Abstract
Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis
INTRODUCTION
Psoriasis is a chronic, systemic inflammatory disorder affecting approximately 1% to 3% of the U.S. population.1 Significant geographic variation has been noted in the prevalence of psoriasis, likely reflecting the fact that environmental and genetic factors influence this disorder.1 Although it is a multisystem disease, the major manifestation of psoriasis is erythematous, scaly patches or plaques on the skin that are often pruritic and/or painful.2 These result from premature maturation of keratinocytes and infiltration of the dermis by dendritic cells, macrophages, and T lymphocytes, leading to hyperproliferation of the epidermal layer.3 These disfiguring skin lesions are often associated with numerous comorbidities, ranging from cardiovascular disease, autoimmune disease, and cancer to psychiatric/psychological disorders.2 Studies have indicated that up to 42% of patients diagnosed with psoriasis develop psoriatic arthritis (PsA), an inflammatory spondyloarthropathy.4 In the majority of such patients, joint disease typically occurs approximately seven to 12 years after the onset of skin disease.4 Patients with PsA present with pain, stiffness, and swelling in and around the joints, similar to other rheumatic diseases. However, distinguishing characteristics of PsA include the presence of enthesitis (ligament and tendon insertions in the bone), spondylitis, dactylitis, nail dystrophy, and seronegativity for the rheumatoid factor.4
Patients with psoriasis experience diminished health-related quality of life (QOL) resulting in physical and mental disability comparable to that seen in patients with other chronic diseases (e.g., diabetes, depression, heart disease).2,4 Compared with psoriasis patients, those with PsA have greater QOL impairment, more work-related disability, and disease-related health care visits and hospitalizations.4,5 Hence the goals of therapy are not only to reduce skin and joint symptoms and prevent further structural damage in those with PsA, but also to make a meaningful impact on health-related QOL.
The current treatment recommendations for patients with psoriasis depend on the severity of disease. Mild disease is treated with topical therapies (e.g., corticosteroids, vitamin D analogues, tazarotene) alone or, with increasing disease severity, in combination with phototherapy and/or traditional nonbiologic systemic therapy (methotrexate, cyclosporine, acitretin).6–8 Moderate-to-severe disease typically necessitates the use of systemic agents, whether it be traditional agents or biologics (e.g., tumor necrosis factor-alpha [TNF-α] inhibitors, ustekinumab).2,6,8,9 In patients presenting with PsA, first-line treatment with nonsteroidal anti-inflammatory drugs is recommended in mild disease with or without the use of local corticosteroid injections.10,11 More advanced disease requires the use of oral disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, sulfasalazine, or leflunomide, and/or TNF-α inhibitors or the interleukin (IL) 12/23 inhibitor ustekinumab.8,10,11 Ultimately, care of patients with psoriasis or PsA must be individualized, taking into account efficacy, adverse effects, availability, ease of administration, and cost of therapy, as well as patient comorbidities and illnesses.8
In March 2014, the Food and Drug Administration (FDA) approved apremilast (Otezla, Celgene Corporation), the first selective inhibitor of phosphodiesterase 4 (PDE4) indicated for adults with active PsA.12 Subsequently, Celgene received FDA approval in September 2014 to further market the drug for the treatment of moderate-to-severe plaque psoriasis in patients for whom phototherapy or systemic therapy is appropriate.13 Since the availability of apremilast adds to the treatment armamentarium of both psoriasis and PsA, the purpose of this article is to review the pharmacological and clinical characteristics of this agent and discuss its implications for use.
CHEMICAL COMPOSITION14
Apremilast is chemically identified as N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]acetamide. The molecular formula and weight of apremilast are C22H24N2O7S and 460.5 g/mole, respectively. The chemical structure is shown in Figure 1.
Figure 1.
