Abstract
Women with hormone receptor–positive, human epidermal growth factor receptor 2− negative breast cancer—the most common subtype—have new options as palbociclib and similar drugs debut. This article outlines the rationale and evidence for their use.
In February 2015, the Food and Drug Administration (FDA) approved palbociclib (Ibrance, Pfizer), in combination with letrozole, as initial endocrine-based therapy for postmenopausal women with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer.1
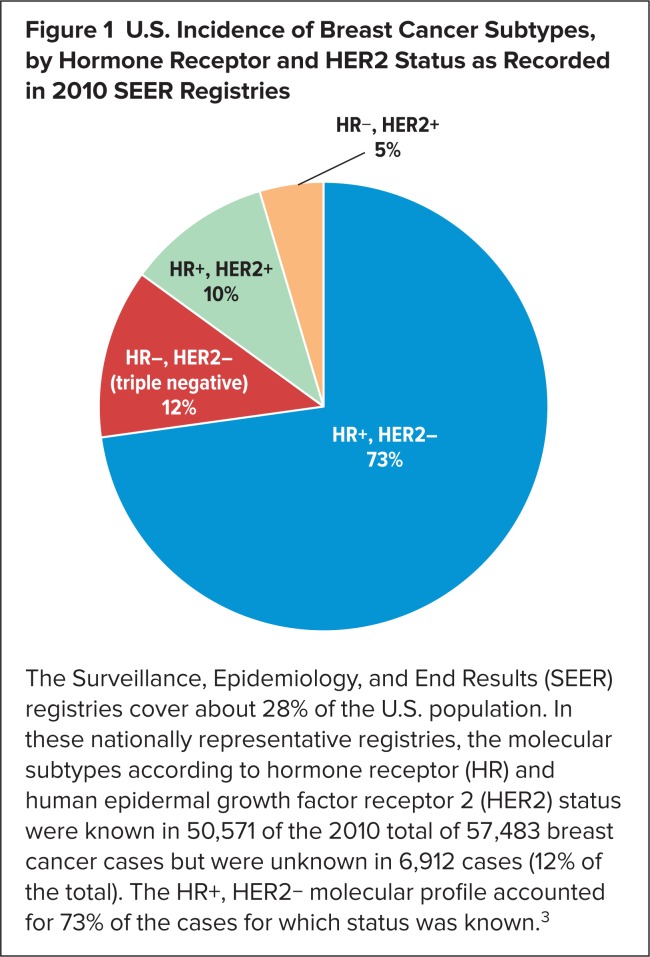
About 20% to 30% of women diagnosed with node-negative breast cancer are believed to progress eventually to metastatic breast cancer.2 Given that hormone receptor (HR)-positive, HER2-negative breast cancer is the most common subtype of breast cancer upon diagnosis in the United States3 (Figure 1), this subtype is also likely to constitute the majority of cases of metastatic breast cancer. An estimated 40,000 U.S. women will die from breast cancer in 2015.4 Over the past several decades, hormonal therapies (Table 1) have emerged as the preferred initial treatment for HR+ disease. Adding palbociclib to one of these drugs, letrozole, nearly doubled median progression-free survival (PFS) versus letrozole alone, from 10.2 to 20.2 months.5
Figure 1.
U.S. Incidence of Breast Cancer Subtypes, by Hormone Receptor and HER2 Status as Recorded in 2010 SEER Registries
The Surveillance, Epidemiology, and End Results (SEER) registries cover about 28% of the U.S. population. In these nationally representative registries, the molecular subtypes according to hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status were known in 50,571 of the 2010 total of 57,483 breast cancer cases but were unknown in 6,912 cases (12% of the total). The HR+, HER2− molecular profile accounted for 73% of the cases for which status was known.3
Table 1.
Hormonal Therapies for HR+ Breast Cancer, by Year of Initial FDA Approval
| Drug | Initial FDA Approval | Class | Indications |
|---|---|---|---|
| Fulvestrant (Faslodex, AstraZeneca) | 2002 | ER antagonist |
|
| Exemestane (Aromasin, Pfizer)* | 1999 | Aromatase inactivator (steroidal) |
|
| Letrozole (Femara, Novartis)* | 1997 | Aromatase inhibitor (nonsteroidal) |
|
| Toremifene (Fareston, ProStrakan) | 1997 | SERM |
|
| Anastrozole (Arimidex, AstraZeneca)* | 1995 | Aromatase inhibitor (nonsteroidal) |
|
| Tamoxifen (Nolvadex, AstraZeneca)* | 1977 | SERM |
|
Generic versions available
SERM = selective estrogen receptor modulator
Palbociclib is the first drug in its class, selective inhibitors of cyclin-dependent kinase (CDK) 4 and CDK6. Pfizer has an ambitious clinical development program in place to investigate other combinations in which palbociclib might benefit women with breast cancer, but Novartis and Eli Lilly also have CDK4/6 inhibitors in late stages of development as novel breast cancer therapies to be used in combination with drugs already on the market or in development. This article will discuss the rationale behind CDK 4/6 inhibition, the evidence supporting the initial indication for palbociclib, and selected phase 2 and phase 3 trials in progress for palbociclib, Lilly’s abemaciclib (LY2832519), and Novartis’s LEE011 (Table 2). CDK4/6 inhibitors are being investigated in other cancers (e.g., gastrointestinal cancer, glioblastoma, head and neck squamous-cell carcinoma, hepatocellular carcinoma, melanoma, non–small-cell lung cancer, prostate cancer, and ovarian cancer), but this article is restricted to their use in breast cancer.
Table 2.
