Abstract
Background
In patients with carotid stenosis, the risk of plaque rupture is related to the composition of the atherosclerotic plaque rather than to its magnitude. In this regard, we evaluated the effects of a supplement, Aterofisiol,® containing omega-3 (EPA [eicosapen acid] DHA [docosahexaenoic acid]), vitamin K2, vitamin B6, vitamin B12, oligomeric proanthocyanidins (OPC) and resveratrol on the composition of atherosclerotic plaque and on neurological symptoms in patients with carotid stenosis undergoing carotid endarterectomy.
Methods
The study was randomized, prospective, and double-blinded. Eligible patients were of both sexes, with carotid stenosis >70% who underwent endarterectomy. Enrolled patients were randomly allocated to receive either one tablet of acetylsalicylic acid 100 mg (Cardioaspirin®) + one tablet of Aterofisiol every 24 hours or one tablet of Cardioaspirin + one tablet of placebo every 24 hours. Each treatment was started 30 days before the surgery and was stopped 5 days before the surgery. The plaques were removed “en bloc” using standard surgical technique.
Results
During the study period, 214 patients (135 men and 79 women) were enrolled for intent-to-treat and randomized in two groups: Group A: 107 patients (68 men and 39 women) were treated with Cardioaspirin + Aterofisiol. Group B: 107 patients (67 men and 40 women) were treated with Cardioaspirin + placebo. At the end of the study, 202 patients participated fully (103 patients in Group A and 99 patients in Group B), making up the protocol evaluation population (94.4%). The mean lipid content of removed plaques was significantly lower (P<0.05) in Group A. We recorded a significantly lower incidence of neurological symptoms in Group A in comparison with Group B (P<0.05).
Conclusion
In the study, Aterofisiol showed to be effective in reducing the amounts of cholesterol and lipids in the plaques and in reducing adverse neurological events in the study group with respect to controls.
Keywords: Aterofisiol®, carotid stenosis, endarterectomy, carotid plaque, neurological symptoms, cholesterol
Introduction
The World Health Organization ranks cardiovascular diseases (CVD) as the leading cause of death worldwide, and showed that CVD constitute 48.6% of the ten main causes of death.1 It has also been estimated that of the 83.6 million American adults with CVD, about 18% have atherosclerosis2 and the presence of atherosclerotic plaque into the carotid artery is a risk factor for transient ischemic attack (TIA) and stroke.3
Usually, atherosclerotic plaque contains a coating of soft material formed by macrophages and lipids and a core of harder fibrous tissue; the size of the nucleus atheroma, the thinness of the cap, and the density of macrophages are the main determinants of the risk of plaque rupture.4,5 This risk is related to the composition of the atherosclerotic plaque rather than to its magnitude; plaques with high concentration of lipids are more susceptible to rupture with subsequent formation of blood clots.6,7 The plaque rupture often occurs on the surface during growth and is related to changes within the plaque as well as to an increase in the circumferential pressure outside the plaque.
To date, several therapeutic strategies are available to reduce both mortality and morbidity in patients with TIA or stroke. In particular, in asymptomatic patients with an atherosclerotic plaque <70% or in symptomatic patients with a plaque <50%, both statins and aspirin are prescribed. Statins are usually used to stabilize plaque, reducing its vulnerability and reducing the inflammation; aspirin is used to inhibit platelet aggregation, reducing the progression of the stenosis.8,9
Several authors10–14 have reported that statins are able to improve clinical symptoms, in several vascular (such as aneurysms and stroke) and nonvascular diseases (such as diabetes, obesity, periodontitis) through the control of inflammation, particularly reducing the matrix metalloproteinase expression,15 and recently, some drugs as well as nutraceuticals have been developed to control vascular symptoms through the control of the same mechanism involved in the inflammation.16–21
Because of the high incidence of atherosclerotic disease, the search for substances that can counter it is always ongoing. Particular attention is being given to substances that reduce the lipid content of plaques and to agents capable of counteracting the local inflammation that seems to be a determining factor for this condition. In fact, some studies have suggested that a chronic systemic inflammation, even of low grade, may be involved in atherosclerosis and its main complications.22–24
In this regard, we evaluated the effects of a food supplement, Aterofisiol® containing omega-3 (EPA [eicosapentaenoic acid] DHA [docosahexaenoic acid]), vitamin K2, vitamin B6, vitamin B12, oligomeric proanthocyanidins (OPC), and resveratrol, on the composition of atherosclerotic plaque and on neurological symptoms and cerebrovascular events in patients undergoing carotid endarterectomy.
