathological component of PVL or perinatal telencephalic leukoencephalopathy, the precise neural basis of this signal remains to be determined. Moreover, relative to ventriculomegaly and cystic focal lesions, inter-rater reliability has been found to be lower for this observation, owing to its more subjective nature [27, 28].
MRI also provides evidence of diffuse white matter injury in preterm infants. Contrary to focal white matter injury, acutely the diffuse form of perinatal white matter injury is difficult to diagnose with conventional MR imaging. It has been proposed that diffuse excessive high-signal intensity (DEHSI) in the periventricular white matter on T2-weighted images represents the diffuse component of white matter injury in preterm infants. Supporting this is the observation that, in the absence of focal white matter necroses, DEHSI has been associated with lower overall development at 18 and 36 months [49, 61, 62]. DEHSI, a common finding in preterm infants [63], has also been associated with a decrease in central gray matter volume [64, 65]. Other studies have demonstrated that approximately 50% of neonates who have DEHSI have a normal developmental outcome at 2 years [49], suggesting that DEHSI has limited predictive utility as a biomarker for white matter injury of prematurity or long-term outcome. More recent studies have also failed to find an association between DEHSI and outcome. Hart and colleagues [66] found no association between DEHSI and outcome at 18 months. Similarly, Skiöld et al. [67] found no relationship between DEHSI and cognitive, language or motor performance at 30 months of age. Finally, a recent study of a large cohort of preterm infants in Korea (n = 126) found that DEHSI alone did not predict poor neurodevelopmental outcome at 2 years of age but that in combination with other white matter lesions it was associated with motor delay [68]. The failure to find an association between DEHSI and long-term neurocognitive outcome might be explained by: (1) low inter-observer agreement for DEHSI, which limits its usefulness in clinical application [66]; (2) poor sensitivity/specificity for the outcome measures selected to detect outcome; and (3) the potential for genetic-environmental factors to moderate or mediate the relationship between DEHSI and outcome. Distinguishing DEHSI from immature white matter development (more frequently seen in congenital heart disease patients) can be difficult. In our experience, DEHSI can be distinguished from immature white matter more readily when the abnormal T2 hyperintense signal is seen extending into the subcortical white matter region and there is associated gray or white matter volume loss (personal observation).
The acute component of the diffuse form of perinatal white matter injury might be reflected by decreased ADC and mean diffusivity, although the evidence for this is limited. The chronic diffuse component of white matter injury can also be detected by both diffusion weighted and diffusion tensor imaging as elevated ADC (mean diffusivity values ranging from 1.4 to 1.6 × 10−3 mm/s) in the periventricular and deep white matter at term-equivalent age [69, 70]. Interestingly, a recent study found that preterm infants without visible abnormality on conventional MRI but higher ADC values in the periventricular white matter at term-equivalent age had poorer developmental outcomes at the age of 2 years [71].
With regard to conventional MRI, on T2-weighted MRI the chronic phase of diffuse white matter injury is associated with ventriculomegaly and increased sulcal size, reflecting both white matter hypoplasia as well as a certain degree of diffuse gray matter volume loss and simplified gyral patterns (Fig. 8). Additionally, preterm infants with moderate to severe disability at 1 year of age have been found to have significantly increased CSF volume and decreased cortical and deep nuclear gray matter volumes demonstrated on term-equivalent MRI [72]. White matter volumes in the sensorimotor and midtemporal regions, especially on the right, have also been strongly correlated with neurodevelopmental outcomes at 18 to 20 months’ corrected age [73]. It should be noted that two specific classifications of white matter injury in the preterm brain have been recently developed including the Inder classification (Table 4) and the UCSF classification (Table 5); these classifications incorporate both focal and diffuse components of premature white matter injury.
Table 4.
Inder classification
| Inder Classification | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| WM Signal abnormalities | Normal | Focal high T1/T2, 2 or fewer regions/hemis | Multiple high T1 or T2 |
| PVWM Volume loss | Normal | Mild reduction, with mild-mod vent increase | Marked, often with marked increase of vent or ex ax space |
| Cystic abnormalities | Normal | Less than 2 mm single focus cyst | Multiple cysts or single larger cyst |
| Ventricular dilatation | Normal | Moderate | More global enlargement |
| Thinning of corpus callosum | Normal | Focal thinning | Global thinning |
Score 5–6: Normal; 7–9: Mild; 10–12: Moderate; 13–15: Severe
Excellent inter- rater agreement: 96%
Inder TE et. Al. J Peds 2003; 143:171–9; Woodward L, et, al. NEJM 2006;355:685–94
Table 5.
WM abnormality classifications
| UCSF Classification | MR Findings |
|---|---|
| Normal | No periventricular WM signal abnormalities |
| Minimal WMI | 3 or fewer areas of T1 signal abnormality of <2 mm |
| Moderate WMI | 3 or more areas, or areas measure >2 mm but <5% of hemisphere |
| Severe WMI | T1 abnormalities with >5% of hemisphere involved |
| Mild / Borderline ventriculomegaly | Ventricular diameter at level of glomus of choroid plexus = 8–10 mm |
| Mod / Severe ventriculomegaly | Ventricular diameter >10mm |
Excellent agreement between readers: 89.5%, kappa = 0.84
Miller SJ, et. al. AJNR 2003; 24:1661–69; Glass HC, et. al. Pediatrics 2008;122:229–305
Specific white matter tract abnormalities
Corpus callosum, cingulum, fornix and anterior commissure
The corpus callosum (CC) is the major fiber bundle connecting the left and right cerebral hemispheres, and because of its composition (white matter) and typical developmental course (i.e. that it begins to myelinate shortly after term) it is susceptible to injury in the perinatal period. CC injury is easily visualized with the sagittal midline view using cranial US or MRI as either thinning or abnormal signal intensity (in addition to frank malformations). Abnormal-appearing CC on cranial US and MRI has been reported in infants with adverse neurodevelopmental outcome, and thinning of the CC on MRI, particularly in the mid- to posterior body, is a common finding in children with cerebral palsy [74, 75]. It has been proposed that the assessment of the thickness of the corpus callosum could be used to estimate the extent of the loss of volume of cerebral white matter in children with a broad spectrum of white matter injury [76]. With regard to primary pathology, neonatal periventricular hemorrhage and ventricular dilatation in preterm infants were associated with greatest decrease in size of the CC [77]. The length of the CC is reproducible on cranial US and a shorter CC at term-equivalent age is associated with poorer neurodevelopmental outcome [78]. Additionally, those with reduced growth of the length of the corpus callosum during the period from 2 weeks to 6 weeks of age were at risk for psychomotor delay and cerebral palsy [79]. It has also been reported that total mid-sagittal CC size and mid-posterior surface area were positively associated with the verbal IQ and verbal fluency scores in very preterm boys [77]. Finally, advanced MR techniques, including DTI, have demonstrated microstructural abnormalities (e.g., decreased fractional anisotropy, or FA) in the splenium of the CC in children with abnormal outcome at 18 months corrected age [80]. FA values of the CC, as well as the cingulum, fornix, anterior commissure and right uncinate fasciculus, have also correlated with the developmental quotient and eye-hand coordination at 2 years of age. [81]
Motor system (descending corticospinal tracts)
The corticospinal tract originates in the pyramidal neurons of the precentral gyrus (and associated motor cortices) and descends through the corona radiata, posterior limb of the internal capsule, cerebral peduncles, basis pontis and ventral medulla before decussating at the lower portion of the brainstem and forming the lateral corticospinal tract (and smaller ipsilateral anterior corticospinal tract). It is well established that brain injury in preterm infants is associated with motor impairments (e.g., cerebral palsy), which can arise from damage to the corticospinal tract and associated motor tracts at many different levels of the central nervous system. Damage involving the basal ganglia (which can arise in the context of germinal matrix or intraventricular hemorrhages or other perinatal pathologies including hypoxic-ischemic encephalopathy or kernicterus) is often associated with a dyskinetic form of cerebral palsy. In contrast, damage involving the corticospinal tract itself (e.g., resulting from focal necroses in the corona radiata superior or lateral to the lateral ventricles) produces upper motor neuron signs, as in spastic cerebral palsy.
MRI at term-equivalent age has demonstrated T1-hyperintense or cystic lesions in the corona radiata, which predict motor function at 3 to 5 years of age. Moreover, the absence of these T1-hyperintense lesions in the corona radiata at term-equivalency predicts normal motor development, irrespective of the presence of ventriculomegaly [80]. Asymmetrical myelination of the posterior limb of the internal capsule (PLIC) demonstrated by MRI in newborn infants with a GMH/IVH and unilateral parenchymal hemorrhage has been found to be strongly associated with subsequent development of hemiplegia and to a lesser extent diplegia and quadriplegia in infants with bilateral cystic white matter injury [82, 83]. Studies using DTI and fiber-tracking suggest that lower FA values in the PLIC or along the motor tract allow earlier detection of microstructural abnormalities in infants at risk for neurological abnormalities and disability [80, 84, 85]. Finally, tractography-based measurements have been found to be more sensitive than arbitrary regions of interest in 3D imaging [86].
Optic radiations and other primary visual pathways
The primary retrogeniculate visual pathways include the optic radiations, which carry visual information from the lateral geniculate nucleus of the thalamus to the primary visual cortices (i.e. area 17/striate cortex in the occipital lobe) and several short-range (i.e. u-fiber) and long-range pathways connecting primary visual cortices to subcortical structures. These include the thalamus (i.e. thalamocortical and corticothalamic connections) and higher-order visual and associated areas in the temporal and parietal lobes (e.g., inferior longitudinal fasciculus). In addition to retinopathy of prematurity, children who are born prematurely have a high incidence of visual disorders ranging from basic visual perceptual disturbances (e.g., difficulty perceiving contrast or color) to difficulty perceiving complex 2- and 3-dimensional visual spatial relations among objects [88]. Neuropathologically, it is well demonstrated that there is a posterior predilection with regard to distribution of the lesions associated with PVL (both the focal and diffuse components). In older children, this is most readily evidenced by a dilation of the lateral ventricles at the level of the trigone and then extending posteriorly (i.e., “squaring off” of the ventricles) while the width and shape of the frontal horns remains largely intact. Given the neuropathology in relation to the organization of the developing central nervous system, it is not surprising that children who are born preterm are at increased risk for visual disturbances.