Selected Ongoing Phase 2 and Phase 3 Clinical Trials of CDK4/6 Inhibitors for ER+, HER2− Breast Cancera
| Acronym (If Any), NCT Number | Sponsor, Collaborator(s) | Interventions | Patient Population (All With HR+, HER2− Breast Cancer) | Phase (N) | Primary Endpoint | Start Date Primary Completionb Completion Date |
|---|---|---|---|---|---|---|
| MONARCH-2 NCT02107703 | Lilly | Abemaciclib + fulvestrant vs. fulvestrant + placebo | Postmenopausal women with locally aBrCa or mBrCa after failure on endocrine therapy | Phase 3 (N = 550) | PFS | July 2014 February 2017 February 2020 |
| MONARCH-3 NCT02246621 | Lilly | Abemaciclib + NSAI, placebo + NSAI | mBrCa in postmenopausal women who have not received systemic therapy | Phase 3 (N = 450) | PFS | November 2014 June 2017 July 2021 |
| NCT01857193 | Novartis | LEE011 + exemestane + everolimus and LEE011 + exemestane vs. everolimus + exemestane | Postmenopausal women with locally aBrCa or mBrCa after adjuvant NSAI failure | Phase 1b/2 (N = 185) | PFS, dose-limiting toxicity | September 2013 May 2016 May 2016 |
| MONALEESA-2 NCT01958021 | Novartis | LEE011 + letrozole vs. placebo + letrozole | Postmenopausal women with locoregionally recurrent or mBrCa and no prior systemic treatment | Phase 3 (N = 650) | PFS | December 2013 August 2017 August 2017 |
| MONALEESA-7 NCT02278120 | Novartis | LEE011 + tamoxifen or NSAI + goserelin vs. placebo + tamoxifen or NSAI + goserelin | Pre- or perimenopausal women with locoregionally recurrent or mBrCa and no prior hormonal therapy for aBrCa | Phase 3 (N = 660) | PFS | November 2014 February 2018 February 2018 |
| NCT02088684 | Novartis | LEE011 + buparlisib + fulvestrant vs. LEE011 + BYL719 + fulvestrant vs. LEE011 + fulvestrant | Postmenopausal women with HR+, HER2− locally aBrCa or mBrCa after endocrine therapy and/or chemotherapy | Phase 1b/2 (N = 216) | PFS (phase 2), dose-limiting toxicity (phase 1b) | May 2014 February 2019 February 2019 |
| PALOMA-3 NCT01942135 | Pfizer, AstraZeneca | Palbociclib + fulvestrant vs. fulvestrant + placebo | HR+, HER2− mBrCa progressing after endocrine therapy in women age ≥ 18 years | Phase 3 (N = 417) | PFS | September 2013 July 2015c January 2017 |
| PALOMA-2 NCT01740427 | Pfizer | Palbociclib + letrozole vs. letrozole + placebo | Postmenopausal women with aBrCa and no prior systemic therapy for aBrCa | Phase 3 N = 650) | PFS | February 2013 October 2015 October 2015 |
| NCT01723774 | Washington University | Palbociclib + anastrozole (+ goserelin if premenopausal) | Early (stage II or III) BrCa | Phase 2 (N = 29) | Complete cell-cycle arrest | June 2013 January 2016 February 2016 |
| PALLET NCT02296801 | NSABP Foundation; Pfizer, others | Letrozole alone vs. palbociclib + letrozole (3 regimens—see text) | Postmenopausal women with newly diagnosed primary BrCa | Phase 2 (N = 306) | Change in Ki67 from baseline; cCR | January 2015 July 2017 Not provided |
| NCT02040857 | Dana-Farber; Pfizer | Palbociclib + endocrine therapy (tamoxifen or AI) vs. endocrine therapy | Invasive BrCa (stage II, except T2N0, or stage III) in men and pre- or post-menopausal women | Phase 2 (N = 120) | Treatment discontinuation rate | January 2014 October 2017 June 2019 |
| PEARL NCT02028507 | Spanish Breast Cancer Research Group; Pfizer | Palbociclib + exemestane vs. capecitabine | mBrCa with NSAI resistance within 12 months after end of adjuvant therapy or 1 month after end of NSAI for aBrCa | Phase 3 (N = 348) | iDFS | March 2014 January 2018 January 2018 |
| PENELOPE-B NCT01864746 | German Breast Group; Pfizer | Palbociclib + endocrine therapy vs. endocrine therapy alone | Early-stage HR+, HER2− normal BrCa in women age ≥ 18 years at high risk of relapse after surgery and chemotherapy | Phase 3 (N = 800) | iDFS | November 2013 December 2019 November 2021 |
Source: ClinicalTrials.gov
Primary completion is the final data collection date for the primary outcome measure.
Terminated early, on April 15, 2015, because of efficacy.
aBrCa = advanced breast cancer; AI = aromatase inhibitor; BrCa = breast cancer; cCR = clinical complete response (number of patients with resolution of measurable lesions or no new lesions or other signs of disease progression); iDFS = invasive disease-free survival (see glossary for definition); mBrCa = metastatic breast cancer; NSAI = nonsteroidal aromatase inhibitor (i.e., anastrozole or letrozole); PFS = progression-free survival
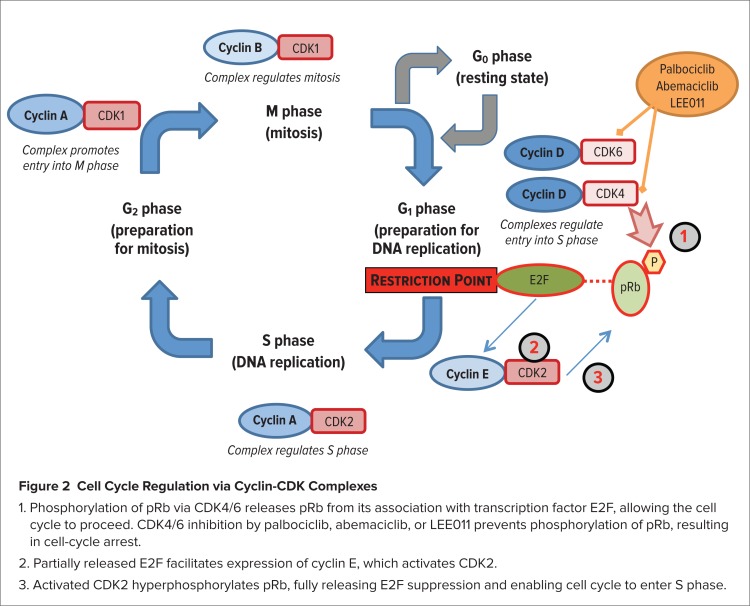
CDK4/6 act as a node within a highly complex intracellular signaling system consisting of positive and negative feedback loops that regulate progression of the cell cycle.6 CDK4/6 are downstream of various mitogenic signals that promote cellular proliferation. CDK4/6 play a key role in regulating progression of the cell cycle from the G1 phase to the S phase, in which DNA synthesis occurs (Figure 2). CDK4/6 form a complex with cyclin D that phosphorylates the retinoblastoma protein (pRb), which suppresses E2F transcription factors that otherwise would enable the cell cycle to progress. The phosphorylated pRb partially ceases its inhibition of E2F, which directs the expression of CDK2. Forming a complex with cyclin E, CDK2 then hyperphosphorylates pRb, resulting in full release of E2F transcription factors, the expression of other proteins, and progression of the cell cycle into the S phase.
Figure 2.
- Phosphorylation of pRb via CDK4/6 releases pRb from its association with transcription factor E2F, allowing the cell cycle to proceed. CDK4/6 inhibition by palbociclib, abemaciclib, or LEE011 prevents phosphorylation of pRb, resulting in cell-cycle arrest.
- Partially released E2F facilitates expression of cyclin E, which activates CDK2.
- Activated CDK2 hyperphosphorylates pRb, fully releasing E2F suppression and enabling cell cycle to enter S phase.