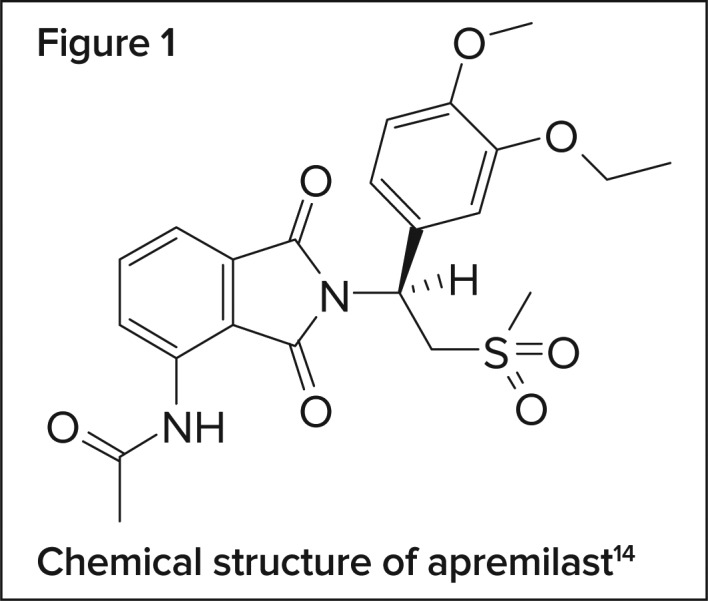
Chemical structure of apremilast14
CLINICAL PHARMACOLOGY
While the exact mechanism by which apremilast exerts its effect in psoriasis and PsA is not clearly defined, many of the cytokine mediators involved in these conditions are influenced by PDE4, which regulates immune and inflammatory processes.15 By inhibiting PDE4, apremilast prevents the degradation of cyclic adenosine monophosphate (cAMP). The subsequent increased level of cAMP results in an antagonistic effect on the production of proinflammatory cytokines such as TNF-α, IL-23, and interferon (IFN)-γ, and an increase in anti-inflammatory mediators (e.g., IL-10).15 Thus, apremilast works intracellularly to interrupt the inflammatory cascade at an early point, unlike biologic agents that target single pro-inflammatory markers (e.g., TNF-α).
PHARMACOKINETICS
The absolute bioavailability of apremilast following oral administration of 20 mg is 73%; food does not alter the extent of absorption.14,16 After the administration of multiple ascending doses in healthy adults, the area under the curve (AUC) and peak plasma concentration (Cmax) increased linearly, with a median time to peak concentration (Tmax) of approximately 2.5 hours.14,16 In patients with PsA, the steady-state Cmax and AUC values are higher by 57% and 38%, respectively.16 Apremilast is readily distributed with a volume of distribution (Vd) of 87 L. Binding to human plasma proteins is approximately 68%.14,16
Hoffman et al. evaluated the disposition, metabolism, and mass balance of radiolabeled apremilast in healthy volunteers.17 Following a single 20-mg dose of an oral suspension, apremilast was noted to undergo extensive metabolism, such that unchanged drug represented 45% of the circulating plasma radioactivity while the inactive metabolite M12 (O-desmethyl apremilast glucuronide) accounted for 39% of the circulating radioactivity. In all, 23 metabolites with negligible pharmacological effects were identified.17 These metabolites are formed via both cytochrome P450 (CYP)-mediated oxidative metabolism (and subsequent glucuronidation) and non–CYP-mediated hydrolysis.14,16 In vitro data indicate that CYP3A4 is the predominant isoenzyme involved in CYP metabolism, followed to a lesser extent by CYP1A2 and CYP2A6.14,16 With respect to the clearance of the drug, Hoffman et al. noted that approximately 58% and 39% of the radioactive apremilast was excreted in the urine and feces, respectively.17
In healthy subjects, the plasma clearance of apremilast is approximately 10 L/hr and the terminal elimination half-life ranges from six to nine hours.14,16 The clearance of the drug is 36% lower in patients with PsA. Notably, in patients with severe renal impairment (an estimated glomerular filtration rate [eGFR] of less than 30 mL/min), the AUC of apremilast is increased by approximately 88% while clearance is diminished by approximately 47%, thereby warranting dosage reductions (see Dosage and Administration).14,16 No other intrinsic factors (age, gender, race/ethnicity, hepatic impairment) influence the pharmacokinetics of apremilast in a way that warrants dosage adjustments.16
CLINICAL STUDIES
The efficacy and safety of apremilast for the treatment of PsA were evaluated in four pivotal phase 3 studies of similar design, three of which formed the basis for the FDA approval of the agent (PALACE 1 to 3).14,18 Notably, only one of these studies was published (PALACE 1).19 Though several phase 2 studies assessing the efficacy and safety of apremilast in patients with plaque psoriasis have been published,20–23 only one of the two phase 3 studies that served as the basis for this added indication is published; the other is described in the prescribing information.24 The phase 3 study data for both indications are described herein.