Materials and methods
Study design
The randomized, prospective, double-blind, and parallel groups study was performed in the Department of Clinical Medicine and Surgery, “Federico II” University of Naples, between April 2013 and November 2014.
This study was approved by the Institutional Review Board – Independent Ethics Committee (IRB-IEC) of the Interuniversity Center of Phlebolymphology-International Research and Educational Program in Clinical and Experimental Biotechnology, and before the beginning of the study, all participants were informed about the aim, procedures, risks, and benefits of the study. Before the beginning of the study, all participants signed written informed consent.
Patients
Eligible patients were women and men older than 20 years with carotid stenosis >70% who had undergone endarterectomy. Patients with embolic cerebrovascular stroke, recent TIA history of drug allergies, psychiatric diseases, creatinine clearance <30 mL/min, or platelet count <100,000/mm3 were excluded. Simultaneous use of dextran, thrombolytic agents, anticoagulants, or heparin or heparinoids for topical use was not allowed during the study and was considered a reason for exclusion.
Experimental protocol
Clinical examination, laboratory findings (blood and urine evaluation), and Doppler ultrasonography were performed in all enrolled patients at the time of admission and during the follow-up. Enrolled patients were randomly allocated to receive either one tablet of acetylsalicylic acid 100 mg (Cardioaspirin®) + one tablet of Aterofisiol every 24 hours or one tablet of Cardioaspirin + one tablet of placebo every 24 hours.
For randomization, a computer program was used to generate a sequence of treatment allocations by block randomization using a random number generator. Investigators were blinded to the block size to avoid selection bias.
Aterofisiol and placebo were made available in identical packages. At the time of randomization, patients received a diary into which to write the tablets taken.
Each treatment was started 30 days before the surgery and was stopped 5 days before the surgery.
Plaques evaluation
204 plaques were removed “en bloc” using standard surgical technique. Particular attention was paid to minimize handling of the plaque during endarterectomy. All the plaques were lyophilized, placed into a saline solution, and stored at −70°C for biochemical analysis.
Lipids were extracted using a chloroform–methanol solution, and dry weight of lipids was determined after evaporation of the solution; lipids are expressed as milligrams of extracted lipid per milligram of plaque dry weight. Cholesterol from an aliquot of the lipid extract was quantified by the manual enzymatic method, and results are expressed as milligrams of cholesterol per milligram dry weight of the plaque. Subsequently, specimens were homogenized in acetic acid and then hydrolyzed using HCl, HCl was removed by vacuum, samples were suspended, and collagen was determined using hydroxyproline colorimetric assay. Plaque calcium was measured by atomic absorption spectroscopy. Plaque lipid extract, after homogenization and hydrolysis, was diluted in lanthanum oxide; calcium absorbance was determined at 422 nm using a calcium/magnesium lamp. Results were expressed as mg Ca/mg dry weight of plaque.
Plaque morphology was assessed preoperatively with the Gray-Wheal classification method.25
Internal carotid artery (ICA) and common carotid artery (CCA) were also assessed by evaluating sonographic indexes such as peak systolic velocity (PSV), end diastolic velocity (EDV), and ICA/CCA PSV ratio.
Efficacy outcome
The efficacy outcome (Figure 1) was identified as a statistically significant difference (P<0.05) in lipid content of plaques between the two groups of treatment.
Figure 1.