Several mechanisms lead to dysregulation of this process in human cancer: amplification or overexpression of cyclin D; amplification of CDK4; loss of p16, a CDK4/6 inhibitor; and, rarely, an activating mutation of CDK4.7 Inhibition of CDK4/6 has the potential to counteract all of these mechanisms. Early CDK inhibitors were nonselective and inhibited CDKs in addition to CDK4 and CDK6, including CDKs outside the cell cycle. Palbociclib, abemaciclib, and LEE011, however, are selective for CDK4/6.8–11
The development of palbociclib can be traced to research in the 1970s and 1980s that identified key molecular regulators of the cell cycle, which led to the 2001 Nobel Prize in physiology or medicine for Leland H. Hartwell, Tim Hunt, and Sir Paul M. Nurse. By 1995, CDK4/6 had been identified as a promising pharmaceutical target and Parke-Davis scientists in Ann Arbor, Michigan, began trying to develop a selective CDK4/6 inhibitor.12 Palbociclib, initially known as PD-0332991, was synthesized in 2001, but corporate acquisitions and mergers delayed its development. In 2000, Pfizer had acquired Warner-Davis/Parke-Lambert, and in 2003 Pfizer acquired Pharmacia and its ample oncology pipeline. Although a phase 1 trial of palbociclib was conducted in 2003, a phase 2 trial didn’t begin until 2009. Research by University of California at Los Angeles oncologist Dennis Slamon, MD, whose earlier work was instrumental in developing trastuzumab (Herceptin, Genentech), helped move palbociclib along. In 2007, Dr. Slamon and colleagues tested palbociclib in breast cancer cell lines, expecting to find that it worked best against triple-negative tumor cells.12 Identified by the absence of staining for estrogen receptors, progesterone receptors, and HER2 (trastuzumab’s target), triple-negative breast cancer constitutes an aggressive but relatively uncommon form of the disease (Figure 1). Instead, the researchers found that palbociclib was most effective against cell lines that were ER+.13 Next, they added palbociclib to standard antiestrogen therapy with letrozole in 12 women with ER+ metastatic breast cancer. The results were sufficiently encouraging to spur Pfizer to launch a phase 2 trial, PALOMA-1, in 2009.
PALOMA-1 (NCT00721409)
The initial indication for palbociclib is based on PALOMA-1, an open-label, phase 2 trial.5 The intent-to-treat population comprised 165 postmenopausal women with ER+, HER2− metastatic or locally recurrent breast cancer not amenable to surgery. Patients were excluded if they had undergone any previous treatment for advanced breast cancer or if they had received letrozole as neoadjuvant or adjuvant treatment 12 months or less prior to study entry. Patients received letrozole 2.5 mg daily plus palbociclib 125 mg (n = 84) or letrozole alone (n = 81); palbociclib was taken in 28-day cycles consisting of three weeks of daily palbociclib followed by one week without palbociclib.
After a median follow-up of 29.6 months, median PFS, the primary outcome, was 10.2 months in the letrozole-alone group versus 20.2 months in the letrozole-plus-palbociclib group (hazard ratio [HR], 0.488; 95% confidence interval [CI], 0.319–0.748; P < .001). The difference in overall survival (OS), a secondary endpoint, was not statistically significant between the palbociclib-plus-letrozole group (37.5 months) versus the letrozole-alone group (33.3 months) (HR, 0.813; 95% CI, 0.492–1.345; P = .42).
Neutropenia, leukopenia, and fatigue were the most common adverse events in the palbociclib-plus-letrozole group. Neutropenia of grades 1–2, 3, and 4 occurred in 20%, 48%, and 6% of patients receiving palbociclib plus letrozole, respectively, versus 4%, 1%, and 0% of patients receiving letrozole alone. No cases of neutropenic fever were reported, however, and no patients discontinued treatment because of neutropenia. Disease progression was the main reason for study discontinuation: 50% in the palbociclib-plus-letrozole group versus 70% in the letrozole-alone group.
PALBOCICLIB CLINICAL TRIALS IN PROGRESS
Palbociclib, in combination with other drugs, is being evaluated in phase 2 and phase 3 clinical trials enrolling patients with a broad range of HR+, HER2− breast cancer: with anastrozole (with or without goserelin depending on menopausal status) as neoadjuvant therapy for early breast cancer; with letrozole as neoadjuvant therapy for postmenopausal women with early breast cancer; with endocrine therapy for stage II or III breast cancer; with fulvestrant for metastatic breast cancer in women of any menopausal status; and with letrozole for postmenopausal women with metastatic breast cancer.
PALOMA-2 (NCT01740427)
The initial indication for palbociclib was granted under accelerated approval, which may be contingent on the results of a confirmatory trial.14 Enrolling 650 patients (and no longer recruiting participants), PALOMA-2 is the confirmatory phase 3 trial for PALOMA-1.15 As such, its inclusion and exclusion criteria are similar—patients must be postmenopausal women with ER+, locoregionally recurrent or metastatic breast cancer not amenable to curative therapy, and they must have received no prior systemic therapy for advanced ER+ breast cancer. Patients who had received letrozole or anastrozole as neoadjuvant or adjuvant treatment were excluded if their disease had progressed within 12 months of completion of that treatment.
Unlike PALOMA-1, an open-label trial, PALOMA-2 is double-blind and placebo-controlled, with patients being randomized to receive either palbociclib plus letrozole or letrozole plus placebo. The drugs are administered as they were in PALOMA-1—letrozole (or placebo) once daily, and a 28-day cycle consisting of three weeks of daily palbociclib and one week without palbociclib. The primary outcome is PFS, defined as the time from the first dose of study treatment to documented tumor progression or death from any cause. OS and quality-of-life (QOL) indicators are among the secondary outcome measures.
PALOMA-3 (NCT01942135)
PALOMA-3 was terminated earlier than expected, on April 15, 2015, rather than in July 2015, because the primary endpoint, PFS, had been met. At the 2015 annual meeting of the American Society of Clinical Oncology, it was reported that median PFS was 9.2 months in the palbociclib-plus-fulvestrant arm versus 3.8 months in the fulvestrant-plus-placebo arm (P < .001).16 PALOMA-3 was a double-blind, phase 3 trial (N = 427) that investigated the use of palbociclib plus fulvestrant versus fulvestrant plus placebo in women 18 years of age and older of any menopausal status with metastatic HR+, HER2− breast cancer or locally advanced disease not amenable to curative therapy.17 Their disease must have progressed within 12 months after adjuvant therapy or within one month after endocrine therapy for advanced/metastatic breast cancer. In conjunction with study treatment, premenopausal and perimenopausal women were required to take goserelin. Patients were excluded if they had received prior treatment with a CDK inhibitor, fulvestrant, or everolimus or any other inhibitor of the PI3K/mTOR pathway.
OS and QOL indicators are among the secondary outcome measures.