Psoriatic Arthritis
The PALACE 1 trial was an international, multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of apremilast in patients with active PsA despite previous use of DMARD and/or biologic therapy.19 A total of 504 patients were randomized, stratified by baseline DMARD use, to placebo (n = 168), apremilast 20 mg twice daily (n = 168), or apremilast 30 mg twice daily (n = 168); the dose of apremilast was titrated over the first week of treatment. At week 16, nonresponders (i.e., patients whose swollen and tender joint count did not improve by at least 20%) were re-randomized to one of the two apremilast-treated groups; patients in the apremilast groups continued receiving their initial therapy. At week 24, all remaining placebo-treated patients were re-randomized to either the apremilast 20-mg arm or the apremilast 30-mg arm. The published report describes the outcomes up to week 24 of the study; however, subjects continued on their assigned dose of apremilast as part of a long-term extension study, which remains ongoing.19
Of the 504 randomized patients (mean age, 50.4 years), 444 (88%) completed 24 weeks of treatment. At baseline, the mean duration of PsA was 7.5 years and 65% of patients were taking DMARDs, most of whom (84%) were on methotrexate. Prior use of a biologic was reported by 24% of patients, while 9% of the overall randomized population had failed biologic therapy.19,24 The primary efficacy endpoint was the proportion of patients achieving a 20% improvement in the modified American College of Rheumatology (ACR) response criteria (ACR20) at week 16, that is, a 20% improvement in tender and swollen joint counts and 20% improvement in three of the five core measures (patient and physician global assessments, pain, physical function, and C-reactive protein).19,24 Based on a prespecified analysis of the per-protocol population (n = 489), significantly more patients achieved this endpoint in the apremilast 20-mg group (31%, P = 0.0140) and in the apremilast 30-mg group (40%, P = 0.0001) compared with placebo-treated patients (19%). These results were consistent with the intent-to-treat analysis (Table 1) and the treatment effect was observed irrespective of prior biologic therapy exposure. Statistically significant improvements were also noted in the key secondary efficacy measure, the change from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) at week 16 (Table 1).19 The HAQ-DI is a patient-reported questionnaire consisting of 20 questions in eight categories—dressing, rising, eating, walking, hygiene, reach, grip, and usual activities. Scores in each category, which range from 0 (no difficulty) to 3 (unable to do), are averaged to provide an overall score.24 Compared with placebo, the proportion of patients achieving a minimally clinically important difference on this measure was only significantly greater than placebo in the apremilast 30-mg group. Significant improvements in additional secondary efficacy measures at week 24 were also noted with apremilast therapy (e.g., ACR20, ACR50, ACR70, physical functioning as measured by the HAQ-DI, and the 36-Item Short Form Health Survey v2 Physical Functioning domain score).19
Table 1.
Results of the Primary and Key Secondary Efficacy Outcome Measures in the PALACE 1–3 Studies19,25,27,28
| Outcome Measures | PALACE 1 | PALACE 2 | PALACE 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PBO | APR20 BID | APR30 BID | PBO | APR20 BID | APR30 BID | PBO | APR20 BID | APR30 BID | |
| ACR20 at week 16a | n = 168 | n = 168 | n = 168 | n = 159 | n = 163 | n = 162 | n = 169 | n = 169 | n = 167 |
| Number (%) | 32 (19) | 51 (30) | 64 (38) | 30 (19) | 61 (37) | 52 (32) | 31 (18) | 48 (28) | 68 (41) |
| P value APR dose vs. PBO | — | 0.0166 | 0.0001 | — | 0.0002 | 0.0060 | — | 0.0295 | < 0.0001 |
| Change from baseline in HAQ-DI at week 16b | n = 165 | n = 163 | n = 159 | n = 153 | n = 159 | n = 154 | n = 163 | n = 163 | n = 160 |
| Mean (± standard error) | −0.09 (0.04) | −0.20 (0.04) | −0.24 (0.04) | −0.05 (0.04) | −0.16 (0.04) | −0.19 (0.04) | −0.07 (0.03) | −0.13 (0.03) | −0.19 (0.03) |
| P value APR dose vs. PBO | — | 0.0252 | 0.0017 | — | 0.0320 | 0.0042 | — | 0.17 | 0.0073 |
Full analysis set consisting of all participants randomized as specified in the protocol. Participants who withdrew early or who did not have sufficient data for a definitive determination of response status at week 16 were counted as non-responders.