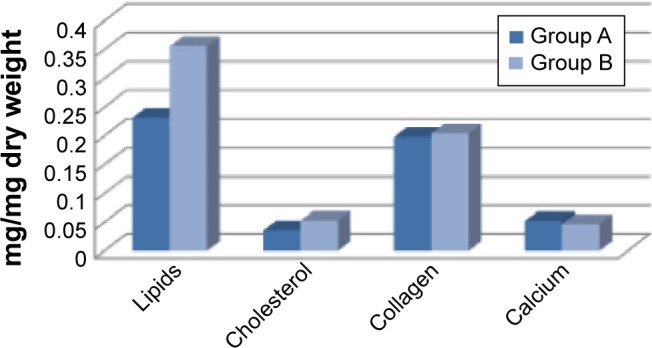
Efficacy outcome in lipid content of plaques between the two groups of treatment.
Notes: Group A patients were treated with Cardioaspirin® and Aterofisiol®. Group B patients were treated with Cardioaspirin® and a placebo.
Drug adherence
At the end of the study, in both groups of patients, the adherence to the treatment was calculated using a formula based on the number of tablets consigned at the time of admission and on the number of tablets returned unused at the end of the study.
Security outcomes
During the follow-up performed 1, 7, and 30 days after the surgery, we evaluated the security outcomes, defined as minor (amaurosis fugax, reversible ischemic neurological deficit [RIND], TIA) or as major (stroke) neurological adverse drug reactions (ADRs) occurring in either the immediate (1st day) or the late (from 7 to 30 days) postoperative period. A secondary security end-point was the development of any ADRs during the study; the development of ADRs was evaluated using both the Naranjo26 scale and the Drug Interaction Probability Scale.27
Statistical analysis
All data are expressed as mean ± standard error medium. Student’s t-test was performed in order to analyze the difference between each group with their control. Analysis of variance (ANOVA) was used to evaluate the differences between the groups. Differences identified by ANOVA were pinpointed by unpaired Student’s t-test. The threshold of statistical significance was set at P<0.05. SPSS (SPSS Inc., Chicago, IL, USA) software was used for the statistical analyses. We defined this study as exploratory, and therefore we did not determine a power calculation. In light of this, these results could only be labeled as exploratory.
Results
Patients
During the study period, 214 patients (135 men and 79 women) were enrolled for intent-to-treat and randomized in two groups:
Group A: 107 patients (68 men and 39 women) were treated with Cardioaspirin + Aterofisiol
Group B: 107 patients (67 men and 40 women) were treated with Cardioaspirin + placebo.
At the end of the study, 202 patients participated fully (103 patients in Group A and 99 patients in Group B), making up the per protocol evaluation population (94.4%).
12 patients (5.6%) did not complete the study: 6 patients (2.8%; 2 patients in Group A – 0.9% – and 4 in Group B – 1.9%) were considered noncompleters due to deviation from protocol, and 6 patients (2.8%; 2 patients in Group A – 0.9% – and 4 in Group B – 1.9%) were lost to follow-up.
Mean age was about 58 years, a previous TIA was detected in about 12% of patients, and CVD in about 4.8% (Table 1).
Table 1.
Population characteristics of enrolled patients
| Characteristics | Group A (Cardioaspirin® + Aterofisiol®) N=103 |
Group B (Cardioaspirin® + placebo) N=99 |
P-value |
|---|---|---|---|
| Age, years (mean±SD) | 58.1±12.2 | 57.1±12.1 | 0.68 |
| Sex, male | 65 (63%) | 62 (62.6%) | 0.94 |
| BMI, kg/m2 | 29.2±5.2 | 29.0±5.4 | 0.72 |
| Pathologies | |||
| Previous TIA | 12 (11.6%) | 12 (12.1%) | 0.91 |
| Previous stroke | 7 (6.8%) | 7 (7.07%) | 0.93 |
| Cardiovascular diseases | 5 (4.8%) | 5 (5.0%) | 0.94 |
| Diabetes mellitus | 5 (4.8%) | 7 (7.07%) | 0.50 |
| Thrombophilia | 5 (4.8%) | 6 (6.0%) | 0.70 |
| Medical therapies at the time of inclusion | |||
| Antihypertensive drugs | 77 (74.7%) | 75 (75.7%) | 0.86 |
| Analgesic drugs | 27 (26.2%) | 26 (26.3%) | 0.99 |
| Antidiabetics | 41 (39.8%) | 40 (40.4%) | 0.93 |
| Anticoagulants | 4 (3.9%) | 3 (3.0%) | 0.74 |
| Statins | 24 (23.3%) | 23 (23.2%) | 0.99 |
| NSAIDs | 3 (2.9%) | 2 (2.0%) | 0.68 |
Abbreviations: TIA, transient ischemic attack; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation.