PEARL (NCT02028507)18
This open-label, phase 3 study (N = 348) compares palbociclib plus exemestane versus chemotherapy with capecitabine in postmenopausal women with HR+, HER2− metastatic breast cancer resistant to the nonsteroidal aromatase inhibitors (NSAIs) anastrozole or letrozole. Recurrence must have occurred while the patient was on adjuvant therapy with the NSAI or within 12 months after its end, or within one month after the end of NSAI treatment for advanced cancer.
The primary outcome is PFS; OS is among the secondary outcome measures.
NCT01723774
This open-label study (N = 29) is half of a pair of phase 2 trials being conducted to investigate the use of either palbociclib or MK-2206, an AKT inhibitor that disrupts the PI3K/AKT/mTOR signaling pathway, in conjunction with anastrozole as neoadjuvant therapy in women 18 years of age and older with stage II or stage III ER+, HER2− breast cancer. Cynthia Ma, MD, PhD, an oncologist at Washington University in St. Louis, Missouri, is the principal investigator in each study. These studies are aimed at expanding the role of oncogene testing to guide breast cancer treatment.19,20
For the most part, a patient’s assignment to the AKT inhibition trial (NCT01776008)21 or the CDK4/6 inhibition trial is determined by genomic evaluation at baseline of tumor PIK3CA. This gene expresses the catalytic subunit of PI3K.
All patients initially take anastrozole for the first 28 days of cycle 0, along with goserelin as estrogen-deprivation therapy if they are premenopausal. In the meantime, the tumor is sequenced. If the tumor is PIK3CA-mutant, the patient begins treatment with the AKT inhibitor plus anastrozole after one month. If the tumor is PIK3CA wild-type, the patient begins treatment with palbociclib plus anastrozole for four 28-day cycles. Immediately before the initiation of combination therapy in cycle 1, the tumor is rebiopsied, and a third biopsy is obtained after a patient has been on combination therapy for two weeks to evaluate an early tumor response biomarker, Ki67, a nuclear protein produced during all phases of the cell cycle except G0. If Ki67 is less than 10%, indicating tumor responsiveness, the patient will receive four months of combination therapy followed by surgery. If Ki67 is greater than 10%, indicating tumor resistance, the patient will go off study to avoid futile treatment.
Ki67 values are used in the primary outcome measures of these studies. In the AKT inhibition study, the primary outcome is pathological complete response (pCR) based on the tumor Ki67 value on, at the latest, day 17 of cycle 1. The primary outcome measure in the study of CDK4/6 inhibition by palbociclib is complete cell-cycle arrest, in women without PIK3CA hot-spot mutation, defined as Ki67 of less than 2.7% following two weeks of neoadjuvant palbociclib. Among the secondary outcome measures in the CDK4/6 inhibition study are the Preoperative Endocrine Prognostic Index (PEPI; see glossary), which incorporates Ki67, at 30 days following the last day of study treatment; and the percentage change in Ki67 from baseline to the completion of two weeks of palbociclib therapy.
NCT0204085722
For women with HR+, HER2− invasive breast cancer (defined in this study as stage II, except T2N0, or stage III breast cancer), endocrine therapy is commonly employed. For premenopausal women the preferred therapy is tamoxifen, and for postmenopausal women it is tamoxifen or an aromatase inhibitor—anastrozole, letrozole, or exemestane. In this open-label phase 2 pilot study to assess the feasibility of using palbociclib in combination with endocrine therapy, participants (N = 120) will enter the study on an endocrine therapy appropriate for their menopausal status, and from that point on they will receive combination therapy consisting of their endocrine treatment plus palbociclib. The primary outcome is the treatment discontinuation rate at two years.
PALLET (NCT02296801)23
Postmenopausal women with ER+, HER2− early invasive breast cancer will be enrolled in this open-label, phase 2 trial (N = 306) to evaluate palbociclib with or without letrozole as neoadjuvant therapy. Patients are being randomized in a 3:2:2:2 ratio to one of four treatment arms: 1) letrozole alone; 2) letrozole for two weeks followed by letrozole plus palbociclib for 12 weeks; 3) palbociclib for two weeks followed by palbociclib plus letrozole for 12 weeks; and 4) letrozole plus palbociclib for 14 weeks. Whenever palbociclib is used, it will be taken on a four-week cycle consisting of three weeks on treatment and one week off. Patients will continue taking letrozole up until the time of surgery, scheduled for 15 to 18 weeks after randomization.
PALLET has two coprimary outcomes: measurement of Ki67 (percentage of positive tumor cells) from baseline to week 14, and complete clinical response, defined as the number of patients with resolution of measurable lesions or no new lesions or other signs of disease progression at 14 weeks compared with baseline.
PENELOPE-B (NCT01864746)24
This phase 3 study (N = 800) is evaluating palbociclib versus placebo in women 18 years of age and older with HR+, HER2-normal primary invasive breast cancer at high risk of relapse after at least 16 weeks of neoadjuvant chemotherapy. High-risk patients are identified through a validated measure that combines four variables: the clinical stage before neoadjuvant therapy (CS), the pathological stage after neoadjuvant therapy (PS), ER status (E), and grade (G)—CPS-EG.25 To be included in this trial, patients must have a CPS-EG score of at least 3 on a scale of 0 to 6. Patients receive palbociclib or placebo for 13 cycles, each consisting of three weeks on treatment and one week off. The primary outcome is invasive disease-free survival (iDFS; see glossary).
MONARCH-2 (NCT02107703)26
This double-blind, phase 3 study (N = 550) compares abemaciclib plus fulvestrant versus placebo plus fulvestrant in postmenopausal women with HR+, HER2− locally advanced or metastatic breast cancer. To be eligible, patients must have met one of the following five sets of criteria: 1) relapsed while on neoadjuvant or adjuvant endocrine therapy; 2) relapsed within one year after completion of adjuvant endocrine therapy; 3) relapsed more than one year after completion of adjuvant endocrine therapy and then relapsed again after first-line endocrine therapy with an antiestrogen or an aromatase inhibitor; 4) presented with de novo locally advanced or metastatic disease without having received any endocrine therapy; or 5) presented with de novo locally advanced or metastatic disease followed by relapse after first-line endocrine therapy.
Patients are assigned to oral placebo or abemaciclib 150 mg every 12 hours in 28-day cycles. They receive intramuscular fulvestrant 500 mg (administered as two 250-mg injections) on day 1 and day 15 of cycle 1, and then on day 1 of subsequent cycles.
PFS is the primary outcome measure. OS and health-related quality of life (HRQOL) measures are among the secondary outcomes.
MONARCH-3 (NCT02246621)27
This double-blind, phase 3 study (N = 450) compares abemaciclib plus an NSAI (anastrozole or letrozole) versus an NSAI plus placebo in postmenopausal women with HR+, HER2− locoregionally recurrent or metastatic breast cancer for which they have not received systemic therapy. Patients receive an NSAI once daily and abemaciclib or placebo every 12 hours.
PFS is the primary outcome. OS and HRQOL indicators are among the secondary outcome measures.