Full analysis set; participants with a baseline value and at least one post-baseline value at or prior to week 16 are included.
ACR20 = American College of Rheumatology 20% response; APR20 = apremilast 20 mg; APR30 = apremilast 30 mg; BID = twice a day; HAQ-DI = Health Assessment Questionnaire-Disability Index; PBO = placebo
Study discontinuation because of adverse events (AEs) was comparable among groups (6% for apremilast 20 mg, 7% for apremilast 30 mg, and 5% for placebo).19 The most frequently reported AEs with apremilast were largely mild to moderate in severity and dose-dependent. These included diarrhea, reported by 11% and 19% of patients in the apremilast 20-mg and 30-mg groups, respectively (versus 2% for placebo), and nausea, reported by 10% of apremilast 20-mg patients and 19% of apremilast 30-mg patients (versus 7% for placebo). These AEs presented early and were self-limiting, accounting for few study discontinuations. In addition to the gastrointestinal side effects, apremilast-treated patients reported more headache (10%, 11%, and 5% in the apremilast 20-mg, apremilast 30-mg, and placebo groups, respectively) and upper respiratory tract infections (6% in the apremilast 20-mg group compared with 4% in both the apremilast 30-mg and placebo groups). The rates of serious AEs and clinically meaningful laboratory abnormalities were low and comparable among groups.19
The 52-week results of the PALACE 1 trial demonstrated that in those patients who continued treatment with apremilast, treatment efficacy was maintained; ACR20 responses of 63% and 55% were reported in the apremilast 20-mg and apremilast 30-mg groups, respectively.26 Consistent results were seen in those patients re-randomized from placebo to apremilast at either week 16 or week 24. The safety profile of apremilast was also similar to that seen in the placebo- controlled phase of the study.26
As previously noted, the PALACE 2 and PALACE 3 trials were very similar in design to the PALACE 1 study, enrolling 488 and 505 patients, respectively.18,27,28 Patients’ baseline characteristics and background disease characteristics were also similar throughout the three studies.18,27,28 Table 1 outlines the results of the primary efficacy outcome and the key secondary efficacy outcome in the PALACE trials. As with the efficacy data, the AE profile of apremilast in PALACE 2 and 3 was comparable to that seen in PALACE 1.27,28
Plaque Psoriasis
The efficacy and safety of apremilast in the treatment of moderate-to-severe plaque psoriasis (Psoriasis Area and Severity Index [PASI] score of 12 or higher, body surface area [BSA] involvement of 10% or more, static Physician Global Assessment [sPGA] of 3 or more, candidates for phototherapy or systemic therapy) were evaluated in two multi-center, randomized, double-blind, placebo-controlled, phase 3 trials of comparable design.14,24,29 In ESTEEM 1 (N = 844) and ESTEEM 2 (N = 413), patients were randomized 2:1 to receive apremilast 30 mg twice daily or placebo for 16 weeks. At 16 weeks, all patients were treated with apremilast through week 32, followed by a randomized withdrawal phase through week 52 and an optional four-year, open-label extension phase to assess safety. The approval of apremilast was based on the data at 16 weeks.14
The primary efficacy endpoint was the percentage of participants who achieved a 75% improvement (reduction) from baseline in the PASI score (PASI-75) at week 16.24,29 The PASI is a measure of psoriatic disease severity that accounts for lesion characteristics (erythema, thickness, and scaling) and degree of skin surface area involvement on defined anatomical regions. Scores range from 0 to 72, with higher scores reflecting greater disease severity.29 Though numerous additional efficacy endpoints were assessed, the key secondary outcome measure was the percentage of participants who achieved a score of 0 (indicating clear) or 1 (almost clear) on the sPGA at week 16, with at least a 2-point reduction from baseline.24,29 The sPGA is an assessment of the severity of the three primary signs of the disease: erythema, scaling, and plaque elevation, with scores ranging from 0 (clear) to 4 (severe).29