We did not detect any difference in blood sugar, blood lipids, and blood pressure between the two groups at the time of inclusion (data not shown).
Ultrasound assessment of plaque morphology and velocimetry
According to the Gray-Wheal classification,25 10 patients of Group A (9.3%) and 6 patients of Group B (6.1%) (P=0.3372) had Type 1 plaques (predominantly echolucent with a thin echogenic cap); 11 patients of Group A (10.7%) and 12 patients of Group B (12.1%) (P=0.7471) had Type 2 plaques (intermediate echolucent lesions with small areas of echogenicity); 38 patients of Group A (36.9%) and 41 patients (41.4%) of Group B (P=0.5104) had Type 3 plaques (intermediate echogenic lesions with small areas of echolucency); 44 patients (42.7%) of Group A and 40 patients (40.4%) of Group B (P=0.7387) had Type 4 (uniformly echogenic lesions).
We did not detect any statistically significant difference in blood-flow rates (ICA – PSV, ICA – EDV, ICA/CCA PSV ratios) between the two groups (data not shown).
Efficacy outcomes
The mean lipid content of removed plaques was significantly lower (P<0.05) in Group A. In particular, the mean of lipids was 0.232±0.018 mg/mg dry weight in Group A and 0.356±0.022 mg/mg dry weight in Group B (P<0.05); the mean of cholesterol was 0.036±0.006 mg/mg dry weight in group A and 0.053±0.007 mg/mg dry weight in Group B (P<0.05) (Table 2). We did not record any statistically significant change in collagen or calcium content of plaques between the groups.
Table 2.
Efficacy outcome evaluation in Aterofisiol® group versus placebo group
| Plaque components | Group A (Cardioaspirin® + Aterofisiol®) N=103 |
Group B (Cardioaspirin® + placebo) N=99 |
P-value |
|---|---|---|---|
| Lipids | 0.232±0.018 | 0.356±0.022 | 0.032 |
| Cholesterol | 0.036±0.006 | 0.053±0.007 | 0.041 |
| Collagen | 0.198±0.012 | 0.204±0.010 | 0.68 |
| Calcium | 0.052±0.006 | 0.047±0.006 | 0.98 |
Notes: Unit of measure: mg/mg dry weight. All values are expressed as mean ± standard error medium.
Security outcomes
We recorded a significantly lower incidence of neurological symptoms in Group A (n=8) compared with Group B (n=20) (P<0.05). In particular, during the study, we recorded the development of amaurosis fugax (4 patients in Group A and 9 in B), TIA (2 patients in Group A and 5 in B), RIND (1 patient in Group A and 4 in B), and stroke (1 patient in Group A and 2 in B). No cases of fatal stroke were recorded (Table 3). No other ADRs appeared during this study.
Table 3.
Security outcome evaluation in Aterofisiol® group versus placebo group
| Cerebrovascular events | Group A (Cardioaspirin® + Aterofisiol®) N=103 |
Group B (Cardioaspirin® + placebo) N=99 |
P-value |
|---|---|---|---|
| Amaurosis fugax | 4 (3.9%) | 9 (9%) | 0.038 |
| TIA | 2 (1.9%) | 5 (5%) | 0.025 |
| RIND | 1 (0.9%) | 4 (4%) | 0.048 |
| Stroke* | 1 (0.9%) | 2 (2%) | 0.125 |
Note:
Indicates non-fatal stroke.
Abbreviations: RIND, reversible ischemic neurological disease; TIA, transient ischemic attack.