LEE011 CLINICAL TRIALS IN PROGRESS
NCT0185719328
This open-label study (N = 185) consists of two parts. The phase 1b part seeks to determine the maximum tolerated dose of LEE011 plus exemestane and everolimus used as triple therapy, as the dose recommended for phase 2 studies, or both. The phase 2 part compares triple therapy with LEE011, exemestane, and everolimus and double therapy with LEE011 plus exemestane versus double therapy with exemestane plus everolimus in postmenopausal women with ER+, HER2− locally advanced or metastatic breast cancer. Patients must have had disease recurrence while on adjuvant treatment with anastrozole or letrozole or within 12 months of the end of such adjuvant treatment, or they must have progressed while on letrozole or anastrozole treatment for locally advanced or metastatic breast cancer or within one month of the end of such treatment. This is the population in which everolimus was studied in BOLERO-2, in combination with exemestane,29 which led to its indication for treatment of postmenopausal women with HR+, HER2− advanced breast cancer after failure of anastrozole or letrozole.
LEE011 is taken orally once daily on a 28-day cycle consisting of 21 days on the drug and seven days off; exemestane and everolimus are taken orally once daily.
The coprimary outcome measures are the incidence of dose-limiting toxicity and PFS. OS is among the secondary outcome measures.
MONALEESA-2 (NCT01958021)30
This double-blind, phase 3 study (N = 650) compares LEE011 plus letrozole versus placebo plus letrozole in postmenopausal women with HR+, HER2− locoregionally recurrent or metastatic breast cancer who have received no prior systemic treatment for advanced disease. Patients who received neoadjuvant or adjuvant therapy for breast cancer are eligible, but if that therapy included letrozole or anastrozole, the disease-free interval must be at least 12 months from the completion of treatment at the time of randomization. Patients will receive LEE011 600 mg once daily or placebo in cycles consisting of three weeks on treatment and one week off. Letrozole will be taken daily.
PFS is the primary outcome. OS and HRQOL are among the secondary outcome measures.
MONALEESA-7 (NCT02278120)31
This double-blind, phase 3 study (N = 660) is comparing LEE011 plus tamoxifen or an NSAI (anastrozole or letrozole) plus goserelin versus placebo plus tamoxifen or NSAI plus goserelin in premenopausal or perimenopausal women 18 to 59 years of age with HR+, HER2− locoregionally recurrent or metastatic breast cancer. Women who have received neoadjuvant or adjuvant therapy for breast cancer are eligible, but women who have received hormonal therapy for advanced breast cancer are not eligible unless they received no more than 14 days of tamoxifen or an NSAI prior to randomization.
LEE011 and placebo will be taken on a four-week cycle consisting of three weeks on treatment and one week off. The NSAI will be taken daily. Goserelin will be administered via subcutaneous injection every 28 days.
The primary outcome measure is PFS. OS and QOL measures are among the secondary outcomes.
NCT0208868432
This two-part study consists of a phase 1b part and an open-label phase 2 part to study three regimens involving LEE011 and two other investigational drugs from Novartis in combination with fulvestrant in postmenopausal women with HR+, HER2− advanced breast cancer. The regimens are LEE011 with fulvestrant; LEE011 with fulvestrant plus buparlisib (BKM120), a pan-class I PI3K inhibitor;33 and LEE011 with fulvestrant plus BYL719, an inhibitor of PI3Kα.34 In either part, patients may have received an unlimited number of lines of endocrine therapy. In phase 1b they must have had no more than two lines of chemotherapy for metastatic disease, and in phase 2 no more than one line of chemotherapy.
The primary outcomes are dose-limiting toxicities in phase 1b and PFS in phase 2. OS is among the secondary outcome measures in phase 2.
DISCUSSION
In 2012, the FDA’s approval of the mTOR inhibitor everolimus, in combination with the hormonal therapy exemestane, provided a regimen offering dual inhibition of the PI3K/AKT/mTOR pathway and the ER pathway as a new option for postmenopausal women with HR+, HER2− advanced breast cancer. This combination is indicated as second-line therapy for patients who have failed on letrozole or anastrozole. Another new option became available in early 2015 when the FDA approved the CDK4/6 inhibitor palbociclib, in combination with letrozole, as initial endocrine-based therapy for women with ER+, HER2− metastatic breast cancer. Following on these developments, it might be reasonable to compare palbociclib plus exemestane with everolimus plus exemestane, or to compare triple-combination therapy with palbociclib, everolimus, and exemestane to dual therapy with everolimus and exemestane. It is unlikely that either Pfizer or Novartis would sponsor such a trial, but since Novartis is developing its own CDK4/6 inhibitor, LEE011, a comparable trial is in progress (NCT01857193) in which the proven dual therapy with everolimus plus exemestane is being compared to dual therapy with LEE011 and exemestane and to triple therapy with LEE011, everolimus, and exemestane. The results of this phase 2 study should be known relatively soon, possibly by the middle of 2016.35
In most of the phase 2 and phase 3 trials of CDK4/6 inhibitors discussed here, the primary endpoint is PFS, a commonly used surrogate endpoint that is often sufficient for regulatory approval. OS is usually being investigated as a secondary endpoint. So far, no statistically significant benefit in OS has been demonstrated in either BOLERO-2, which led to the indication for everolimus in combination with exemestane, or in PALOMA-1, which led to the initial FDA approval of palbociclib. In BOLERO-2, median OS was 31.0 months in the everolimusexemestane group versus 26.6 months in the exemestane-placebo group (HR, 0.89; 95% CI, 0.73–1.10; P = .14);36 in PALOMA-1, median OS was 37.5 months in the palbociclibletrozole group versus 33.3 months in the letrozole-alone group (HR, 0.81; 95% CI, 0.49–1.34; P = .42).5 Perhaps a meaningful survival rate for palbociclib plus letrozole eventually will be demonstrated in the large PALOMA-2 clinical trial, and perhaps it will be demonstrated for palbociclib, abemaciclib, and LEE011 in some of the trials in progress.
In the absence of an OS benefit shown thus far for the addition of everolimus or palbociclib to an endocrine therapy, health-related quality-of-life (HRQOL) measures gain added importance in the treatment of patients with advanced breast cancer. In PALOMA-1, no HRQOL data were reported, but they are being collected in phase 3 studies.5 In BOLERO-2, the median time to definitive deterioration (TDD) in HRQOL, defined as a 5% decrease in HRQOL versus baseline, was 8.3 months in the everolimus-exemestane group versus 5.8 months in the placebo-exemestane group (HR, 0.74; 95% CI, 0.58–0.95; P = .008).37 Using a more stringent measure, a minimally important decrease of 10 points in the global health status score, median TDD was 11.7 months in the everolimus-exemestane group versus 8.4 months in the placebo-exemestane group (HR, 0.8; 95% CI, 0.61–1.06; P = .10). The question of whether a modest improvement in TDD is important to patients, physicians, and payers remains unresolved in the absence of an OS benefit and in light of the high cost of these drugs (per Red Book pricing, palbociclib costs $11,820 for four weeks of treatment [21 days on treatment, seven days off], while a 28-day supply of everolimus has a wholesale price of about $12,645).38 In the phase 2 trial NCT01857193, in which everolimus plus exemestane is being compared with LEE011 plus exemestane with and without everolimus, QOL data are not being collected, but if this study leads to a larger phase 3 clinical trial, HRQOL data presumably would be generated for secondary outcome measures showing how mTOR inhibition compares with CDK4/6 inhibition in this important dimension.