For both ESTEEM 1 and ESTEEM 2, the overall median age of enrolled patients was 46 years (range, 18 to 83 years), while the mean baseline BSA involvement and PASI scores were 25.2% and 19.1, respectively. Almost all patients had a baseline score on the sPGA of 3 (70.0%) or 4 (29.8%), and 54% had received prior systemic therapy with conventional agents and/or biologics.14 At week 16, the proportion of patients achieving a PASI-75 response was significantly greater (P < 0.0001) in the apremilast-treated group than in the placebo group in both studies (ESTEEM 1: 33% versus 5%, respectively; ESTEEM 2: 29% versus 6%, respectively). Compared with placebo, significantly more apremilast-treated patients achieved a score of 0 or 1 on the sPGA in ESTEEM 1 (4% versus 22%, respectively; P < 0.0001) and in ESTEEM 2 (4% versus 20%; P < 0.0001).14,24,29 Significant results were also noted in favor of apremilast in the percent change from baseline in affected BSA and PASI score and in the percentage of patients achieving a PASI-50.24,29 In the patients re-randomized to placebo at week 32, the median time to loss of PASI-75 response was 5.1 weeks in ESTEEM 1.14,24 Overall, in both studies apremilast was well tolerated. As in the PsA studies, in both ESTEEM 1 and ESTEEM 2 apremilast was predominantly associated with diarrhea (19% and 16%, respectively) and nausea (16% and 18%, respectively) in terms of AEs. Tension headache, headache, nasopharyngitis, and upper respiratory infection were also reported frequently.24,29 Study discontinuation due to any AEs was similar between the apremilast-treated patients and the placebo-treated patients and no new significant AEs were noted with continued apremilast exposure for up to 52 weeks.24,29
SAFETY PROFILE
While the long-term extension studies and post-marketing reports will ultimately shed more light on the overall safety of apremilast, the data from the phase 3 clinical studies in patients with PsA and psoriasis suggest that apremilast is generally well tolerated. The gastrointestinal side effects associated with the agent largely occurred within the first month of treatment and subsequently subsided.18 Based on the mechanism of action of apremilast, known PDE4 class effects, comorbidities of PsA, and other factors, several adverse drug reactions of special interest were also assessed in the clinical studies. These included, but were not limited to, the risk of serious infections (e.g., tuberculosis), malignancies, major adverse cardiovascular events, and vasculitis. Importantly, no imbalances were observed between apremilast and placebo for any of these AEs, suggesting that apremilast does not increase their risk.18
Contraindications, Warnings, And Precautions
The labeling of apremilast cites no major contraindications to its use, other than avoidance of the agent in patients with a history of hypersensitivity to apremilast or any excipients found in the formulation.14 However, the label does warn about the potential for an increased risk of depression in apremilast-treated patients. As is the case with the PDE4 inhibitor roflumilast, used for chronic obstructive pulmonary disease,30 apremilast has been associated with an increased frequency of depression. Depression or depressed mood was reported by 1.0% of apremilast-treated patients in the PsA studies and 1.3% in the psoriasis studies (versus 0.8% and 0.4% with placebo, respectively).14 While the overall incidence is low, it is nevertheless recommended that the risks and benefits of apremilast be evaluated carefully prior to initiating therapy in patients with a history of depression and/or suicidal thoughts or behavior; close monitoring for worsening of such events during therapy is also advised.14 The potential for apremilast to cause weight loss is also highlighted as a warning/precaution in the product labeling. In the PsA studies, 10% of apremilast 30-mg patients lost between 5% and 10% of body weight compared with 3% of those receiving placebo.14,18 Likewise, during the psoriasis trials, 12% of patients in the apremilast group experienced 5% to 10% weight loss versus 5% in the placebo group.14 As a result, regular monitoring of weight during apremilast therapy is warranted. As further discussed in the Drug Interactions section, an additional warning for apremilast concerns its use with strong CYP 450 enzyme inducers; concomitant use with such agents (e.g., rifampin, phenytoin) is not recommended because of a reduction in the systemic exposure of apremilast and the potential for a subsequent loss of efficacy.14