Research has demonstrated strong correlations between visual dysfunction in preterm children and a variety of metrics derived from MRI. For example, reduction in the inferior occipital regional volumes in preterm infants at term-corrected age has been associated with later impaired oculomotor function control [88]. Fractional anisotropy, a metric derived from diffusion tensor imaging (DTI) reflecting microstructural integrity of the white matter, increases with gestational age in the optic radiations and correlates significantly with visual fixation tracking scores [89]. Moreover, FA values within the optic radiations have been found to independently predict visual assessment scores regardless of the gestational age at birth, postmenstrual age at scan, or the presence of lesions on conventional MRI. Finally, using tract-based spatial statistics (TBSS), group-level comparisons of white matter have found that only FA in the optic radiations is associated with overall visual functioning [90]. Advances in DTI processing including the use of TBSS and probabilistic tractography will improve our ability to delineate white matter connectivity and plasticity in the preterm brain at different ages (Fig. 9).
Fig. 9.
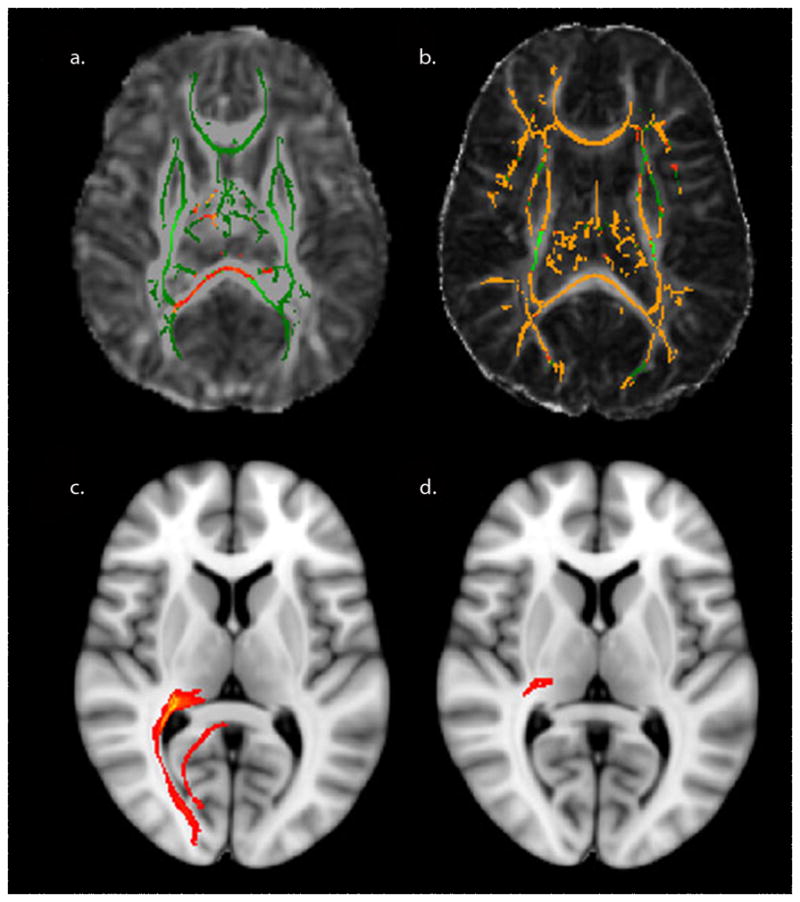
Recent postprocessing tools for DTI analysis. a TBSS of a preterm neonatal cohort shows differences in fractional anisotropy in the thalamus and splenium compared to age-matched controls. b In contrast, TBSS of a cohort of preterm children shows more widespread differences in fractional anisotropy compared to age-matched controls. c Probabilistic tractography of the posterior thalamic radiation in a control child is compared to (d) a preterm child with PVL
Gray matter injury
Gray matter includes both the developing cerebral mantle and the central nuclei deep in the human brain (e.g., basal ganglia, thalamus). Preterm infants are at risk for damage involving gray matter regions via both primary (e.g., direct hypoxia-ischemia) and secondary (e.g., deafferentation following damage to sensory or motor axons) mechanisms, which can lead to long-term adverse neurocognitive outcomes.
Studies examining gray matter structures in infants and children who were born preterm have shown that at term-corrected age the preterm infants demonstrate a reduction in cortical and deep nuclear GM volumes in comparison to the term-born infants and that the alteration is associated with moderate to severe neurodevelopmental disability at 1 year of age [72,73]. Additionally, the growth of the cerebral cortex (e.g., cortical surface volume) has been found to be reduced in preterm infants relative to term-born comparison infants and is proportional to later neurodevelopmental impairment [91]. The decreased volumes in cortical gray matter and smaller cortical surface area have also been shown to persist into adolescence [92]. Moreover, intrauterine growth restriction appears to moderate the relation between premature birth and cortical surface area such that preterm infants with intrauterine growth-restriction have been shown to have a more pronounced reduction in cortical surface area compared to premature newborns with adequate intrauterine growth and poorer long-term outcomes [93]. Finally, gray matter volume has been found to correlate with IQ as well as performances across cognitive domains, including working memory, even after adjustment for relevant perinatal, sociodemographic and developmental factors [94–98].
Cerebellum
The cerebellum is heavily interconnected with the overlying cerebral hemispheres and plays an important role in cognitive and emotional processes, as well as motor functions. Cerebellar hemorrhage (CBH) is as an important and under-recognized complication of extreme prematurity. It is associated with increased mortality and adverse neurodevelopmental outcome and often occurs concomitantly with supratentorial hemorrhage [99–102]. Unexplained motor agitation has often been reported in the days preceding the diagnosis of cerebellar hemorrhage [103]. Cranial US is typically less sensitive for detecting cerebellar hemorrhage, particularly if the hemorrhage is small, because the cerebellum is less easily visualized via the anterior fontanel and because it can be difficult to distinguish infarction and hemorrhage from the echogenic cerebellar vermis and tentorium. Careful examination for areas of asymmetrical echogenicity via the mastoid and posterior fontanels is essential to improve the accuracy of cranial US for detecting cerebellar hemorrhage [22–24, 104,105]. MRI can depict petechial hemorrhages, but the clinical relevance of this finding is not well-defined. In one study, clinically detected neurological abnormalities were present in two-thirds of the isolated cerebellar hemorrhagic injuries at the age of 32 months (diagnosed by cranial US and confirmed by MRI). It has also been shown that these infants had significantly impaired expressive language, delayed receptive language, cognitive deficits, severe motor disabilities, severe functional limitations in day-to-day activities and increased risk for autism. Abnormal outcomes were more common and profound in preterm infants with injury to the vermis [8]. Cerebellar hemorrhages related to blood disorders in babies without white matter injury were less frequently related to motor deficits [99,100]. MRI-detected cerebellar hemorrhages have recently been associated with long-term poor neurodevelopmental outcomes [102]. With regard to cerebellar volume, studies have not found decreased cerebellar volumes in preterm infants in the absence of cerebral white matter damage [106, 107]. Additionally, cerebellar volume has not been found to predict outcome at 2 years of age [106], but data are controversial [108]. More recent studies have demonstrated relationships between cerebellar structure and supratentorial development injury [109,110].
Value of MRI and cranial US to predict neurodevelopmental outcome
As mentioned in the sections before, several brain imaging abnormalities have been shown to be correlated with neurodevelopmental outcome using both cranial US and MRI (tables 1–3). MRI can also be complementary to cranial US in predicting neuromotor impairments, delineating abnormalities in the posterior limp of the internal capsule (PLIC) [82, 83]. In a large study, the appearance of white matter injury on MRI was very sensitive to predict severe cognitive delay, severe motor delay, cerebral palsy and neurosensory impairment (89%, 88%, 94% and 89%, respectively), but the specificity was moderate to low (31%, 30%, 31% and 30%, respectively); abnormalities seen on cranial US had low sensitivity values (15%, 18%, 18% and 16%, respectively), but high specificity (95%, 95%, 95%, and 95%). When only moderate-to-severe white matter injury on MRI was included, the specificity to predict severe cognitive delay, severe motor delay, CP and neurosensory impairment was close to that of cranial US (84%, 85%, 84% and 82%, respectively) with sensitivity values of 41%, 65%, 65% and 82%, respectively [111]. Another study reported higher specificity (79%) of MRI parenchymal lesions to predicted CP and high sensitivity (100%) to predict cerebral palsy, but again cranial US was more specific (85%) and less sensitive (67%). [112]
MRI in the evaluation of brain development in older survivors of prematurity
It is not uncommon for survivors of prematurity to be referred for MRI evaluations later to screen for underlying brain pathology in the context of clinical evidence of motor impairment, developmental delay or seizures. Most often, conventional MR imaging conducted at this time will be largely unremarkable, with the exception of increased ventricular size (particularly in the trigone and posterior horns of the lateral ventricles) and concomitant decreased white matter volume. Additionally, on occasion scarring is apparent in the white matter in areas corresponding to the clinical deficits (e.g., subadjacent to the precentral gyrus in the corona radiata).