In PEARL (NCT02028507), palbociclib plus exemestane is being investigated in postmenopausal patients with HR+, HER2− metastatic breast cancer whose disease showed resistance to adjuvant anastrozole or letrozole. This trial is similar to BOLERO-2, except that instead of using exemestane as the comparator it substitutes capecitabine, a chemotherapy agent regarded as a preferred single agent for recurrent or metastatic breast cancer.39 Meanwhile, in MONALEESA-2, LEE011 plus letrozole is being compared with letrozole plus placebo in postmenopausal women who have not received systemic therapy for their advanced breast cancer, making this study similar to PALOMA-1. Together with the MONARCH studies of abemaciclib, these studies should clarify whether a hormonal therapy combined with mTOR inhibition or CDK4/6 inhibition is the better approach to advanced HR+, HER2− breast cancer. In the meantime, the early termination of PALOMA-3 positioned palbociclib for FDA approval of its second indication: treatment, in combination with fulvestrant, of women of any menopausal status who have HR+, HER2− metastatic breast cancer that has progressed during or after endocrine therapy.
Several of these studies are notable for their attempts to use biomarkers to refine therapy by identifying poor responders at an early point after the initiation of therapy. This approach has the potential to provide two benefits: avoiding the expenditure of time and money on a treatment likely to be futile, and speedily enabling the patient to begin treatment with a different therapy that may be more helpful.
CONCLUSION
In the relatively near future, the phase 2 and 3 trials of CDK4/6 inhibitors now in progress are likely to result in the availability of three CDK4/6 inhibitors for the treatment of breast cancer, with multiple indications for each agent. This will substantially increase the options available for patients with HR+, HER2− breast cancer, along with provoking discussions among members of P&T committees as they determine the appropriate formulary placement for each agent. All lines of therapy, from neoadjuvant and adjuvant therapy to third-line metastatic cancer, and in premenopausal or postmenopausal women, probably will be covered by one or more of these drugs, used in combination with one or more agents.
Owing to demographic changes, the incidence of female breast cancer in the United States is predicted to reach 268,000 cases by 2020—an increase of 17.8% compared with the 227,000 cases in 2010.40 The emergence of a new class of targeted therapy for the most common subtype of breast cancer therefore would appear to be a timely development, especially if the use of CDK4/6 inhibition to treat patients with early breast cancer is demonstrated to delay or prevent disease progression.
GLOSSARY
- Advanced breast cancer
metastatic breast cancer or locally recurrent breast cancer not amenable to surgery, or both. Because advanced breast cancer is incurable, goals of therapy are to prolong survival, palliate symptoms, and optimize quality of life. Therapy usually is systemic–hormonal treatment, chemotherapy, or targeted therapy
- AI
aromatase inhibitor. See also nonsteroidal aromatase inhibitor (NSAI)
- Allred score
scoring system to stratify estrogen receptor status to predict response to hormone therapy with tamoxifen or an aromatase inhibitor. Based on the proportion of cells testing positive for estrogen receptors (no ER+ cells, 0; 1% or less, 1; 1% to 10%, 2; 11% to 33%, 3; 34% to 66%, 4; more than 67%, 5) and the intensity of immunohistochemical staining (negative, 0; weak, 1; intermediate, 2; strong, 3). Adding scores for ER+ proportion and intensity yields a scale of 0 to 8, with higher scores indicating increasing percentages and greater intensity. A total score of 0–1 predicts no benefit from hormone therapy; 2–3, slight chance of benefit; 4–6, moderate chance; 7–8, good chance
- Aromatase inhibitors (AI)
drugs that block the enzyme aromatase, which converts adrenal androgens into estrone and estradiol; this is the primary pathway by which estrogens are produced in postmenopausal women. Blocking aromatase therefore may help control tumor growth that is stimulated or maintained by estrogens. AIs are classified as nonsteroidal (NSAI–anastrozole, letrozole) or steroidal (exemestane)
- Buparlisib (BKM120)
an investigational, orally available, selective pan-class I PI3K inhibitor being developed by Novartis. It inhibits the four class I PI3K isoforms as well as the most common PI3Kα somatic mutations. In breast cancer, in addition to the phase 1b/2 study with LEE011 mentioned in this article, two phase 2 studies in combination with fulvestrant (BELLE-2 [NCT01610284] and BELLE-3 [NCT01633060]), and a phase 2/3 study in combination with paclitaxel (BELLE-4 [NCT01572727]) are in progress
- BYL719
an investigational, orally available PI3Kα-specific inhibitor being developed by Novartis
- CDK
cyclin-dependent kinases, a family of serine/threonine protein kinases. Four CDKs (1, 2, 4, and 6) are activated, by cyclins and sometimes by phosphorylation, at different points of the cell cycle. Upon activation, CDKs phosphorylate certain proteins. CDK levels remain constant during the cell cycle
- CDK1
forms complex with cyclin A that facilitates transition from the G2 phase to the M phase of the cell cycle; also forms complex with cyclin B that is activated during mitosis
- CDK2
forms complex with cyclin E that facilitates transition from the G1 phase to the S phase of the cell cycle; forms complex with cyclin A that is activated during S phase
- CDK4
forms complex with cyclin D1, D2, and D3 that is activated during G1 phase. Highly homologous with CDK6
- CDK4/6
shorthand for “CDK4 and CDK6.”