Special Populations
Studies on the use of apremilast in pregnant women have not been conducted; as a consequence, apremilast is classified as pregnancy category C. As a post-marketing requirement, the FDA has mandated a pregnancy exposure registry to monitor pregnancy outcomes related to apremilast.12 In the PsA and psoriasis studies, the number of participants 65 years of age and older was low; overall, however, no differences were noted in the efficacy and safety of apremilast in such patients compared with adults younger than 65 years of age.14 As is typical with newly approved agents, the use of apremilast in patients younger than 18 years of age has not been evaluated.14 Although apremilast is significantly metabolized, an open-label, single-dose study assessing the pharmacokinetics of apremilast in subjects with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment demonstrated no significant effects on apremilast pharmacokinetics in such individuals.16 Thus, dose adjustment in this population is not necessary. In contrast, the results of a single-dose study in subjects with severe renal impairment (creatinine clearance [CrCl] of less than 30 mL/min) provided justification for a dosage reduction in this population (see Dosage and Administration).16
DRUG INTERACTIONS
Since in vitro data revealed that apremilast is metabolized at a high rate by CYP3A4 and is a substrate for P-glyco-protein (P-gp), studies were conducted to assess whether apremilast interacts with CYP3A4 substrates, the CYP3A4 and P-gp inhibitor ketoconazole, and the CYP3A4 inducer rifampin.14,31 The only clinically relevant interactions found involved rifampin. Following the administration of multiple doses of rifampin (600 mg once daily for 15 days) and a single 30-mg dose of apremilast, the AUC and Cmax of apremilast were reduced by 72% and 43%, respectively.14,31 Importantly, there is no interaction with methotrexate, an agent frequently used in patients with PsA and plaque psoriasis.14,32
DOSAGE AND ADMINISTRATION14
The recommended maintenance dose of apremilast, irrespective of indication, is 30 mg twice daily administered without regard to meals. To reduce gastrointestinal side effects associated with the start of therapy, a five-day titration is recommended. The initial dose on day 1 is 10 mg in the morning; this is increased to 10 mg in the morning and evening on day 2. The evening dose is further increased by 10 mg (to 20 mg) on day 3. On day 4, the morning dose is increased to 20 mg, so that 20 mg is taken twice daily, and on day 5 the evening dose is increased to 30 mg. The maintenance dose of 30 mg twice daily begins on day 6. A two-week titration pack containing 10-mg, 20-mg, and 30-mg tablets is available to facilitate the initiation of therapy. Patients with severe renal impairment (CrCl of less than 30 mL/min as estimated by the Cockcroft–Gault equation) require a modified dosing schedule with a maintenance dose of 30 mg once daily. When titrating the initial dosage in such individuals, it is recommended that the evening dose be eliminated (i.e., 10 mg in the morning on days 1, 2, and 3, followed by 20 mg on days 4 and 5 and 30 mg on day 6).
COST
The average wholesale price for a 30-day supply (60 tablets) of apremi-last 30 mg is $2,666.33 This is significantly greater than traditional systemic DMARDs, which are available generically, but less than the cost of biologics. A cost comparison of agents commonly used in the treatment of PsA and/or plaque psoriasis is provided in Table 2.
Table 2.