Advanced neuroimaging techniques, now and in the next decade
In vivo imaging of brain metabolism using MR spectroscopy
The signal used by MRI to create anatomical maps is generated primarily by the hydrogen nuclei (1H) of water molecules (H2O). In contrast, 1H MRS analyzes the signal of protons attached to other molecules, including small molecular-weight amino acids, carbohydrates, fatty acids and lipids, many of which are involved in complex and well-regulated biochemical processes in the human brain. The most common metabolites to be examined in the preterm brain include N-acetylaspartate (which is present in axons and therefore is often considered to be a marker for neurons), creatine (which represents contributions from free creatine and phosphocreatine and is strongly coupled with ATP synthesis), choline (which is strongly related to membrane metabolism) and lactate (typically thought of as a marker for impaired metabolism because of interruptions in cerebral blood flow, but in preterm infants elevations in lactate might be normal because of normal differences in metabolism in the preterm brain). It is important to note that because 1H-MRS measures protons attached to larger molecules, it is theoretically possible to measure any molecule that contains protons that is present in sufficient quantity in either the cytoplasm of neurons or other cells (e.g., astroglia) or the extracellular matrix of the brain.
There are many age-related differences in metabolites as measured by MRS in the perinatal period extending into early childhood. However, absolute brain metabolite content in premature infants at term was not different from that in full-term infants [113]. As noted above, it has been reported that a lactate peak can be seen in preterm and very low-birth-weight infants [114]. Pentobarbital commonly used in neonates can lower lactate:choline and lactate:N-acetylaspartate (NAA) ratios in the basal ganglia of premature neonates [115].
To date, the utility of MRS for risk-stratifying preterm infants in relation to their potential for long-term adverse outcomes is not well-established. MRS failed to reveal differences in brain metabolites in small-for-gestational-age premature infants compared to normal-size-for-gestational-age preterm infants [116]. Additionally, MRS has not been found to be a good predictor of outcome at 18 months to 24 months of age, when outcome is measured as an overall developmental quotient on the Bayley scales of infant and toddler development [117]. However, there have been methodological limitations to many of the studies involving MRS to date. For example, relatively few studies have used absolute quantitation of metabolites and rely instead on ratios of NAA and lactate to creatine and choline. It is possible that absolute quantitation will be a more sensitive predictor of outcome. Second, outcome has been typically measured in a general sense (e.g., overall developmental quotient). It is possible that MRS will become a much more sensitive tool when applied to more specific contexts. For example, it is possible that MRS applied to the posterior limb of the internal capsule will be a very sensitive predictor of dysfunction within the motor system (and of cerebral palsy). Similarly, MRS applied to the parietal-occipital white matter might be a very sensitive predictor of visual dysfunction. And last, examination of very specific metabolites (e.g., markers of ischemia or inflammation) within periventricular white matter might prove to be a very sensitive indicator of white matter injury of prematurity and provide a more precise understanding of the processes that contribute to the death or damage to neurons and pre-oligodendrocytes in white matter injury of prematurity (Fig. 10).
Fig. 10.
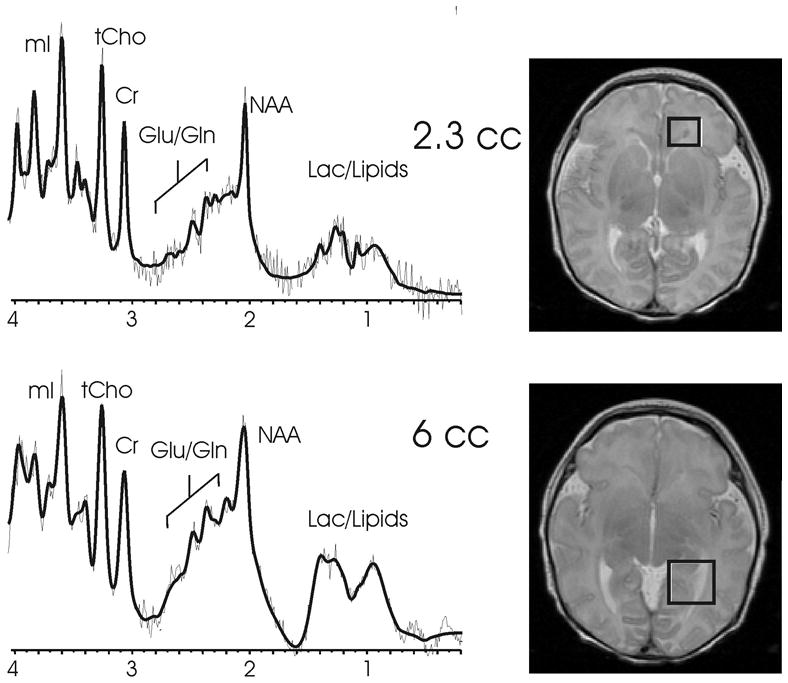
Short echo proton MR spectroscopy of focal white matter necrosis in the frontal lobes (top) and the optic radiation (bottom) in a preterm neonate imaged near term-equivalent age show slightly reduced NAA and elevated glutamate/glutamine, lactate and lipids. The T2-weighted images on the right depict the voxel location for each proton MRS acquisition.
Blood flow
Blood flow velocities in the basal cerebral arteries on Doppler cranial US progressively increase with birth weight and gestational age [118, 119]. Autoregulation of cerebral blood flow can be impaired in preterm neonates and in neonates who have suffered a hypoxic-ischemic insult. Moreover, impaired cerebral autoregulation, including fluctuating pattern of cerebral blood-flow velocity or absent end-diastolic flow, has been associated with intraventricular hemorrhage [120, 121].
Several measures of cerebral blood flow derived from Doppler cranial US have been found to be helpful in clinical situations. The resistive index (RI) increases with significant post-hemorrhagic hydrocephalus and decreases following cerebrospinal fluid (CSF) drainage, suggesting that it might be useful to assess shunt function [122]. Significant change in RI with anterior fontanelle compression is associated with increased intracranial pressure and need of surgical intervention [123].
Advanced imaging techniques, including MRI acquisitions with arterial spin labeling or Near-infrared spectroscopy (NIRS), might provide further information regarding cerebral blood flow dynamics in preterm infants. No current studies have evaluated the use of these tools (with or without gadolinium contrast agents) in the preterm brain. Thus it is important as these techniques are applied increasingly in clinical contexts across institutions to evaluate their effectiveness for detecting injury and predicting patterns of outcome in preterm infants.
Volumetric and brain metric analysis
A number of programs allow for segmentation and quantification of gray, white and cerebral spinal fluid volumes based on volumetric T1-weighted MR data. However, most of these algorithms have been developed for data from adults, and the tissue contrast between gray and unmyelinated white matter is inverted relative to the contrast between gray and myelinated white matter Thus, the existing algorithms typically fail when applied to neonatal T1-weighted MR data. As the field moves toward developing additional algorithms toward the application of neonatal MR data, other methods have been applied toward the assessment of neonatal brain volume. These methods often combine manual segmentation with volumetric statistical analyses and include surface-based and tensor-based morphometry (Fig. 11). Our laboratory has recently extended tensor-based morphometry to the study of subcortical structures in preterm infants (Fig. 11). Additionally, linear measurements, or “brain metrics,” have been developed and validated in the preterm brain and can be applied manually to 3D and 2D datasets. The 15 standard head and brain measurements include: bifrontal diameter, left and right frontal height, brain and bone biparietal diameter, frontal-occipital diameter, length of corpus callosum, surface of the vermis and transverse cerebellar diameter and “fluid” measures of the pericerebral space (interhemispheric distance, cranial caudal left and right interopercular distances) and the intracerebral spaces (diameter of the right and left lateral ventricles, third ventricle diameter) as manually measured on four selected slices. Slices and landmarks have been described [124]. These simple brain metric measurements have been validated as a method to quantify brain growth and assess brain atrophy in the neonatal and infant population and have been shown to correlate with outcome [125]. As we move forward, it will be important to develop techniques that not only will be sensitive to detecting injury and monitoring brain development in preterm infants, but that will allow us to begin to examine development at a different scale — at the level of a neural system. Today, most work examines macroscopic differences (e.g., whole brain volume, CSF volume). Future work needs to examine changes in the context of the systems-level organization of the human brain.
Fig. 11.
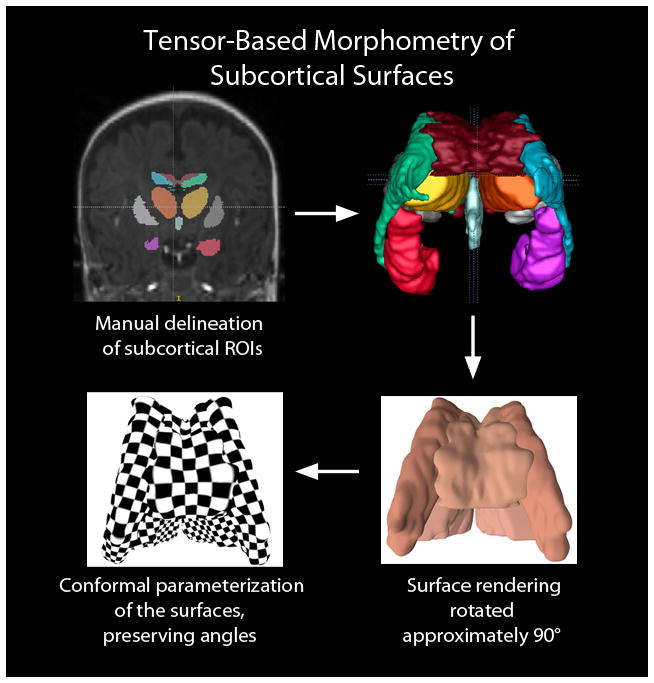
Application of tensor-based surface morphometry to the subcortical structures of a developing preterm infant brain derived from coronal SPGR 3D-volumedatasets. The first step in data processing (top left) involves manual delineation of subcortical structures. From there, 3D surfaces can be generated (top right) representing the corpus callosum (dark red), lateral ventricles (green and blue, representing the left and right lateral ventricles, respectively), hippocampus (orange red and purple, representing the left and right hippocampi, respectively), and third ventricle (light blue). The putamen, which were manually delineated in the coronal SPGR have not been included in this 3D rendering. The bottom left illustrates the computed conformal parameterization of the surfaces. The checkerboard texture map shows the angle-preserving property (right angles on the plane are mapped to right angles on surfaces). The surface conformal parameterization provides an ideal canonical space to register subcortical structures and analyze their interaction-related morphometry changes
Resting bold/functional connectivity analysis
Functional connectivity is method of data analysis applied to fMRI datasets during postprocessing to reveal patterns of interconnection among distributed neural regions. The method relies on correlations in low-frequency spontaneous fluctuations in blood oxygen level dependent (BOLD), and when applied to neonatal and other pediatric datasets, functional connectivity analyses allow for monitoring the establishment and maturation of neuronal networks [126–129]. Resting state networks (RSN) involving the sensorimotor, anterior and posterior cingulate, occipital, medial and lateral prefrontal and temporal cortices, as well as the thalamus and cerebellum have been identified in preterm infants [130–132]. Additionally, the method has suggested that the formation of thalamocortical connections, which are believed to be critical for cortical organization, might be disrupted in preterm compared to term-born controls [130–133] (Fig. 12). Additionally, in the basal ganglia, increased spectral energy in the low frequency range (0.01–0.06 Hz) has been reported for preterm infants at 36 months of age and stronger connectivity has been reported between RSNs in term infants [134]. Additional research is necessary to determine the clinical utility of resting state functional connectivity analyses in the evaluation of preterm infants as well as the potential for the method to reveal the anatomical substrate for cognitive deficits in preterm infants who do not appear to have abnormalities on conventional neuroimaging.