Discussion
Recently, it has been reported that inflammation plays a role in the destabilization of the plaque preceding the rupture, and therefore, statins as well as substances with anti-inflammatory actions could be used to improve the inflammation.15–21 In fact, the generation of atherosclerotic plaques starts with a concentration-dependent transport of low-density lipoproteins (LDL) into the arterial wall, followed by modification of these lipids by oxidation and/or inflammation, invasion of macrophages and their transformation to foam cells, infiltration of smooth muscle cells, and production of fibrin and extracellular matrix.28 The anti-inflammatory role of resveratrol and omega-3 fatty acids, in combination with the effects of vitamin K2, B6, and B12, seems to have a stabilizing effect on the atheromatous plaque opposing that mechanism. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a nonflavonoid polyphenolic compound belonging to the stilbene group able. It has been reported in cultured endothelial cells that resveratrol shows anti-inflammatory effects through the inhibition of cyclo-oxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9).29,30 Moreover, resveratrol has been shown to protect endothelial cells by inhibiting NADPH-oxidase and attenuating reactive oxygen species production, thereby showing antioxidant effects.31,32 In contrast, several clinical studies have failed to report a clinical effect of resveratrol on plasma lipids,33–37 but another in patients treated with statins and at high risk for CVDs documented that daily ingestion of 350 mg resveratrol-enriched grape extract containing 8 mg resveratrol for 6 months caused a 20% reduction of oxidized LDL cholesterol, but only a modest 4.5% reduction in LDL cholesterol,38 suggesting that the antioxidant effect of resveratrol may be more important than its LDL cholesterol-lowering effect. Therefore, it may be postulated that resveratrol could increase the antiatherosclerotic effect of traditional lipid-lowering medications that work mainly by lowering the cholesterol and/or triglycerides levels.
Polyunsaturated fatty acids omega-3 long-chain (n–3 LCPUFA), such as EPA and DHA, play an important role in the regulation of blood pressure, kidney function, blood clotting, and inflammatory and immunological reactions. In fact, the European Food Safety Authority (EFSA) established that to obtain the effects described for the reduction of blood pressure and triglyceride levels, there must be an intake of EPA and DHA of between 2 and 4 g/d, while for the maintenance of a normal cardiac function an intake of 250 mg is sufficient. Furthermore, as regards the dietary reference values, the group of experts concluded that the intake of 250 mg/d is adequate for the maintenance of overall cardiovascular health in adults and healthy children. Moreover, an additional daily intake of 5 g of omega-3 long-chain does not raise safety concerns for the general population.39,40
Hyperhomocysteinemia increases the risk of atherosclerosis as well as venous disease.41 It may result from folate deficiency or a genetic metabolic defect. The pathophysiologic mechanism is unknown, but may involve direct endothelial injury, stimulation of monocyte and T-cell recruitment, LDL uptake by macrophages, and smooth muscle cell proliferation. Epidemiological studies suggest that mild to moderate elevations in plasma total homocysteine (tHcy) concentrations are associated with an increased risk of atherosclerotic vascular diseases.42–45 Elevated tHcy was independently associated with plaque morphology and increased plaque area, subclinical markers of stroke risk.46 Lowering tHcy ought to slow the progression of atherosclerosis and prevent atherothrombotic events. Folate (folic acid), vitamins B6, and B12 have been used to treat hyperhomocysteinemia.47
Menaquinone-7 (MK-7) is part of a family of vitamin K that are essential cofactors for the enzyme γ-glutamyl carboxylase, which is involved in the activation of γ-carboxy glutamate (Gla) proteins in the body. Gla proteins are important for normal blood coagulation and normality of bones and arteries. Menaquinones or vitamin K2 (VK2) are formed usually by bacteria. Humans may obtain VK2 from fermented dietary sources, such as curd cheese and natto, a Japanese food.48 The potential beneficial effects of menaquinones is being uncovered, and some studies suggest that VK2 reduces vascular calcification and the advancement of atherosclerosis in hypercholesteremic rabbits.49 VK2 improves lipid profile by increasing high-density lipoprotein levels and decreasing total cholesterol levels and by playing an important role in the process of vascular calcification and plaque formation in atheroma, as shown in the Rotterdam study.50 Vascular tissue and calcified plaques contain MGP, a vitamin K–dependent protein known to prevent excessive calcium deposition in bone.51 Lack of vascular MGP resulted in excessive aortic and coronary calcification in knockout mice.52 Calcified plaques are more prone to rupture, which will elicit a thrombotic response, thereby increasing the risk of a coronary event. Another vitamin K–dependent protein found in the vessel wall is protein S. Together with activated protein C, this anticoagulant plays an important role in preventing clot formation at the inner surface of the vessel wall.53,54 In recognition of the effect of VK2 on reducing the risk of coronary heart disease, the International Life Sciences Institute (ILSI Europe) recently recommended taking VK2, in addition toVK1.50
The combination of these substances may have a beneficial effect on atherosclerosis by reducing the lipid content of the plaque, the reduction of homocysteinemia, the anti-inflammatory effect, and favoring the stability of the wall.