Cost of Therapy With Systemic Agents Used in the Treatment of Psoriatic Arthritis and/Or Plaque Psoriasis
| Drug | Usual Adult Maintenance Dose9,34 | Costa |
|---|---|---|
| Phosphodiesterase 4 (PDE4) Inhibitor | ||
| Apremilast (Otezla, Celgene) | 30 mg PO twice daily | $2,666 |
| Disease-Modifying Antirheumatic Drugs (DMARDs) | ||
| Acitretin (generic, multiple manufacturers) | 25–50 mg PO once daily | $1,154–$2,308 |
| Cyclosporine, modified (generic, multiple manufacturers) | 2.5–5 mg/kg/day PO, divided, twice daily | $412b |
| Leflunomide (generic, multiple manufacturers) | 20 mg PO once a day | $197 |
| Methotrexate (generic, multiple manufacturers) (Otrexup, Antares Pharma) (Rasuvo, Medac Pharma) |
10–25 mg PO, IM, IV, SQ once weekly | $57–$142 (PO)c,d $658 (SQ)d $538 (SQ)d |
| Sulfasalazine (generic, multiple manufacturers) | 1–4 g/day PO, divided, twice daily | $13–53 |
| Interleukin 12/23 Inhibitor | ||
| Ustekinumab (Stelara, Janssen Biotech) | 45 mg SQ every 12 weeks | $9,828e |
| Interleukin 17A Inhibitor | ||
| Secukinumab (Cosentyx, Novartis) | 300 mg SQ every four weeks | $4,104d |
| Tumor Necrosis Factor-Alpha (TNF-α) Inhibitors | ||
| Etanercept (Enbrel, Amgen USA, Inc.) | 50 mg SQ once weekly | $3,843d |
| Adalimumab (Humira, AbbVie) | 40 mg SQ every other week | $3,843d |
| Infliximab (Remicade, Janssen Biotech) | 5 mg/kg IV every eight weeks | $4,674f |
| Golimumab (Simponi, Janssen Biotech) | 50 mg SQ once a month | $3,929 |
| Certolizumab (Cimzia, UCB Inc.) | 200 mg SQ every other week | $3,652d |
Cost is calculated for a 30-day supply unless otherwise specified. Cost is based on average wholesale price (AWP) at the usual adult maintenance dose and rounded to the nearest dollar. The lowest AWP noted in Red Book Online is provided for generic products.
Cost is based on a 70-kg patient requiring a dose of 125 mg twice daily (3.5 mg/kg/day; 60 capsules of both 100 mg and 25 mg)
Cost is based on tablets; cost of solution for IM and IV injection is not provided because these routes of administration are not easily administered at home.
Cost for a 28-day supply
Cost is based on a patient weighing ≤ 100 kg; 12-week supply
Cost is based on a 70-kg patient requiring four single-dose vials containing 100 mg; eight-week supply
IM = intramuscular; IV = intravenous; PO = by mouth; SQ = subcutaneous
P&T COMMITTEE CONSIDERATIONS
Advantages to the use of apremilast include its oral administration, minimal drug interaction potential, and what appears to be a fairly safe AE profile, particularly as it compares to those of methotrexate and biologics. The cost of apremilast is also lower than that of biologics. Nevertheless, the twice-daily dosing might not be advisable if nonadherence is a concern, and the gastrointestinal side effects may be troublesome. Furthermore, although comparative studies are lacking, the efficacy of apremilast (based on the percentage of patients with PsA and psoriasis achieving ACR20 and PASI-75, respectively) appears to be lower than that of TNF-α inhibitors and ustekinumab.8 Given that treatment of psoriasis and PsA is highly individualized, apremilast will likely be of value to patients who cannot tolerate and/or are unresponsive to conventional systemic nonbiologic agents and to those who may not be candidates for biologics. It will be interesting to see how the American Academy of Dermatology delineates the specific role of apremilast in clinical practice in its next iteration of the guidelines for care of patients with plaque psoriasis and PsA.
CONCLUSION
Psoriasis and PsA are chronic disorders with significant morbidity. As the first PDE4 inhibitor approved for use in these conditions, apremilast provides clinicians with a new tool in the arsenal for fighting psoriatic diseases. The drug has been shown to be effective, even in patients with prior use of DMARDs and/or biologics, and is generally well tolerated. Additional long-term studies are needed to further elucidate its place in therapy.
Footnotes
Disclosure: The authors report no commercial or financial relationships in regard to this article.
REFERENCES
- 1.Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Palfreeman AC, McNamee KE, McCann FE. New developments in the management of psoriasis and psoriatic arthritis: a focus on apremilast. Drug Des Devel Ther. 2013;7:201–210. doi: 10.2147/DDDT.S32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehncke WH, Menter A. Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2013;14(5):377–388. doi: 10.1007/s40257-013-0032-x. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence Psoriasis: the assessment and management of psoriasis. NICE clinical guideline 153. Oct, 2012. Available at: https://www.nice.org.uk/guidance/cg153/resources/guidance-psoriasis-pdf. Accessed July 6, 2015. [PubMed]
- 7.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174. doi: 10.1016/j.jaad.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 9.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71(1):4–12. doi: 10.1136/annrheumdis-2011-200350. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864. doi: 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration FDA news release: FDA approves Otezla to treat psoriatic arthritis. Mar 21, 2014. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm390091.htm. Accessed July 6, 2015.