- CDK6
forms complex with cyclin D1, D2, and D3 that is activated during G1 phase of cell cycle. Highly homologous with CDK4
- Cell cycle
cycle of four phases through which a cell progresses during proliferation: M (mitosis), G1. S (DNA replication), and G2. After mitosis, a cell may enter the senescent G0 phase before entering G1. See Choi 20146 for an updated overview
- Cyclins
proteins that activate CDK throughout the cell cycle. Levels of most cyclins fluctuate periodically during the cycle; cyclin D is an exception. At the end of a cell-cycle phase, cyclins are degraded via proteolysis
- Cyclin A
forms complex with CDK2 that is active during the S phase of the cell cycle; also forms complex with CDK1 that facilitates transition from the G2 phase to the M phase
- Cyclin B
forms complex with CDK1 that is active during mitosis
- Cyclin D
three types of cyclin D (D1, D2, and D3) bind to CDK4 and CDK6, creating complexes necessary for entry into the G1 phase of the cell cycle. The cell synthesizes cyclin D whenever growth factors are present
- Cyclin E
forms complex with CDK2 that regulates progression from G1 to S phase of cell cycle
- Early-stage breast cancer
invasive breast cancer without distant metastases (i.e., American Joint Committee on Cancer stage I–III). In this context, high-risk refers to patients with early-stage breast cancer who have a high risk of distant disease recurrence and death despite use of optimal local and systemic adjuvant therapy
- Everolimus (Afinitor, Novartis)
inhibitor of mTOR activity indicated in combination with exemestane for treatment of postmenopausal women with ER+, HER2− advanced breast cancer after the failure of letrozole or anastrozole. The mTOR pathway (PI3K/AKT/mTOR) is dysregulated in several human cancers. Everolimus binds to an intracellular protein, FKBP-12, which leads to formation of a complex that inhibits mTOR kinase activity
- Exemestane (Aromasin, Pfizer)
a steroidal aromatase inactivator that acts as a false substrate for aromatase and is processed to an intermediate that binds irreversibly to the active site of the enzyme, causing its inactivation. Lowers circulating estrogen concentrations in postmenopausal women
- Fulvestrant (Faslodex, AstraZeneca)
estrogen receptor antagonist indicated for treatment of postmenopausal women with ER+ metastatic breast cancer with disease progression following antiestrogen therapy
- G0
a quiescent state that a cell can enter prior to entering the G1 phase of the cell cycle. Most cells are in G0 most of the time
- G1
phase of cell cycle during which cell prepares for DNA replication during the S phase
- HER2-negative (HER2−)
breast cancer tissue classified as HER2− does not overexpress these receptors. Patients with HER2− tumors are unlikely to respond to therapies that target HER2 (e.g., Herceptin). About 80% of patients with advanced breast cancer are HER2− (sometimes described as HER2-normal)
- HR-positive (HR+)
breast cancer tumors classified as HR+ are estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), or both
- iDSF
invasive disease-free survival. Composite endpoint used in early-stage (I to IIIa) breast cancer adjuvant trials. Includes ipsilateral recurrence in the same breast parenchyma as the original primary tumor, regional invasive recurrence (invasive breast cancer in axilla, regional lymph nodes, chest wall, or skin of ipsilateral breast), distant recurrence, death from any cause, death from unknown cause, invasive contralateral breast cancer, and second primary nonbreast invasive cancer41
- Ki67
a nuclear protein expressed in all phases of the cell cycle except G0, and therefore used as a biomarker of cell proliferation. Usually measured via immunohistochemical assays, Ki67 levels may be prognostic in early breast cancer and predictive of treatment benefit
- Mitosis
the process by which a cell divides into two new daughter cells, each with a complete set of chromosomes from the parent cell
- mTOR
mammalian target of rapamycin, a serine-threonine kinase that is a link in the PI3K/AKT/mTOR signaling pathway, which is dysregulated in breast cancer and other cancers. Everolimus inhibits mTOR activity
- NSAI
nonsteroidal aromatase inhibitor (anastrozole, letrozole)
- p16
a regulatory protein (along with p15, p18, and p19, all known as INK4 proteins) that inhibits catalytic activity of CDK4/6 by blocking the binding site of cyclin D but does not directly affect other CDK complexes; pRb suppresses expression of p16 and other INK4 proteins
- Pathological complete response (pCR)
a validated predictor of disease-free and overall survival. Often used as an endpoint to support accelerated approval in clinical trials of neoadjuvant systemic therapy for breast cancer
- PEPI (Preoperative Endocrine Prognostic Index)
a tool to evaluate relapse-free survival (RFS) or breast cancer-specific survival (BCSS), comprising four variables: tumor size (T1/2, 0 points; T3/4, 3 points), node status (negative, 0 points; positive, 3 points), ER status (Allred score 0–2, 3 points; Allred score 3–8, 0 points); Ki67 level (2.7% or less, 0 points; more than 2.7% to 7.3%, 1 point; more than 7.3% to 19.7%, 1 point if RFS, 2 points if BCSS; 19.7% to 53.1%, 2 points if RFS, 3 points if BCSS; more than 53.1%, 3 points)
- PI3K
phosphatidylinositol 3-kinases. Family of kinases involved in signal transduction, cell metabolism, and cell survival. Divided into three classes, I, II, and III, based on substrate specificity and structure. Class I PI3K, most often implicated in cancer because of its involvement in cell growth, survival, and metabolism, is a heterodimeric enzyme consisting of a p110 catalytic subunit and a p85 regulatory subunit. Class I is further divided into class IA (p110, p110β, p110δ) and class IB (p110γ)
- PIK3CA
gene encoding p110α, the catalytic subunit of PI3K. PI3K is a link in the PI3K/AKT/mTOR signaling pathway. PIK3CA mutations are associated with many cancers, including breast cancer. PIK3CA is second only to the suppressor gene TP53 as the most frequently mutated gene in breast cancer; “hot spots” accounting for 80% of PIK3CA mutations are found in exons 9 and 20
- pRb
retinoblastoma protein. A tumor suppressor that, in concert with CDK4/6, inhibits cell proliferation by binding to E2F transcription factors
- SERM
selective estrogen receptor modifier. A drug that acts like an antiestrogen in some tissues, notably breast cancer, but like an estrogen in other tissues (e.g., uterus and bone). Tamoxifen and toremifene are SERMs.
REFERENCES
- 1.Food and Drug Administration FDA approves Ibrance for post-menopausal women with advanced breast cancer. Feb 3, 2015. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm432871.htm. Accessed May 22, 2015.
- 2.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society Cancer facts and figures, 2015. 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed May 22, 2015.
- 5.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 6.Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014;33(15):1890–1903. doi: 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 7.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20(13):3379–3382. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- 8.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1437. [PubMed] [Google Scholar]
- 9.Dempsey JA, Chan EM, Burke TF, Beckmann RP. LY2835219, a selective inhibitor of CDK4 and CDK6, inhibits growth in pre-clinical models of human cancer. Presentation at the American Association of Cancer Research Annual Meeting; April 6–10, 2013; Washington, D.C.. Abstract published in Cancer Res 2013;73(8 suppl):LB-122. [Google Scholar]
- 10.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Loo A, Chopra R, et al. LEE011: an orally bioavailable, selective small molecule inhibitor of CDK4/6–reactivating Rb in cancer. Presentation at the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; October 19–23, 2013; Boston, Massachusetts. Abstract published in Mol Cancer Ther 2013;12(11 suppl):PR02. [Google Scholar]
- 12.Garber K. The cancer drug that almost wasn’t. Science. 2014;345(6199):865–867. doi: 10.1126/science.345.6199.865. [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfizer. Pfizer receives U.S. FDA accelerated approval of Ibrance (palbociclib). February 3, 2015. Available at: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_u_s_fda_accelerated_approval_of_ibrance_palbociclib. Accessed May 22, 2015.