Fig. 12.
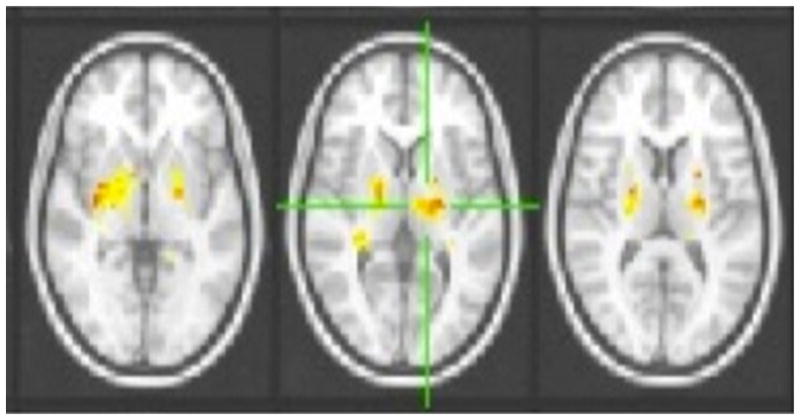
Functional connectivity data from a preterm cerebral palsy cohort vs. controls show stronger correlation within the thalamus and basal ganglia using a group-level independent components analysis and dual-regression approach
Finally, functional connectivity analyses have transformed fMRI analysis in cognitive neuroscience — moving away from subtraction-based, phrenology-like designs and toward a model of interconnected brain function that more closely approximates actual brain activity. At the same time, these analyses have helped generate a model of the systems-level neural architecture that sub-serves higher-order cognitive functions. Extension of these methods into the pediatric population, particularly preterm infants and fetuses, will help reveal the systems-level neural architecture sub-serving cognitive functions in the neonate as well as aberrant patterns of connectivity. Longitudinal analyses will allow for discovery of the manner in which those processes mature over time.
Discussion
Neuroimaging has played a central role in the evaluation of the preterm infant. Both cranial US and MRI have been found to be sensitive to patterns of brain injury. Cranial US is easy, relatively inexpensive and can be performed serially at the bedside. MRI provides better visualization of the brain parenchyma, subtle patterns of injury involving the developing white matter, and when advanced MR techniques (DWI, DTI, 3D acquisition, etc.) are applied, in vivo physiological data. However, MRI is more expensive, involves transportation of fragile infants out of the NICU and in certain instances requires sedation or general anesthesia, making it potentially higher risk. Given the latter, current practice models typically use cranial US as a broad-based screening method (because of its high sensitivity to blood products) in the NICU. In contrast, MRI is often reserved for the neonates who are found to have abnormalities on cranial US or who demonstrate severe or persistent neurological deficits.
Multiple recent reviews and commentaries have concluded that using MRI for general screening for brain injury in preterm infants is not indicated, and we agree with this assessment and the current practice parameter that supports this [135–138]. Moreover, the utility of cranial US or MRI in the neonatal period to predict neurocognitive outcomes in school-age children in the setting of white matter injury of prematurity has not been established. In contrast, cranial US and MRI both predict long-term neurocognitive outcome in the setting of IVH and cerebral palsy [139]. It is important to note that longitudinal data examining the utility of MRI as a biomarker of long-term neurocognitive function are limited, particularly with regard to the school-age period and beyond, and are notable for variability across time. For example, 25–30% of the toddlers in the cohort of infants followed by Woodward and colleagues [111] with normal or mild injury evidenced on the neonatal MRI were classified as having neurodevelopmental impairment at 18 months of age. In contrast, approximately 40% of the infants with moderate or severe white matter injury in the neonatal period were classified within the normal range in the neurodevelopmental assessment at 18 months of age [111]. These initial findings raised serious concerns regarding the sensitivity and specificity of MRI at term-equivalency for detecting infants at risk for long-term adverse neurodevelopmental outcomes. However, subsequent follow-up of this cohort into school age has demonstrated stronger correlations between the neonatal MRI and neurocognitive outcome, with attention to specific domains [140, 141]. These recent results stress the importance of longitudinal study design combined with more specific neurocognitive batteries in the evaluation of neuroimaging biomarkers of preterm brain injury.
Another emerging theme in the cranial US vs. MRI debate is the concept of combining these imaging biomarkers to improve clinical detection of preterm brain injury, which has been recently demonstrated [142, 143]. A multi-center study sponsored by the NICHD Neonatal Research Network called “THE SUPPORT neuroimaging and neurodevelopmental outcomes (NEURO) study,” which is a prospective study of early cranial US (4–14 days), late cranial US (35–42 weeks PMA), and brain MRI (within 5 days of late cranial US), is designed to predict neurodevelopmental outcomes at 18–22 months and early school age in a subcohort of extremely preterm infants. All survivors will receive comprehensive neurodevelopmental assessments at 18 to 22 months of age, corrected for prematurity, and at early school age. This study will utilize central readers for interpretation of cranial US and MRI data. Predictive modeling analysis will be used to assess the value of early cranial US, late cranial US and term MRI both alone and in combination to predict outcome at 18–22 months and early school age (6–7 years). Because of the large number of patients, ancillary studies will be possible to evaluate specific risk groups.
Another issue that needs consideration is that most studies that compare cranial US with MRI target high-risk, early gestational age and extremely low-birth-weight infants. There is emerging evidence that late preterm neonates are also at risk for brain injury and poor neurocognitive outcome, and the results from the studies of extremely low-birth-weight infants might not be generalizable to the late preterm infants [144].
Given the increased sensitivity of conventional MRI to more subtle patterns of brain injury, as well as the potential for advanced imaging techniques to provide detailed physiological information, it also might be useful to reconsider when MRI is utilized for screening for brain injury in high-risk preterm infants. Although typically MRI is applied in situations when abnormalities are suggested by cranial US, MRI might also provide diagnostic or predictive information in cases where cranial US is negative. The rationale for this is that cranial US might not be sensitive enough to aspects of neonatal brain injury that can be best detected by MRI conducted in the perinatal period in high-risk preterm neonates. Additionally, the information pertaining to the underlying biochemical and maturational processes might not only be sensitive markers of brain dysfunction, they might provide important road maps for targeted therapies. For example, based upon the high specificity of moderate-to-severe brain abnormality on cranial US for cerebral palsy, it is possible to determine high-risk preterm infants using this modality. If there are no additional clinical concerns, further imaging might not be needed, as moderate-to-severe brain abnormalities on cranial US have excellent correlation with severe abnormalities on MRI, and the results from cranial US are more highly specific than those from MRI. Accordingly, if cranial US suggests an infant is at high risk for cerebral palsy, it would be very unlikely that an MRI exam would change this determination. On the other hand, a normal cranial US or a normal conventional MRI cannot completely exclude later adverse neurological outcome. Imaging decisions are particularly crucial in preterm infants with high-risk factors for brain injury and mild or no abnormality demonstrated on cranial US. MRI of these infants might help to demonstrate clinically significant white matter injury, which imparts increased risk for CP and language, visual perceptual and memory impairments, and autism. Theoretically, early identification and risk stratification could lead to early intervention for high-risk infants and ultimately more favorable outcomes (Fig. 13). The major risk factors associated with adverse outcomes included pneumothorax, prolonged exposure to mechanical ventilation, major cranial US abnormalities, bronchopulmonary dysplasia, lower birth weight, male gender, racial differences and educational and economic disadvantages [145–147]. Sepsis and nectrotizing enterocolitis (NEC) have also been associated with higher rates of white matter injury on MRI and poorer psychomotor development at 2 years [148]. Recurrent postnatal infection is a significant risk factor for progressive white matter injury on serial MRI exams [149]. Chorioamnionitis was not directly associated with white matter injury visible on conventional on MRI; however, postnatal infection with hypotension was a significant risk factor for white matter injury [150]. These reports are consistent with emerging evidence that white matter injury is attributable to oligodendrocyte precursor susceptibility to inflammation, hypoxia and ischemia [117]. Most centers now use conventional MRI for preterm infants in clinical situations as a complementary tool to cranial US. For instance, in high-risk groups (recurrent sepsis, NEC, suspected hypoxic-ischemic insult in premature infants), suspected congenital brain malformation, high-grade IVH to evaluate posterior limb of the internal capsule (PLIC), and cerebellar injury, MRI provides useful information that can guide individual patient management.