As atherosclerosis is a multifactorial disease with endothelial dysfunction and the alteration of several molecular systems, it justifies the identification and modulation of various therapeutic targets as several studies have done in past and recent years.19–21,55–58
In this context, our study evaluated the effects of a food supplement, Aterofisiol based on omega-3 (EPA and DHA), vitamin K2, vitamin B6, vitamin B12, OPC, and resveratrol, on atherosclerotic plaque composition and on neurological assessment in patients undergoing carotid endarterectomy.
The study found that carotid plaques removed from patients in Group B (treated with Cardioaspirin and placebo) contain higher amounts of cholesterol and lipids than the plaques removed from patients in group A (treated with Cardioaspirin and Aterofisiol), and the difference was statistically significant. Moreover, patients in group A developed a lower incidence of neurological accidents. The patients in our study are a representative sample of patients seen in clinical practice, with a clear predominance of men, with diabetics and smokers.
The study population was quite homogeneous with respect to patient demographics, clinical characteristics, and plaque composition, and this allows us to state that the evidence of better outcomes in patients treated with Aterofisiol were not biased by any factors related to patients or plaques variables.
Conclusion
In this study, we documented the efficacy of a new substance on atherosclerotic plaque and its constitution and on neurological assessment in patients with atherosclerosis and carotid stenosis.
Acknowledgments
This work received no funding.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization (WHO) The 10 Leading Causes of Death in the World, 2000 and 2012. Geneva, Switzerland: WHO; [Accessed June 30, 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/ [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Merwick A, Albers GW, Amarenco P, et al. Addition of brain and carotid imaging to the ABCD(2) score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol. 2010;9:1060–1069. doi: 10.1016/S1474-4422(10)70240-4. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ, Richardson PD, Woolf N, et al. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno PR, Falk E, Palacios IF, et al. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 6.Falk E. Why do plaques rupture? Circulation. 1992;86(Suppl III):III30–III42. [PubMed] [Google Scholar]
- 7.Davies MJ, Woolf N. Atherosclerosis: what is it and why does it occur? Br Heart J. 1993;69(Suppl 1):S3–S11. doi: 10.1136/hrt.69.1_suppl.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juul-Muller S, Edvardsson N, Jahnmatz B, et al. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet. 1992;340(8833):1421–1425. doi: 10.1016/0140-6736(92)92619-q. [DOI] [PubMed] [Google Scholar]
- 9.Zukowski AJ, Nicolaidas AN, Lewis RT, et al. The correlation between carotid plaque ulceration and cerebral infarction seen on CT. J Vasc Surg. 1994;1:782. [PubMed] [Google Scholar]
- 10.Schweitzer M, Mitmaker B, Obrand D, et al. Atorvastatin modulates matrix metalloproteinase expression, activity, and signaling in abdominal aortic aneurysms. Vasc Endovasc Surg. 2010;44(2):116–122. doi: 10.1177/1538574409348352. [DOI] [PubMed] [Google Scholar]
- 11.Zhao HD, Zhang YD. The effects of previous statin treatment on plasma matrix metalloproteinase-9 level in Chinese stroke patients undergoing thrombolysis. J Stroke Cerebrovasc Dis. 2014;23(10):2788–2793. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Kadoglou NP, Sailer N, Fotiadis G, Kapelouzou A, Liapis CD. The impact of type 2 diabetes and atorvastatin treatment on serum levels of MMP-7 and MMP-8. Exp Clin Endocrinol Diabetes. 2014;122(1):44–49. doi: 10.1055/s-0033-1358762. [DOI] [PubMed] [Google Scholar]
- 13.Andrade VL, do Valle IB, Sandrim VC. Simvastatin therapy decreases MMP-9 levels in obese women. J Clin Pharmacol. 2013;53(10):1072–1077. doi: 10.1002/jcph.146. [DOI] [PubMed] [Google Scholar]