- 15. ClinicalTrials.gov. A study of palbociclib (PD-0332991) + letrozole vs. letrozole for 1st line treatment of postmenopausal women with ER+/HER2− advanced breast cancer (PALOMA-2). NCT01740427. Available at: https://clinicaltrials.gov/ct2/show/NCT01740427. Accessed May 22, 2015.
- 16.Turner NC, Ro J, Andre F, et al. PALOMA3: A double-blind, phase III trial of fulvestrant with or without palbociclib in pre- and post-menopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer that progressed on prior endocrine therapy. Presentation at the 2015 American Society of Clinical Oncology Annual Meeting; Chicago, Illinois. May 29–June 2, 2015; Abstract published in J Clin Oncol 2015;33(15 suppl):LBA502 Available at: http://meetinglibrary.asco.org/print/1982781. Accessed July 7, 2015. [Google Scholar]
- 17. ClinicalTrials.gov. Palbociclib (PD-0332991) combined with fulvestrant in hormone receptor+ HER2-negative metastatic breast cancer after endocrine failure (PALOMA-3). NCT01942135. Available at: https://clinicaltrials.gov/ct2/show/NCT01942135. Accessed May 22, 2015.
- 18. ClinicalTrials.gov. Phase III study of palbociclib (PD-0332991) in combination with exemestane versus chemotherapy (capecitabine) in hormonal receptor (HR) positive/HER2 negative metastatic breast cancer (MBC) patients with resistance to non-steroidal aromatase inhibitors (PEARL). NCT02028507. Available at: https://clinicaltrials.gov/ct2/show/NCT02028507. Accessed May 22, 2015.
- 19.Tabchny A, Ma CX, Bose R, Ellis MJ. Incorporating genomics into breast cancer clinical trials and care. Clin Cancer Res. 2013;19(23):6371–6379. doi: 10.1158/1078-0432.CCR-13-0837. [DOI] [PubMed] [Google Scholar]
- 20. ClinicalTrials.gov. PD 0332991 and anastrozole for stage 2 or 3 estrogen receptor positive and HER2 negative breast cancer. NCT01723774. Available at: https://clinicaltrials.gov/ct2/show/NCT01723774. Accessed May 22, 2015.
- 21. ClinicalTrials.gov. Akt inhibitor MK-2206 and anastrozole with or without goserelin acetate in treating patients with stage II–III breast cancer. NCT01776008. Available at: https://clinicaltrials.gov/ct2/show/NCT01776008. Accessed May 22, 2015.
- 22. ClinicalTrials.gov. A study of palbociclib in combination with adjuvant endocrine therapy for hormone receptor positive, HER2 negative invasive breast cancer. NCT02040857. Available at: https://clinicaltrials.gov/ct2/show/NCT02040857. Accessed May 22, 2015.
- 23. ClinicalTrials.gov. A phase II randomized study evaluating the biological and clinical effects of the combination of palbociclib with letrozole as neoadjuvant therapy in post-menopausal women with estrogen-receptor positive primary breast cancer (PALLET). NCT02296801. Available at: https://clinicaltrials.gov/ct2/show/NCT02296801. Accessed May 22, 2015.
- 24. ClinicalTrials.gov. A study of palbociclib in addition to standard endocrine treatment in hormone receptor positive HER2 normal patients with residual disease after neoadjuvant chemotherapy and surgery (PENELOPE-B). NCT01864746. Available at: https://clinicaltrials.gov/ct2/show/NCT01864746. Accessed May 22, 2015.
- 25.Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29(15):1956–1962. doi: 10.1200/JCO.2010.31.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ClinicalTrials.gov. A study of abemaciclib (LY2835219) combined with fulvestrant in women with hormone receptor positive HER2 negative breast cancer (MONARCH 2). NCT02107703. Available at: https://clinicaltrials.gov/ct2/show/NCT02107703. Accessed May 22, 2015.
- 27. ClinicalTrials.gov. A study of nonsteroidal aromatase inhibitors plus abemaciclib (LY2835219) in postmenopausal women with breast cancer (MONARCH 3). NCT02246621. Available at: https://clinicaltrials.gov/ct2/show/NCT02246621. Accessed May 22, 2015.
- 28. ClinicalTrials.gov. A study of palbociclib (PD-0332991) + letrozole vs. letrozole for 1st line treatment of postmenopausal women with ER+/HER2− advanced breast cancer (PALOMA-2). NCT01740427. Available at: https://clinicaltrials.gov/ct2/show/NCT01740427. Accessed May 22, 2015.
- 29.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ClinicalTrials.gov. Study of efficacy and safety of LEE011 in postmenopausal women with advanced breast cancer (MONALEESA-2). NCT01958021. Available at: https://clinicaltrials.gov/ct2/show/NCT01958021. Accessed May 22, 2015.
- 31. ClinicalTrials.gov. Study of efficacy and safety in premenopausal women with hormone receptor positive, HER2-negative advanced breast cancer (MONALEESA-7). NCT02278120. Available at: https://clinicaltrials.gov/ct2/show/NCT02278120. Accessed May 22, 2015.
- 32. ClinicalTrials.gov. Study of LEE011 with fulvestrant and BYL719 or BKM120 in advanced breast cancer. NCT02088684. Available at: https://clinicaltrials.gov/ct2/show/NCT02088684. Accessed May 22, 2015.
- 33.Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11(2):317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 34.Fritsch CM, Schnell C, Chatenay-Rivauday C, et al. NVP-BYL719, a novel PI3K-alpha selective inhibitor with all the characteristics required for clinical development as an anti-cancer agent. Presentation at the Annual Meeting of the American Association for Cancer Research; March 31–April 4, 2012; Chicago, Illinois. Abstract published in Cancer Res 2012;72(8 suppl):3748. [Google Scholar]
- 35. ClinicalTrials.gov. A study of palbociclib (PD-0332991) + letrozole vs. letrozole for 1st line treatment of postmenopausal women with ER+/HER2− advanced breast cancer (PALOMA-2). NCT01740427. Available at: https://clinicaltrials.gov/ct2/show/NCT01740427. Accessed May 22, 2015.
- 36.Piccart M, et al. Everolimus plus exemestane for hormone receptor-positive (HR+), human epidermal growth factor receptor-2-negative (HER2−) advanced breast cancer (BC): overall survival results from BOLERO-2. Presentation at the European Breast Cancer Conference; Glasgow, Scotland. March 19, 2014; Abstract LBA.1. [Google Scholar]
- 37.Burris HA, 3rd, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 38.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Accessed July 6, 2015. [Google Scholar]
- 39.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology, Breast Cancer. Version 2.2015. Available at: http://www.nccn.org. Accessed July 5, 2015.
- 40.Weir HK, Thompson TD, Soman A, et al. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121(11):1827–1837. doi: 10.1002/cncr.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]