Fig. 13.
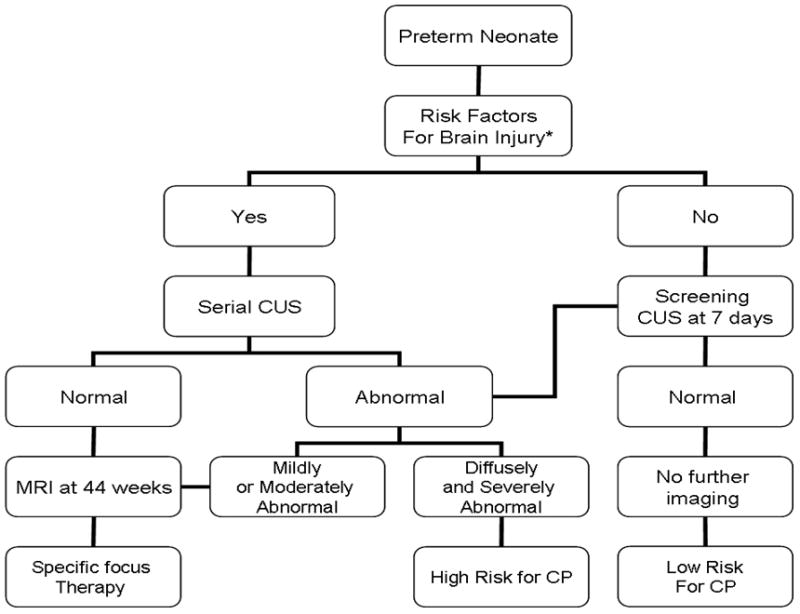
Flow chart of the rational utilization of neuroimaging in very high-risk preterm infants using both cranial US and conventional MRI. This chart is used for extreme preterm cases: (< 29 weeks GA), very low birth weight (< 1.5 g), pneumothorax, mechanical ventilation, hypotension, sepsis, recurrent postnatal infection, necrotizing enterocolitis and chorioamnionitis. We do not advocate screening MRI for all preterm infants. CUS cranial MRI, CP cerebral palsy
Two issues would probably drive MR and advanced MR techniques to become more routine in the preterm brain: development of neural-specific behavioral therapy (i.e. cognitive visual impairment) or initiation of specific therapy (hypothermia or pharmacological, stem cells) (151–154). Since high-risk preterm infants would be the beneficiaries of these therapies, integrating and optimizing MR advanced neuroimaging techniques within this group is warranted.
Perspective
As we move forward, multiple unanswered questions remain concerning the role of neuroimaging biomarkers in the evaluation of the preterm brain. For example, can neonatal MRI identify brain injury that might predict childhood outcomes? Can we develop neuroimaging biomarkers with sufficient sensitivity and specificity as to risk-stratify infants not only by risk for general adverse outcome but by risk of adverse outcome along specified neurocognitive domains? Could MRI help to define proper target groups for trials of neuroprotective strategies in preterm infants or evaluate efficacy of neonatal therapies? Some themes are emerging as we move into another decade of preterm research: (1) the need for further research into determining predictive value of cranial US and MRI in the assessment of preterm brain injury with modeling that incorporates both serial cranial US and MRI findings, (2) the importance of following neurocognitive development into school age and beyond as we examine potential neuroimaging biomarkers for preterm brain injuries, (3) the need for determining a more reliable definition of and neuropathological correlation for DEHSI;, (4) the importance of developing further practice parameters with regard to neuroimaging mechanisms for screening preterm infants for brain injury, (5) the potential insight to be gained from the incorporation of advanced neuroimaging techniques into conventional imaging practices and (6) the need to incorporate mechanisms of injury and brain development/plasticity into longitudinal models of neurocognitive outcome in children who are born preterm.
Toward the preterm connectome
Recently, adult neuroscience has provided a model of the human connectome, a comprehensive structural description of the network of elements and connections forming the human brain. This has been a marked shift from more than two decades of research aimed at identifying the loci of particular cognitive functions in the human brain and has provided a framework for new models of brain function and new paradigms in neuroscience research. Is it time for us to develop similar models for the preterm brain? Multiple reasons to do so exist, including (1) the importance of delineating neural networks in the context of brain injury and brain recovery of premature neonates; (2) the possibility of inter-relating single imaging biomarkers with complex imaging biomarkers, i.e. cranial US/conventional MR with more sophisticated combined neural system imaging (the cognitive visual system); (3) the importance of inter-relating both imaging and non-imaging biomarkers together into comprehensive models of risk to predict longitudinal neurocognitive outcome, (see tables 6 and 7 for examples of the battery of neurocognitive tests that can be administered to preterm infants and children as part of a research protocol); (4) the potential utility of the preterm connectome to be used a surrogate imaging biomarker of brain injury in future neuron-protective clinical trials aimed at reducing brain injury in the preterm infant.
Table 6.
Developmental testing for children ages 1 year, 6 months to 3 years, 11 months
| DOMAIN | ENGLISH | SPANISH |
|---|---|---|
| Cognition, Language, and Motor Functioning | Battelle Developmental Inventory, 2nd Edition (1 hour) | Battelle Developmental Inventory, 2nd Edition Spanish Version (1 hour) |
| Psychosocial Functioning | Modified Checklist for Autism in Toddlers (M-CHAT) | Modified Checklist for Autism in Toddlers (M-CHAT) (Spanish) |
| Adaptive Functioning | Adaptive Behavior Assessment System-II (ABAS-II) | Adaptive Behavior Assessment System-II (ABAS-II) Spanish Version |
Table 7.
Developmental testing for children ages 6–8 years
| DOMAIN | ENGLISH | SPANISH |
|---|---|---|
| General Cognitive and Intellectual Functions | Wechsler Intelligence Scale for Children (WISC-IV) Standard Subscales (90 minutes to 2 hours) | Wechsler Intelligence Scale for Children (WISC-IV) Standard Subscales Spanish Version (90 minutes to 2 hours) |
| Memory and Learning Verbal Learning & Memory Visual Learning & Memory Working Memory |
CVLT-C (20 min.) CMS-Faces (10 min.) WISC-IV DS, LNS (inc. above.) |
WISC-IV DS, LNS (inc. above.) |
| Attention & Executive Functions Response Inhibition & Inhibition of Over-learned Information Verbal Fluency Attention & Vigilance Reasoning & Problem Solving Attention/Divided Attention |
Trails (5 minutes) D-KEFS Verbal Fluency (5 min.) DS-CPT (15 min.) WISC-IV Subtests (inc. above) D-KEFS Tower Test (10 min.) WISC-IV DS and LN (inc. above) |
WISC-IV Subtests (inc. above) WISC-IV DS and LN (inc. above) |
| Visual Perceptual/Visual Motor Functions | WISC-IV Performance subtests (included above) Rey-Osterreith Complex Figure Copy (10 minutes) The Purdue Pegboard (10 min.) |
WISC-IV Performance subtests (included above) Rey-Osterreith Complex Figure Copy (10 minutes) The Purdue Pegboard (10 min.) |
| Academic Achievement Math & Computation Skills Reading Fluency & Reading Comprehension |
Woodcock-Johnson-III, Calculation and Applied Problems (20 min.) Passage Comprehension and Reading Fluency (20 min.) |
Woodcock-Munoz, Bateria, 3rd Edition, Calculo & Problemas Applicados (20 min.) Woodcock-Munoz, Bateria, 3rd Ed., Fluidez en la lectura & Comp. de Textos (20 min.) |
| Psychosocial Functioning | CBCL-Parent Report CBCL-Teacher Report Conners Parent Rating Scale–Parent Long Version Peds QL |
CBCL-Parent Report CBCL-Teacher Report Conners Parent Rating Scale–Parent Long Version PcdsQL |
| Adaptive Functioning | ABAS-II | ABAS-II (Spanish Version) |
Conclusion
A rational utilization of cranial US and MRI is crucial to optimize neuroimaging strategies in preterm infants to predict neurodevelopmental outcome. Cranial US remains the gold standard for evaluation of IVH and associated complications and is comparable to MRI in the prognosis for cerebral palsy. Current research and practice parameters do not support the use of routine scanning of preterm neonates with conventional MRI. However, as the field moves to incorporate advanced imaging techniques toward the prediction of neurocognitive outcome well into school-age time points, it is likely that new ways integrating cranial US with MRI using predictive modeling will work better than either cranial US or MRI alone in predicting neurocognitive outcome. Moreover, optimizing MRI to image preterm neonates with certain clinical risk factors is reasonable. In the future, advanced neuroimaging techniques might also provide the specific neural phenotypes relevant to specific neurocognitive outcomes in preterm neonates (i.e. visual spatial, learning and memory, etc.) Research is needed to understand how more basic neuroimaging biomarkers (e.g., cranial US and conventional MRI) can be combined with more advanced neuroimaging biomarkers (e.g., functional connectivity) to yield specificity and sensitivity for diagnosis, prognosis and treatment effects in preterm brain injury. Finally, development of a preterm connectome will provide a detailed roadmap of the developing preterm brain and a framework by which future research can ultimately pave the way for good outcomes for even the most high-risk preterm infants.
Table 2.