- 14.Estanislau IM, Terceiro IR, Lisboa MR, et al. Pleiotropic effects of statins on the treatment of chronic periodontitis – a systematic review. Br J Clin Pharmacol. 2015;79(6):877–885. doi: 10.1111/bcp.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siniscalchi A, Gallelli L, Maleferrari G, et al. Cerebral stroke injury: the role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol. 2014;25(2):131–137. doi: 10.1515/jbcpp-2013-0121. [DOI] [PubMed] [Google Scholar]
- 16.Serra R, Grande R, Butrico L, et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J. 2014 Feb 25; doi: 10.1111/iwj.12240. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra R, Grande R, Buffone G, Gallelli L, de Franciscis S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Act Phlebol. 2013;14:99–107. [Google Scholar]
- 18.Serra R, Gallelli L, Buffone G, et al. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J. 2015;12:179–184. doi: 10.1111/iwj.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serra R, Gallelli L, Conti A, et al. The effects of Sulodexide on both clinical and molecular parameters in patients with mixed arterial and ulcers of lower limbs. Drug Des Devel Ther. 2014;8:519–527. doi: 10.2147/DDDT.S61770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Franciscis S, Gallelli L, Battaglia L, et al. Cilostazol prevents foot ulcers in diabetic patients with peripheral vascular disease. Int Wound J. 2015;12:250–253. doi: 10.1111/iwj.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra R, Grande R, Buffone G, et al. Effects of glucocorticoids and TNF-alfa inhibitors on both clinical and molecular parameters in patients with Takayasu Arteritis. J Pharmacol Pharmacother. 2014;5(3):193–196. doi: 10.4103/0976-500X.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Arterial stiffness and ischemic stroke in subjects with and without metabolic syndrome. Atherosclerosis. 2012;225(1):216–219. doi: 10.1016/j.atherosclerosis.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Pinto A, Di Raimondo D, Tuttolomondo A, Buttà C, Milio G, Licata G. Effects of physical exercise on inflammatory markers of atherosclerosis. Curr Pharm Des. 2012;18(28):4326–4349. doi: 10.2174/138161212802481192. [DOI] [PubMed] [Google Scholar]
- 24.Pinto A, Tuttolomondo A, Di Raimondo D, et al. Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metabolism. 2008;57(5):676–682. doi: 10.1016/j.metabol.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Gray-Weale AC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: comparison of preoperative B mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg. 1988;29:676–681. [PubMed] [Google Scholar]
- 26.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 27.Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother. 2007;41(4):674–680. doi: 10.1345/aph.1H423. [DOI] [PubMed] [Google Scholar]
- 28.Berliner JA, Navab M, Fogelman AM, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1195;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 29.Scoditti E, Calabriso N, Massaro M, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelia cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–89. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Annabi B, Lord-Dufour S, Vezina A, et al. Resveratrol targeting of carcinogen-induced brain endothelial cell inflammation biomarkers MMP-9 and COX-2 is Sirt1-independent. Drug Target Insights. 2012;6:1–11. doi: 10.4137/DTI.S9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen F, Qian LH, Deng B, et al. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci Ther. 2013;19:675–681. doi: 10.1111/cns.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow SE, Hshu YC, Wang JS, et al. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino J, Conte C, Fontana L, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujitaka K, Otani H, Jo F, et al. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res. 2011;31:842–847. doi: 10.1016/j.nutres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Wong RH, Berry NM, Coates AM, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–1827. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- 36.Dash S, Xiao C, Morgantini C, et al. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33:2895–2901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- 37.Poulsen MM, Vestergaard PF, Clasen BF, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tome-Carneiro J, Gonzalvez M, Larrosa M, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. 2012;56:810–821. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- 39.European Food Safety Authority (EFSA) Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J. 2015;13(1):3982. [Google Scholar]