- 13.Celgene Corporation Oral Otezla (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis. Sep 23, 2014. Available at: http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed July 6, 2015.
- 14.Otezla (apremilast) prescribing information. Summit, New Jersey: Celgene Corp.; Dec, 2014. [Google Scholar]
- 15.Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi: 10.1016/j.bcp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration Center for Drug Evaluation and Research. Nov, 2013. Application number: 205437Orig1s000: clinical pharmacology and biopharmaceutics review(s). Available at: http://www.accessdata.fda.gov/drug-satfda_docs/nda/2014/205437Orig1s000ClinPharmR.pdf. Accessed July 6, 2015.
- 17.Hoffmann M, Kumar G, Schafer P, et al. Disposition, metabolism and mass balance of [(14)C]apremilast following oral administration. Xenobiotica. 2011;41(12):1063–1075. doi: 10.3109/00498254.2011.604745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration Center for Drug Evaluation and Research. Feb, 2014. Application number: 205437Orig1s000: medical review(s). Available at: http://www.access-data.fda.gov/drugsatfda_docs/nda/2014/205437Orig1s000MedR.pdf. Accessed July 6, 2015.
- 19.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–1026. doi: 10.1136/annrheumdis-2013-205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strand V, Fiorentino D, Hu C, et al. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health Qual Life Outcomes. 2013;11:82. doi: 10.1186/1477-7525-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papp KA, Kaufmann R, Thaçi D, et al. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol. 2013;27(3):e376–383. doi: 10.1111/j.1468-3083.2012.04716.x. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12(8):888–897. [PubMed] [Google Scholar]
- 23.Papp K, Cather JC, Rosoph L, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–746. doi: 10.1016/S0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- 24.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 25. ClinicalTrials.gov. Efficacy and safety study of apremilast to treat active psoriatic arthritis (PALACE-1). NCT01172938. July 24, 2014. Available at: http://clinicaltrials.gov/ct2/show/NCT01172938?term=palace+1&rank=1. Accessed July 6, 2015.
- 26.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42(3):479–488. doi: 10.3899/jrheum.140647. [DOI] [PubMed] [Google Scholar]
- 27. ClinicalTrials.gov. PALACE 2: Efficacy and safety study of apremilast to treat active psoriatic arthritis (PALACE2). NCT01212757. July 24, 2014. Available at: http://clinicaltrials.gov/ct2/show/study/NCT01212757?term=palace+2&rank=1. Accessed July 6, 2015.
- 28. ClinicalTrials.gov. PALACE 3: Efficacy and safety study of apremilast to treat active psoriatic arthritis. NCT01212770. July 29, 2014. Available at: http://clinicaltrials.gov/ct2/show/NCT01212770?term=palace+3&rank=1. Accessed July 6, 2015.
- 29. ClinicalTrials.gov. Study to evaluate safety and effectiveness of oral apremilast (CC-10004) in patients with moderate to severe plaque psoriasis (ESTEEM 2). NCT01232283. November 3, 2014. Available at: http://clinicaltrials.gov/ct2/show/study/NCT01232283?term=Esteem+2&rank=1. Accessed July 6, 2015.
- 30.Daliresp (roflumilast) prescribing information. Wilmington, Delaware: AstraZeneca Pharmaceuticals LP; Jul, 2015. [Google Scholar]
- 31.Liu Y, Zhou S, Wan Y, et al. The impact of co-administration of ketoconazole and rifampicin on the pharmacokinetics of apremilast in healthy volunteers. Br J Clin Pharmacol. 2014;78(5):1050–1057. doi: 10.1111/bcp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Zhou S, Nissel J, et al. The pharmacokinetic effect of coadministration of apremilast and methotrexate in individuals with rheumatoid arthritis and psoriatic arthritis. Clin Pharmacol Drug Dev. 2014;3(6):456–465. doi: 10.1002/cpdd.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Accessed July 6, 2015. [Google Scholar]
- 34. Micromedex Solutions Website. Available at: http://0-www.micromedexsolutions.com.liucat.lib.liu.edu/micromedex2/librarian. Accessed July 6, 2015.