Neuroimaging finding and associated neurological outcome in the preterm brain
| Imaging Findings | Neurological Deficit Studies | Study |
|---|---|---|
| GMH/IVH Grade 3 and 4 |
Monoplegia, hemiplegia, diplegia, triplegia, quadriplegia, and hypotonic CP. | Hack et al[6], Laptook AR at al, [146] |
| Ventricular Dilatation | Post-hemorrhagic Ventricular dilatation were associated with 17 times more likely to have quadriparesis or hemiparesis | Kuban KC et al, [47] |
| Abnormally dilated occipital horns were associated with low scores on the Denver and the Bayley tests at the age of 1 year | Skranes et al [48] | |
| Focal White Matter Disease of Prematurity | Focal WM Injury strongly associated with spastic diplegia or quadriparesis and CP. | Kuban KC et al [ 47], Nanba et al [52], Aida N et al, [53], Olsen P et al [54] |
| 52% of infants with echolucency developed cerebral palsy and 24 times more increased risk of quadriparesis and 29 times of hemiparesis. | Kuban KC et al [47] | |
| Diffuse White Matter Injury | Abnormal cerebral structure is present at term in premature infants. | Inder TE et al. [72] |
| WM volumes in the sensorimotor and midtemporal regions, especially on the right, were strongly correlated with neurodevelopmental outcome. | Peterson BS et al [73] | |
| Reduction in the inferior occipital regional volumes at term corrected age was associated with later impaired oculomotor function control. | Shah DK et al [88] | |
| Higher white matter ADC values at term-quivalent age were associated with suboptimal developmental performance at the age of 2 years. | Krishnan ML et al [71] | |
| Corpus Callosum | Thinner CC on MRI is a common finding in children with CP. Shorter CC on CUS at term equivalent age was associated with poorer neurodevelopmental outcome. Reduced growth of the length of the corpus callosum during the period from 2 to 6 week of age was associated with increased risk for later psychomotor delay and cerebral palsy. FA values of the splenium of CC derived from MRI using DTI at term-equivalent age were lower in children with abnormal outcome at 18 months’ corrected age. FA values of the CC have also been correlated with the developmental quotient and eye-hand coordination at 2 years of age. Cingulum, fornix, anterior commissure, and uncinate fasciculus | Rademaker KJ et al [74], Iai M et al [74]; Anderson NG et al [78] Nosarti C 2004 et al [77] Counsell SJ et al. [88] |
| Specific White Matter Tracts Motor Tract |
T1 hyperintensity or cystic lesions in the corona radiata seen of preterm neonates on MRI coronal view obtained at term predicted motor prognosis at 3 to 5 years. Asymmetrical myelination of the PLIC on MRI in newborn infants with a GMH/IVH, unilateral parenchymal hemorrhage and bilateral cystic WMI strongly predicted subhemiplegia and to a lesser extent diplegia or quadriplegia. Lower FA values DTI in the PLIC or along the motor tract allow earlier detection in infants at risk for neurologic abnormalities and disability. | Rose J et al [85] [80]; De Vries LS et al [82], Roelants-van Rijn AM et al [83];, Arzoumanian Y 2003 et al [84]; Murakami A 2008. Et al [86] |
| Optic Radiation | Reduction in regional inferior occipital volumes was associated with later impaired oculomotor function control. FA in the optic radiations was correlated with visual assessment score |
Bassi L et al [90] |
| Gray Matter Injury | Reduction in cortical and deep nuclear GM volumes was associated with moderate to severe neurodevelopmental disability at 1 year of age. The growth rate of cortical surface area was proportional to later neurodevelopmental impairment. Bilateral GM volume middle temporal and postcentral gyri volumes positively correlated with in the IQ. Volumes of sensorimotor and midtemporal cortices at 8 years of age were associated positively with verbal and performance IQ scores. Volume loss of dorsolateral prefrontal cortex, sensorimotor, parietooccipital, premotor regions and hypocampus were correlated with working memory performance. |
Inder TE et al [72], Peterson BS et al [73] Nosarti C et al [77] Soria-Pastor et al [94] Woodward LJ et al [97] Beauchamp MH et al [98] |
| Cerebellar Hemorrhage | Associated with increased mortality and adverse neurodevelopmental outcome and increased risk for autism. The vermis was more commonly associated with Impaired language, cognitive deficits, severe motor disabilities, and increased risk for autism |
Limperopoulos C et al [99,100], Bednarek N et al [101], Tam EW et al [102] |
| Cerebellum Volume | Highly associated with supratentorial lesion. No direct correlation has been reported between cerebellar volumes at term and outcome at 2 years of age. | Shah DK et al [106], Srinivasan L et al [107] |
| MR Spectroscopy | Metabolite ratios from near-term MRS were not predictive of Bayley scores at 18 to 24 months’ corrected age | Augustine EM et al [117] |
| Abnormal Cranial Dopper | Fluctuating pattern of cerebral blood-flow velocity or absent end-diastolic flow are associated with intraventricular hemorrhage. The RI increases with significant post-hemorrhagic hydrocephalus and decreases following CSF drainage. Less clear correlation with outcome. |
Perlman JM et al [120], Julkunen M et al [121] Westra SJ et al [122] |
Abbr: GMH, germinal matrix hemorrhage; IVH, intraventricular Hemorrhage; CP, cerebral palsy; CC, corpus callosum; CSF, cerebrospinal fluid; WM, white matter; ADC, apparent diffusion coefficient; CC, corpus callosum; PLIC, posterior limb of the internal capsule; GM, gray-matter; IQ, intelligence coefficient.
Acknowledgments
The authors thank Dr. Susan Hintz (Stanford) for comments and edits of the manuscript. The authors thank Julie Castro, Arabhi Nagasunder, Anita Hamilton, Hesham Mahmoud and Sandra Figueroa (Children’s Los Angeles) and Rafael Ceschin and Fern Wasco (Pittsburgh Children’s) and the MR technologists at Children’s Los Angeles and Children’s Pittsburgh for their commitment toward the multisite perinatal white matter injury project. We acknowledge Anita Hamilton for helping with the development of the preterm neuropsychological battery. We would also like to acknowledge Dr. Bharat Biswal for the functional connectivity collaboration and analysis and Dr. Yalin Wang for the collaboration and analysis with the surface base morphometric analysis. We also thank Drs. Hannah Kinney, Robin Haynes, Hanna Damasio, Marvin Nelson and Floyd Gilles for insight and advice. We thank Melanine Gierltowski for manuscript preparation. This study is funded by: K23NS063371 (AP), P50NS01962 (JLW), and Rudi Schulte Foundation (SB), R21EB012177 (NL) and USC-CHLA CTSI grant 1UL1RR031986 (AP, JLW, LP).
Footnotes
Disclaimer The supplement this article is part of is not sponsored by the industry. Dr. Panigrahy, Dr. Wisnowski, Dr. Furtado, Dr. Lepore, Dr. Paquette, and Dr. Bluml have no financial interest, investigational or off-label uses to disclose.
Contributor Information
Ashok Panigrahy, Email: panigrahya@upmc.edu, Department of Radiology, Children’s Hospital Los Angeles, Los Angeles, CA, USA. Department of Pediatric Radiology, Children’s Hospital of Pittsburgh of UPMC, 45th Street and Penn Avenue, Pittsburgh, PA 15201, USA.
Jessica L. Wisnowski, Department of Radiology, Children’s Hospital Los Angeles, Los Angeles, CA, USA. Brain and Creativity Institute, University of Southern California, Los Angeles, CA, USA
Andre Furtado, Department of Pediatric Radiology, Children’s Hospital of Pittsburgh of UPMC, 45th Street and Penn Avenue, Pittsburgh, PA 15201, USA.
Natasha Lepore, Department of Radiology, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Lisa Paquette, Center for Fetal and Neonatal Medicine, Children’s Hospital Los Angeles Los Angeles, CA, USA.
Stefan Bluml, Department of Radiology, Children’s Hospital Los Angeles, Los Angeles, CA, USA. Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, USA.