- 40.European Food Safety Authority (EFSA) Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA.) EFSA J. 2012;10(7):2815. [Google Scholar]
- 41.de Franciscis S, De Sarro G, Longo P, et al. Hyperhomocysteinaemia and chronic venous ulcers. Int Wound J. 2015;12(1):22–26. doi: 10.1111/iwj.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boushey CJ, Beresford AA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 43.Held C, Sumner G, Sheridan P, et al. Correlations between plasma homocysteine and folate concentrations and carotid atherosclerosis in high-risk individuals: baseline data from the Homocysteine and Atherosclerosis Reduction Trial (HART) Vasc Med. 2008;13:245–253. doi: 10.1177/1358863X08092102. [DOI] [PubMed] [Google Scholar]
- 44.Mierzecki A, Kłoda K, Bukowska H, et al. Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors. Med Sci Monit. 2013;19:733–739. doi: 10.12659/MSM.889087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potter K, Hankey GJ, Green DJ, Eikelboom J, Jamrozik K, Arnolda LF. The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: a randomized controlled trial and meta-analysis. BMC Cardiovasc Disord. 2008;8:24. doi: 10.1186/1471-2261-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsulaimani S, Gardener H, Elkin MS, Cheung K, Sacco RL, Rundek T. Elevated homocysteine and carotid plaque area and densitometry in the Northern Manhattan Study (NOMAS) Stroke. 2013;44(2):457–461. doi: 10.1161/STROKEAHA.112.676155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller T, Furtmueller B, Aigelsdorfer J, Luft C, Poelz W, Haltmayer M. Total serum homocysteine – a predictor of extracranial carotid artery stenosis in male patients with symptomatic peripheral arterial disease. Vasc Med. 2001;6:163–167. doi: 10.1177/1358836x0100600307. [DOI] [PubMed] [Google Scholar]
- 48.Beulens JWJ, Booth SL, Van den Heuvel EGHM, et al. The role of menaquinones (vitamin K2) in human health. Br J Nutr. 2013;110:1357–1368. doi: 10.1017/S0007114513001013. [DOI] [PubMed] [Google Scholar]
- 49.Kawashima H, Nakajima Y, Matubara Y, et al. Effects of vitamin K2 (menatetrenone) on atherosclerosis and blood coagulation in hypercholesterolemic rabbits. Jpn J Pharmacol. 1997;75(2):135–143. doi: 10.1254/jjp.75.135. [DOI] [PubMed] [Google Scholar]
- 50.Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134(11):3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 51.Wallin R, Schurgers L, Wajih N, et al. Effects of the blood coagulation vitamin K as an inhibitor of arterial calcification. Thromb Res. 2008;122(3):411–417. doi: 10.1016/j.thromres.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 53.El Asmar MS, Naoum JJ, Arbid EJ. Vitamin K dependent proteins and the role of vitamin K2 in the modulation of vascular calcification: a review. Oman Med J. 2014;29(3):172–177. doi: 10.5001/omj.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D Status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167(3):313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strano A, Hoppensteadt D, Walenga JM, et al. Plasma levels of the molecular markers of coagulation and fibrinolysis in patients with peripheral arterial disease. Semin Thromb Hemost. 1996;22(1):35–40. [PubMed] [Google Scholar]
- 56.de Franciscis S, Serra R. Matrix metalloproteinases and endothelial dysfunction: the search for new prognostic markers and for new therapeutic targets for vascular wall imbalance. Thromb Res. 2015;136(1):5–6. doi: 10.1016/j.thromres.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Crepaldi G, Fellin R, Calabrò A, et al. Double-blind multicenter trial on a new medium molecular weight glycosaminoglycan. Current therapeutic effects and perspectives for clinical use. Atherosclerosis. 1990;81(3):233–243. doi: 10.1016/0021-9150(90)90071-p. [DOI] [PubMed] [Google Scholar]
- 58.De Caridi G, Massara M, Stilo F, et al. Effectiveness of Prostaglandin E1 in patients with mixed arterial and venous ulcers of lower limbs. Int Wound J. 2014 Aug 5; doi: 10.1111/iwj.12334. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]