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) Acta Paediatrica. 2010;99:978–992. doi: 10.1111/j.1651-2227.2010.01846.x. [DOI] [PubMed] [Google Scholar]
- 3.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 4.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, et al. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Hoult LA, Streiner DL, et al. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- 6.Hack M. Young adult outcomes of very-low-birth-weight children. Semin Fetal Neonatal Med. 2006;11:127–137. doi: 10.1016/j.siny.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Sie LT, van der Knaap MS, van Wezel-Meijler G, et al. Early MR features of hypoxic-ischemic brain injury in neonates with periventricular densities on sonograms. AJNR. 2000;21:852–861. [PMC free article] [PubMed] [Google Scholar]
- 8.Maalouf EF, Duggan PJ, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm neonates. Pediatrics. 2001;107:719–727. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 9.Miller SP, Cozzio EE, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR. 2003;24:1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 10.Inder TE, Anderson NJ, Spencer C, et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR. 2003;24:805–809. [PMC free article] [PubMed] [Google Scholar]
- 11.Childs A-M, Cornette L, Ramenghi LA, et al. Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants. Clin Radiol. 2001;56:647–655. doi: 10.1053/crad.2001.0754. [DOI] [PubMed] [Google Scholar]
- 12.Hagmann P, Cammoun L, Gigandet X, et al. MR connectomics: principles and challenges. J Neurosci Methods. 2010;194(1):34–45. doi: 10.1016/j.jneumeth.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Sporns O. The human connectome: a complex network. Ann NY Acad Sci. 2011;1224(1):109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 14.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- 15.Rubinov M, Bassett DS. Emerging evidence of connectomic abnormalities in schizophrenia. J Neurosci. 2011;31(17):6263–6265. doi: 10.1523/JNEUROSCI.0382-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 17.Volpe JJ, Kinney HC, Jensen FE, et al. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29(4):423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill A, Melson GL, Clark HB, et al. Hemorrhagic periventricular leukomalacia: diagnosis by real time ultrasound and correlation with autopsy findings. Pediatrics. 1982;69:282–284. [PubMed] [Google Scholar]
- 20.Manger MN, Feldman RC, Brown WJ, et al. Intracranial ultrasound diagnosis of neonatal periventricular leukomalacia. J Ultrasound Med. 1984;3:59–63. doi: 10.7863/jum.1984.3.2.59. [DOI] [PubMed] [Google Scholar]
- 21.Fawer CL, Calame A, Perentes E, et al. Periventricular leukomalacia: a correlation study between real-time ultrasound and autopsy findings. Periventricular leukomalacia in the neonate. Neuroradiology. 1985;27:292–300. doi: 10.1007/BF00339560. [DOI] [PubMed] [Google Scholar]
- 22.Correa F, Enriquez G, Rossello J, et al. Posterior fontanelle sonography: an acoustic window into the neonatal brain. AJNR. 2004;25:1274–1282. [PMC free article] [PubMed] [Google Scholar]
- 23.Luna JA, Goldstein RB. Sonographic visualization of neonatal posterior fossa abnormalities through the posterolateral fontanelle. AJR. 2000;174:561–567. doi: 10.2214/ajr.174.2.1740561. [DOI] [PubMed] [Google Scholar]
- 24.Anderson N, Allan R, Darlow B, et al. Diagnosis of intraventricular hemorrhage in the newborn: value of sonography via the posterior fontanelle. AJR. 1994;163:893–896. doi: 10.2214/ajr.163.4.8092030. [DOI] [PubMed] [Google Scholar]
- 25.Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR. 2003;24(8):1661–1669. [PMC free article] [PubMed] [Google Scholar]
- 26.Inder TE, Anderson NJ, Spencer C, et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR. 2003;24(5):805–809. [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shea TM, Allred EN, Dammann O, et al. ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hintz SR, Slovis T, Bulas D, et al. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150(6):592–596. doi: 10.1016/j.jpeds.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barkovich AJ. MR imaging of the neonatal brain. Neuroimaging Clin N Am. 2006;16:117–135. doi: 10.1016/j.nic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Blüml S, Friedlich P, Erberich S, et al. MR imaging of newborns by using an MR-compatible incubator with integrated radiofrequency coils: initial experience. Radiology. 2004;231(2):594–601. doi: 10.1148/radiol.2312030166. [DOI] [PubMed] [Google Scholar]
- 31.Wilson-Costello D, Friedman H, Minich N, et al. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 32.Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weight less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 33.Vohr BR, Wright LL, Poole WK, et al. Neurodevelopmental outcomes of extremely low birth weight infants < 32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–643. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 34.Marlow N, Wolke D, Bracewell MA, et al. EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 35.Hack M, Wilson-Costello D, Friedman H, et al. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1,000 g: 1992–1995. Arch Pediatr Adolesc Med. 2000;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 36.Patra K, Wilson-Costello D, Taylor HG, et al. Grades I–II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149(2):169–173. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Leijser LM, Srinivasan L, Rutherford MA, et al. Structural linear measurements in the newborn brain: accuracy of cranial ultrasound compared to MRI. Pediatr Radiol. 2007;37:640–648. doi: 10.1007/s00247-007-0485-2. [DOI] [PubMed] [Google Scholar]
- 38.Horsch S, Bengtsson J, Nordell A, et al. Lateral ventricular size in extremely premature infants: 3D MRI confirms 2D ultrasound measurements. Ultrasound Med Biol. 2009;35:360–366. doi: 10.1016/j.ultrasmedbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Levene MI, Starte DR. A longitudinal study of post-haemorrhagic ventricular dilatation in the newborn. Arch Dis Child. 1981;56:905–910. doi: 10.1136/adc.56.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.London DA, Carroll BA, Enzmann DR. Sonography of ventricular size and germinal matrix hemorrhage in premature infants. AJR. 1980;135:559–564. doi: 10.2214/ajr.135.3.559. [DOI] [PubMed] [Google Scholar]
- 41.Grasby DC, Esterman A, Marshall P. Ultrasound grading of cerebral ventricular dilatation in preterm neonates. J Paediatr Child Health. 2003;39:186–190. doi: 10.1046/j.1440-1754.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 42.Maunu J, Lehtonen L, Lapinleimu H, et al. Ventricular dilatation in relation to outcome at 2 years of age in very preterm infants: a prospective Finnish cohort study. Dev Med Child Neurol. 2011;53:48–54. doi: 10.1111/j.1469-8749.2010.03785.x. [DOI] [PubMed] [Google Scholar]
- 43.El-Dib M, Massaro AN, Bulas D, et al. Neuroimaging and neurodevelopmental outcome of premature infants. Am J Perinatol. 2010;27:803–818. doi: 10.1055/s-0030-1254550. [DOI] [PubMed] [Google Scholar]
- 44.Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- 45.Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12:639–649. [PubMed] [Google Scholar]
- 46.Del Bigio MR, da Silva MC, Drake JM, et al. Acute and chronic cerebral white matter damage in neonatal hydrocephalus. Can J Neurol Sci. 1994;21:299–305. doi: 10.1017/s0317167100040865. [DOI] [PubMed] [Google Scholar]
- 47.Kuban KC, Allred EN, O’Shea TM, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skranes JS, Vik T, Nilsen G, et al. Cerebral magnetic resonance imaging (MRI) and mental and motor function of very low birth weight infants at one year of corrected age. Neuropediatrics. 1993;24:256–262. doi: 10.1055/s-2008-1071553. [DOI] [PubMed] [Google Scholar]
- 49.Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 50.Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116(1):221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- 51.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1–6. doi: 10.1016/s0166-4328(05)80189-5. [DOI] [PubMed] [Google Scholar]
- 52.Nanba Y, Matsui K, Aida N, et al. Magnetic resonance imaging regional T1 abnormalities at term accurately predict motor outcome in preterm infants. Pediatrics. 2007;120:e10–19. doi: 10.1542/peds.2006-1844. [DOI] [PubMed] [Google Scholar]
- 53.Aida N, Nishimura G, Hachiya Y, et al. MR imaging of perinatal brain damage: comparison of clinical outcome with initial and follow-up MR findings. AJNR. 1998;19:1909–1921. [PMC free article] [PubMed] [Google Scholar]
- 54.Olsen P, Paakko E, Vainionpaa L, et al. Magnetic resonance imaging of periventricular leukomalacia and its clinical correlation in children. Ann Neurol. 1997;41:754–761. doi: 10.1002/ana.410410611. [DOI] [PubMed] [Google Scholar]
- 55.Rutherford MA, Supramaniam V, Ederies A, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology. 2010;52(6):505–521. doi: 10.1007/s00234-010-0700-y. [DOI] [PubMed] [Google Scholar]
- 56.Niwa T, de Vries LS, Benders MJ, et al. Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology. 2011 May 7;53(9):669–679. doi: 10.1007/s00234-011-0872-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramenghi LA, Fumagalli M, Righini A, et al. Magnetic resonance imaging assessment of brain maturation in preterm neonates with punctate white matter lesions. Neuroradiology. 2007;49(2):161–167. doi: 10.1007/s00234-006-0176-y. [DOI] [PubMed] [Google Scholar]
- 58.Bassi L, Chew A, Merchant N, et al. Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res. 2011;69(6):561–566. doi: 10.1203/PDR.0b013e3182182836. [DOI] [PubMed] [Google Scholar]
- 59.Cornette LG, Tanner SF, Ramenghi LA, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Arch Dis Child Fetal Neonatal Ed. 2002;86(3):F171–177. doi: 10.1136/fn.86.3.F171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 61.Volpe JJ. Cerebral white matter injury of the premature infant -- more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 62.Domizio S, Barbante E, Puglielli C, et al. Excessively high magnetic resonance signal in preterm infants and neuropsychobehavioural follow-up at 2 years. Int J Immunopathol Pharmacol. 2005;18:365–375. doi: 10.1177/039463200501800218. [DOI] [PubMed] [Google Scholar]
- 63.Skiöld B, Horsch S, Hallberg B, et al. White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Paediatr. 2010;99(6):842–849. doi: 10.1111/j.1651-2227.2009.01634.x. [DOI] [PubMed] [Google Scholar]
- 64.Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007;119(4):759–765. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- 65.Boardman JP, Counsell SJ, Rueckert D, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006;32(1):70–78. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 66.Hart AR, Smith MF, Rigby AS, et al. Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatr Radiol. 2010;40:1390–1396. doi: 10.1007/s00247-010-1633-7. [DOI] [PubMed] [Google Scholar]
- 67.Skiöld B. Brain imaging and outcome in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010;95(5):F310–314. doi: 10.1136/adc.2009.161547. [DOI] [PubMed] [Google Scholar]
- 68.Kim Hye, et al. Pediatric Radiology. 2011;(41) supplement:S250–S310. abstract. [Google Scholar]
- 69.Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 71.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120:e604–609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 72.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 73.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 74.Rademaker KJ, Lam JN, Van Haastert IC, et al. Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol. 2004;28:279–287. doi: 10.1053/j.semperi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Iai M, Tanabe Y, Goto M, et al. A comparative magnetic resonance imaging study of the corpus callosum in neurologically normal children and children with spastic diplegia. Acta Paediatr. 1994;83:1086–1090. doi: 10.1111/j.1651-2227.1994.tb12991.x. [DOI] [PubMed] [Google Scholar]
- 76.Panigrahy A, Barnes PD, Robertson RL, et al. Quantitative analysis of the corpus callosum in children with cerebral palsy and developmental delay: correlation with cerebral white matter volume. Pediatr Radiol. 2005;35:1199–1207. doi: 10.1007/s00247-005-1577-5. [DOI] [PubMed] [Google Scholar]
- 77.Nosarti C, Rushe TM, Woodruff PW, et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- 78.Anderson NG, Laurent I, Cook N, et al. Growth rate of corpus callosum in very premature infants. AJNR. 2005;26:2685–2690. [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson NG, Laurent I, Woodward LJ, et al. Detection of impaired growth of the corpus callosum in premature infants. Pediatrics. 2006;118:951–960. doi: 10.1542/peds.2006-0553. [DOI] [PubMed] [Google Scholar]
- 80.Rose J, Butler EE, Lamont LE, et al. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol. 2009;51:526–535. doi: 10.1111/j.1469-8749.2008.03231.x. [DOI] [PubMed] [Google Scholar]
- 81.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 82.De Vries LS, Groenendaal F, van Haastert IC, et al. Asymmetrical myelination of the posterior limb of the internal capsule in infants with periventricular haemorrhagic infarction: an early predictor of hemiplegia. Neuropediatrics. 1999;30:314–319. doi: 10.1055/s-2007-973511. [DOI] [PubMed] [Google Scholar]
- 83.Roelants-van Rijn AM, Groenendaal F, Beek FJ, et al. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics. 2001;32:80–89. doi: 10.1055/s-2001-13875. [DOI] [PubMed] [Google Scholar]
- 84.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 85.Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49:745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 86.Murakami A, Morimoto M, Yamada K, et al. Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics. 2008;122:500–506. doi: 10.1542/peds.2007-2816. [DOI] [PubMed] [Google Scholar]
- 87.O’Connor AR, Fielder AR. Visual outcomes and perinatal adversity. Semin Fetal Neonatal Med. 2007;12:408–414. doi: 10.1016/j.siny.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Shah DK, Guinane C, August P, et al. Reduced occipital regional volumes at term predict impaired visual function in early childhood in very low birth weight infants. Invest Ophthalmol Vis Sci. 2006;47:3366–3373. doi: 10.1167/iovs.05-0811. [DOI] [PubMed] [Google Scholar]
- 89.Berman JI, Glass HC, Miller SP, et al. Quantitative fiber tracking analysis of the optic radiation correlated with visual performance in premature newborns. AJNR. 2009;30:120–124. doi: 10.3174/ajnr.A1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bassi L, Ricci D, Volzone A, et al. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain. 2008;131:573–582. doi: 10.1093/brain/awm327. [DOI] [PubMed] [Google Scholar]
- 91.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nosarti C, Al-Asady MH, Frangou S, et al. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 93.Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131:2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:e1161–1170. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- 95.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 96.Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 97.Woodward LJ, Edgin JO, Thompson D, et al. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 98.Beauchamp MH, Thompson DK, Howard K, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 99.Limperopoulos C, Bassan H, Gauvreau K, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 100.Limperopoulos C, Benson CB, Bassan H, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116:717–724. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 101.Bednarek N, Akhavi A, Pietrement C, et al. Outcome of cerebellar injury in very low birth-weight infants: 6 case reports. J Child Neurol. 2008;23:906–911. doi: 10.1177/0883073808318063. [DOI] [PubMed] [Google Scholar]
- 102.Tam EW, Rosenbluth G, Rogers EE, et al. Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatri. 2011;158:245–250. doi: 10.1016/j.jpeds.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ecury-Goossen GM, Dudink J, Lequin M, et al. The clinical presentation of preterm cerebellar haemorrhage. Eur J Pediatr. 2010;169:1249–1253. doi: 10.1007/s00431-010-1217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merrill JD, Piecuch RE, Fell SC, et al. A new pattern of cerebellar hemorrhages in preterm infants. Pediatrics. 1998;102:E62. doi: 10.1542/peds.102.6.e62. [DOI] [PubMed] [Google Scholar]
- 105.Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, et al. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. 2009;252:190–199. doi: 10.1148/radiol.2521081525. [DOI] [PubMed] [Google Scholar]
- 106.Shah DK, Anderson PJ, Carlin JB, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60:97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 107.Srinivasan L, Allsop J, Counsell SJ, et al. Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. AJNR. 2006;27:573–579. [PMC free article] [PubMed] [Google Scholar]
- 108.Lind A, Parkkola R, Lehtonen L, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953–961. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- 109.Tam EW, Miller SP, Studholme C, et al. Differential effects of intraventricular hemorrhage and white matter injury on cerebellar growth. J Pediatr. 2011;158(3):366–371. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Limperopoulos C, Chilingaryan G, Gulzard N, et al. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res. 2010;68(2):145–150. doi: 10.1203/PDR.0b013e3181e1d032. [DOI] [PubMed] [Google Scholar]
- 111.Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 112.Valkama AM, Paakko EL, Vainionpaa LK, et al. Magnetic resonance imaging at term and neuromotor outcome in preterm infants. Acta Paediatr. 2000;89:348–355. [PubMed] [Google Scholar]
- 113.Kreis R, Hofmann L, Kuhlmann B, et al. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48:949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 114.Leth H, Toft PB, Pryds O, et al. Brain lactate in preterm and growth-retarded neonates. Acta Paediatr. 1995;84:495–499. doi: 10.1111/j.1651-2227.1995.tb13681.x. [DOI] [PubMed] [Google Scholar]
- 115.Wang ZJ, Vigneron DB, Miller SP, et al. Brain metabolite levels assessed by lactate-edited MR spectroscopy in premature neonates with and without pentobarbital sedation. AJNR. 2008;29:798–801. doi: 10.3174/ajnr.A0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roelants-van Rijn AM, van der Grond J, Stigter RH, et al. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr Res. 2004;56:285–290. doi: 10.1203/01.PDR.0000132751.09067.3F. [DOI] [PubMed] [Google Scholar]
- 117.Augustine EM, Spielman DM, Barnes PD, et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol. 2008;28:611–618. doi: 10.1038/jp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child. 1988;63:606–611. doi: 10.1136/adc.63.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kehrer M, Krageloh-Mann I, Goelz R, et al. The development of cerebral perfusion in healthy preterm and term neonates. Neuropediatrics. 2003;34:281–286. doi: 10.1055/s-2003-44663. [DOI] [PubMed] [Google Scholar]
- 120.Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 1983;309:204–209. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]
- 121.Julkunen M, Parviainen T, Janas M, et al. End-diastolic block in cerebral circulation may predict intraventricular hemorrhage in hypotensive extremely-low-birth-weight infants. Ultrasound Med Biol. 2008;34:538–545. doi: 10.1016/j.ultrasmedbio.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 122.Westra SJ, Lazareff J, Curran JG, et al. Transcranial Doppler ultrasonography to evaluate need for cerebrospinal fluid drainage in hydrocephalic children. J Ultrasound Med. 1998;17:561–569. doi: 10.7863/jum.1998.17.9.561. [DOI] [PubMed] [Google Scholar]
- 123.Taylor GA, Madsen JR. Neonatal hydrocephalus: hemodynamic response to fontanelle compression -- correlation with intracranial pressure and need for shunt placement. Radiology. 1996;201:685–689. doi: 10.1148/radiology.201.3.8939216. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen The Tich S, Anderson PJ, Shimony JS. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR. 2009;30(1):125–131. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tich SN, Anderson PJ, Hunt RW, et al. Neurodevelopmental and perinatal correlates of simple brain metrics in very preterm infants. Arch Pediatr Adolesc Med. 2011;165(3):216–222. doi: 10.1001/archpediatrics.2011.9. [DOI] [PubMed] [Google Scholar]
- 126.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 127.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 128.Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fransson P, Skiold B, Horsch S, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Doria V, Beckmann CF, Arichi T, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 2010;107(46):20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 134.Damaraju E, Phillips JR, Lowe JR, et al. Resting-state functional connectivity differences in premature children. Front Syst Neuro. 2010;4:23. doi: 10.3389/fnsys.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Vries LS, Cowan FM. Should cranial MRI screening of preterm infants become routine? Nat Clin Pract Neurol. 2007;3(10):532–533. doi: 10.1038/ncpneuro0608. [DOI] [PubMed] [Google Scholar]
- 136.Hintz SR, O’Shea M. Neuroimaging and neurodevelopmental outcomes in preterm infants. Semin Perinatol. 2008;32(1):11–19. doi: 10.1053/j.semperi.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 137.Hart AR, Whitby EW, Griffiths PD, et al. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50(9):655–663. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 138.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 139.Nosarti C, Walshe M, Rushe TM, et al. Neonatal ultrasound results following very preterm birth predict adolescent behavioral and cognitive outcome. Dev Neuropsychol. 2011;36(1):118–135. doi: 10.1080/87565641.2011.540546. [DOI] [PubMed] [Google Scholar]
- 140.Clark CA, Woodward LJ. Neonatal cerebral abnormalities and later verbal and visuospatial working memory abilities of children born very preterm. Dev Neuropsychol. 2010;35(6):622–642. doi: 10.1080/87565641.2010.508669. [DOI] [PubMed] [Google Scholar]
- 141.Woodward LJ, Clark CA, Pritchard VE, et al. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 2011;36(1):22–41. doi: 10.1080/87565641.2011.540530. [DOI] [PubMed] [Google Scholar]
- 142.Munck P, Haataja L, Maunu J, et al. Cognitive outcome at 2 years of age in Finnish infants with very low birth weight born between 2001 and 2006. Acta Paediatr. 2010;99(3):359–366. doi: 10.1111/j.1651-2227.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- 143.Horsch S, Skiold B, Hallberg B, et al. Cranial ultrasound and MRI at term age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;95:19. doi: 10.1136/adc.2009.161547. [DOI] [PubMed] [Google Scholar]
- 144.Kinney HC. The near term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–88. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 145.O’Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. 1998;147:362–369. doi: 10.1093/oxfordjournals.aje.a009458. [DOI] [PubMed] [Google Scholar]
- 146.Laptook AR, O’Shea TM, Shankaran S, et al. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 147.Wood NS, Costeloe K, Gibson AT, et al. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 149.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 150.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 151.Doyle LW, Cheong J, Hunt RW. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68(5):734–742. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- 152.Murphy BP, Inder TE, Huppi PS, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107(2):217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 153.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 154.Milgrom J, Newnham C, Anderson PJ, et al. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatric Res. 2010;67(3):330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]